Abstract
Background. Plasmodium falciparum invades human erythrocytes by using an array of ligands that interact with several receptors, including sialic acid (SA), complement receptor 1 (CR1), and basigin. We hypothesized that in malaria-endemic areas, parasites vary invasion pathways under immune pressure. Therefore, invasion mechanisms of clinical isolates collected from 3 zones of Ghana with different levels of endemicity (from lowest to highest, Accra, Navrongo, and Kintampo) were compared using standardized methods.
Methods. Blood samples were collected from children aged 2–14 years in whom malaria was diagnosed, and erythrocyte invasion phenotypes were determined using the enzymes neuraminidase, chymotrypsin, and trypsin, which differentially cleave receptors from the erythrocyte surface. In addition, antibodies against CR1 and basigin were used to determine the contributions of these receptors to invasion. Gene expression levels of P. falciparum invasion ligands were also examined.
Results. The parasites generally expressed SA-independent invasion phenotypes across the malaria-endemic areas, with parasites from Kintampo showing the highest invasion rates in neuraminidase-treated erythrocytes. CR1 was a major mediator of SA-independent invasion, while basigin was essential for both SA-dependent and SA-independent invasion mechanisms. Furthermore, expression of the basigin ligand PfRh5 was the best predictor of donor parasitemia.
Conclusions. Erythrocyte invasion phenotypes expressed by P. falciparum are influenced by endemicity levels, and the PfRh5-basigin pathway is a potential vaccine target.
Keywords: Plasmodium falciparum, malaria, erythrocyte invasion, endemicity, complement receptor 1, basigin, ligand gene expression
As a result of concerted efforts toward malaria control, the global incidence of severe life-threatening malaria in malaria-endemic areas has decreased significantly over the last decade [1]. However, the disease remains a significant cause of morbidity and mortality in children in sub-Saharan Africa, accounting for 163 million cases and 437 000 deaths in children <5 years old [1]. Thus, the development of an effective deployable vaccine remains the optimal strategy for solving the menace of malaria. Although the parasites initially infect liver cells, clinical symptoms of malaria are caused only after the parasites enter the bloodstream and invade erythrocytes. Therefore, targeting the erythrocyte invasion mechanism is a logical approach for the design of vaccines for preventing clinical manifestations and reducing disease severity of malaria. A complete understanding of the molecular mechanisms of erythrocyte invasion is hampered because Plasmodium falciparum has a repertoire of ligands that interact with a variety of erythrocyte receptors during invasion.
Sialic acid (SA) on glycophorins is the major receptor used by P. falciparum for invasion of erythrocytes [2–6]. Therefore, invasion pathways have often been categorized as SA dependent or SA independent. Recent studies have begun to clarify the molecular interactions involved in SA-independent invasion, with the identification of complement receptor 1 (CR1) as a key receptor [7, 8]. Furthermore, the Ok blood group antigen, basigin, has been identified as playing an essential role in erythrocyte invasion by many strains of P. falciparum [9, 10]. Plasmodium falciparum interacts with the various erythrocyte receptors by using a range of ligands, including erythrocyte binding antigen 175 (EBA-175) and EBA-140, which bind glycophorins A and C, respectively [2–6], and reticulocyte binding protein homologues PfRh4 and PfRh5, which bind CR1 and basigin, respectively [7–10]. Additional parasite proteins such as EBA-181, PfRh1, PfRh2a, and PfRh2b have also been shown to play roles in invasion [11], but the erythrocyte receptors they interact with remain unclear.
Plasmodium falciparum appears to deploy these ligands strategically, such that alternative pathways are only activated if the preferred pathway is blocked [12]. In malaria-endemic areas, immune pressure likely influences the choice of ligands that the parasites deploy and, hence, their erythrocyte receptor preferences. Furthermore, there is evidence that antibodies to the SA-dependent ligands are acquired before immunity to the SA-independent ligands is achieved [13]. Therefore, we hypothesize that the prevailing invasion phenotypes expressed by parasites in a given P. falciparum–endemic area will be determined by the strength of immune-mediated selection, which in turn is influenced by the intensity of malaria transmission. Previous studies have shown that field isolates of P. falciparum from various geographical areas with different malaria endemicities, including The Gambia, Senegal, Brazil, India, and Kenya, use different invasion pathways [14–20]; however, the relationship between intensity of transmission and invasion phenotypes has not been examined.
In this study, we investigated invasion receptor preferences of parasites collected from children aged 2–14 years residing in 3 regions in Ghana (Accra, Kintampo, and Navrongo) where malaria transmission is endemic but differs in pattern and intensity. Standardized methods were used for sample collection, processing, storage, culturing, and invasion assays at the 3 distinct locations. Erythrocyte invasion phenotypes were determined using a combination of enzymes with differential activities against the major receptors: neuraminidase cleaves SA, chymotrypsin digests glycophorin B and CR1 but not glycophorin A and C, and trypsin removes most receptors, including glycophorins A and C, and CR1 [6, 21]. Further resolution of receptor preferences was achieved by competitive inhibition of the 2 newly identified receptors CR1 and basigin. In addition, the relative gene expression levels of P. falciparum invasion ligands were examined, and their relationship with receptor preferences and clinical parasitemia was investigated.
MATERIALS AND METHODS
Ethical Statement
The research presented here was approved by the ethics committees of the Ghana Health Service, Navrongo Health Research Centre, Kintampo Health Research Centre, and Noguchi Memorial Institute for Medical Research, University of Ghana, Legon. All samples were collected after obtaining written informed consent from the parents/guardians of participating children who were aged ≤10 years. For children older than 10 years, additional assent was obtained from the donor, following receipt of parental consent.
