Abstract
Children in sub-Saharan Africa continue to acquire and die from cerebral malaria, despite efforts to control or eliminate the causative agent, Plasmodium falciparum. We present a quantitative histopathological assessment of the sequestration of parasitized erythrocytes in multiple organs obtained during a prospective series of 103 autopsies performed between 1996 and 2010 in Blantyre, Malawi, on pediatric patients who died from cerebral malaria and controls. After the brain, sequestration of parasites was most intense in the gastrointestinal tract, both in patients with cerebral malaria and those with parasitemia in other organs. Within cases of histologically defined cerebral malaria, which includes phenotypes termed “sequestration only” (CM1) and “sequestration with extravascular pathology” (CM2), CM1 was associated with large parasite numbers in the spleen and CM2 with intense parasite sequestration in the skin. A striking histological finding overall was the marked sequestration of parasitized erythrocytes across most organs in patients with fatal cerebral malaria, supporting the hypothesis that the disease is, in part, a result of a high level of total-body parasite sequestration.
Keywords: autopsy, cerebral malaria, pediatrics, Africa, Malawi, pathology
Despite massive, large-scale programs for the elimination and possible eradication of malaria, 200 million people are infected annually; 655 000 deaths still occur each year, almost exclusively in sub-Saharan African children <5 years of age [1]. Cerebral malaria (CM) is defined clinically as coma (based on a Blantyre coma score of ≤2), parasitemia, and no evidence of hypoglycemia, meningitis, or postictal state. We previously modified this definition to include signs of malaria retinopathy on retinal examination, which eliminated approximately 25% of diagnoses that were incorrect at autopsy [2]. When patients meeting the modified definition are examined at autopsy, histological evidence of intense parasite sequestration is found in the brain [3].
In this study, we investigated the quantitative levels of sequestration in organs other than the brain. We performed histological analyses of organs obtained during 103 autopsies and tested 2 hypotheses regarding the quantitative parasite pathology in CM. The first hypothesis was that CM is a specific condition associated with a high number of sequestered parasites throughout the organs of the body, including the brain, and that, conversely, comatose patients with parasitemia who do not meet our modified clinical case definition have little to no sequestered parasites. Our second hypothesis was that the median sequestered parasite numbers differ between 3 patient groups that underwent autopsy—those with CM, those with severe malarial anemia (SMA), and parasitemic controls with nonmalarial causes of coma—and that the highest burden is in patients with histologically defined CM.
MATERIALS AND METHODS
Patients
Study design, ethical approval, patient recruitment, clinical management, autopsy protocols, and laboratory investigations have been previously described [2–6]. Briefly, if, after enrollment into an ongoing clinical study of malaria pathogenesis, the subject died, and informed consent for an autopsy was obtained from a parent/guardian, a standardized autopsy was carried out. Cases were subjects meeting the standard clinical case definition of cerebral malaria (CCM). Controls were subjects with severe malarial anemia, coma without parasitemia, parasitemia with nonmalarial coma, as well as noncomatose, nonparasitemic patients. The brain and all organs were dissected immediately, without fixation.
Histological Classification
All histological tissue specimens used in the study were fixed in fresh 10% neutral buffered formalin, processed, embedded in paraffin, cut into 3–4-μm sections, and stained with hematoxylin and eosin per routine protocols. Review of brain histological findings by 2 pathologists for the overall features of CM allowed for histological classification of patients who met the clinical case definition (CCM) during life into 3 distinct categories: CM1, sequestration of parasitized red blood cells (PRBCs) in the brain, no additional cerebral histopathological changes, and no other cause of death; CM2, sequestration of PRBCs and the presence of cerebral microthrombi, ring hemorrhages, and extraerythrocytic malaria pigment, and no other cause of death; and CM3, no sequestration of PRBCs in the brain, and another cause of death identified [2]. Use of the modified clinical case definition (ie, the addition of malaria retinopathy to the classical case definition) would eliminate the CM3 designation, but for the purpose of testing the hypotheses proposed, we here use the CM1, CM2, and CM3 designations.
