Summary
In transfusional iron overload, extra-hepatic iron distribution differs, depending on the underlying condition. Relative mechanisms of plasma non-transferrin bound iron (NTBI) generation may account for these differences. Markers of iron metabolism (plasma NTBI, labile iron, hepcidin, transferrin, monocyte SLC40A1 [ferroportin]), erythropoiesis (growth differentiation factor 15, soluble transferrin receptor) and tissue hypoxia (erythropoietin) were compared in patients with Thalassaemia Major (TM), Sickle Cell Disease and Diamond-Blackfan Anaemia (DBA), with matched transfusion histories. The most striking differences between these conditions were relationships of NTBI to erythropoietic markers, leading us to propose three mechanisms of NTBI generation: iron overload (all), ineffective erythropoiesis (predominantly TM) and low transferrin-iron utilization (DBA).
Keywords: iron overload, non-transferrin bound iron, NTBI, hepcidin, erythropoiesis, inflammation
Transfused β-Thalassaemia Major (TM), Sickle Cell Disease (SCD), and Diamond Blackfan Anaemia (DBA) patients are at risk of organ damage from iron overload but have fundamentally different erythropoietic patterns: haemolytic in SCD, dyserythropoietic in TM and hypoplastic in DBA. Although not comprehensively described, these conditions also have different patterns of iron distribution: in SCD, consequences of iron overload appear later and at lower frequencies than in TM (Vichinsky et al, 2005), whereas in DBA, extra-hepatic iron overload, appears early and at high frequency (Berdoukas et al, 2013). We wished to understand whether differences in non-transferrin bound iron (NTBI) generation could account for different patterns of extra-hepatic iron distribution.
Plasma NTBI may be present when transferrin saturation exceeds about 60–70% and has been implicated in determining tissue iron distribution. Although plasma NTBI levels correlate loosely with markers of iron overload, additional factors, such as ineffective erythropoiesis (Wickramasinghe et al, 1999), suspension of erythropoiesis (Bradley et al, 1997) or recent blood transfusion (Hod et al, 2010) are also implicated with NTBI levels. High levels of ineffective erythropoiesis (IE), such as in TM, suppress hepcidin levels (Origa et al, 2007) through bone marrow-derived factors (Kautz et al, 2014), thereby potentially increasing transferrin saturation and NTBI generation. Conversely, inflammation increases hepcidin, potentially lowering NTBI. However, hepcidin interaction with membrane SLC40A1 (ferroportin), the key conduit for cellular iron export (Theurl et al, 2008) may be insufficient to withhold cellular iron, in iron burdened cells and thus limit transferrin saturation and NTBI generation in severe transfusional iron overload. Still, it is unclear how the summation of these factors impacts net NTBI generation in different disease processes. In order to gain insight into the mechanisms of NTBI generation, we have compared plasma NTBI, labile plasma iron (LPI, a redox-active subspecies of NTBI), markers of erythroid activity and erythropoiesis (growth differentiation factor 15 [GDF15], soluble transferrin receptors [sTfR]), hypoxia (plasma erythropoietin [EPO]) and inflammation (high-sensitivity C-reactive protein [hsCRP]) in three contrasting disorders of erythropoiesis. Plasma hepcidin and SLC40A1 mRNA expression in circulating monocytes have also been measured.
Patients and methods
Fifteen chronically transfused patients (5 each of TM, SCD and DBA), iron overloaded with serum ferritin (SF) levels >1500 μg/l or liver iron concentration (LIC) >7 mg/g dry weight (but free of pathogenic inflammation), age >16 years, age 0 to 9 years at initiation of transfusion with 10–20 years of transfusion exposure, were enrolled from three sites in the USA and Europe. There was no significant difference in age, years of transfusion and years of chelation between the groups. All patients were currently prescribed chelation therapy and had been using chelation for a similar number of years. Chelation was withheld for 72 h prior to each sample. Five non-transfused healthy controls were also enrolled. Fasting, early morning blood samples were obtained within three days prior to blood transfusion. LIC was measured by magnetic resonance imaging (R2* and R2) with central analysis of all measures (Wood et al, 2005). Plasma measurements were performed either as per manufacturers enzyme-linked immunosorbent assays: sTfR (Oxford Biosystems, Oxford, UK), Transferrin (Source BioScience plc, Nottingham, UK), EPO, & GDF15 (R&D Systems Ltd, Minneapolis, MN, USA); or as described elsewhere; plasma hepcidin (Bansal et al, 2009), transferrin saturation, ferritin, NTBI and LPI (Walter et al, 2006) (Lal et al, 2013). SLC40A1 mRNA analysis was completed as described (Theurl et al, 2008). All participants provided written informed consent.