Study Sites and Sample Collection
Three study sites in different ecological zones of Ghana (Supplementary Figure 1) with different transmission intensities were chosen for the collection of blood samples: Ledzokuku-Krowor Municipal Assembly Hospital in Teshie, Accra (Greater Accra Region), Navrongo Health Research Centre in Navrongo (Upper East Region), and Kintampo Health Research Centre in Kintampo (Brong Ahafo Region). Kintampo is within the forest transition zone in a malaria-holoendemic region where malaria transmission is high all year round, with entomological inoculation rates (EIRs) of >250 infective bites/person/year [22], whereas Navrongo is in the savannah area with hyperendemic transmission that is very seasonal and dependent on rainfall, with EIRs of <250 infective bites/person/year [23]. Teshie is a suburb of the capital city, Accra, where malaria transmission is generally lower than that in Navrongo and Kintampo (EIR, <50 infective bites/person/year) [24] but has a peak during the early rainy season, from June to August. Samples were collected during the rainy seasons at the respective study sites between September 2011 and September 2013. Children aged 2–14 years presenting with symptoms of malaria were screened for malaria with rapid diagnostic tests, using blood specimens obtained by finger prick. Children who tested positive for malaria were further confirmed for malaria by means of microscopy, and the parasitemia level was determined by counting the number of parasites per 200 white blood cells (WBCs) and multiplying by the total WBC count obtained from an automated hematology analyzer. About 5 mL of venous blood from children with confirmed parasitemia was collected into heparinized tubes from which erythrocytes were cryopreserved in liquid nitrogen, using the glycerolyte method [25].
Enzymatic Treatments
To determine the optimal enzyme concentrations for removal of specific erythrocyte receptors, group O+ erythrocytes were treated with 250 mU/mL and 500 mU/mL of neuraminidase from Vibrio cholera (Sigma Aldrich, St. Louis, Missouri); 1, 2, and 4 mg/mL of α-chymotrypsin (pretreated with 1-chloro-3-tosylamido-7-amino-2-heptanone, to remove trypsin activity) from bovine pancreas (Sigma Aldrich); and 1, 2, and 4 mg/mL of trypsin from bovine pancreas (Sigma Aldrich). After incubation for 1 hour at 37°C with gentle shaking, erythrocytes were washed, resuspended in complete parasite medium, and stored refrigerated for a maximum of 1 week. The efficiency of enzymatic cleavage was determined by examining the surface expression of SA, which is sensitive to neuraminidase, and of CR1, which is sensitive to both trypsin and chymotrypsin. Erythrocytes were stained with SA-specific mouse anti-human glycophorin A (clone E3, 10 µg/mL; Sigma Aldrich) and chicken anti-human CR1 (12 µg/mL; Gallus Immunotech, Fergus, Canada) in cold phosphate-buffered saline containing 1% bovine serum albumin, followed by goat anti-mouse immunoglobulin G (IgG) conjugated to phycoerythrin (4 µg/m; Invitrogen, Eugene, Oregon) or goat anti-chicken IgG conjugated to fluorescein isothiocyanate (10 µg/mL; KPL, Gaithersburg, Maryland). Flow cytometry was performed using a BD FACS Calibur (BD, San Jose, California), and results were analyzed using FlowJo (Tree Star, Ashland, Oregon). These analyses revealed that 250 mU/mL and 500 mU/mL of neuraminidase resulted in similar removal of SA (Supplementary Figure 2A); therefore, 250 mU/mL was selected as optimal. Also, 1 mg/mL was selected for both chymotrypsin and trypsin because all concentrations of these enzymes removed CR1 to a similar extent (Supplementary Figures 2B and 2C).
Invasion Assays
Cryopreserved samples from the various study sites were removed from cold storage and thawed using NaCl solutions as described elsewhere [25]. To determine the ex vivo invasion phenotypes of clinical parasite isolates, the efficiency of invasion into untreated and enzyme-treated erythrocytes was investigated during the first 3 consecutive parasite cycles in culture. In addition, to determine the relative dependency of field isolates on CR1 and basigin as receptors for invasion, experiments were set up in the presence of chicken anti-CR1 antibodies or immunoglobulin Y (IgY) control (12 µg/mL; Gallus Immunotech, Fergus, Canada) [8]; and mouse monoclonal anti-basigin antibodies (clone MEM-M6/6) or control mouse IgG (10 μg/mL; Sigma-Aldrich) [9]. The erythrocytes used as targets for invasion assays were labeled with 7-hydroxy-9H-(1,3-dichloro-9, 9-dimethylacridin-2-one) succinimidyl ester (DDAO-SE; 10 μM; Invitrogen), a far red cell stain, to differentiate them from the uninfected erythrocytes in the parasite inoculum, using previously described procedures [26].
Parasite isolates were incubated for about 1 day after thawing, to ensure that they were growing and developing into schizonts before invasion assays were set up. For each donor sample, the schizont stage parasite isolate was added to DDAO-SE–stained erythrocytes in a 1:1 ratio at 2% hematocrit in a 96-well titer plate. All experiments were set up in duplicate wells and repeated 3 times. The plates were incubated at 37°C for 18–20 hours in a blood gas atmosphere of 2% O2, 5.5% CO2, and 92.5% N2 (Air Liquide, Birmingham, United Kingdom). SYBR Green I (Invitrogen) stain was used to differentiate parasitized erythrocytes from uninfected erythrocytes. Invasion levels were then determined using flow cytometry (FACS Calibur, BD), and the percentage of cells expressing DDAO that was positive for SYBR Green was recorded as the invasion rate.
