Abstract
Medial temporal lobe brain structures, such as the amygdala, play an important role in the normal perception and generation of emotional behavior. Little research, however, has assessed the role of such structures across the neurodevelopmental trajectory. We assessed emotional behavioral responses of rhesus macaques that received bilateral ibotenic acid lesions of the amygdala or hippocampus at two weeks of age and sham-operated controls. At 9 and 18 months of age, animals interacted with novel objects that varied in visual complexity as a means of varying emotional salience. All animals behaved differently in the presence of visually simple, as compared to complex, objects, suggesting that they were sensitive to variation in emotional salience. Across both experiments, amygdala-lesioned animals appeared to be less behaviorally inhibited insofar as they explored all objects most readily. Interestingly, hippocampus-lesioned animals’ propensity for exploration mirrored that of control animals in some contexts but amygdala-lesioned animals in other contexts. At 18 months of age, both amygdala-lesioned and hippocampus-lesioned animals were judged to be less fearful than controls during the testing procedure. Implications for understanding the neurobiology of emotional behavior are discussed.
Keywords: emotional behavior, novelty, nonhuman primate, neurodevelopment, Macaca mulatta, Rhesus macaque, amygdala, hippocampus
There is a long history of studying the role of medial temporal lobe brain structures in emotional processing (e.g., Klüver & Bucy, 1939; Weiskrantz, 1956, etc.). Particular attention has been paid to the function of the amygdala in the perception and generation of appropriate emotional behavior. For example, nonhuman primate research has demonstrated that the amygdala plays a critical role in generating appropriate social responses during interactions with conspecifics (e.g., Kling & Brothers, 1992; Emery et al., 2001; Machado & Bachevalier, 2006) and modulating appropriate behavioral inhibition in the presence of novel and emotionally provocative objects (e.g., Machado et al., 2009; Mason et al., 2006; Stefanaci, Clark & Zola, 2003; Aggleton & Passingham, 1981; Zola-Morgan et al., 1991). Amygdala lesion experiments are typically conducted with adult animals. As such, there is a paucity of work investigating how amygdala damage influences behavior across animals’ early neurodevelopmental trajectory. Evaluating the development of normal emotional responding is germane to understanding the etiology of, and developing appropriate treatment for, neurodevelopmental and psychiatric disorders in which normal emotional responding is disrupted, such as autism and anxiety.
Much is known about how damage to the amygdala in adult animals influences emotional processing (for reviews Phelps, 2006; LeDoux, 2000). Monkeys that receive amygdala lesions as adults consistently demonstrate atypical emotional responses in the presence of provocative objects. These atypical responses are often characterized by increased propensity to approach and examine objects (Mason et al., 2006; Stefanaci, Clark & Zola, 2003; Aggleton & Passingham, 1981; Zola-Morgan et al., 1991). Animals with damage to the amygdala also consistently show fewer emotional behaviors related to fear processing (Kalin et al., 2001; Aggleton & Passingham, 1981; Machado, Kazama, & Bachevalier, 2009) and increased “tameness” (Zola-Morgan et al., 1991). Amygdala-lesioned animals, furthermore, do not modulate their behavioral responses based on the variation in the emotional salience of object stimuli (Mason et al., 2006). Animal-like objects used by Mason et al. (2006) were selected to differ in visual complexity to vary the extent to which they were emotionally provocative. While control subjects’ latency to take the food from in front of the objects increased with the level of object salience, that amygdala-lesioned subjects’ latency did not increase.
Although the hippocampus has been widely implicated in declarative memory processes (for a review see Squire, Stark, & Clark, 2004), little is known about its role in emotional processing and, in particular, its role in generating responses to emotionally salient objects. Zola et al. (1991), demonstrated that hippocampus-lesioned macaques were comparable to control subjects in their willingness to approach and examine provocative objects. Like control subjects, hippocampus-lesioned subjects were more aggressive and fearful of the objects than amygdala-lesioned animals (Zola-Morgan, et al., 1991). In contrast, recent evidence indicates that hippocampus-lesioned monkeys are less behaviorally inhibited than controls in the presence of emotionally provocative stimuli insofar as they spent more time in proximity to the objects, retrieved a food item placed near the object more quickly and displayed less defensive and avoidance behavior (Chudasama, Wright, & Murray, 2008). Clearly, further investigation of the role of the hippocampus in normative responding to objects of emotional significance is warranted.
Even less experimental work has investigated the role of the amygdala or hippocampus in the development of normal emotional responding. In one study, nine month old infant macaques with neonatal lesions to a large portion of the medial temporal lobe (including amygdala, hippocampus, and surrounding cortex), compared to age-matched control subjects, were less active and more withdrawn but otherwise showed similar patterns of behavior (e.g., decreased touching of novel objects) in the presence of novel and familiar objects (Meunier, Nalwa & Bachevalier, 2003). Preliminary research from our laboratory demonstrated that three rhesus macaque infants (6-8 months of age) that received bilateral amygdala lesions at two weeks of age spent more time exploring novel objects and were faster to retrieve food in the presence of a emotionally evocative stimuli (e.g., a rubber snake) as compared to three unoperated control animals (Prather et al., 2001). These findings essentially replicate findings in adult animals suggesting that abnormal emotional processing resulting from amygdala damage may arise at any point during development. To further and more thoroughly investigate this claim, the present experiments sought to confirm and extend the results of Prather et al. (2001) using a larger group of amygdala-lesioned animals as well as a second group of animals that received bilateral lesions to the hippocampus.
We tested affective responsivity in the same group of subjects at two time points— 9 months of age (8.5 months post lesion) and 18 months of age (17.5 months post lesion)—to investigate whether abnormal responsivity persists over time and varies based on the emotional salience of objects. Previous research with young neurologically intact macaques who varied in age (ranging from 20 days to 27 ½ months) demonstrated that while the mean number of emotion-related behaviors generated in the presence of animal-like objects was consistent across age groups, older animals (6 ½ to 27 ½ months), as compared with younger animals (20 to 105 days) generated more behaviors directed at the objects (i.e., facial expressions and vocalizations) (Bernstein & Mason, 1962). These finding suggest that while the general magnitude of responsivity may be consistent across age, the specific pattern of behaviors generated may change with development. Interestingly, in this same study, animals from all age groups were more responsive to complex as compared to simple objects (Bernstein & Mason, 1962) indicating that the ability to differentiate between objects based on their visual properties is present early in development.
In the present study, we hypothesized that animals with early damage to the amygdala, as compared to neurologically intact controls, would be less avoidant and more interactive with novel and emotionally salient objects during both the 9- and 18-month time points. Although we anticipated that the hippocampus and control animals would both respond to novel objects and differentiated between levels of complexity, we had no specific expectations regarding differences between these two groups. Based on the previous literature in adult animals, we suspected that hippocampal lesioned animals would respond in a manner quite similar to control animals.
Animals for Experiments 1 and 2
All experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center (CNPRC). All protocols were approved by the UC Davis Institutional Animal Care and Use Committee.
