Abstract
Background. Telomeres provide a key mechanism for protecting the integrity of chromosomes and their attrition after cell division and during aging are evident in lymphocytes. However, the significance of telomere shortening in age-associated decline of immune function is unknown.
Methods. We selected 22 HLA-A2–positive healthy older adults who have relatively short or long telomere lengths to compare their antibody response against the influenza vaccine, and their CD8+ T-cell response against an influenza antigen.
Results. B cells from individuals with a robust antibody response to the influenza vaccine had significantly longer telomeres than those with a poor antibody response. Monocyte-derived antigen-presenting cells of both short and long telomere groups induced similar expansions of influenza M1–specific CD8+ T cells. Vaccination did not increase M1-specific CD8+ T cells in blood, but M1-specific CD8+ T cells from the long telomere group exhibited significantly greater expansion in vitro than those from the short telomere group. Finally, M1-specific CD8+ T cells that underwent more expansions had significantly longer telomeres than cells with fewer divisions.
Conclusions. Telomere length is positively associated with a robust lymphocyte response, and telomere attrition may contribute to the age-associated decline of adaptive immunity.
Keywords: telomere, influenza vaccine, CD8 T cells, antibody, CMV, CD28− T cells
The decline in immune function with age is characterized by an increased susceptibility to infectious disease and decreased vaccine efficacy [1, 2]. Studies on the efficacy of the seasonal influenza vaccine show a decline of both humoral and cellular immune responses in older adults [3, 4]. At the cellular level, aging is associated with a decline in the thymic output of naive T cells, in B-cell generation [5], and in an expansion of incompetent memory T cells [6]. The accumulation of T cells with a reduced proliferative capacity in response to an antigenic challenge is one hallmark of an aging immune system [7, 8] and has been shown in reduced immune response to the influenza vaccine in the elderly [9–11]. Some explanations have been suggested in regulating T-cell proliferation in aging [12] but the molecular mechanisms underlying the age-associated deficiency of lymphocyte proliferation and function are not fully understood.
Telomeres are the tips of linear chromosomes composed of tandem TTAGGG repeats and telomere binding proteins [13–15]. Because conventional DNA polymerase cannot replicate the very ends of chromosomes, each cell division results in a loss of small portions of telomeric DNA. The shortening of telomeres is observed in lymphocytes with cumulative cell divisions [16, 17], and in advancing age [18, 19]. A substantial telomere loss results in critically shortened telomeres in the cell and is associated with the cessation of cell division or apoptosis in vitro [20]. Because an effective adaptive immune response relies on the clonal expansion to produce large numbers of effector lymphocytes, the role of telomeres in regulating lymphocyte proliferation has become a topic of great interest [21, 22].
Studies of altered telomere length and its relationship with telomerase, an enzyme that synthesizes telomeres, in lymphocytes have revealed novel insights. Germinal center B cells derived from the activation of naive B cells express higher levels of telomerase activity and have longer telomere lengths than naive B cells [23, 24]. Telomere attrition occurs in actively dividing and long-term cultured T cells, and shortened telomeres limit the proliferative capacity of cultured T cells [25, 26]. Furthermore, patients infected with cytomegalovirus (CMV) or Epstein-Barr virus have more differentiated T cells in the blood; these T cells have shorter telomeres compared with the healthy controls [27, 28]. Finally, genetic disorders negatively affect telomerase, resulting in short telomeres and defects in the replication of hematopoietic stem cells in vivo [29–33]. Collectively, these findings point to the critical role of telomere length in lymphocyte function. However, it remains to be elucidated whether age-associated telomere shortening actually contributes to the decline of the immune response in the elderly.
Peripheral blood mononuclear cells (PBMCs) are commonly used to assess telomere length in humans. Lymphocytes, part of PBMCs, are a heterogeneous population of cells including B and T cells. Furthermore, the lymphocytes that respond to a specific pathogen during an immune response are only a small fraction of lymphocytes. Although the telomere lengths of different types of tissues in an individual seem to be correlated [34–36], it is uncertain whether telomere lengths of PBMCs are the same as those of the antigen-specific lymphocytes. Owing to the paucity of antigen-specific lymphocytes in the blood of healthy adults, the impact of telomere length on the antigen-specific lymphocyte response has not been adequately assessed. This is of particular importance, because the decline of a specific immune response to a vaccine or pathogen resulting from the short telomeres of antigen-specific lymphocytes could be one of the earliest signs of overall immune functional decline during aging.
We conducted a comparative study of immune response to the influenza vaccine in healthy older adults with either relatively long or short telomeres. Our aim was to determine whether telomere length in older persons indicates their immune competency to respond to influenza. We hypothesized that the telomere length of antigen-specific lymphocytes is a determining factor for the robustness of lymphocyte proliferation and function.
PARTICIPANTS, MATERIALS, AND METHODS
Study Design and Subjects
This study protocol was approved by the National Institute on Aging Institutional Review Board. Study subjects were participants of the Baltimore Longitudinal Study of Aging and were selected based on the following criteria: HLA-A2 positive, aged ≥70 years, generally healthy, willing to receive influenza vaccination, and telomere length of PBMCs in either the top or bottom third in telomere lengths for our study cohort [19]. All participants were provided a description of the study and signed an informed consent document. Participants were excluded if they had any of the following: kidney or liver enzyme abnormalities, recent illnesses, inflammatory or immune deficiencies, Guillain-Barré syndrome, regular use of immunosuppressive medications, regular use of anti-inflammatory medications within the past 2 years, or previous allergic responses to the influenza vaccine or egg products. At each visit (before vaccination and at a mean [standard deviation] of 21 [1] and 84 [3] days after vaccination), 43 mL of blood was drawn by venipuncture either at the hospital or during a home visit.