Statistical analysis
Differences between medians (±first and third quartile values) were tested using non-parametric analysis of variance (Kruskal-Wallis) with a Dunn’s post-test (because the majority of variables were not normally distributed) and considered significant at P ≤ 0·05. All statistical analyses were performed using Minitab version 17 (State College, PA, USA).
Results
(Table I) NTBI and LPI were at least two-fold greater in DBA and TM patients than controls. Particularly high NTBI levels were found in DBA (previously unreported) even in those with low LIC (3 DBA patients had low LIC and high NTBI). DBA patients also had virtually absent sTfR (three patients had none detected) and very high EPO values, despite similar pre-transfusion Hb compared to SCD and TM (Hb data not shown). SCD had the lowest NTBI levels, transferrin saturation and LPI, as well as lower GDF15 and sTfR than TM, where these were highest, consistent with the greatest expansion of erythropoiesis (IE) in TM as has been reported elsewhere. Hepcidin levels were increased in all three conditions relative to control but were less increased in TM than SCD and DBA, presumably due to IE suppressing hepcidin through factors, such as erythroferrone. However the low NTBI in SCD is unlikely to be accounted for by high plasma hepcidin levels, as these were similar in SCD and TM patients when adjusted for the level of iron overload using the hepcicin/ferritin ratio (Origa et al, 2007). Plasma transferrin was decreased in all patient groups by up to a half that of healthy controls.
Table I.
Median measurements of iron status and erythropoiesis in patients with thalassaemia major (TM), sickle cell disease (SCD), Diamond-Blackfan anaemia (DBA): differences between medians (±first and third quartile values) were tested using non-parametric analysis of variance (ANOVA; Kruskal-Wallis) with a Dunn’s post-test.
| Pre-transfusion Values Median ± 1st & 3rd quartile values |
TM (n = 5) | SCD (n = 5) | DBA (n = 5) | Control (n = 5) | ANOVA P |
|---|---|---|---|---|---|
| NTBI (μmol/l) | 1·68 (1·21, 1·79)a | −0·23 (−1·83, 0·71)b | 2·50 (1·96, 3·17)a | −2·63 (−2·71, −2·63)c | 0·001 |
| Transferrin saturation (%) | 100 (100, 100)a | 49·7 (38·2, 91·4)b | 100 (94·7, 100)a | 36·0 (29·4, 39·6)c | 0·002 |
| LPI (μmol/l) | 1·30 (0·86, 2·10)a | 0·05 (−0·05, 0·79)a,b | 0·86 (0·33, 1·67)a,b | 0·01 (−0·05, 0·10)b | 0·037 |
| LIC (R2*) (mg/g dry weight) | 18·3 (8·3)a | 26·0 (8·0)a | 6·1 (24·2)a | 1·7* | 0·5 |
| Transferrin (g/l) | 1·94 (1·69, 1·94)a | 2·44 (2·10, 3·06)a | 2·67 (2·32, 3·22)a | 4·45 (3·99, 5·8)b | 0·008 |
| Ferritin (μg/l) | 3251 (4680)a,b | 12000 (27168)a | 2150 (13984)a,b | 32 (109)b | 0·003 |
| sTfR (nmol/l)† | 14·9 (6·30, 19·7)a | 8·4 (7·70, 14·3)a,b | 0·00 (0·0, 0·55)* c | 3·2 (2·75, 3·70)b,c | 0·001 |
| GDF15 (pg/ml) | 5504 (2965, 11067)a | 634 (527, 3690)a,b | 467 (332, 3078)b | 279 (272, 307)b | 0·012 |
| Erythropoietin (miu/ml) | 41·0 (11·0, 108·0)b | 28·0 (21·0, 38·0)b | 2004 (1162, 3474)a | 7·0 (5·0, 8·5)b | 0·002 |
| Hepcidin (nmol/l) | 3·97 (21·2)b | 24·3 (28·5)a | 28·7 (36·3)a | 0·81 (2·57)b | 0·002 |
| SLC40A1/RPL27 (mRNA)‡ ×1000) | 41 (32·2, 195)a | 25 (5, 848)a | 75 (18, 165)a | 56 (21, 476)a | 0·820 |
| Hepcidin/Ferritin‡ | 1·7 (0·71, 5·57)a | 1·6 (1·06, 2·9)a | 12·4 (3·2, 66·4)b | 23·2 (15·2, 93·0)b | 0·023 |
| hsCRP (mg/l) | 0·9 (0·25, 3·35)a,b | 3·2 (2·31, 5·25)a | 1·8 (0·3, 9·05)a,b | 0·34 (0·21, 0·75)b | 0·041 |
NTBI, non-transferrin bound iron; LPI, labile plasma iron; LIC, liver iron concentration; sTfR, soluble transferrin receptor; GDF15, growth differentiation factor 15; hsCRP, high-sensitivity C-reactive protein.