Animals and Living Conditions
Twenty-four infant rhesus monkeys (Macaca mulatta) were randomly assigned to one of three lesion conditions: bilateral amygdala lesions (five females, three males), bilateral hippocampus lesions (five females, three males) or sham-operated controls (four females, four males). All surgeries were performed at 12-16 days after birth. The animals were returned to their mothers following surgery and housed in standard home cages (61 cm W × 66 cm D × 81 cm H). Following a brief recovery period, each mother-infant pair was assigned to a socialization group consisting of six mother-infant pairs and one adult male. Socialization groups met for a minimum of three hours per day, five days per week in a large group cage. Each socialization group included two subjects from each lesion condition. The age range across the group was no more than two months. When the youngest member of a socialization group reached six months of age, the animals were weaned and separated from their mothers. Weaning of each socialization group happened on a single occasion according to standard protocols at the CNPRC. Briefly, mothers were sedated and removed from the home cages. Infants continued to live in the same cage in which they had lived with their mothers. Immediately following weaning, infants participated in an experiment to investigate their attachment to their mothers (Bauman, et al., 2004a). Infants continued to participate in group socialization on the same schedule as before separation from their mothers. The same adult male remained in each group and a new adult female was added to each group to provide continued exemplars of normal adult female social behavior. Experiment 1 occurred at approximately 9 months of age. At 1 year of age, each rearing cohort became permanently socially housed (24 hours per day, seven days per week) with their original socialization cohort in an indoor chain link enclosure (2.13m W × 3.35m D × 2.44m H). Experiment 2 occurred while infants were living 24-hours per day in their socialization groups.
One male amygdala-lesioned animal was humanely euthanized at approximately one year of age because of his quickly and severely deteriorating health related to a congenital heart defect. He was subsequently replaced with an alternative amygdala-lesioned male. The substitute subject was the same age as subjects in the test group, received an amygdala-lesion at two weeks of age, and was reared with his mother only for the first year of life. At 1 year of age, the animal was weaned and pair housed with an age-matched female until being introduced to his socialization cohort at approximately 1 year and 3 months of age. The original subject participated in Experiment 1 and the replacement subject participated in Experiment 2.
Surgical Procedures
The surgical procedures summarized below are detailed in previous publications (Bauman et al., 2004a, 2004b). On the day of surgery, each infant was anesthetized with ketamine hydrochloride (15 mg/kg i.m.) and medatomidine (30μg/kg), and then placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). The infant's brain was imaged using a General Electric 1.5 T Gyroscan magnet, 1.0 mm thick coronal sections were taken using a T1-weighted Inversion Recovery Pulse sequence (TR = 21, TE =7.9, NEX 3, FOV = 8cm, Matrix, 256 × 256). From these images, we determined the location of the amygdala or hippocampus and calculated the coordinates for the ibotenic acid injections. Infants were ventilated and vital signs monitored throughout the surgery. A stable level of anesthesia was maintained using a combination of isoflurane (1.0% - varied as needed to maintain an adequate level of anesthesia) and intravenous infusion of fentanyl (7-10 μg/kg/hour). Following a midline incision, the skin was laterally displaced to expose the skull, two craniotomies were made over the amygdala or the hippocampus, depending on the pre-determined lesion condition, and the dura was reflected to expose the surface of the brain. Ibotenic acid (IBO, Biosearch Technologies Inc., 10 mg/ml in 0.1 M phosphate buffered saline) was injected simultaneously bilaterally into the amygdala or hippocampus using 10 μl Hamilton syringes (26 gauge beveled needles) at a rate of 0.2 μl/min. Sham-operated controls underwent the same pre-surgical preparations, received a midline incision and the skull was exposed. The control animals were maintained under anesthesia for the average duration of the lesion surgeries and the fascia and skin were sutured in two separate layers. Following the surgical procedure, all infants were monitored by a veterinarian and returned to their mothers once they were fully alert.
Lesion Analysis
Although the animals are continuing behavioral testing and have therefore not been euthanized, T2-weighted MR images acquired ten days after surgery were used to examine the extent of the edema associated with the lesion. Hyper-intense T2-weighted images allow for the visualization of edema caused by ibotenic acid induced cell death. The hyper-intense T2-weighted signal for each of the sixteen lesion animals (eight amygdala lesion, eight hippocampus lesion) was evaluated to confirm the general target and extent of the lesions (i.e., amygdala lesion sparing the hippocampus or hippocampus lesion sparing the amygdala). Imaging occurred using a General Electric 1.5 T Gyroscan magnet; 1.5 mm thick sections were taken using a T2 weighted Inversion Recovery Pulse sequence (TR = 4000, TE = 102, NEX 3, FOV = 8cm, Matrix, 256 × 256). T2-weighted images of coronal sections through the mid portion of the amygdala are illustrated in previous publications (Lavenex, Banta Lavenex, & Amaral, 2007; Bauman et al., 2004a, 2004b), providing substantial reassurance that the ibotenic acid was injected and was focused in the amygdaloid complex or hippocampal formation. Lesion extent was further characterized in T1-weight MRI images when animals were four years of age (Machado, Snyder, Cherry, et al., 2008). The mean percentage of tissue loss for amygdala-lesioned animals was 71.9% (minimum 67.0%, maximum 80.7%) and for hippocampus-lesioned animals was 76.6% (minimum 66.0%, maximum 86.8%) (Machado et al., 2008).
The extent of the targeted lesion was confirmed in one amygdala-lesioned animal that died due to an unrelated illness and was then used for histological evaluation of lesion (Bauman et al., 2004a, Figure 3; Bauman et al., 2004b, Figure 2).
Experiment 1: Object Responsiveness Testing at 9-Months of Age
Method
Animals were tested in their home cages (61 cm W × 66 cm D × 81 cm H) once they turned 9 months old. During the testing, animals were in auditory contact with other experimental animals but had no visual access to them. In the week prior to testing, they were acclimated to the testing procedure by placing a small gray plastic cup containing banana slices in their cage twice a day for five consecutive days. All subjects retrieved the banana during this acclimation phase.
The general procedure was based on a previous experiment conducted in our laboratory (Prather, et al., 2001). Testing for responsiveness to objects was completed on four consecutive days. Each test day included two types of trials and there were five trials total per day. The first trial was intended to assess animals’ propensity to explore completely novel nonbiological objects. Objects were selected that could be easily manipulated, but that were unlike any objects the animals had experienced before, and did not have biological features (i.e., without eyes and/or mouths). The first trial consisted of a 60-second presentation of a novel nonbiological object suspended from a small chain. Animals remained free (unconstrained) during the placement of the objects. Objects were placed in each animal's cage by an experimenter who was familiar to the animals. On each novel object trial, the experimenter reached into the cage and attached the chain to the top of the cage so that it was equidistant from each side of the cage. The object was therefore suspended in the center of the cage, approximately 6-inches from the cage floor. A different object was presented each day in the same order for all animals: toy plastic keys, a plastic golf ball, a toy for birds, and a luggage tag. Examples of objects are illustrated in Figure 1.
Figure 1.
Objects used for Experiments 1 and 2. Note: Examples of novel objects used in Experiment 1 (a) and Experiment 2 (b). Example of simple (c) and complex (d) animal-like objects used in Experiments 1 and 2. Examples of objects presented either stationary or in motion. For the motion condition, the “Barbie Disco Ball” (e) spun while flashing lights, while the truck (f) drive across the object platform.