Influenza Vaccine
Seasonal influenza vaccines (2009–2010 and 2010–2011) were made by Sanofi-Pasteur (Fluzone). The 2009–2010 vaccine consisted of an A/Brisbane/59/2007 (H1N1)–like virus, an A/Brisbane/10/2007 (H3N2)–like virus, and a B/Brisbane/60/2008-like virus. The 2010–2011 vaccine consisted of an A/California/7/2009 (H1N1)–like virus, an A/Perth/16/2009 (H3N2)–like virus, and a B/Brisbane/60/2008–like virus. The influenza vaccination was administered via intramuscular injection.
Isolation and Culture of Lymphocytes and Monocytes From Blood
PBMCs were isolated from approximately 40 mL of blood by Ficoll-gradient centrifugation. CD8+ T cells, B cells (CD19+), monocytes (CD14+), and CD4+ T cells were sequentially isolated from PBMCs by positive selection with Dynal magnetic beads (Life Technologies) according to the manufacturer's protocol, and were used for subsequent analysis. 0.5 × 106 isolated CD4+ and CD8+ T cells were seeded in a 48-well plate with anti-CD3 pan T Dynal beads at a 1:1 cell to bead ratio and 20 U/mL of interleukin 2 (Peprotech) at 37°C in a 5% carbon dioxide incubator. On day 3, cells were harvested for cell count and anti-CD3 beads were removed via magnets for fluorescence-activated cell sorting analysis.
Flow Cytometric Analysis of PBMCs
PBMCs of each participant were stained at each visit to determine the immune cell composition with following antibodies: CD4, CD8, CD14, CD19, CD16/56, CD45RA, CD62L, CCR7, CD28, Foxp3, CD25, HLA-DR, CD123, and CD11c (Biolegend). Staining of influenza M1 and CMV pp65 dextramer (Immudex) was carried out before all other surface stains for 10 minutes at room temperature. Stained cells were collected on a Canto II flow cytometer (BD) and analyzed using FlowJo software version 7.6.5.
Analysis of Influenza M1–Specific CD8+ T-Cell Response in Vitro
The procedure for generating antigen-presenting cells (APCs) was described elsewhere [37]. For APC function, monocytes were isolated from PBMCs at each participant visit and cultured for 5 days in the presence of interleukin 4 (20 ng/mL) and granulocyte-macrophage colony-stimulating factor (10 ng/mL) and then another day after addition of lipopolysaccharide (100 ng/mL). Mature APCs were pulsed with M158–66 (GILGFVFTL) peptide (5 µg/mL), irradiated, and incubated for 7 days with CD8+ T cells isolated from a healthy HLA-A2–positive adult. Cells were harvested for counts, and M1-specific CD8+ T cells were analyzed with flow cytometry. For M1-specific CD8+ T-cell expansion, CD8+ T cells were isolated from PBMCs at each visit and incubated with APCs (plus M1 peptide) derived from a healthy HLA-A2–positive adult. Culture time and analysis were identical to those used for APC function. This nonautologous system allows the defects of APCs and CD8+ T cells to be distinguished but has the potential HLA-B and HLA-C loci mismatching problem; this possible mismatching was tested by comparing the expansion of M1-specific CD8+ T cells with that of nonautologous APCs and the artificial APC system; the degree of expansion was well correlated between these 2 methods (r = 0.696).
Measurement of Anti-influenza Virus Antibodies
World Health Organization hemagglutination inhibition (HAI) test kits for the 2009–2010 and 2010–2011 seasons were obtained from the Centers for Disease Control and Prevention, including the standard reference antisera and influenza antigens. Serum samples (3 mL) were collected at each visit and stored at −20°C. Participant and reference sera were treated with receptor destroying enzyme (Denka Seiken) overnight and diluted to an initial titer of 1:10. Serum samples were diluted 2-fold, starting at 1:10 to 1:1280. Appropriate antigen and Turkey red blood cells (Fitzgerald Industries International) were added to each well, and plates were monitored for inhibition of agglutination after 30 minutes.
Stimulation of M1-Specific CD8+ T Cells With Artificial Antigen Presentation System
Specific CD8+ T-cell expansion was monitored through the artificial APC system, as described elsewhere [37]. Briefly, CD8+ T cells were isolated through negative selection, stained for M1-specific CD8+ T cells, and cultured using the artificial APC system. After 7 days of culture, cells were harvested, counted, and checked for M1-specific CD8+ T-cell expansion via the M1 dextramer (Immudex). Expanded M1-specific CD8+ T cells were sorted with an iCyt cell sorter (Sony) for telomere measurement.
Telomere Length Analysis With Southern Blot Hybridization and Quantitative Polymerase Chain Reaction
Telomere length in PBMCs was determined using the Southern blot hybridization method, as described elsewhere [38]. Telomere lengths for B cells and M1-specific CD8+ T cells were measured using the quantitative polymerase chain reaction (qPCR) method, as described elsewhere [39]. Telomere length was recoded as the T/S values from the qPCR method and then converted to the Kb values by using a standard fit equation for samples, in which telomere length was measured with both Southern blot hybridization and qPCR (n = 31).
Statistical Analysis
Two-tailed Student t tests were used for analysis, and differences were considered significant at P < .05. Because there was a modest difference in age between the short and long telomere groups, age adjustments were applied to all subjects when these groups were compared using analysis of covariance. Pearson correlation was used to compare telomere length with the antibody response and the M1-specific CD8+ T-cell expansion and to compare M1-specific CD8+ T-cell expansion between the 2 methods.
RESULTS
Association of the Robust Anti-influenza Antibody Titers and Longer Telomere Length in B Cells
We sought to ascertain the impact of telomere length on immune function by comparing the antibody response to the influenza vaccine of healthy old humans. Based on our study of telomere length of PBMCs [19], we selected 22 healthy participants whose telomere length was in the bottom third of the cohort as short telomeres (≤5.6 kb; n = 9) and in the top third as long telomeres (≥6.3 kb; n = 13) (Figure 1A). All of them were aged >70 years and HLA-A2 positive and had no obvious chronic illness. The general demographic information for these subjects is summarized in Table 1. All subjects were given the trivalent inactivated influenza vaccine. Blood samples were obtained before and 21 and 84 days after vaccination; all measurements were conducted in an observer-blind manner.