Ligand Gene Expression Assays
For isolates with sufficient sample volume available, parasites were cultured to the schizont stage, and RNA was extracted using TRIzol reagent (Ambion/Life Technologies, Carlsbad, California) as described previously [18]. RNA was extracted using an RNAeasy Micro kit (Qiagen Inc., Valencia, California) and reverse transcribed using the TaqMan RT system (Applied Biosystems/Life technologies), and complementary DNA was amplified using Kapa Probe Fast qPCR reagents (Kapa Biosystems, Cape Town, South Africa). Transcript levels for eba140, eba175, eba181, PfRh1, PfRh2a, PfRh2b, PfRh4, and PfRh5 were determined using gene specific primer/probe sets (Sigma Aldrich) [18, 27] in a fluorogenic 5′-nuclease assay performed on a Rotor-Gene 3000 system (Corbett Life Sciences, Mortlake, Australia).
Statistical Analyses
Statistical analyses were performed with Minitab, version 16, and SigmaPlot, version 12, software. Variables that passed the Kolmogorov-Smirnov normality test were analyzed using parametric methods; otherwise, nonparametric procedures were used. The protected comparisons procedure was used to minimize errors due to multiple comparisons in all analyses. Invasion rates were compared across transmission areas using Kruskal–Wallis tests; when significant differences were observed, post hoc pairwise comparisons were performed using Mann–Whitney U tests. Relationships between various invasion-related variables were examined using Pearson correlation or Spearman rank correlation, based on the normality of the data. For all analyses, a P value of < .05 was considered statistically significant.
RESULTS
Clinical and Demographic Characteristics of Study Participants
Parasites were collected from participating children in Accra (n = 25), Navrongo (n = 22), and Kintampo (n = 31) after a complete clinical evaluation, including determination of parasitemia and hemoglobin levels. Clinical and demographic characteristics of study participants whose parasite isolates grew for at least 1 cycle are listed in Table 1. Comparison of the demographic and clinical characteristics of the children across the 3 malaria-endemic areas showed no statistically significant differences in sex distribution (P = .449) and age (P = .865). However, parasitemia levels differed significantly across the sites (P < .001), with the lowest parasitemia levels observed in Accra and the highest levels observed in Kintampo (Table 1), which mirrored the relative transmission intensities in the areas. Furthermore, hemoglobin levels were lower in participants recruited in Navrongo and Kintampo, compared with those recruited from Accra; however, these differences were not statistically significant (P = .106).
Table 1.
Clinical and Demographical Characteristics of Study Participants Aged 2–14 Years Who Received a Diagnosis of Malaria in Accra, Kintampo, and Navrongo, Ghana
| Characteristic | Accra | Navrongo | Kintampo | Overall | P Valuea |
|---|---|---|---|---|---|
| Donors, no. | 20 | 15 | 17 | 52 | … |
| Female sex, no. (%) | 9 (45.0) | 9 (60.0) | 11 (64.7) | 28 (53.8) | .449b |
| Age, y, mean ± SEM | 5.9 ± 0.8 | 5.2 ± 0.8 | 5.1 ± 0.7 | 5.5 ± 0.4 | .865 |
| Parasitemia level, parasites/µL, mean ± SEM | 26 942 ± 5289 | 71 370 ± 11 394 | 354 334 ± 75 904 | 122 210 ± 32 264 | <.001 |
| Hemoglobin level, g/dL, mean ± SEM | 11.1 ± 0.2 | 9.7 ± 0.5 | 10.2 ± 0.4 | 10.4 ± 0.4 | .106 |
Abbreviation: SEM, standard error of the mean.
a By the Kruskal–Wallis test, unless otherwise indicated.
b By the χ2 test.