The next four trials of the daily test session were intended to compare responses to emotionally salient animal-like objects paired with food and to food alone. The objects resembled animals insofar as they included visible eyes and/or mouths. Similar objects have been shown to generated behavioral responses in macaques in previous experiments (e.g., Mason et al. 2006; Bernstein & Mason, 1962). On each 30-second trial, a slice of banana was placed in the familiar food cup located on the floor at the front and center of the cage. On trials 1 and 4 (food only) no other object was present. On trials 2 and 3 (food + object), an animal-like object attached to a small wooden base was placed immediately behind the food cup so that the base of the object touched the front center of the cage. A different object was used on each test day. Each object had a simple form and a complex form. Simple objects were the same size and shape as complex objects, but lacked specific details (see Figure 1 for examples of objects). The complex objects—a Dalmatian dog puppet, a Sylvester-the-Cat doll, a Mr. Potato Head, and a rubber snake with realistic features—were presented to all animals in the order given, with simple objects always presented before complex.
For both types of trials, a trial began when an experimenter placed the object and/or cup with banana in the appropriate location in the cage. Objects were shielded from the animals’ view until being placed in the cage so that neither the test animal nor any other animal in the room could see the object. A second experimenter, seated approximately 2 meters from the cage, recorded latency to contact the object and/or take the food, as well as the frequency and duration of exploration. Exploration was defined as any physical contact with or manipulation of the object. Other behaviors that were recorded are listed in the included ethogram (Supplemental Materials). Behaviors were recorded using the Observer software (Noldus, Sterling, VA). Both experimenters avoided staring at the subject during the trial.
Data Analysis Strategy
Means for latency to first response and frequency and duration of exploration were computed for each trial type. Data were log10(x+1) transformed in cases where they were not normally distributed as indicated in the Results Section. For the purposes of interpretation, raw data (means and variance indices) are presented in the text and tables; log transformed data are available upon request. On trials during which animals did not take the food or explore the objects, missing latency data was replaced with the length of the trial (30 seconds). Data were subjected to traditional ANOVA. Lesion condition was the between-subjects factor for all analyses. For data from the 30-second trials during which food was presented with or without an object, object complexity was the repeated measure. In the analyses of food retrieval there were three levels of the repeated measure (no object, simple objects, complex objects); in the analyses of object exploration there were two levels of the repeated measure (simple objects v. complex objects). In order to document the specific pattern of effects between lesion groups, we ran a series of non-parametric mean comparisons using Mann-Whitney tests. We elected to use non-parametric post-hoc tests because such tests are particularly well suited to compare means when data are not normally distributed and when sample sizes are small. Because our a priori hypothesis was that amygdala-lesioned animals’ affective responses to objects would differ significantly from hippocampus-lesioned and control animals (i.e., pair-wise group differences) we completed the Mann-Whitney comparisons to evaluate group differences even in cases where the p-values associated with the overall ANOVA did not reach conventional levels of significance.
Results
60-second Novel Nonbiological Object Trials
As predicted, amygdala-lesioned monkeys were faster to first explore and explored novel nonbiological objects more frequently and for longer than either hippocampus-lesioned or control monkeys. Hippocampus-lesioned and control animals’ frequency, duration, and latency to first explore did not differ. See Table 1.
Table 1.
Exploration of Novel Objects
| C | A | H | Lesion Effect | Mann-Whitney | |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Frequency (rate)† | .20 ± .13 | .77 ± .33 | .06 ± .13 | F(2, 24)=3.59, p<.05, ηp2=.255 | A>C: p<.042 A>H: p<.013 H v. C: n.s. |
| Duration (seconds)† | .33 ± .22 | 2.22 ± 1.21 | .09 ± .06 | F(2, 24)=5.08, p<.02, ηp2=.326 | A>C: p<.050 A>H: p<.006 H v. C: n.s. |
| Latency (seconds) | 56.99 ± 2.11 | 48.70 ± 4.05 | 58.13 ± 1.27 | F(2, 24)=3.29, p<.06, ηp2=.238 | A<C: p<.050 A<H: p<.028 H v. C: n.s. |
| Experiment 2 | |||||
| Frequency (rate)† | .13 ± .07 | .71 ± .43 | .44 ± .27 | F(2, 23)=1.95, p<.17, ηp2=.163 | A>C: p<.058 A v. H: n.s. H v. C: n.s. |
| Duration (seconds)† | .14 ± .08 | 1.90 ± .87 | 1.64 ± 1.28 | F(2, 23)=2.44, p<.11, ηp2=.196 | A>C: p<.014 A v. H: n.s. H v. C: n.s. |
| Latency (seconds) | 58.00 ± 1.32 | 47.42 ± 4.38 | 50.39 ± 4.09 | F(2, 23)=2.47, p<.11, ηp2=.198 | A<C: p<.020 A v. H: n.s. H v. C: n.s. |
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
data used in ANOVA was log transformed.
30-second Animal-Like Object and/or Food Reward Trials
Retrieval of food reward
Food retrieval behavior did not differ between lesion groups. All animals retrieved the food most frequently and fastest when no object was present and least frequently and slowest when a complex object was present (with simple objects intermediate) (see Table 2).
Table 2.
Experiment 1—Food Retrieval Behavior in the Presence of Animal-Like Objects
| Frequency(rate) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Object Type | No Object | .74 ± .17 | .89 ± .05 | .86 ± .07 | F(2, 42)=11.63, p<.0001, ηp2=.356 |
| Simple Object | .59 ± .18 | .72 ± .14 | .75 ± .14 | ||
| Complex Object | .53 ± .16 | .56 ± .13 | .75 ±. 16 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 21)=.42, p<.66, ηp2=.038 | F(4, 42)=1.04, p<.40, ηp2=.090 | |||
| A v. C, n.s.; H v. C, n.s; A v. H, n.s. | |||||
| Latency (seconds) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Object Type | No Object | 19.15 ± 4.48 | 6.99 ± 2.26 | 10.15 ± 2.54 | F(2, 42)=23.96, p<.0001, ηp2=.533 |
| Simple Object | 13.69 ± 5.01 | 12.02 ± 3.64 | 13.69 ± 3.92 | ||
| Complex Object | 15.86 ± 4.29 | 15.95 ± 3.25 | 15.86 ± 3.41 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 21)=.20, p<.82, ηp2=.019 | F(4, 42)=.39, p<.82, ηp2=.036 | |||
| A v. C, n.s.; H v. C, n.s; A v. H, n.s. | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
Object exploration
As with the novel objects, amygdala-lesioned monkeys explored objects most readily in terms of frequency, duration and latency. Strikingly, control animals did not explore objects at all and hippocampus-lesioned animals only explored simple objects. There was a trend level difference between hippocampus-lesioned and control animals driven by hippocampus-lesioned animals very minimal exploration of simple objects (see Table 3).
Table 3.
Experiment 1— Exploration of Animal-Like Objects
| Frequency (rate) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Simple Object | 0 ± 0 | .16 ± .09 | .13 ± .07 | F(1, 21)=.00003, p<.99, ηp2=.000 |
| Complex Object | 0 ± 0 | .32 ± .15 | 0 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 21)=3.32, p<.06, ηp2=.240 | F(2, 21)=4.21, p<.03, ηp2=.286 | |||
| A>C, p<.011; H>C, p<.064; A v. H, n.s. | |||||
| Duration (seconds) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Object Type | Simple Object | 0 ± 0 | 1.23 ± .73 | .27 ± .17 | F(1, 21)=.082, p<.78, ηp2=.004 |
| Complex Object | 0 ± 0 | 1.39 ± .67 | 0 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 21)=5.58, p<.01, ηp2=.347 | F(2, 21)=.43, p<.65, ηp2=.040 | |||
| A>C, p<.011; H>C, p<.064; A v. H, n.s. | |||||
| Latency(seconds) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Object Type | Simple Object | 30 ± 0 | 27.36 ± 1.48 | 28.69 ± .85 | F(1, 21)=.27, p<.61, ηp2=.013 |
| Complex Object | 30 ± 0 | 25.13 ± 2.21 | 30 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 21)=3.78, p<.04, ηp2=.26 | F(2, 21)=3.11, p<.07, ηp2=.229 | |||
| A>C, p<.011; H>C, p<.064; A v .H, n.s. | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
data used in ANOVA was log transformed.