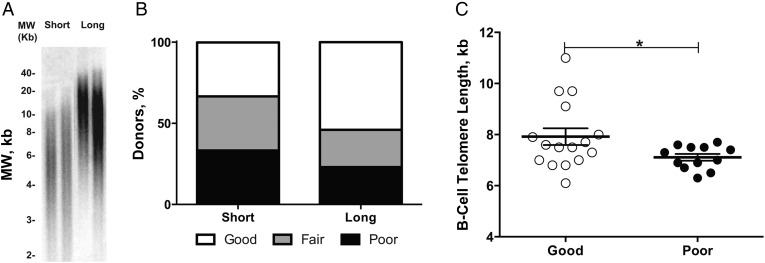
Figure 1.
Telomere length and anti-influenza virus titers. A, Telomere length of peripheral blood mononuclear cells from 2 representative subjects in the short and long telomere groups. B, Percentage of subjects who exhibited a different type of antibody response in the short and long telomere groups. The responder grouping is described in “Participants, Materials, and Methods” section; 33% of subjects in the short versus 54% in the long telomere group were categorized as robust responders. C, B-cell telomere length of robust responders (mean age, 83.4 years) and poor responders (mean age, 85.3 years). The mean (standard error of the mean) telomere length of B cells from robust responders was 7.9 (0.3) kb for robust responders (n = 16) and 7.1 (0.1) kb for poor responders (n = 12). *P < .05. Abbreviation: MW, DNA molecular weight.
Table 1.
General Parameters for Study Participantsa
| Category | Short Telomere Group (n = 9) | Long Telomere Group (n = 13) | P Valueb |
|---|---|---|---|
| Age, y | 84.4 (5.9) | 80.5 (4.8) | .12 |
| Male-female ratio, No. | 6:3 | 8:5 | … |
| Telomere length of PBMCs, kbc (mean [SEM]) | 5.3 (0.4) | 7.2 (0.6) | |
| CD4+ | 28.2 (10.8) | 28.3 (8.6) | .87 |
| CD8+ | 8.8 (3.3) | 8.2 (4.4) | .66 |
| Proportion of blood cells in PBMCs, % | |||
| CD19+ | 4.5 (1.9) | 4.3 (1.7) | .66 |
| CD16/56+ | 21.6 (8.1) | 14.1 (9.1) | .27 |
| CD14+ | 19.6 (7.3) | 13.8 (5.3) | .22 |
Abbreviation: PBMCs, peripheral blood mononuclear cells.
a Unless otherwise specified, values represent means (standard deviation).
b Age-adjusted P values.
c Measured by Southern blot hybridization and quantitative polymerase chain reaction.
We first compared the influenza-specific antibody response between short and long telomere groups. Influenza-specific antibodies were measured from the serum samples of subjects at each visit using World Health Organization HAI test kits (years 2010 and 2011). Postimmunization HAI titers compared with preimmunization HAI titers of H1, H3, and B strains were categorized into 3 groups: (1) those having a seroconversion with a ≥4-fold increase from day 0 to day 21 or 84 (for any of the 3 influenza strains tested) were considered robust responders; (2) those with a 2–4-fold increase were considered fair responders; and (3) those with a <2-fold increase were considered poor responders (Supplementary Table 1). In parallel, telomere lengths from both PBMCs and B cells collected from each visit were measured. Based on the telomere lengths of PBMCs, 33% of the subjects in the short telomere group compared with 54% in the long telomere group had a robust antibody response (Figure 1B).
Because B cells accounted for approximately 5% of the cells in PBMCs of the study subjects (Table 1), we also compared telomere length of B cells between the robust and poor responders from this study plus 9 healthy nonfrail older subjects from a study of vaccine-induced immunity against influenza in frail older adults [40]. B cells from the robust antibody responders had significantly longer telomeres (mean length, 7.9 [0.3] kb; n = 16) than those of the poor responders (7.1 [0.1] kb; n = 12; P < .05). Because the mean ages of these 2 groups were not identical, we also adjusted for subject age, and the difference remained statistically significant (Figure 1C). Overall, the telomere length of B cells was positively correlated with antibody response (r = 0.328). These data show that subjects whose B cells have longer telomere lengths have better antibody response against the influenza vaccine.