The control LIC is a calculated value, determined from Wood et al (2005).
In 3 out of 5 DBA patients, sTfR levels were below detection limit (<0·5 nmol/l).
Monocyte SLC40A1/RPL27 mRNA and Hepcidin/Ferritin are reported at ×1000.
Within a row, groups not sharing the same letter are significantly different from each other (5% procedure wise error rate).
Discussion
These findings relate NTBI to erythropoietic markers across three distinct underlying mechanisms of anaemia: DBA, TM and SCD. The findings in DBA are of particular interest, suggesting that hypoplasia of late erythroid precursors (using sTfR as a marker) is a key factor in NTBI genesis, due to lack of utilization of transferrin iron by the erythron. This mechanism has previously been implicated in NTBI generation following myeloablative chemotherapy (Bradley et al, 1997). The high NTBI in DBA cannot be explained by IE, as markers for this were no higher than in SCD (GDF15) or were virtually absent (sTfR); consistent with erythron maturation arrest in DBA. The high EPO in DBA has been previously reported (Horvathova et al, 2012), but not with concomitant measurement of hepcidin and sTfR. The high EPO with concomitant high hepcidin (and hepcidin/ferritin ratio) in DBA are consistent with a lack of direct suppression of hepcidin by EPO and the relative absence of IE in the erythron, in contrast to TM. The very high EPO levels in DBA, despite similar Hb values as SCD patients, are consistent with decreased EPO clearance by the erythron. Equally, the high NTBI in the context of low sTfR in DBA is consistent with low clearance of transferrin-bound iron due to low tissue TfR expression in the erythron. It would be of interest to determine whether aplastic anaemia patients show the same high NTBI in relation to low sTfR as in DBA. The decreased transferrin levels in all disease groups, parallels findings in hereditary haemochromatosis (Macedo et al, 2005). This may exacerbate NTBI levels where a primary mechanism for NTBI generation already exists.
In SCD, the low NTBI and LPI, both relative to the degree of iron overload and to TM patients, are consistent with previous findings (Walter et al, 2006). It is likely that the lower IE in SCD than TM, together with lower bone marrow expansion (Table I) allows greater efficiency of transferrin iron utilization and hence lower transferrin saturation. Increased hepcidin synthesis is an attractive potential mechanism for low NTBI in SCD, (Porter & Garbowski, 2013), which could result from either a greater inflammatory state in SCD (Walter et al, 2006) or from less hepcidin suppression because of less erythron expansion than in TM. However, our findings do not support this as a key mechanism differentiating NTBI generation in SCD from TM because the hepcidin/ferritin ratio is similar in the two conditions. The hepcidin/ferritin ratio may not be the most sensitive way of adjusting hepcidin for iron overload because ferritin can be modulated by other factors.