Enzyme Sensitivities of P. falciparum Clinical Isolates Across Malaria-Endemic Sites
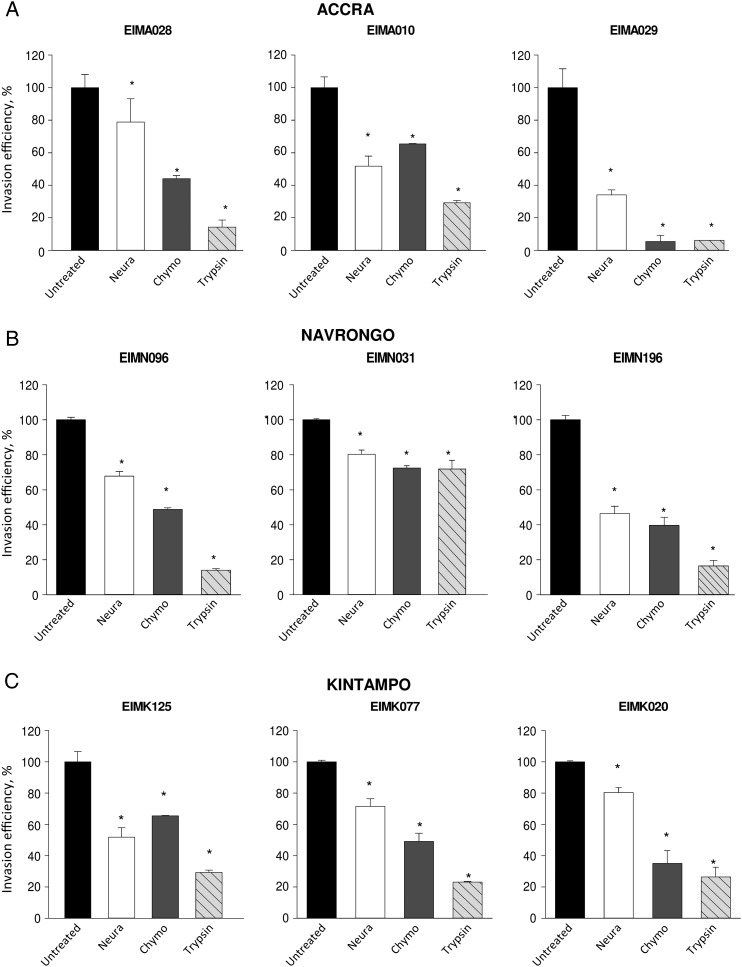
Invasion receptor dependency of the clinical parasite isolates across the 3 sites was investigated by determining their ability to invade erythrocytes treated with neuraminidase, trypsin, or chymotrypsin. Altogether, the clinical isolates across the 3 sites invaded neuraminidase-treated erythrocytes at an efficiency of 20%–90% (mean, 65.5%) relative to untreated cells (Figure 1), suggesting the use of SA-independent mechanisms. Furthermore, the parasites generally showed sensitivity to chymotrypsin and trypsin, which cleave SA-independent receptors, invading enzyme-treated erythrocytes at efficiencies of only 2%–70% (mean, 39%) and 2%–60% (mean, 25%), respectively (Figure 1).
Figure 1.
Representative invasion phenotypes of Plasmodium falciparum clinical isolates from across 3 malaria-endemic areas of Ghana. Erythrocyte invasion assays were set up in duplicates on 96-well plates to determine the ability of parasite isolates from Accra (A), Navrongo (B), and Kintampo (C) to invade erythrocytes treated with neuraminidase (Neura; 250 mU/mL), chymotrypsin (Chymo; 1 mg/mL), and trypsin (1 mg/mL). Invasion rates were determined by flow cytometry as the percentage of ring-stage-infected erythrocytes after 18–20 hours of incubation and are expressed as invasion efficiency relative to invasion of untreated erythrocytes. Data are presented as means ± standard errors of 3 independent experiments performed in duplicate. *P < .05, by the Student t test, compared with untreated erythrocytes.
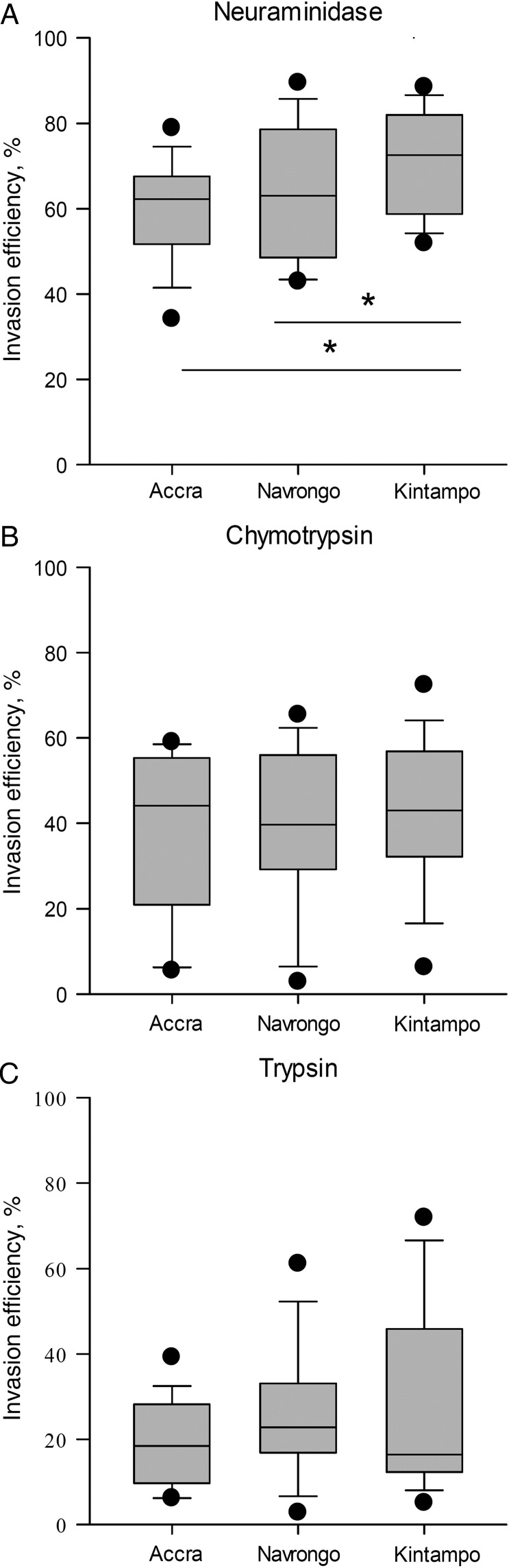
When compared across the transmission sites, sensitivity to neuraminidase differed significantly in parasites from the 3 areas (P = .02; Figure 2A), showing a pattern that suggests an inverse correlation with transmission intensity. Parasites collected in the Kintampo area showed the lowest sensitivity to neuraminidase and thus retained a higher invasion efficiency in enzyme-treated erythrocytes than those from Accra (P = .01) and Navrongo (P = .02; Figure 2A). However, there appeared to be no such trend in the sensitivities of parasite invasion into erythrocytes treated with chymotrypsin (P = .78; Figure 2B) and trypsin (P = .08; Figure 2C) across the 3 areas. These findings suggest that there may be differences in immune pressures exerted on parasites in the areas with varying endemicities, which may be eliciting the expression of different invasion phenotypes.