M1-Specific CD8+ T-Cell Expansion Induced by Monocyte-Derived APCs in Short and Long Telomere Groups
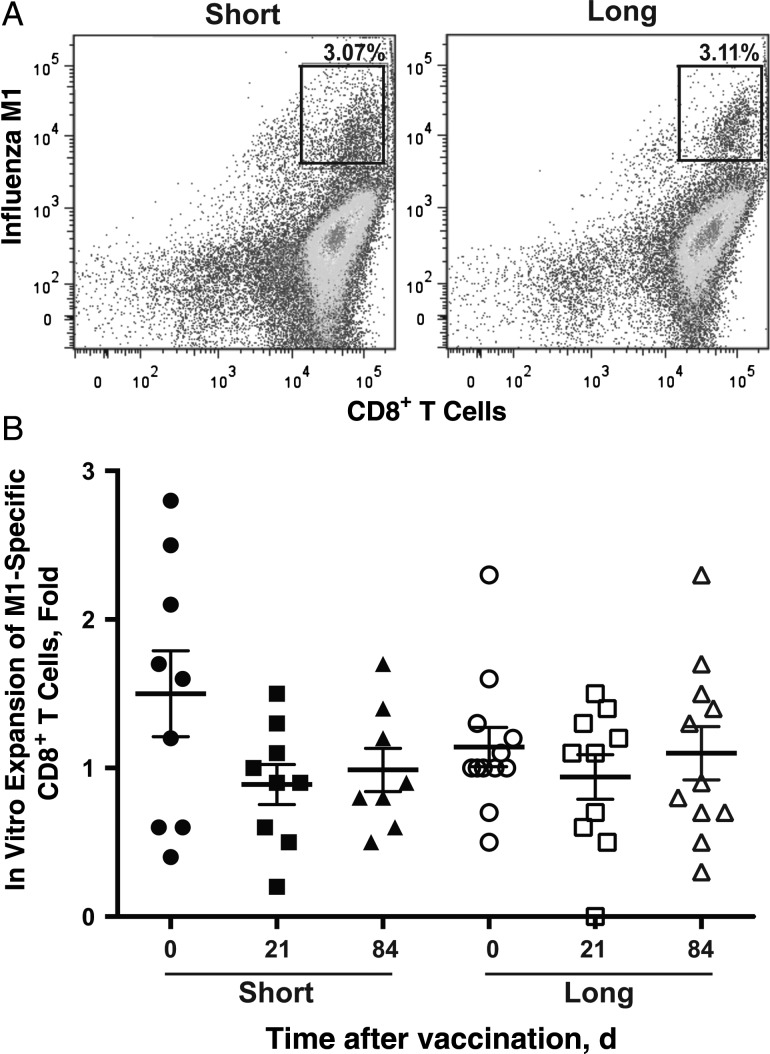
To assess T-cell functions, we first analyzed the ability of APCs to induce an influenza-specific CD8+ T-cell proliferative response in vitro. Monocytes were isolated from the blood of the participants and differentiated into APCs in vitro. APCs were then pulsed with an influenza-specific antigen (matrix peptide, M1-61-65) and incubated with the control CD8+ T cells from a healthy HLA-A2–positive participant for 7 days. The induced CD8+ T-cell responses were measured by their expansion using flow cytometry and cell counts. We found no significant difference in the expansion of M1-specific CD8+ T cells between the short and the long telomere groups before or after vaccination (Figure 2). Monocytes are terminally differentiated cells, and their differentiation to APCs does not require cell division; thus, it is not surprising that the function of monocyte-APC function is comparable between the short and long telomere subjects.
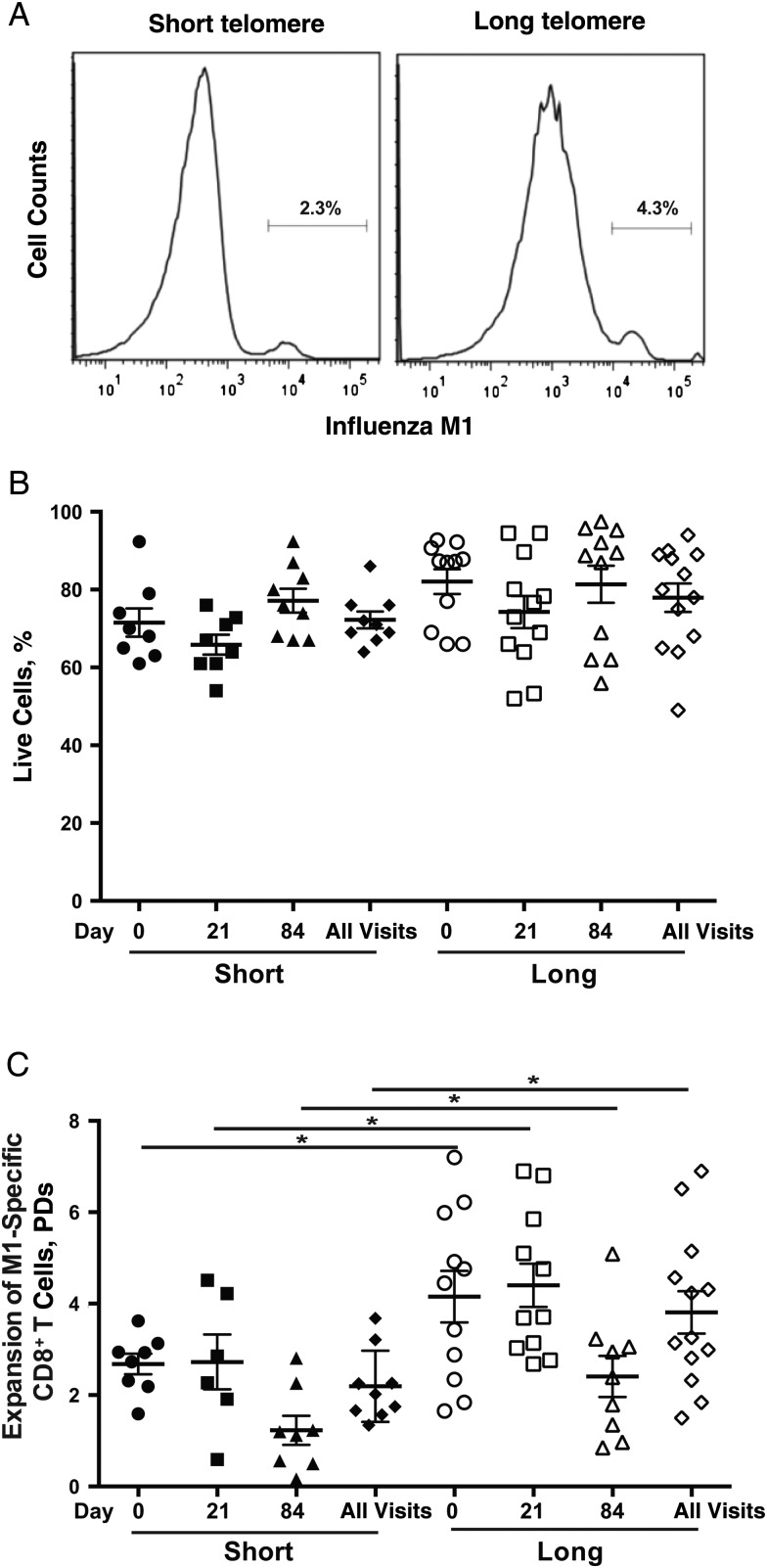
Figure 2.