The limited role of hepcidin in preventing iron entry into plasma is further supported by the findings in DBA, where high hepcidin/ferritin ratios are seen (Table I) with high NTBI values. Thus in the context of iron overload and low utilization of transferrin iron, hepcidin is not sufficiently upregulated to limit NTBI generation in DBA. While several inflammatory markers are greater in SCD than TM (Walter et al, 2006), in this study the increase in the CRP was not significantly different between SCD and TM (Table I). Monocyte SLC40A1 mRNA was measured here as an estimate of cellular iron export capacity because SLC40A1 mRNA expression was shown to correlate well with its protein expression in human samples of iron deficiency and inflammatory anaemia (Theurl et al, 2008). If the relatively low monocyte SLC40A1 mRNA found here in SCD were also accompanied by low membrane SLC40A1 in the macrophage system, this would provide a mechanism for lowering NTBI in SCD and requires further exploration. Additional factors, such as high levels of intravascular haemolysis with induction of tissue haemoxygenase (HO1), may also modulate distribution and tissue retention of iron in SCD (Porter & Garbowski, 2013). Additional factors may, in principle, influence NTBI uptake by tissues, such as advanced liver disease decreasing the hepatic capacity to clear NTBI, or heterogeneity of NTBI species in different underlying disease states.
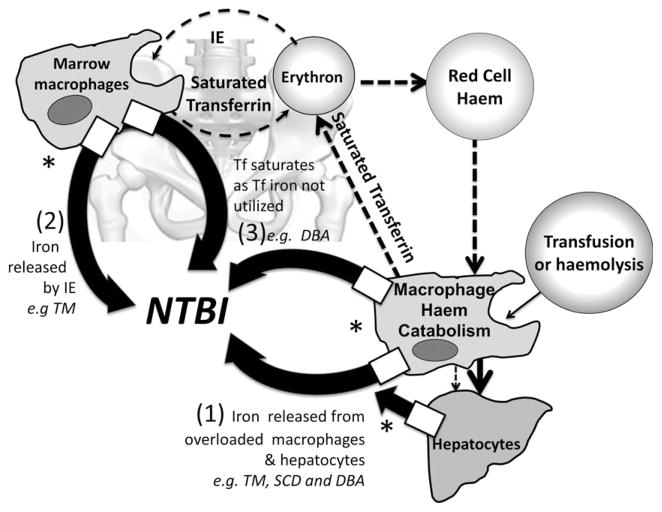
This pilot study was intended to generate hypotheses about factors responsible for variability of NTBI and extra-hepatic iron distribution in transfusional iron overload. On the basis of these findings, we propose three mechanisms by which NTBI is generated but only one of these (Mechanism 1) relates directly to iron overload and is shared by TM, DBA and transfusionally overloaded SCD patients (Fig 1). The remaining mechanisms for NTBI generation are: Mechanism 2) massive iron release from destruction of haemoglobinized cells in the bone marrow due to IE, which may also suppress hepcidin thereby further increasing NTBI (mainly TM), and Mechanism 3) low utilization of transferrin iron (mainly DBA). Other patient groups with low transferrin iron utilization may also be susceptible to this mechanism of NTBI generation, exposing them to the risk of extra-hepatic iron distribution, as with DBA. The effect of transfusion policy on suppression of erythropoiesis in TM also requires further systematic investigation as excessive hyper-transfusion regimes may inhibit erythropoiesis to an extent where further NTBI is generated by reduced clearance of transferrin iron, as in DBA.
Fig 1.
Physiological iron turnover is shown by dashed arrows, bold lines represent pathways of NTBI generation. (1) Iron overload as a mechanism for NTBI generation (shared by TM, SCD and DBA): For iron derived from blood transfusions, excess iron accumulates first in the macrophage system and then in splenic and hepatic macrophages and/or in hepatocytes. *Iron release from these cells requires membrane SLC40A1 (ferroportin) (boxes showing iron release (thick arrows) from macrophages and hepatocytes) but upregulation of hepcidin synthesis by iron overload is insufficient to abrogate cellular iron egress and thus NTBI is formed when the cellular iron release exceeds the binding capacity of transferrin. (2) In IE, massive intra-marrow catabolism of red cells: IE results in intramarrow destruction of up to 60–80% of erythroid precursors with iron release exceeding the rate at which iron can bind to transferrin and hence NTBI is generated. This mechanism can occur in the absence of iron overload in dyserythropietic conditions (Wickramasinghe et al, 1999) (3) Low transferrin iron utilization: when erythropoiesis is suspended (as in DBA patients), iron utilization by the erythron from transferrin is decreased but iron continues to be released by red cell catabolism, causing transferrin saturation and NTBI formation. TM, thalassaemia major; SCD, sickle cell disease; DBA, Diamond-Blackfan anaemia, IE, ineffective erythropoiesis; NTBI, non-transferrin bound iron; LPI, labile plasma iron; LIC, liver iron concentration; sTfR, soluble transferrin receptor; GDF15, growth differentiation factor 15; hsCRP, high-sensitivity C-reactive protein; Tf, transferrin.