Figure 2.
Comparison of erythrocyte invasion phenotypes of Plasmodium falciparum isolates from 3 areas of Ghana with different malaria endemicities. The erythrocyte invasion receptor preferences of P. falciparum clinical isolates from 3 areas of Ghana with differing endemicities (lowest, Accra; intermediate, Navrongo; highest, Kintampo) were compared by using their abilities to invade erythrocytes treated with neuraminidase (250 mU/mL; A), chymotrypsin (1 mg/mL; B), and trypsin (1 mg/mL; C). The box plots show the invasion efficiencies in enzyme-treated erythrocytes relative to untreated erythrocytes for isolates from Accra (n = 20), Navrongo (n = 15), and Kintampo (n = 17). *P < .05, by the Mann–Whitney U test.
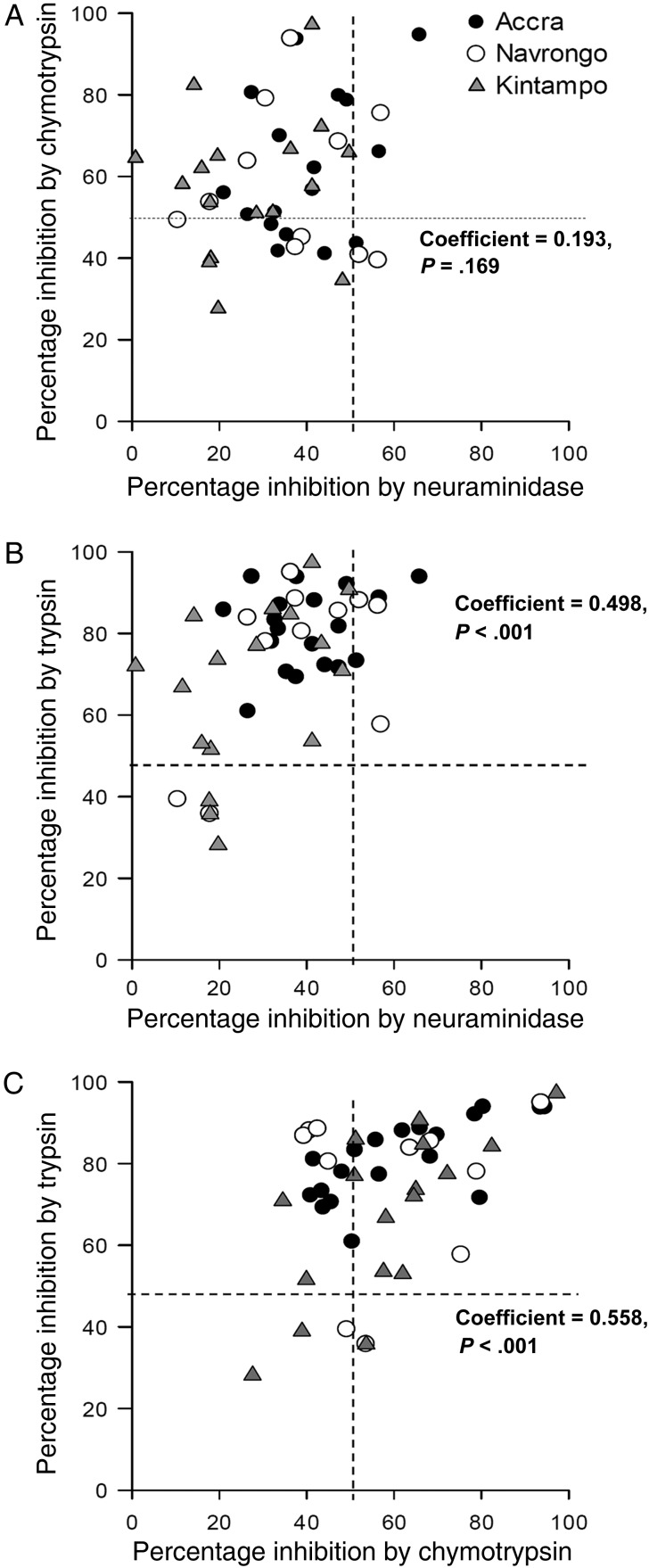
Overall, parasite sensitivity to neuraminidase treatment of erythrocytes was not related to chymotrypsin sensitivity (coefficient, 0.193; P = .169; Figure 3A) but was positively correlated with trypsin sensitivity (coefficient, 0.498; P < .001; Figure 3B). Furthermore, sensitivities of parasite isolates to chymotrypsin and trypsin correlated significantly (coefficient, 0.558; P < .001; Figure 3C), consistent with their common specificity for receptors such as CR1 and basigin.
Figure 3.