No obvious differences were noted in antigen-presenting cell (APC) function between subjects in the short (n = 9) and long (n = 13) telomere groups. A, M1-specific CD8+ T cells after in vitro stimulation (% of total CD8 T cells). The differentiation of monocytes to APCs in vitro was described in “Participants, Materials, and Methods” section. Representative fluorescence-activated cell sorting plots are presented for short and long telomere groups. B, Expansion of M1-specific CD8+ T cells after 7 days in vitro. Monocytes were isolated from peripheral blood mononuclear cells from each visit (day 0, 21, or 84 after vaccination, as indicated) of study subjects, cultured for 5 days in the presence of interleukin 4 (20 ng/mL) and granulocyte-macrophage colony-stimulating factor (10 ng/mL), and then cultured for another day after lipopolysaccharide was added (100 ng/mL) at day 5. Mature APCs were pulsed with M158−66 (GILGFVFTL) (5 µg/mL), irradiated, and then incubated with CD8+ T cells from a healthy HLA-A2–positive adult for another 7 days. At the end of 7 days, cells were harvested for cell count, and M1-specific CD8+ T cells were analyzed with flow cytometry. Individual symbols represent values for individuals subject at a given visit (days 0, 21, and 84); means and standard errors of the mean are also shown.
CD4+ and CD8+ Profiles and Proliferative Response in Influenza-Specific CD8+ T Cells in Long and Short Telomere Group
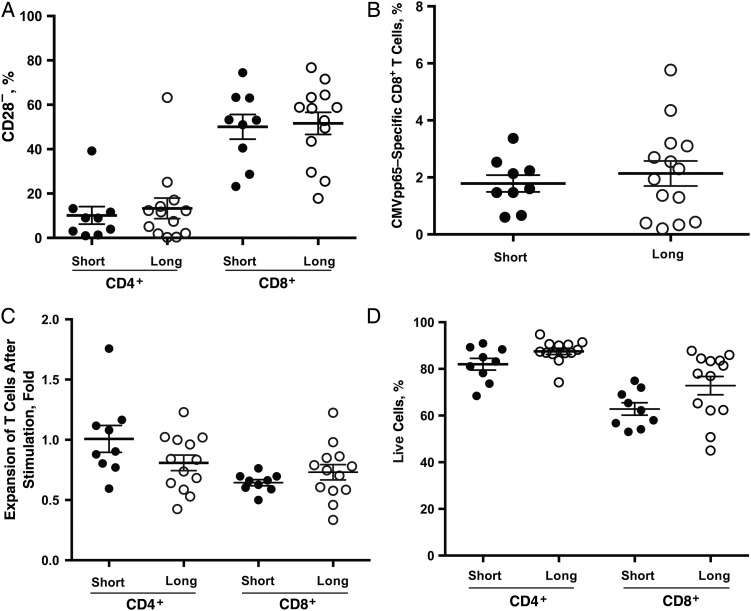
Reduced immune response to the influenza vaccine has been linked with an increase of CD28− T cells and CMV-reactive CD8+ T cells [9, 10, 41]. However, we did not find any substantial differences in the percentages of CD28− T cells and CMV pp65-reactive CD8+ T cells in the blood of short and long telomere groups (Figure 3A and 3B). To assess activation-induced proliferation, we stimulated CD4+ and CD8+ T cells isolated at each visit from the participants in vitro with anti-CD3 antibody in the presence of interleukin 2 for 3 days. Proliferation was measured by the live cell count before and after in vitro stimulation. We observed a similar proliferative response of CD4+ and CD8+ T cells in short and long telomere groups (Figure 3C), and no obvious differences in cell death between these groups, as judged by the percentage of live cells at harvest (Figure 3D).
Figure 3.
Comparable general profiles of CD4+ and CD8+ T cells in short (n = 9) and long (n = 13) telomere groups. A, B, Percentages of CD28− (A) and cytomegalovirus (CMV) pp65–specific CD8+ (A) T cells in both telomere groups. CD28− and CMV pp65–specific CD8+ T cells were determined with flow cytometry, as described in “Participants, Materials, and Methods” section. C, Expansion of CD4+ and CD8+ T cells in response to anti-CD3 plus interleukin 2 (20 U/mL) for 3 days in vitro. Live cells were counted using Trypan blue dye. Each circle represents a mean of 3 visits per subject; group means (with standard error of the mean) are also shown. D, Percentages of live CD4+ and CD8+ T cells after 3-day stimulation; live cells were identified based on the fluorescence-activated cell sorting profile with forward and side scatter. Each circle represents a mean of 3 visits per subject.