Acknowledgments
This work was funded by the NIH NIDDK grant 2R01DK057778-06A1 and also supported in part by NIH CTSA grant UL1 RR024131. MG was supported by BJH Research Fellowship Grant May 2009. JBP is supported by NIHR University College London Hospitals Biomedical Research Centre.
Footnotes
Authorship information
JBP recruited and administered procedures to patients, designed the research, analysed the data and wrote the paper; PBW designed and performed the research, analysed the data and wrote the paper; LDN recruited and administered procedures to patients, designed the research, analysed the data and wrote the paper; PE designed and performed the research and wrote the paper; SB designed the research, analysed data and wrote the paper; MG analysed the data and reviewed the paper, MGW designed the research, performed the research, analysed data and wrote the paper; PRH designed the research, recruited patients and wrote the paper; JCW designed the research, analysed data and wrote the paper; JLM analysed data and wrote the paper; CB performed the research and analysed the data; GW designed the research, analysed the data and wrote the paper; MS performed the research and analysed the data; RG designed the research, recruited and administered procedures to patients, and wrote the paper; RF designed the research, analysed data and wrote the paper; PN designed the research, recruited and administered procedures to patients, analysed the data and wrote the paper, CN designed the research, recruited and administered procedures to patients and wrote the paper, EV designed the research, recruited and administered procedures to patients and wrote the paper. All authors gave final approval for the manuscript to be published.
Disclosures
JBP, PBW, and EV have received research funding from Novartis Pharmaceuticals Corp. PH has received an educational grant and research support from Novartis Pharmaceuticals Corp.
References
- Bansal SS, Halket JM, Fusova J, Bomford A, Simpson RJ, Vasavda N, Thein SL, Hider RC. Quantification of hepcidin using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry: RCM. 2009;23:1531–1542. doi: 10.1002/rcm.4033. [DOI] [PubMed] [Google Scholar]
- Berdoukas V, Nord A, Carson S, Puliyel M, Hofstra T, Wood J, Coates TD. Tissue iron evaluation in chronically transfused children shows significant levels of iron loading at a very young age. American journal of hematology. 2013;88:E283–E285. doi: 10.1002/ajh.23543. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Gosriwitana I, Srichairatanakool S, Hider RC, Porter JB. Non-transferrin-bound iron induced by myeloablative chemotherapy [see comments] British journal of haematology. 1997;99:337–343. doi: 10.1046/j.1365-2141.1997.4143221.x. [DOI] [PubMed] [Google Scholar]
- Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvathova M, Kapralova K, Zidova Z, Dolezal D, Pospisilova D, Divoky V. Erythropoietin-driven signaling ameliorates the survival defect of DMT1-mutant erythroid progenitors and erythroblasts. Haematologica. 2012;97:1480–1488. doi: 10.3324/haematol.2011.059550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nature genetics. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Porter J, Sweeters N, Ng V, Evans P, Neumayr L, Kurio G, Harmatz P, Vichinsky E. Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells, Molecules & Diseases. 2013;50:99–104. doi: 10.1016/j.bcmd.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo MF, Cruz E, Lacerda R, Porto G, de Sousa M. Low serum transferrin levels in HFE C282Y homozygous subjects are associated with low CD8 (+) T lymphocyte numbers. Blood cells, molecules & diseases. 2005;35:319–325. doi: 10.1016/j.bcmd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology Education Program. 2013;2013:447–456. doi: 10.1182/asheducation-2013.1.447. [DOI] [PubMed] [Google Scholar]
- Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H, Weiss G. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392–2399. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. American journal of hematology. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. British journal of haematology. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe SN, Thein SL, Srichairatanakool S, Porter JB. Determinants of iron status and bilirubin levels in congenital dyserythropoietic anaemia type I. British journal of haematology. 1999;107:522–525. doi: 10.1046/j.1365-2141.1999.01745.x. [DOI] [PubMed] [Google Scholar]
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]