Quantification
Tissue sections from all organs sampled from 103 cases were reviewed for adequacy of preparation and morphological quality, and sequestration was determined quantitatively in brain, lung, heart, stomach, jejunum, ileum, colon, kidney, skin, and spleen. This subset of organs was selected because they had the most-consistent sectioning and sampling, represented a diversity of vascular blood flow patterns, and consistently had sequestration appreciable in qualitative review [7]. The heart section examined was the left (predominantly) and right ventricle; the lung section examined was from the right upper or lower lobe (inflated with formalin prior to sectioning). PRBCs in each organ other than the brain were counted using the same protocol, in which the total number of PRBCs seen in all vessels in at least 10 high-power fields were counted and reported as the number of PRBCs per 10 high-power fields. The methods used for counting PRBCs in the brain were as previously published [3], and the brain counts were converted for the purposes of the present study to the per 10 high-power field nomenclature, using the average number of capillaries per high-power field from a test set. For the intestinal tract, counting was limited to the capillary network of the lamina propria. The 2 tissue blocks representing the intestinal tract contained 2 pieces of tissue each (stomach and ileum; jejunum and colon). When only 1 section was available for review in a block with adequate lamina propria, the count for the available one was imputed (by assumption) for the other. In addition to routine processing, a section of spleen was soaked in picric acid for 48 hours to remove all pigments (ie, hemozoin, iron, and formalin) and then stained with Giemsa stain. Splenic PRBC counts were measured in the red pulp of the spleen containing the sinusoids and littoral cell network. Parasites in skin are found largely in vessels supplying subcutaneous fat; skin counts were taken exclusively from those vessels.
Statistical Analysis
To control for the variability in vascular bed density across organs, counts of parasites between organs were compared using the nonparametric Kruskal–Wallis and trend tests to test the hypothesis that the parasite burden would decrease in the following order, based on our a priori hypothesis: CM1>CM2>>SMA>CM3>other. The term “other” included all patients who did not meet the clinical case definition of CM in life, except for patients with SMA, a discrete category of severe malaria. The total number of sequestered parasites across measured organs was summed (excluding those from the brain) and compared by the Kruskal–Wallis and trend tests between CM and non-CM cases.
RESULTS
Of the 103 patients who underwent autopsy, the final anatomical diagnoses were distributed as follows: CM1, 13; CM2, 41; CM3, 20; SMA, 4; nonmalarial coma, 17; incidental parasitemia, 3; indeterminate, 4; and nonmalarial death, 1. On review, 2 of the 4 indeterminate cases met the histological criteria for CM, but insufficient clinical data were gathered to make a premortem diagnosis. The single nonmalarial, noncomatose patient who died was aparasitemic and had pneumonia.
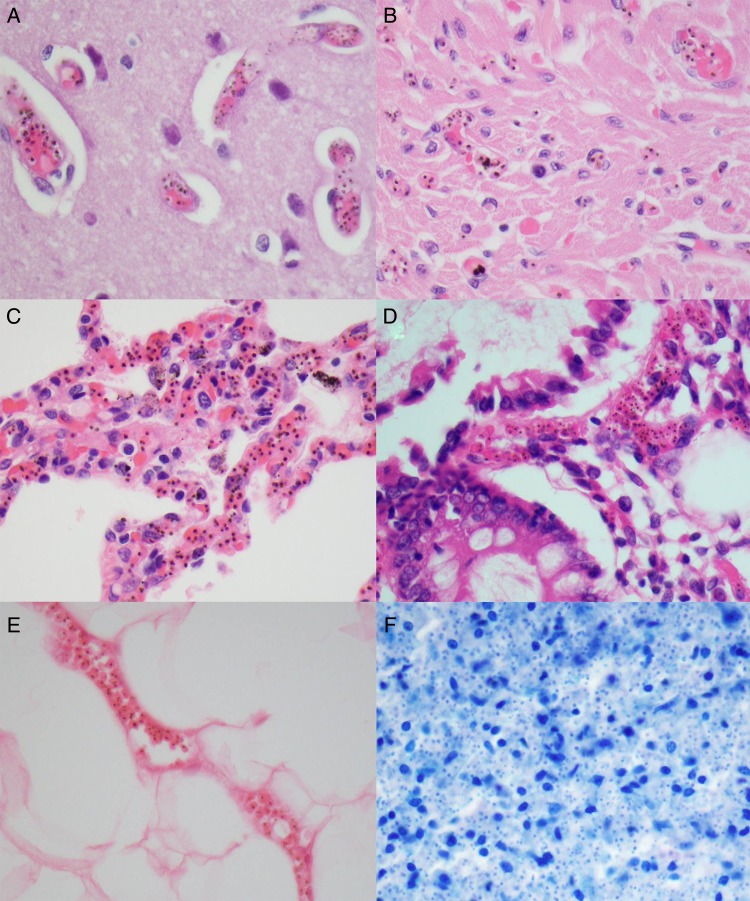
Sequestration of PRBCs occurred in central nervous system capillaries in all CM1 and CM2 cases but rarely if at all in SMA, CM3, or control cases. The quantity of parasites per organ in the CM1 group was higher than that in the CM2 and other groups, with a significant trend holding across the groups (Table 1). Although the quantities within different organs were similar between the CM1 and CM2 groups, trends across organs, including heart, lung, and spleen, showed higher numbers in CM1 cases, compared with CM2 cases. The gut was similar in both categories, and the skin showed more intense sequestration in CM2 cases, compared with CM1 cases (Table 1). In a few CM3 cases, SMA cases, and controls, sparsely scattered parasites were observed (Table 1 and Supplementary Table 1). Representative examples of tissue sequestration across the organs that were counted are shown in Figure 1.