Phenotypic diversity of Plasmodium falciparum clinical isolates and interrelationships between sensitivities to enzyme treatment. The diversity of invasion phenotypes of parasite isolates from Accra (closed circles), Navrongo (open circles), and Kintampo (closed triangles) are illustrated by the relative magnitudes by which their invasion of erythrocytes was inhibited after treatment by neuraminidase (250 mU/mL), chymotrypsin (1 mg/mL), and trypsin (1 mg/mL). The correlations between the inhibitory effects of neuraminidase and chymotrypsin (A), neuraminidase and trypsin (B), and chymotrypsin and trypsin (C) were also determined using Spearman correlations tests. The broken lines indicate 50% inhibition of invasion, which demarcates the threshold for categorization as enzyme sensitive.
Diversity of Invasion Phenotypes Across Transmission Areas
To quantify the extent of heterogeneity in invasion phenotypes in the 3 malaria-endemic areas and to determine the relationship with transmission intensity, we categorized parasites as either sensitive (s) or resistant (r) to neuraminidase (N), chymotrypsin (C), or trypsin (T) treatment, using an invasion efficiency of 50% as a threshold. This categorization yielded 6 distinct invasion phenotypes: NrCsTs (63%), NrCrTs (17%), NsCsTs (8%), NsCrTs (6%), NrCsTr (2%), and NrCrTr (4%). This pattern was nearly consistent across the 3 transmission areas, with a majority of isolates belonging to the NrCsTs category (Figure 3A–C), confirming the predominance of SA-independent invasion pathways among Ghanaian isolates. However, the NsCrTs phenotype was observed only among Accra isolates, whereas NrCsTr, which is a rare phenotype previously reported in Brazil [20], was unique to isolates from Navrongo. Significantly, there were only 3 distinct invasion phenotypes observed among Kintampo isolates, compared with 4 phenotypes in Accra and 6 in Navrongo, suggesting that isolates from Navrongo had the highest diversity of invasion pathways and that Kintampo had the lowest.
Roles of CR1 and Basigin
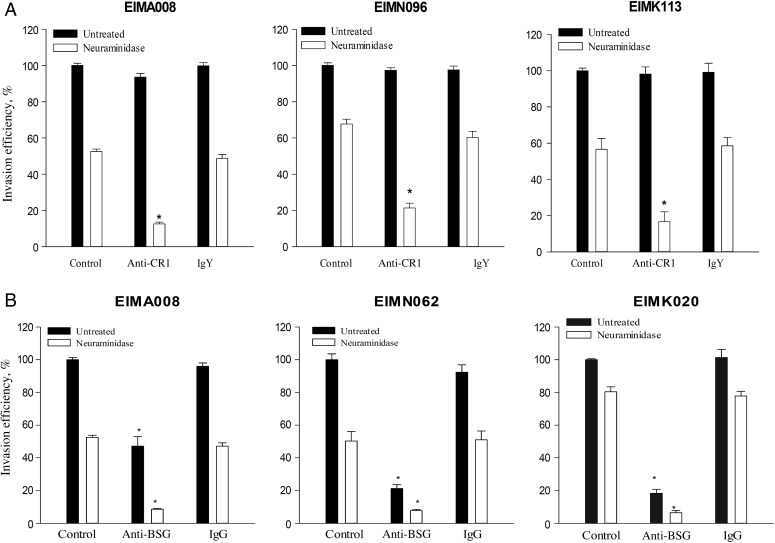
Given the predominance of SA-independent phenotypes among the isolates from all the sites and the demonstrated roles of CR1 and basigin in mediating SA-independent invasion in both clinical and laboratory-adapted P. falciparum parasites [7, 9], we investigated the use of these receptors among the isolates. While anti-CR1 antibodies had no significant effects on invasion of untreated erythrocytes, invasion of neuraminidase-treated erythrocytes was significantly reduced (by approximately 40%–95%) by anti-CR1 relative to IgY isotype control antibodies (P < .05 for all isolates; Figure 4A). The effects of anti-CR1 were consistent across Accra, Navrongo, and Kintampo, with no significant differences in the magnitude of the inhibition at the different sites (P = .057 by the Kruskal–Wallis test; data not shown).
Figure 4.
Complement receptor 1 and basigin are important receptors for erythrocyte invasion in Ghanaian Plasmodium falciparum field isolates. Invasion of untreated and neuraminidase-treated erythrocytes was determined in the presence of antibodies against complement receptor 1 (anti-CR1; 12 µg/mL) or isotype control antibodies (immunoglobulin Y [IgY]; 12 µg/mL; A) and anti-basigin (anti-BSG; 10 µg/mL) or isotype control antibodies (immunoglobulin G [IgG]; 10 µg/mL; B). Invasion rates were expressed relative to invasion in untreated erythrocytes (control). Data are presented as means ± standard errors of 3 independent experiments performed in duplicate and are representative of 52 isolates tested for anti-CR1 and 27 isolates tested for anti-BSG. *P < .05, by the Student t test, compared with isotype control antibodies.
On the other hand, anti-basigin antibodies significantly inhibited invasion in both untreated and neuraminidase-treated erythrocytes, compared with IgG isotype control antibodies (P < .05 for all comparisons), reducing invasion by 50%–85% in untreated erythrocytes and by 70%–95% in neuraminidase-treated erythrocytes (Figure 4B). The magnitude of the invasion inhibition by anti-basigin in both untreated and neuraminidase-treated erythrocytes varied significantly in isolates collected from the 3 sites (P = .03), with isolates from Navrongo more sensitive than isolates from Accra (P = .03 for untreated erythrocytes, and P < .01 for neuraminidase-treated erythrocytes) and those from Kintampo (P = .05 for untreated erythrocytes, and P < .01 for neuraminidase-treated erythrocytes).