Experiment 1: Summary of Findings
The results of Experiment 1 demonstrate marked behavioral differences between amygdala-lesioned subjects as compared to hippocampus-lesioned and control subjects. In the presence of both completely novel objects and animal-like objects, amygdala-lesioned subjects were faster to first explore objects, explored objects more frequently and for longer.
All animals differentiated between simple and complex animal-like objects as indicated by how frequently and how quickly they took food in the presence of objects. All animals took food less frequently and were slower to take food when objects were complex as compared to simple. This suggests that the increase of exploration exhibited by the amygdala-lesioned was not driven by an inability to differentiate between simple and complex objects. Despite the differentiation between simple and complex objects in the take-food measure, we did not see a robust effect of stimulus complexity for object exploration.
Observed differences between control and hippocampus-lesioned animals’ propensity to explore were driven by low rates and durations of exploration of simple objects. Importantly, while hippocampus-lesioned animals did explore objects, the durations of those explorations was short. In the presence of complex objects, control and hippocampus-lesioned animals behaved identically—both groups showed behavioral inhibition and did not manipulate the objects at all. This suggests that the hippocampus is not as critical for regulating appropriate (species typical) behavioral responsiveness to objects.
Experiment 2: Object Responsiveness Testing at 18-Months of Age
The goal of Experiment 2 was to confirm the findings from Experiment 1 at a later point in our subjects’ neurological development. To that end, animals completed a similar experiment in which they were presented with novel objects and animal-like objects that were presented in either simple or complex form. Additionally, we sought to test a hypothesis that amygdala-lesioned animals might be particularly sensitive to object motion. In data collected from social interactions (Bauman et al., 2004b), amygdala-lesioned subjects showed increased social fear in the presence of conspecifics. One possible explanation for this finding is that amygdala-lesioned subjects are particularly sensitive to the movements of other animals. To address this question, we included both stationary and moving objects as stimuli in this experiment.
Method
At the time of testing, subjects were approximately 18 months of age (age range 16.8-22.8 months, no differences in age between lesion groups). Testing occurred in a specialized testing cage that was separated from the animals’ home cages with a large metal divider. As in Experiment 1, animals completing the testing were in auditory contact with other experimental animals but had no visual access to the other experimental animals. Other animals in the room could not see the test cage nor the objects being presented. The testing cage was an adapted lab care cage (83.32 cm D × 101.6 cm H × 80.01 cm W) with a clear plastic front window (80.01 cm D × 98.3 cm H × 1.016 cm W) which had two vertical openings (25 cm H × 5 cm W) separated by 5.08 cm and centered 32.25 cm from the left and right sides of the cage. An opaque guillotine door (80.01 cm D × 98.3 cm H × 1.016 cm W) in front of the clear window could be controlled by a rope-and-pulley system. In front of the cage there was a platform containing a central food well (2.54 cm D × 2.54 cm W) situated 5.18 cm from the front of the cage and a frame directly behind the food well (22.86cm D × 15.24cm W) for securing the bases attached to the test objects.
Five days before the experiment began, all animals were acclimated to the test cage. On each day, each animal was placed in the test cage and presented with a single food reward (mini-marshmallow) on ten 30-second trials. Each trial began when the opaque guillotine door was lifted and ended when the opaque door was closed 30 seconds later. Criterion to move to the test phase was retrieval of the food item eight out of ten times on two consecutive days.
Testing for responsiveness to objects was similar to that used in Experiment 1. As in Experiment 1, testing occurred on four consecutive test days, each including two types of trials. In contrast to Experiment 1, each test day of Experiment 2, however, included 7 trials. Trial 1 was identical to Trial 1 in Experiment 1—a novel nonbiological object was suspended from the center of the cage by a chain for 60-seconds in order to assess animals’ propensity to explore completely novel nonbiological (i.e., without biological features like eyes and mouths) objects. A different object was presented each day in the same order for all animals: a Koosh ball, a dog chew toy, women's hair accessories, and a wheel. As in Experiment 1, the objects were easily manipulatable, completely novel to the animals, and lacking any biological features. Examples of objects can be found in Figure 1.
The next six trials of the session were intended to compare responses to emotionally salient objects (i.e., animal-like objects that were potentially fear inducing or objects presented either stationary or in motion) paired with food and to food alone. On each 30-second trial, a food reward (mini marshmallow) was placed in the food well on the object platform. On trials 1 and 6 (food only) no other object was present. On trials 2 through 5 (food + object), an object from one of the two object series was presented: animal-like objects or stationary or mobile objects. As in Experiment 1, animal-like objects were presented either in simple or complex form; simple objects were the same size and shape as complex objects, but lacked details. Objects in the animal-like series were selected from the objects used in Experiment 1. Objects in the stationary/mobile series were selected on the basis that they could be easily set in motion (by a switch or remote control), could move on their own, and could be easily controlled as they moved on the object platform. Objects from the stationary/mobile series were presented either still or in motion. All objects in the stationary/mobile object series were electric children's toys that could be left powered off for the stationary presentation or powered on for the mobile presentation. In the mobile presentation, objects spun (e.g., the “Barbie Disco Ball”) or moved across the object platform (e.g., the toy truck). All objects were presented on the object platform.
All trials began when the opaque door was raised and ended when it was lowered. The opaque door remained lowered during the inter-trial interval which was approximately 30 seconds long. Two experimenters operated the opaque door and recorded behaviors; they sat approximately 1.5 meters from the test cage and were therefore in visual contact with the subject. One experimenter recorded the latency to contact the object and/or take food, as well as the frequency and duration of contact with the object (exploration) using the Observer software (Noldus). As in Experiment 1, exploration was defined as any physical contact with or manipulation of the object. Other behaviors that were recorded are listed in the included ethogram (Supplemental Materials). Both experimenters avoided staring at the subject during the trial. All trials were recorded on video with a tripod-mounted video camera positioned directly in front of the cage.
Temperament Assessments
Following the final test day of the experiment, the animals’ temperamental responses to the objects were assessed using a four-factor index. Temperament assessments were made by a trained coder while watching the videotapes of each trial recorded during the testing sessions. The coder was blind to the animal's lesion condition, had no previous contact with the animals, and was unfamiliar with the animals’ previous testing history. Thus, the coder was able to provide an unbiased assessment of each animal's temperament for each trial of the experiment. The coder reached 90% reliability with an established coder (JT). For each trial, each animal's behavior was assessed on four factors: 1) Confidence, 2) Nervousness, 3) Fearfulness, 4) Activity. Each factor was scored on a scale of 1 to 5, where 1 indicated that the factor was not at all descriptive of the animal's behavior and 5 indicated that the factor was very descriptive of the animal's behavior (see definitions in Table 4).
Table 4.