Table 1.
Quantitative Distribution of Parasites Across the Brain and Other Organs Among Patients Who Underwent Autopsy
| Organ, Metric | Cases, No. | Diagnosisa |
||||
|---|---|---|---|---|---|---|
| CM1 | CM2 | SMA | CM3 | Other | ||
| Brain | ||||||
| Parasitized vessels, %b | 99 | 87 (76.6–95.8) | 84.6 (61.4–93.0) | 0.5 (0–2.85) | 0 (0–2.6) | 0 (0–0) |
| Unpigmented parasites, no./100 vessels | 99 | 11 (0–50) | 6.6 (0–102) | 0 (0–2) | 0 (0–0) | 0 (0–0) |
| Pigmented parasites, no./100 vessels | 99 | 201 (147–396) | 202 (47–314) | 0 (0–0.5) | 0 (0–0) | 0 (0–0) |
| Parasites, no./10 HPFc | 99 | 62 (58–102) | 59 (35–84) | 0.1 (0–0.5) | 0 (0–0.5) | 0 (0–0) |
| Heart, parasites, no./10 HPFc | 82 | 42 (2–123) | 14 (5–43) | 18 (0–19) | 0 (0–4) | 0 (0–2) |
| Lung, parasites, no./10 HPFc | 94 | 43 (26–115) | 29 (17–71) | 3 (1–23) | 8 (5–22) | 7 (2–20) |
| Stomach, parasites, no./10 HPFc | 87 | 49 (16–375) | 52 (13–209) | 10 (0–27) | 3 (1–13) | 1 (0–4) |
| Jejunum, parasites, no./10 HPFc | 82 | 93 (22–368) | 87 (31–542) | 27 (11–42) | 18 (4–54) | 0 (0–26) |
| Ileum, parasites, no./10 HPFc | 87 | 86 (3–265) | 51 (13–108) | 11 (2–43) | 11 (1–26) | 2 (0–6) |
| Colon, parasites, no./10 HPFc | 83 | 142 (10–379) | 69 (17–405) | 38 (6–70) | 13 (0–28) | 0 (0–7) |
| Spleen, parasites, no./10 HPFc | 92 | 63 (47–113) | 10 (3–115) | 9 (5–15) | 3 (2–9) | 7 (2–11) |
| Skin, parasites, no./10 HPFc | 48 | 19 (5–138) | 109 (40–316) | 0 (0–2.7) | 0 (0–3) | 0 (0–0) |
| Overalld | … | 488 (217–1237) | 354 (159–1598) | 51 (16–179) | 68 (35–106) | 34 (15–47) |
Data are median (interquartile range), unless otherwise indicated. For all rows, findings from the Kruskal–Wallis test (differences among groups, nonparametric) and the nonparametric trend test (decreasing value from left to right) were highly significant (P ≤ .0001).
Abbreviations: CM1, sequestration only; CM2, sequestration with extravascular pathology; CM3, no sequestration of parasitized red blood cells in the brain, and another cause of death identified; HPF, high-power field; SMA, severe malarial anemia.
a All diagnoses but SMA had ≥6 cases per organ. Indeterminate cases were not used in calculations.
b Out of at least 100 counted vessels.
c Data were calculated by dividing the total number of parasites/100 vessels by 4, which approximates the number of parasites in capillaries per 10 HPF (1000× original magnification; oil immersion). However, because this counting method only includes capillaries, these data underestimate the total number of parasites present in any section.
d Data denote the sum of all counts (per 10 HPF) for each organ, where available. If no count was available, 0 was used. Thus, these data underestimate the true range, because cases with missing organs may be undercounted.
Figure 1.
Representative images of the organs surveyed in this study for quantitation of parasites are shown at 600× original magnification. A–E, Hematoxylin and eosin–stained sections of brain (A), heart (B), lung (C), gastrointestinal tract (D), and skin (E). F, Giemsa-stained section of spleen after depigmentation. The vascularity of each organ varies but prominent parasites with distinctive pigment are seen easily.
DISCUSSION
We proposed, on the basis of our previous qualitative data, that CM is a specific condition associated with a large numbers of sequestered parasites throughout the organs of the body, including the brain [7]. Our quantitative data demonstrate that, in parasitemic, comatose patients, the brain and other organs have a higher number of parasites than controls with similar peripheral parasitemia levels before death [2]. Conversely, comatose patients with parasitemia who did not meet our modified clinical case definition (and lacked significant brain sequestration at <20% of vessels) lacked a significant burden of sequestered parasites [3]. Further, we looked at the median organ parasite numbers between our patient groups coming to autopsy—those with CM, those with SMA, and controls with other diagnoses—and found the highest numbers in histologically defined CM (Table 1).