Gene Expression of Invasion Ligands
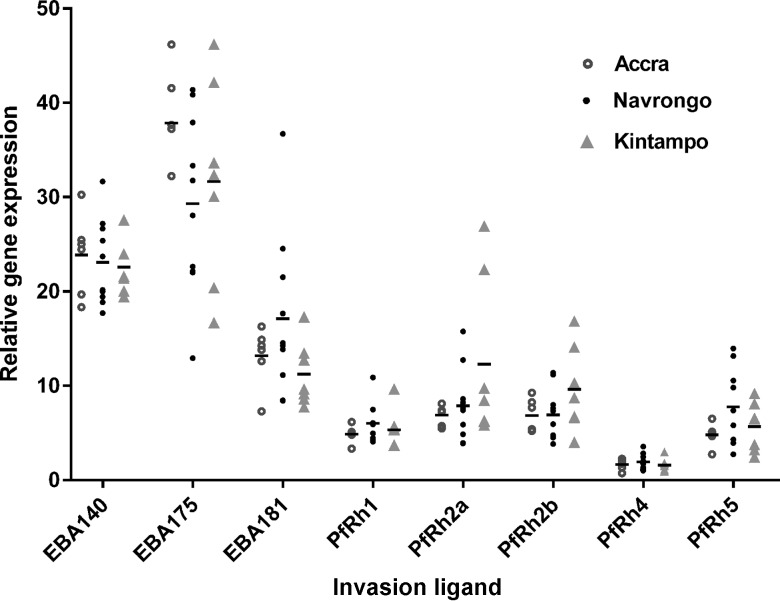
To further investigate the relative importance of the various erythrocyte invasion pathways used by the P. falciparum isolates, gene expression of the major ligands, including EBA-140, EBA-175, EBA-181, PfRh1, PfRh2a, PfRh2b, PfRh4, and PfRh5, was examined in schizont stage parasites. Eba175 was the most abundant gene expressed, constituting 32.3% of the combined expression of all 8 genes, while PfRh4 was the least expressed, contributing only 1.7% of the total transcripts. The eba gene family were generally expressed at higher levels relative to the PfRh genes (P < .001; Figure 5). When compared across the 3 study sites, expression of eba175 appeared to be higher in parasites collected from Accra, while expression of PfRh2a was elevated in Kintampo isolates, but these differences were not statistically significant (P > .05 for all comparisons; Figure 5).
Figure 5.
Relative expression levels of genes encoding 8 invasion ligands in Plasmodium falciparum clinical isolates across 3 malaria-endemic areas of Ghana. Transcript levels of P. falciparum invasion ligand genes eba140, eba175, eba181, PfRh1, PfRh2a, PfRh2b, PfRh4, and PfRh5 were determined by quantitative polymerase chain reaction analysis in 23 isolates from Accra (open circles), Navrongo (closed circles), and Kintampo (closed triangles). The relative abundance of each gene was expressed as a percentage of the total transcripts of all 8 genes. Transcript levels for individual isolates are shown by the symbols, and the dashes indicate the positions of the group means for the respective study sites.
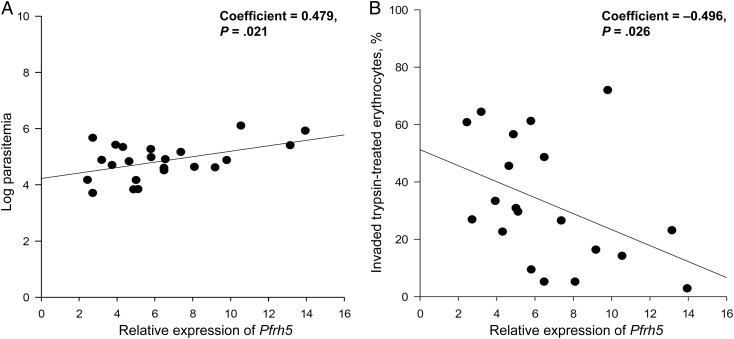
Relational analyses between ligand gene expression levels and age and parasitemia levels of donors at enrollment showed that PfRh5 had the strongest correlation with parasitemia level (coefficient, 0.479; P = .021), while PfRh1 expression was the best correlate of age (Figure 6A and Supplementary Table 1). Furthermore, PfRh5 expression was also negatively correlated with the rate of invasion into trypsin-treated erythrocytes (coefficient, −0.496; P = .026; Figure 6B and Supplementary Table 2), suggesting that sensitivity to trypsin in these isolates was significantly influenced by the contribution to invasion of the basigin-PfRh5 pathway. There was no significant relationship between ligand gene expression levels and sensitivity to any of the other enzymatic treatments (Supplementary Table 2).
Figure 6.
Correlation of Pfrh5 expression levels with donor parasitemia level and parasite invasion efficiency in trypsin-treated erythrocytes. Relative expression of Pfrh5 in Ghanaian field isolates correlated positively with donor parasitemia at enrollment (A) and negatively with parasite invasion efficiency in trypsin-treated erythrocytes relative to untreated isolates (B). Correlations were statistically significant by the Pearson test.