Temperament Rating Definitions
| Traits | Definition |
|---|---|
| Confidence | Animal's movements are fluid, not furtive. Animal is not tentative and spends the majority of time in the front of cage, takes the food with little or no hesitation and may touch the object or touch the object/reward presentation platform. |
| Nervousness | Animal fidgets, yawns, scratches, or displays stereotypies. Movements may be jerky. Animal appears overly vigilant. |
| Fearfulness | Animal appears anxious and readily grimaces, screams, cowers, and/or moves away from object. Noticeably averts gaze from object or front of cage. |
| Activity | Animal actively moving around the cage and remains stationary only for short periods of time. An active animal will spend time in many different locations. |
Data Analysis Strategy
Data were analyzed as they were for Experiment 1. Means for latency to first response, and frequency and duration of response were computed for each trial type. Data were log10(x+1) transformed in cases where they were not normally distributed as indicated in the results section. For the purposes of interpretation, raw data (means and variance indices) are presented in the text and tables; log transformed data are available upon request. Data were subjected to traditional ANOVA. Lesion condition was the between subjects factor for all analyses. For data from the 30-second trials in which food was presented with or without an object, object complexity was the repeated measure. In the analyses of food retrieval there were three levels of the repeated measure (no object, simple object, complex object); in the analyses of object exploration there were two levels of the repeated measure (simple object v. complex object, or, stationary object v. mobile object). As in Experiment 1, lesion group differences were evaluated using a series of non-parametric mean comparisons (Mann-Whitney tests). Because our a priori hypothesis was that amygdala-lesioned animals’ affective responses to objects would differ significantly from hippocampus-lesioned and control animals (i.e., pair-wise group differences) we completed the Mann-Whitney comparisons to evaluate group differences even in cases where the p-values associated with the overall ANOVA did not reach conventional levels of significance.
Results
Test Procedure Acclimation
With the exception of one amygdala-lesioned animal, all animals met the criterion to retrieve a single food reward (a miniature marshmallow) within 30 seconds on 8 out of 10 trials over two consecutive days. The animal that did not meet criterion was not included in these analyses.
60-second Novel Nonbiological Object Trials
While there were not significant overall differences between the groups as revealed by an ANOVA, between group non-parametric comparisons showed that amygdala-lesioned animals, compared to control animals, tended to explore objects more frequently, explored objects for significantly longer and were significantly faster to first explore objects. As in Experiment 1 hippocampus-lesioned and control animals did not differ in their propensity to explore; however in contrast to Experiment 1, the differences between amygdala-lesioned and hippocampus-lesioned animals were not significant (see Table 1).
30-second Animal-Like Object and/or Food Reward Trials
Retrieval of food reward
As in Experiment 1, there were no group differences in the frequency or latency of food retrieval. All groups retrieved food most frequently and fastest when no object was present and least frequently and slowest when a complex object was present (with simple objects intermediate) (see Table 5).
Table 5.
Experiment 2—Food Retrieval Behavior in the Presence of Animal-Like Objects
| Frequency(rate) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | No Object | .97 ± .02 | 1 ± 0 | .94 ± .06 | F(2, 40)=10.41, p<.0001, ηp2=.342 |
| Simple Object | .75 ± .11 | .93 ± .05 | .91 ± .07 | ||
| Complex Object | .59 ± .16 | .82 ± .11 | .81 ± .10 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=1.04, p<.37, ηp2=.094 | F(4, 40)=1.32, p<.28, ηp2=.117 | |||
| A v. C, n.s.; H v. C, n.s; A v. H, n.s. | |||||
| Latency (seconds) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | No Object | 4.40 ± 1.25 | 3.48 ± .51 | 5.41 ± 2.15 | F(2, 40)=19.07, p<.0001, ηp2=.488 |
| Simple Object | 10.25 ± 3.05 | 6.22 ± 1.29 | 7.83 ± 2.34 | ||
| Complex Object | 16.08 ± 4.19 | 9.39 ± 2.52 | 10.05 ± 2.69 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=.78, p<.47, ηp2=.072 | F(4, 40)=1.69, p<.17, ηp2=.145 | |||
| A v. C, n.s.; H v. C, n.s; A v. H, n.s. | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
Object exploration
The overall pattern established in Experiment 1—that amygdala-lesioned animals, compared to controls, had an increased propensity to explore s—was essentially replicated (see Table 6). Amygdala-lesioned animals, compared to control animals, explored objects for significantly longer and tended to explore objects more frequently and faster. It is important to note that there was a good deal of variability in the patterns of behavior within the amygdala-lesioned group; this large magnitude of variance likely masked group differences. In contrast to Experiment 1, hippocampus-lesioned animals also explored objects for longer and tended to explore objects more frequently and more quickly than control animals.
Table 6.
Experiment 2— Exploration of Animal-Like Objects
| Frequency (rate) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Simple Object | .06 ± .06 | .54 ± .22 | .22 ± .12 | F(1, 20)=.96, p<.34, ηp2=.046 |
| Complex Object | .03 ± .03 | .61 ± .40 | .41 ± .16 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=2.09, p<.15, ηp2=.173 | F(2, 20)=.70, p<.51, ηp2=.065 | |||
| A>C, p<.060; H>C, p<.050; A v. H, n.s. | |||||
| Duration (seconds) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Simple Object | .06 ± .06 | 2.06 ± .87 | .33 ± .19 | F(1, 20)=.19, p<.67, ηp2=.009 |
| Complex Object | .03 ± .03 | 2.84 ± 1.75 | .72 ± .33 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=3.24, p<.06, ηp2=.245 | F(2, 20)=1.27, p<.30, ηp2=.113 | |||
| A>C, p<.046; H>C, p<.035; A v. H, n.s. | |||||
| Latency(seconds) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Simple Object | 29.43 ± .57 | 20.28 ± 4.19 | 27.86 ± 1.25 | F(1, 20)=.37, p<.55, ηp2=.018 |
| Complex Object | 29.58 ± .42 | 22.49 ± 4.16 | 25.78 ± 2.07 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(1, 21)=2.19, p<.14, ηp2=.180 | F(2, 20)=1.65, p<.22, ηp2=.142 | |||
| A<C, p<.063; H<C, p<.061; A v .H, n.s. | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
data used in ANOVA was log transformed.
Object complexity did not influence object exploration as measured by the frequency of contact, the duration of contact, or the latency to first contact. Similarly, there were no significant interactions between lesion group and stimulus complexity.
30-second Stationary/Mobile Object and/or Food Reward Trials
Retrieval of food reward
While there were not overwhelming lesion group differences in food retrieval behavior, amygdala-lesioned and hippocampus-lesioned animals tended to retrieve food more frequently than did control animals across all trial types (see Table 7).
Table 7.