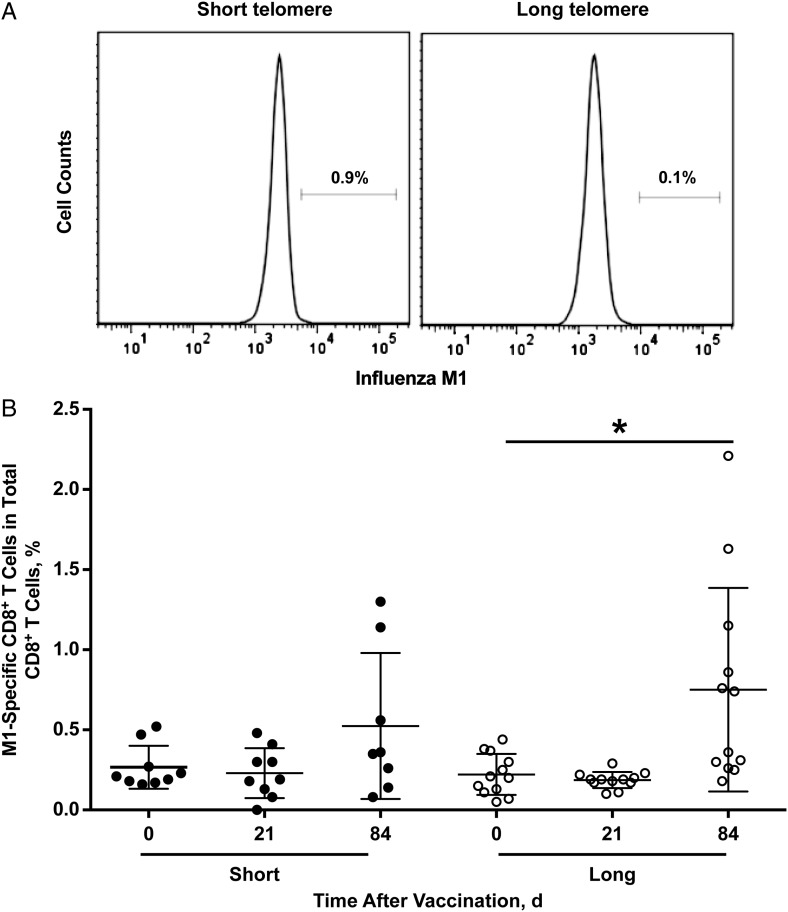
To assess influenza-specific (M1)-CD8+ T cells after vaccination, we analyzed the M1-specific CD8+ T cells in the blood and found that the percentages of M1-specific CD8+ T cells were similar before and 21 day after vaccination in both telomere groups. A slight increase in the percentage of M1-specific T cells was found at day 84 in the long telomere but not the short telomere group (Figure 4A and 4B). When 2 postvaccine time points were combined, the frequency of M1-specific CD8+ T cells did not differ significantly between telomere groups.
Figure 4.
Frequency and in vitro expansion of M1-specific CD8+ T cells. A, Frequency of M1-specific CD8+ T cells in short (n = 9) and long (n = 13) telomere groups, displayed as representative histograms. B, Frequency of M1-specific CD8+ T cells from participants in short and long telomere group before and after influenza vaccination. Each circle represents a single subject; group means and standard deviations are also shown. *P < .05 (day 0 vs day 84 in the long telomere group).
To determine whether the proliferative capacity of M1-specific CD8+ T cells was related to the telomere length, we applied the APCs/M1 peptide system (APCs were derived from monocytes of a healthy HLA-A2–positive subject, irradiated, ad then pulsed with the M1 peptide) to stimulate isolated CD8+ T cells from participants at each visit. M1-specific CD8+ T cells were analyzed with flow cytometry at day 7, and the percentages of live cells after stimulation were comparable in the long and short telomere groups (Figure 5A). However, M1-specific CD8+ T cells from the long telomere group displayed significantly greater expansion at each visit and as a whole than the short telomere group (mean [SEM] population doubling [PD], 3.8 [1.6] vs (2.2 [0.8]) (Figure 5B). Overall, the telomere length of PBMCs was positively correlated with the degree of M1-specific CD8+ T-cell expansion in vitro (r = 0.425). This finding shows that overall longer telomere lengths (for PBMCs) are correlated with greater expansion of M1-specific CD8+ T cells in response to M1 peptide in vitro.
Figure 5.
Expansion of M1-specific CD8+ T cells after in vitro stimulation. A, A representative histogram of the frequency of M1-specific CD8+ T cells after in vitro expansion in short (n = 9) and long (n = 13) telomere groups. Activation of M1-specific CD8+ T cells using monocyte-derived antigen-presenting cells (APCs), as described in “Participants, Materials, and Methods” section. B, Percentage of live M1-specific CD8+ T cells after 7-day in vitro stimulation by APCs plus M1 peptide. Circles represent results for single subjects; group means and standard errors of the mean are also shown. Live cells were identified based on the fluorescence-activated cell sorting profile with gating from forward and side scatter. C, Expansion of M1-specific CD8+ T cells after in vitro stimulation by APCs plus M1 peptide. M1-specific-CD8+ T cells were stained with M1 dextramer before and after culture and analyzed by means of flow cytometry. Population doublings (PDs) were calculated based on the live M1-specific CD8+ T cells before and after 7-day culture. Group means and standard errors of the mean are shown. *P < .05 (age adjusted).