Between CM1 and CM2 cases, we noted differences in the median values, with similar ranges. After depigmentation of spleen sections in picric acid, individual PRBCs were sometimes noted on careful examination in CM2, CM3, SMA, and other groups, but large numbers of splenic parasites were a feature predominantly of patients with CM1. Sequestration of PRBCs in the skin only occurred in the capillaries and small venules and arterioles of the subcutaneous adipose tissue, sparing the dermis. The skin had more parasites in CM2 cases, compared with CM1 cases. Skin tissue sections were also the most difficult to assess, with less than half of the cohort being analyzed, because of technical issues of quality and section adequacy. Findings for the gut, overall, were similar in CM1 and CM2 cases but quite different from findings for controls, which leads us to conclude, on the basis of the large total volume of gut microvasculature, that this represents a large and very important proportion of total body sequestration; how this manifests clinically is unclear, but gastrointestinal complaints are commonly associated with malaria infection.
Previous clinical data from our cohort and this complete quantitative cohort demonstrate one of the remarkable difference between CM1 and CM2 at autopsy: death occurs at variable time points in the course of the infection [3, 7]. That one particular unified parasite “event” causes death (other than the common features of dense full-body sequestration and increase in brain volume) is unlikely. CM1, the less common pattern, appears to represent an overall more rapid disease with higher levels of parasite sequestration in the spleen (the primary organ of parasite clearance) but little end organ pathology (ie, ring hemorrhages). In contrast, the pathology associated with CM2, the more common of the two, appears to be associated with a slightly prolonged illness with the development of end organ pathology in the brain. The lack of end organ damage outside of the brain in our pediatric cohort (and the rarity of multiorgan failure as seen in adult patients) is possibly explained by the difference in duration of illness and the rapid onset of the cerebral dysfunction that leads to death in children [8–10]. The pathology of CM in adults in areas of low malaria endemicity has been studied quantitatively by others across several organs [11–14]. Adults survive (usually with support) for protracted periods, but death, when it occurs, is due to the development of multiorgan failure [8].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the parents and families in Malawi, for their continued support of this ongoing project, as well as the children of Malawi, for their participation; Sam Wassmer, Jacqui Montgomery, Dumizulu Tembo, Jimmy Vareta, Fingani Mphande, Wales Namanya, Johanness Kaliwambe, and Laston James Mbewe, for their indispensable effort in making the autopsy study possible in Malawi; Sebastian Lucas, for wise advice; and the many pathologists who volunteered their services in Malawi to perform autopsies during our transitional period and capacity building.
Financial support. This work was supported by the National Institutes of Health (grants RO1 AO34969 and K23 AI072033), the Wellcome Trust (grant 042390/Z/94), and Brigham and Women's Hospital (medical student pathology fellowship program).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO Global Malaria Program. World malaria report 2011. Geneva: World Health Organization, 2011. [Google Scholar]
- 2.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 2004; 10:143–5. [DOI] [PubMed] [Google Scholar]
- 3.Milner DA, Jr, Valim C, Carr RA, et al. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar J 2013; 12:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner DA, Taylor TE. Continuing study of pediatric fatal malaria in Blantyre, Malawi. Int J Parasitol 2008; 38(supp 1):S22. [Google Scholar]
- 5.Seydel KB, Milner DA, Jr, Kamiza SB, Molyneux ME, Taylor TE. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis 2006; 194:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitten R, Milner DA, Jr, Yeh MM, Kamiza S, Molyneux ME, Taylor TE. Liver pathology in Malawian children with fatal encephalopathy. Hum Pathol 2011; 42:1230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milner DA, Jr, Whitten RO, Kamiza S, et al. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol 2014; 4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medana IM, Day NP, Sachanonta N, et al. Coma in fatal adult human malaria is not caused by cerebral oedema. Malar J 2011; 10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponsford MJ, Medana IM, Prapansilp P, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 2012; 205:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White NJ, Turner GD, Day NP, Dondorp AM. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis 2013; 208:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg 2003; 69:345–59. [PubMed] [Google Scholar]
- 12.Prommano O, Chaisri V, Turner GD, et al. A quantitative ultrastructural study of the liver and the spleen in fatal falciparum malaria. Southeast Asian J Trop Med Public Health 2005; 36:1359–70. [PubMed] [Google Scholar]
- 13.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol 1999; 155:395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban BC, Hien TT, Day NP, et al. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect Immun 2005; 73:1986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.