Experiment 2—Food Retrieval Behavior in the Presence of Stationary-Mobile Objects
| Frequency(rate) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | No Object | .97 ± .02 | 1 ± 0 | .94 ± .06 | F(2, 40)=17.88, p<.0001, ηp2=.472 |
| Stationary Object | .75 ± .11 | .93 ± .05 | .91 ± .07 | ||
| Mobile Object | .41 ± .12 | .75 ± .09 | .75 ± .09 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=2.16, p<.14, ηp2=.178 | F(4, 40)=2.48, p<.06, ηp2=.199 | |||
| A>C, p<.088; H>C, p<.098; A v. H, n.s. | |||||
| Latency (seconds) | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | No Object | 4.40 ± 1.25 | 3.48 ± .51 | 5.41 ± 2.15 | F(2, 40)=33.25, p<.0001, ηp2=.624 |
| Stationary Object | 10.25 ± 3.04 | 6.22 ± 1.29 | 7.83 ± 2.35 | ||
| Mobile Object | 20.92 ± 2.93 | 13.81 ± 3.18 | 13.97 ± 1.78 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=1.61, p<.22, ηp2=.139 | F(4, 40)=1.85, p<.14, ηp2=.156 | |||
| A v. C, n.s.; H v. C, n.s; A v. H, n.s. | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
All lesion groups differentiated between the levels of object motion (and therefore complexity) as measured by the frequency and latency to retrieve food. The three groups retrieved the food most frequently and fastest on trials with no object and least frequently and slowest on trials with mobile objects (with stationary objects intermediate). Evaluation of the marginal means indicated that amygdala-lesioned animals retrieved food more frequently in the presence of both stationary and mobile objects as compared to controls; hippocampus-lesioned animals also retrieved food more frequently as compared to controls but only on trials with mobile objects.
Object exploration
Replicating the previous patterns of object exploration, amygdala-lesioned animals, compared to the other groups, explored objects more frequently and for longer and tended to be fastest to first explore (see Table 8). Amygdala-lesioned animals explored objects significantly more frequently, for longer and tended to be faster to first explore than control animals. In contrast to the pattern of effects observed with the animal-like objects from this experiment, there were also differences between amygdala-lesioned and hippocampus-lesioned animals—amygdala-lesioned animals tended to explore objects more, for longer and faster than hippocampus-lesioned animals. Hippocampus-lesioned and control animals did not differ; no animals from either group explored the mobile objects. All animals explored objects in their stationary, as compared to mobile, form more frequently and for longer, and were faster to first explore stationary objects.
Table 8.
Experiment 2-- Exploration of Stationary-Mobile Objects
| Frequency (rate) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Stationary Object | .13 ± .09 | .54 ± .23 | .16 ± .08 | F(1, 20)=11.98, p<.002, η2=.375 |
| Mobile Object | 0 ± 0 | .25 ± .11 | 0 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=4.20, p<.030, η2=.296 | F(2, 20)=2.94, p<.75, η2=.029 | |||
| A>C, p<.050; H v. C, n.s.; A>H, p<.076 | |||||
| Duration (seconds) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Stationary Object | .06 ± .06 | 2.06 ± .87 | .33 ± .19 | F(1, 20)=10.94, p<.004, ηp2=.354 |
| Mobile Object | 0 ± 0 | 1.14 ± .56 | 0 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=4.74, p<.021, ηp2=.322 | F(2, 20)=.81, p<.460, ηp2=.075 | |||
| A>C, p<.045; H v. C, n.s.; A>H, p<.088 | |||||
| Latency(seconds) † | |||||
|---|---|---|---|---|---|
| Lesion Group |
|||||
| C | A | H | Effect of Object Complexity | ||
| Trial Type | Stationary Object | 27.96 ± 1.47 | 23.39 ± 3.12 | 28.20 ± .94 | F(1, 20)=4.78, p<.04, ηp2=.193 |
| Mobile Object | 30 ± 0 | 25.63 ± 1.80 | 30 ± 0 | ||
| Lesion X Object Complexity | |||||
| Effect of Lesion | F(2, 20)=2.73, p<.09, ηp2=.214 | F(2, 20)=3.91, p<.68, ηp2=.038 | |||
| A<C, p<.060; H v. C, n.s.; A<H, p<.067 | |||||
Note: C: control group; A: amygdala-lesioned group; H: hippocampus-lesioned group. Means ± standard errors. Lesion groups were compared using Mann-Whitney tests.
data used in ANOVA was log transformed.
Temperament Assessments
One set of ANOVAs (one for each attribute of temperament) was completed for the animal-like object series and one set of ANOVAs was completed for the stationary/mobile object series. See Figure 2 for means and Table 9 for statistics.
Figure 2.
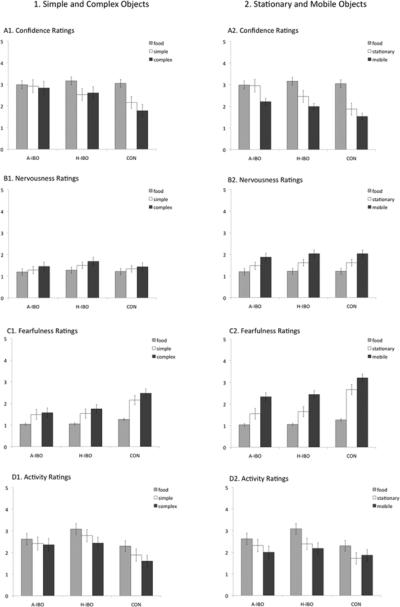
Experiment 2: Temperament Assessments. Note: Mean temperament assessment across object series. Panel 1: Simple/Complex object series. Panel 2: Stationary/Mobile object series. A-IBO: Amygdala-lesioned animals; H-IBO: Hippocampus-lesioned animals; CON: Control animals
Table 9.
Experiment 2—Temperament Assessment Statistics
| Simple/Complex Animal-Like Object Series |
Stationary/Mobile Object Series |
|||||||
|---|---|---|---|---|---|---|---|---|
| Confidence | Nervousness | Fearfulness | Activity | Confidence | Nervousness | Fearfulness | Activity | |
| Statistical Effects | ||||||||
| Lesion Effect d.f.=(2,20) |
F=2.06 p<.15 ηp2=.170 |
F=.44 p<.66 ηp2=.041 |
F=5.03 p<.02 ηp2=.334 |
F=2.67 p<.09 ηp2=.211 |
F=3.33 p<.06 ηp2=.250 |
F=.18 p<.84 ηp2=.017 |
F=9.06 p<.002 ηp2=.475 |
F=1.57 p<.23 ηp2=.334 |
|
Mann-Whitney
Comparisons |
A<C, p<.018 H<C, p<.040 |
H>C, p<.012 | A>C, p<.064 | A<C, p<.011 H<C, p<.018 |
||||
| Object Complexity Effect d.f.=(4,20) |
F=10.60 p<.0001 ηp2=.346 |
F=8.16 p<.001 ηp2=290 |
F=33.94 p<.0001 ηp2=.629 |
F=30.10 p<.0001 ηp2=.601 |
F=32.65 p<.0001 ηp2=.620 |
F=30.75 p<.0001 ηp2=.606 |
F=96.36 p<.0001 ηp2=.828 |
F=19.34 p<.0001 ηp2=.492 |
| Lesion X Object Complexity Interaction d.f.=(4,40) |
F=2.63 p<.05 ηp2=.208 |
F=.37 p<.83 ηp2=.036 |
F=2.09 p<.10 ηp2=.173 |
F=2.00 p<.11 ηp2=.163 |
F=2.70 p<.04 ηp2=.213 |
F=.44 p<.77 ηp2=.042 |
F=3.53 p<.02 ηp2=.261 |
F=1.39 p<.26 ηp2=.122 |
Note: Lesion groups were compared using Mann-Whitney tests.
Confidence
In general, object complexity affected confidence more than did lesion condition. There were not overall differences between lesion groups in confidence during the animal-like object series, but all animals were most confident when no object was present and least confident when complex objects were present. Amygdala-lesioned animals were equally confident across all trial types, but hippocampus-lesioned and control subjects were most confident when no object was present and least confident when complex objects were present.