Telomere Length of M1-Specific CD8+ T Cells Correlated With In Vitro Expansion
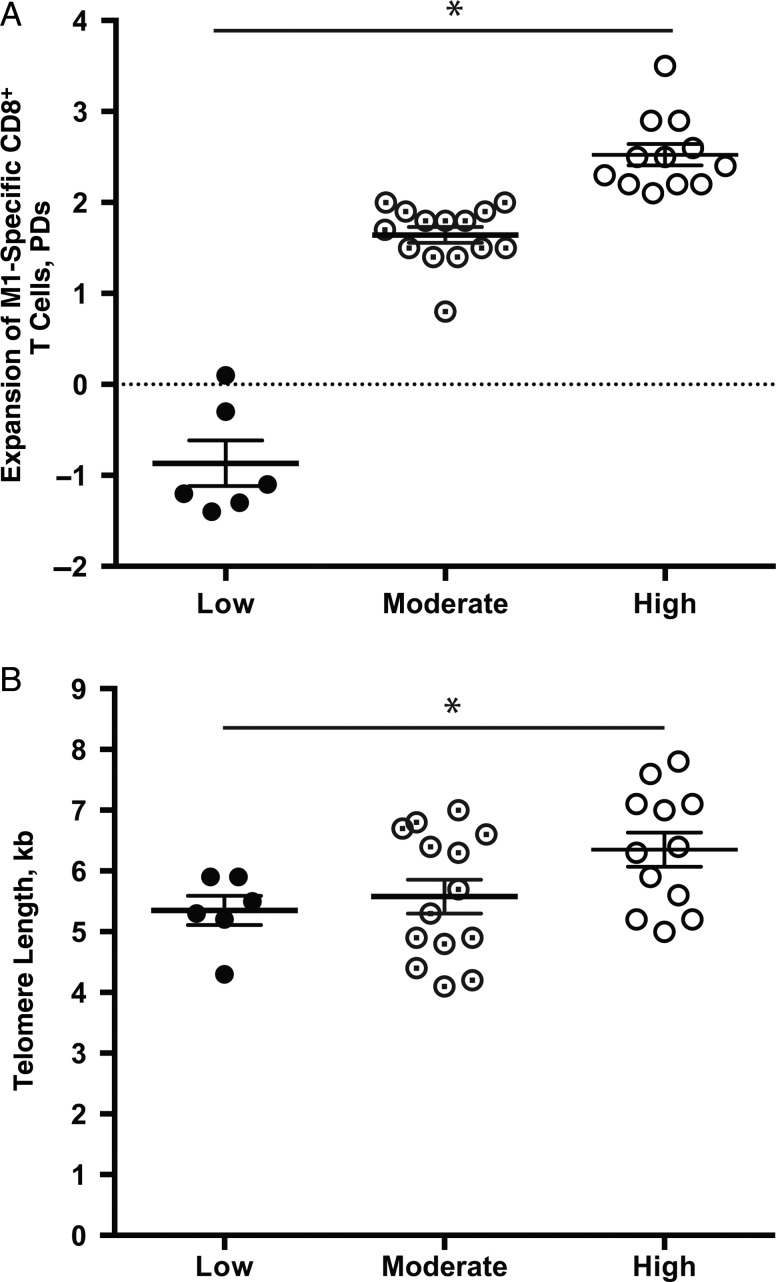
M1-specific CD8+ T cells account for <0.1% of PBMCs. Using an artificial APC system [37], we directly measured the telomere length of M1-specific CD8+ T cells from 10 participants who had enough CD8+ T cells as well as 20 other healthy adults. The expansion of M1-specific CD8+ T cells was calculated based on the live cell counts and flow cytometric analysis, presented as PD, and the expanded M1-specific CD8+ T cells were sorted according to telomere length. Based on the expansion, we divided them into low-expansion (mean [SEM] PD, −0.9 [0.3]; n = 6), modest-expansion (mean [SEM] PD, 1.6 [0.1]; n = 14), and high-expansion (mean [SEM] PD, 2.5 [0.1]; n = 12) groups (Figure 6A). There were no significant differences in live cell percentages among these 3 groups (Supplementary Figure 1), but telomeres were significantly longer in expanded M1-specific CD8+ T cells from the high-expansion compared with the low-expansion group (mean [SEM] length, 6.4 [0.3] vs 5.3 [0.3] kb) (Figure 6B). This finding confirms that telomere length is positively correlated with the proliferation of M1-specific CD8+ T cells in vitro and suggests that the robustness of expansion of M1-specific CD8+ T cells may be partially determined by telomere length.
Figure 6.
Telomere length of M1-specific CD8+ T cells correlated with the in vitro antigen-induced cell expansions in vitro. A, Expansion of M1-specific CD8+ T cells in vitro. The expansion of M1-specific CD8+ T cells was determined by means of cell counts and flow cytometric analysis using M1–HLA-A2 dextramer. Based on the number of cell divisions, influenza M1–specific CD8+ T cells or naive or memory CD8+ T cells were divided into 3 expansion groups: low (mean population doubling [PD], −0.9 [0.3]; n = 6), modest (mean PD, 1.6 [0.1]; n = 14), and high (mean PD, 2.5 [0.1]; n = 12). Values represent means and standard errors of the mean. B, Mean telomere length of expanded M1-specific CD8+ T cells in each expansion group: low expansion, 5.3 (0.3) kb (n = 6); modest expansion, 5.6 (0.3) kb (n = 14); and high expansion, 6.4 (0.3) kb (n = 12). *P < .05.
DISCUSSION
This study aims to assess the role of telomere length in lymphocyte function by analyzing antibody response to the influenza vaccine and CD8+ T-cell response to an influenza antigen (M1) in healthy older subjects with relatively short or long telomeres. We found that (1) the antibody titer against the influenza vaccine is significantly higher in subjects whose B cells had longer telomeres; (2) there was no differences in monocyte-derived APC function or in the frequency of M1-specific CD8+ T cells in the blood before or 21 days after vaccination between short and long telomere groups; (3) M1-specific CD8+ T cells from long telomere subjects had a mean of 1.6 more PDs than those from the short telomere subjects; and (4) robustly expanded M1-specific CD8+ T cells had significantly longer telomeres than little-expanded M1-specific CD8+ T cells. Collectively, these findings demonstrate that telomere length is positively associated with B- and T-cell responses to the influenza vaccine/antigen in older subjects and suggest that telomere attrition with age may contribute to the age-associated decline of adaptive immune functions.
Old humans display a great heterogeneity in immune competency against various infections. Characterizing the immune phenotype of aging will be valuable for improving the efficacy of vaccination and predicting the potential outcome of the immune response to infection. Studies of elderly individuals with a reduced antibody response to influenza vaccine show an association with increased CD28−CD8+ T cells and expanded CMV reactive CD8+ T cells in blood [9–11]. In the current study, we did not find such changes between the long and the short telomere groups. Instead, we observed that the titers of anti-influenza antibody after vaccination were positively correlated with the telomere length of B cells. Although there was no significant difference in frequency of M1-specific CD8+ T cells in blood after vaccination between the long and the short telomere groups, we observed that the expansion of CD8+ T cells specific to influenza M1 antigen in vitro was also positively correlated with the telomere length of these CD8+ T cells. Because CD28−CD8+ T cells have relatively short telomeres [42, 43] whereas the expanded CMV-specific CD8+ T cells in older persons are often CD28− [44] and have short telomeres [27], telomere length is probably an earlier indicator of immune competency than the increased CD28−CD8+ T cells and expanded CMV-specific CD8+ T cells.