During the stationary/mobile object series, amygdala-lesioned animals were rated most confident. All lesion groups were most confident when no object was present and least confident when mobile objects were present. A significant lesion X complexity interaction, indicated that both hippocampus-lesioned and control subjects were most confident when no object was present and least confident when mobile objects were present. In contrast, amygdala-lesioned animals were least confident when mobile objects were present but they were equally confident when no object was present and when stationary objects were present.
Nervousness
There were no lesion group differences in nervousness in either object series, but all animals were least nervous when no object was present and most nervous when either the complex or mobile.
Fearfulness
Amygdala-lesioned animals were significantly least fearful in both object series. Overall, all animals were least fearful in the presence of no object and most fearful in the presence of the complex or mobile objects. Finally, the lesion X complexity interaction was marginally significant for the animal-like objects and was significant for the stationary/mobile objects. The pattern of means were similar across both object series—control animals showed the greatest differences, and amygdala-lesioned animals showed the smallest differences.
Activity Ratings
Hippocampus-lesioned animals tended to be most active during the animal-like object series. All groups were equally active during the stationary/mobile object series. In both object series, there was a significant main effect of object such that all animals were rated to be most active when no object was present and least active when complex or mobile objects were.
Experiment 2: Summary of Findings
As in Experiment 1, amygdala-lesioned animals were not behaviorally inhibited in the presence of objects with which they had no prior experience. Specifically, in the presence of novel objects, amygdala-lesioned as compared to control subjects, were fastest to first explore, explored for longer and tended to explore more frequently. Similarly, amygdala-lesioned subjects were faster to first explore animal-like objects than control subjects and explored more frequently than control subjects; interestingly hippocampus-lesioned animals also demonstrated an increased propensity for exploration of these objects. These behavioral measures were complemented by the observer temperament ratings of confidence and fearfulness. Overall, amygdala-lesioned animals were rated to be the most confident and the least fearful but both amygdala-lesioned and hippocampus-lesioned subjects were significantly less fearful as compared to controls. These differences were most pronounced when animals were presented with the most complex objects.
All animals were faster to explore and explored objects longer when the objects were presented stationary as opposed to in motion. As with stationary objects, amygdala-lesioned subjects exhibited a lack of inhibition in the presence of objects in motion. Amygdala-lesioned subjects, as compared to hippocampus-lesioned and control subjects, lacked behavioral inhibition insofar as they explored objects more, for longer and were faster to first explore. Taken together, these results suggest that motion of objects did not influence amygdala-lesioned subjects lack of behavioral inhibition.
One remarkable finding from Experiment 2 is that amygdala-lesioned and hippocampus-lesioned animals behaved more similarly than they did in Experiment 1. Specifically, both groups explored novel objects as frequently and for the same duration, although amygdala-lesioned animals differed from control animals while hippocampus-lesioned animals did not. Like amygdala-lesioned animals, hippocampus-lesioned animals explored animal-like objects more frequently, for longer, and faster and were rated to be less fearful than controls. These findings are consistent with evidence indicating that macaques that received hippocampus-lesions as adults, as compared to controls, were less behaviorally inhibited in the presence of emotionally provocative objects (Chudasama, Wright, & Murray, 2008). Interestingly, the exploration finding did not hold for objects from the stationary/mobile series during which the behavior of hippocampus-lesioned animals did not differ from that of the control animals. It appears that hippocampus-lesioned animals, but not control animals, benefited uniquely from experience with general object types (novel toys and animal-like objects) that were experienced during Experiment 1 and then again during Experiment 2. During their second interaction with an object type, hippocampus-lesioned animals were less inhibited.
Experiment 2 replicated the “take food-reward” effect documented in Experiment 1; all animals differentiated between the two levels of complexity of the object stimuli. Animals retrieved the food reward more frequently and were faster to retrieve it when objects were simple (or stationary) as compared to when objects were complex (or in motion). While there was not an overall effect of lesion condition, both amygdala-lesioned and hippocampus-lesioned animals tended to retrieve food more frequently than controls. As in Experiment 1, there were no lesion condition differences in frequency or latency to take food reward in the presence of objects from the animal-like set.
Comparison of Experiment 1 and Experiment 2
We assessed changes in responsivity to novel and animal-like objects across Experiment 1 and Experiment 21 using a series of repeated measure ANOVAs with time (Experiment 1 or Experiment 2) as the only repeated measure for Novel Object data and time (Experiment 1 or Experiment 2) and object complexity (simple or complex) as the repeated measures for the Animal-Like Object trials.2 For the sake of brevity, only effects that supplement the previously presented findings are discussed. All other statistics are available upon request.
60-second Novel Nonbiological Object Trials
Across both experiments, amygdala-lesioned animals explored novel nonbiological objects most frequently and for the longest duration and were fastest to first explore the objects. Patterns of frequency, latency and duration to explore objects were consistent across experiments. Interestingly, all animals tended to be faster to explore novel objects during Experiment 2 as compared with Experiment 1, F(1, 19)=3.60, p<.07, ηp2=.159.
30-second Animal-Like Objects and/or Food Reward Trials
Retrieval of food reward
Food retrieval behavior was consistent across Experiments—lesion condition had no impact on the propensity to retrieve food rewards but stimulus complexity did. All animals took food most frequently and fastest when no object was present and least frequently and slowest when the complex objects were present.
Object exploration
All animals explored more frequently during Experiment 2 as compared to Experiment 1, F(1, 19)=6.45, p<.02, ηp2=.254. This was qualified by a significant time X complexity X lesion effect, F(2, 19)=4.85, p<.02, ηp2=.338. The difference in exploration between Experiment 2 and Experiment 1 was most robust for amygdala- and hippocampus-lesioned animals. Amygdala-lesioned animals explored simple objects significantly more during Experiment 2 as compared to Experiment 1. Hippocampus-lesioned animals explored all objects more during Experiment 2 as compared to Experiment 1.
All animals were faster to first explore (log transformed) during Experiment 2 as compared to Experiment 1, F(1, 19)=4.47, p<.05, ηp2=.190. As in the frequency data this was qualified by a significant time X complexity X lesion effect on latency to first explore object, F(2, 19)=3.90, p<.04, ηp2=.291. The latency to first explore did not differ between Experiments 1 and 2 for control subjects. In contrast, amygdala and hippocampus-lesioned subjects were faster to first explore in Experiment 2 as compared to Experiment 1. Amygdala-lesioned subjects tended to be faster to explore complex objects during Experiment 1 and simple objects during Experiment 2. The pattern was reversed for hippocampus-lesioned subjects.
General Discussion
Across two experiments, we demonstrated that young rhesus macaques with early bilateral amygdala lesions are behaviorally uninhibited when tested at two points in their development in the presence of novel objects and emotionally salient objects that were potentially fear inducing. This lack of behavioral inhibition was characterized by an increased propensity to physically explore objects. Diminished behavioral inhibition was observed with different types of objects—novel nonbiological objects, simple and complex animal-like objects and objects presented either stationary and in motion. Furthermore, amygdala-lesioned subjects were rated as being more confident and less fearful of objects. This pattern of findings was remarkably stable over time. These data are consistent with previously published studies that show that adult amygdala-lesioned nonhuman primate subjects explore objects of emotional significance more than controls and are less fearful and more relaxed or “tame” in the presence of such objects (Mason et al., 2006; Zola-Morgan et al., 1991).
Based on similar previous testing in our laboratory (Mason et al., 2006), we expected, but did not find, lesion differences in the frequency and latency to retrieve a food reward in proximity to an object of emotional significance. While we did not find significant lesion differences in the food retrieval data, we did find that all animals retrieved food most frequently and fastest when no object was present and least frequently and slowest when complex (or mobile) objects were present. In contrast to the adult amygdala-lesioned animals tested by Mason et al. (2006), young amygdala-lesioned macaques, similar to hippocampus-lesioned and control subjects, showed evidence of differentiating between levels (or intensity) of emotional salience as evidenced in their food retrieval latencies. One possibility is that food reward retrieval behavior is a better index of behavioral inhibition in adults because adults, as compared to young animals, are less prone to manipulate objects in their environments (Reinhardt, 1990). Another possibility is that because the lesions were created early in their development, emotional processing in our young amygdala-lesioned macaque subjects was less compromised (in contrast to adult lesioned animals) because other areas of the brain were able to compensate for some, but not all, of the typical amygdala functions. A final possibility is that the levels of stimulus complexity that were tested in this study (two—simple and complex) were not sufficient to capture variation in food reward retrieval behavior related to lesions. These are all avenues of potentially fruitful areas for future research.
The lack of behavioral inhibition observed in our amygdala-lesioned subjects in this experiment is consistent with previous observations that the same amygdala-lesioned subjects’ behavior is also uninhibited in social contexts. For example, following weaning from their mothers at six months of age, each subjects’ behavior was observed while it was in a large testing cage in the presence of its mother (constrained on one end of the cage) and a familiar adult female (constrained on the opposite end of the cage) (Bauman et al., 2004a). In this emotionally provocative social testing environment, hippocampus-lesioned and control subjects positioned themselves in proximity to their mothers while amgydala-lesioned animals did not and rather explored the cage (Bauman et al., 2004a). Amygdala-lesioned animals also made fewer distress vocalizations (Bauman et al., 2004a) suggesting that they were less emotionally reactive to the testing context. Importantly, in that testing environment, as in the present experiment, hippocampus-lesioned and control subjects’ behavior did not differ markedly.
One of the strengths of this series of experiments is that we were able to compare the behavior of amygdala-lesioned subjects to that of age-matched hippocampus-lesioned subjects and to non-operated control subjects. In the present report, hippocampus-lesioned animals behaviorwere intermediate between amygdala-lesioned and control animals, suggesting that their emotional processing was not as impaired as amygdala-lesioned animals but not necessarily normal either. Examining the trajectory of hippocampus-lesioned animals’ behavior over time supports the idea that their emotional processing is compromised, although less than that of amygdala-lesioned animals. While hippocampus-lesioned animals behaved most similarly to controls at 9 months of age, their behavior was comparable to amygdala-lesioned animals at 18 months of age when tested with objects similar to those that they saw before (the novel and animal-like objects). Notably, at 18 months of age, hippocampus-lesioned animals behaved like controls in the presence of objects that were completely different from those with which they had previous experience (objects from the stationary/mobile series). This pattern of effects suggests that multiple exposures to the same type of objects led to a behavioral habituation for hippocampus-lesioned animals (but not amygdala-lesioned or control animals). We know from other testing with these animals that their brains underwent reorganization allowing for intact spatial memory in the absence of hippocampi (Lavenex, et al., 2008) and it is possible that this reorganization could have subserved such habituation. Further inquiry is needed to investigate, characterize and justify this claim. By 18 months of age, hippocampus-lesioned animals were more active than control subjects. This is consistent with the hypermotoric behavior that we have documented with these animals in social settings (Bauman et al., 2008; Lavenex, et al., 2008) and with evidence from other research groups who have demonstrated hyperactivity in hippocampus-lesioned adult macaques (e.g., Machado & Bachevalier, 2006).
Future research should investigate the development of more naturalistic emotional responding which occurs when particular affective challenges are presented (e.g., interacting with a novel but dominating conspecific), or interaction with a broader diversity of objects. While it is possible that the effects observed were specific to the types of stimuli used, it is more likely that variation between lesion conditions is stimulus general—in other words, it is most likely that amygdala-lesioned animals’ behavioral inhibition system is compromised such that they inappropriately approach and engage all sorts of stimuli, not just complex animal-like children's toys. Similarly, it would be interesting to vary the time window in which animals have to respond and interact with the objects. In general, durations of exploration were short and the latency to first exploration was long. It is possible, even probable, that the length of our trials may have artificially truncated meaningful variation in behavior that would be apparent at longer trial lengths.
In closing, the two experiments presented in this paper provide evidence that early damage to the amygdala, and to a lesser extent the hippocampus, of nonhuman primates disrupts normative emotional processing. This behavioral disruption—decreased behavioral inhibition—observed in our young subjects was similar to that observed after similar brain damage imposed later in life. These findings therefore suggest that, despite reorganization of neural networks following damage (Machado, et al., 2008), early damage to regions of the medial temporal lobe, and the amgydala in particular, may have life-long consequences for emotional processing.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R37MH57502) and by the base grant of the California National Primate Research Center (RR00169). This work was also supported through the Early Experience and Brain Development Network of the MacArthur Foundation. We thank the veterinary and husbandry staff of the California National Primate Research Center for excellent care of the animal subjects. We thank Dr. Pierre Lavenex, Jeffrey Bennett and Pamela Tennant for assistance with surgical procedures, and Melissa Marcucci for assistance with behavioral data collection. Finally, we thank two anonymous reviewers for their helpful comments on a previous draft of the manuscript.
Footnotes
One amygdala-lesioned animal in the original cohort died after Experiment 1 and was replaced with a different experimental animal for Experiment 2. Data from those two animals have been removed from the present analyses. For the sake of completeness, we repeated all analyses from Experiments 1 and 2 without these two subjects; patterns of means, standard errors, and effects did not change notably. For that reason, we have not included additional figures comparing Experiment 1 and 2.
It is important to note that the test cages differed between Experiments 1 and 2. Given this, we are unable to directly evaluate the extent to which behavioral differences observed across the two time points are a result of neurodevelopmental trajectory alone.
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behavioral Neuroscience. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein S, Mason WA. The effects of age and stimulus conditions on the emotional responses of rhesus monkeys: Responses to complex stimuli. Journal of Genetic Psychology. 1962;101:279–297. doi: 10.1080/00221325.1962.10533632. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in Rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amgydala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelly AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amgydala and social behavior. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction. Wiley-Liss; Hoboken, NJ: 1992. pp. 353–377. [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. Spatial relational learning persists following neonatal lesions in macaque monkeys. Nature Neuroscience. 2007;10:234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in Rhesus monkeys (Macaca mulatta). Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbitofrontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imagining study. Neuroimage. 2008;15:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in Rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meunier M, Nalway V, Bachevalier J. Reactions to familiar and novel objects in infant monkeys with neonatal temporal lobe lesions. Hippocampus. 2003;13:489–493. doi: 10.1002/hipo.10143. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Time budget of caged rhesus monkeys exposed to a companion, a PVC perch and a piece of wood for an extended time. American Journal of Primatology. 1990;20:51–56. doi: 10.1002/ajp.1350200108. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alverez-Royo P, Clower RP. Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