Telomere lengths in lymphocytes differ between B and T cells [19] and among different T-cell subsets [25, 42, 45]. Although the telomere length of PBMCs is useful for identifying general telomere lengths in an individual, determining telomere length in defined cell populations enables a close assessment of the role of telomere length in the proliferative response of that lymphocyte population. It is interesting to note that the correlation of telomere length between PBMCs and lymphocytes (B cells or M1-specific CD8+ T cells) was weaker than the that between B cells and M1-specific CD8+ T cells (data not shown). This highlights the importance of directly measuring telomere length for the defined type of cells when a specific lymphocyte response is under study.
Difficulty in measuring telomere length in low-frequency antigen-specific lymphocytes has been a major barrier for assessing the role of telomeres in the age-associated decline of adaptive immune functions. Here we showed that the telomere length in B cells and in antigen-specific CD8+ T cells is indicative of the competency of the B- and T-cell response. However, it remains to be determined whether B cells or plasma cells that produce antibodies against the influenza virus/vaccine have longer telomeres and how various lengths of telomeres of influenza-specific T cells affect their response during a real influenza infection. Such information should reveal a more detailed portrait of T-cell response to different pathogens and could provide a framework for choosing different clinical interventions as a basis for personalized medicine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Richard Hodes for critically reading the manuscript, the National Institute on Aging clinical core laboratory for collecting blood samples, and Robert Wersto of the flow cytometry core laboratory for cell sorting.
Financial support. This research was supported by the Intramural Research Program of the National Institute on Aging and the Radiation and Nuclear Countermeasures Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013; 14:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 2006; 24:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011; 121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev 2011; 10:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol 2013; 25:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng NP. Telomere and adaptive immunity. Mech Ageing Dev 2008; 129:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decman V, Laidlaw BJ, Dimenna LJ, et al. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol 2010; 184:5151–9. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Fisher EM, Murasko DM. Intrinsic defects in CD8 T cells with aging contribute to impaired primary antiviral responses. Exp Gerontol 2013; 48:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 2002; 168:5893–9. [DOI] [PubMed] [Google Scholar]
- 10.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 2001; 75:12182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol 2002; 2:982–7. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn EH. Telomere states and cell fates. Nature 2000; 408:53–6. [DOI] [PubMed] [Google Scholar]
- 14.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19:2100–10. [DOI] [PubMed] [Google Scholar]
- 15.Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol 1998; 8:R178–81. [DOI] [PubMed] [Google Scholar]
- 16.Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A 1992; 89:10114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 1995; 92:11190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with Age. J Immunol 2000; 165:1191–6. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Damjanovic A, Metter EJ, et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci (Lond) 2015; 128:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345:458–60. [DOI] [PubMed] [Google Scholar]
- 21.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol 2002; 2:699–706. [DOI] [PubMed] [Google Scholar]
- 22.Weng NP. Telomeres and immune competency. Curr Opin Immunol 2012; 24:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng NP, Granger L, Hodes RJ. Telomere lengthening and telomerase activation during human B cell differentiation. Proc Natl Acad Sci U S A 1997; 94:10827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norrback KF, Dahlenborg K, Carlsson R, Roos G. Telomerase activation in normal B lymphocytes and non-Hodgkin's lymphomas. Blood 1996; 88:222–9. [PubMed] [Google Scholar]
- 25.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A 1995; 92:11091–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Effros RB, Boucher N, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol 1994; 29:601–9. [DOI] [PubMed] [Google Scholar]
- 27.van de Berg PJ, Griffiths SJ, Yong SL, et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol 2010; 184:3417–23. [DOI] [PubMed] [Google Scholar]
- 28.van Baarle D, Nanlohy NM, Otto S, Plunkett FJ, Fletcher JM, Akbar AN. Progressive telomere shortening of Epstein-Barr virus-specific memory T cells during HIV infection: contributor to exhaustion? J Infect Dis 2008; 198:1353–7. [DOI] [PubMed] [Google Scholar]
- 29.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet 2009; 10:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med 2010; 12:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim Biophys Acta 2009; 1792:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batista LF, Pech MF, Zhong FL, et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 2011; 474:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010; 2:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler MG, Tilburt J, DeVries A, et al. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet 1998; 105:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev 2000; 119:89–99. [DOI] [PubMed] [Google Scholar]
- 36.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res 2002; 52:377–81. [DOI] [PubMed] [Google Scholar]
- 37.Ndhlovu ZM, Angenendt M, Heckel D, Schneck JP, Griffin DE, Oelke M. Development of an artificial-antigen-presenting-cell-based assay for the detection of low-frequency virus-specific CD8(+) T cells in whole blood, with application for measles virus. Clin Vaccine Immunol 2009; 16:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hathcock K, Hodes R, Weng NP. Methods of analysis of telomere length and telomerase activity. New York: John Wiley & Sons, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 2011; 29:5015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derhovanessian E, Maier AB, Hahnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol 2014; 193:3624–31. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol 1996; 156:3587–90. [PubMed] [Google Scholar]
- 43.Allsopp R, Chiu CP, Hausner MA, et al. Shortened telomeres in the expanded CD28- CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 1996; 10:F17–22. [DOI] [PubMed] [Google Scholar]
- 44.Ouyang Q, Wagner WM, Wikby A, et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 2003; 23:247–57. [DOI] [PubMed] [Google Scholar]
- 45.Rufer N, Brummendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 1999; 190:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.