DISCUSSION
Previous studies have shown that field isolates of P. falciparum from various malaria-endemic areas, including The Gambia, Brazil, India, and Kenya, use different invasion pathways that can be classified on the basis of the enzyme susceptibility of the erythrocyte receptors involved [14–18]. To directly investigate the relationship between levels of malaria transmission and the invasion phenotypes expressed by the infecting parasites, we sought to minimize confounding factors by examining parasites from 3 distinct areas with differing malaria endemicities within the same country. Furthermore, because age in malaria-endemic areas is essentially a surrogate marker of accumulated exposure to parasites and development of immunity, we selected children aged 2–14 years, an age range that spans from non-immune infants to semi-immune older children.
Using standardized methods for sample collection, processing, storage, and culture, we observed a range of invasion phenotypes across the 3 malaria-endemic areas, characterized by varying invasion rates in erythrocytes treated with neuraminidase, chymotrypsin, or trypsin. Consistent with studies of parasites collected in Kenya [7, 15], erythrocyte invasion by the clinical isolates was generally resistant to neuraminidase treatment but very sensitive to trypsin and moderately sensitive to chymotrypsin. This pattern suggests the use of SA-independent receptors, which is in contrast to findings for field parasites collected in Gambia, where the parasites' ability to invade neuraminidase-treated erythrocytes was much lower [14, 18]. Significantly, our data showed that the prevalence of SA-independent phenotypes appears to correlate with levels of endemicity, whereby isolates collected from Kintampo, which has the highest transmission intensity, invaded neuraminidase-treated erythrocytes at significantly higher rates than parasites collected from Navrongo and Accra. Previous studies suggest that immunity to the SA-dependent parasite ligands are acquired prior to development of antibodies to SA-independent ligands [13]. Therefore, our data showing that 100% of the parasites from Kintampo used SA-independent invasion pathways may indicate that high immune pressure on parasites in Kintampo or competition among parasite variants may have driven a complete switch away from the use of SA-dependent receptors.
Despite the significant differences in the ability to invade neuraminidase-treated erythrocytes, the majority of parasites from each of the malaria-endemic areas invaded using a receptor that is neuraminidase resistant, trypsin sensitive, and chymotrypsin sensitive (NrCsTs). This pattern of invasion is characteristic of the pathway mediated by interactions between PfRh4 and its erythrocyte receptor, CR1 [8, 28]. In agreement with our previous studies showing that CR1 is the major SA-independent receptor used by P. falciparum field isolates [7], anti-CR1 antibodies inhibited the majority of invasion of neuraminidase-treated erythrocytes in parasites from all of the malaria-endemic areas. Using a similar approach, we investigated the role of the basigin-PfRh5 invasion pathway by measuring the ability of the parasites to invade erythrocytes in the presence of anti-basigin antibodies. These experiments clearly demonstrated the importance of the basigin-Rh5 interaction in mediating erythrocyte invasion, since anti-basigin antibodies reduced invasion by an average of 70% in all parasite isolates tested from all 3 malaria-endemic areas. These results confirm previous studies that used similar reagents and showed comparable inhibitory effects in both laboratory-adapted and fields strains of P. falciparum [9].
Our data further demonstrated that, while anti-CR1 antibodies inhibited invasion only in neuraminidase-treated erythrocytes, anti-basigin antibodies significantly inhibited invasion into both untreated and neuraminidase-treated erythrocytes. These findings suggest that CR1 is predominantly required only in the absence of SA, whereas basigin is required for both SA-dependent and SA-independent invasion mechanisms. However, it was clear from the data that inhibition of basigin plus treatment with neuraminidase caused a greater reduction of invasion than basigin inhibition alone. It has been shown that antibodies against the basigin ligand PfRh5 synergize with antibodies against other parasite ligands to enhance invasion inhibitory activity [29], suggesting that a potential vaccine designed against the basigin-PfRh5 invasion pathway alone may not be sufficient to completely block invasion. Furthermore, sensitivity to basigin inhibition appears to vary across transmission areas, as we found parasites from Navrongo to be significantly more sensitive to anti-basigin antibodies, compared with those from Accra and Kintampo. Taken together, these data demonstrate that, while the basigin-PfRh5 pathway is an extremely promising target for vaccine design, formulation of such a vaccine must incorporate other ligand components to ensure effectiveness across a range of transmission areas.
Analysis of relative gene expression levels of invasion ligands in the clinical isolates provided further support for a pivotal role for PfRh5 in erythrocyte invasion. Of all the ligand genes examined, PfRh5 expression was the strongest predictor of parasitemia levels in donor blood. This correlation between PfRh5 expression and parasitemia level was previously reported in a Gambian study [18] and also supports recent data from Mali [30] and Papua New Guinea [31], which demonstrated that antibodies against PfRh5 protected against clinical malaria and reduced parasite density. In addition, while our data indicated that ligand gene expression generally did not differ significantly when compared across the transmission areas, invasion sensitivity to trypsin treatment correlated significantly with PfRh5 expression levels.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the children and their parents or guardians who volunteered for the study in Accra, Navrongo, and Kintampo; and the directors, clinical staff, and technicians at Ledzokuku-Krowor Municipal Assembly Hospital in Accra, War Memorial Hospital in Navrongo, the Navrongo Health Research Center, the Kintampo Municipal hospital, and the Kintampo Health Research Center.
Financial support. This work was supported by the UK Royal Society–Leverhulme African Award Scheme (grant AA110050 to G. A. A. and D. J. C.); the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant 1R01AI102848 to G. A. A.); and the European Research Council (advanced award AdG-2011-294428 to D. J. C.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. World malaria report 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 2.Deas JE, Lee LT. Competitive inhibition by soluble erythrocyte glycoproteins of penetration by Plasmodium falciparum. Am J Trop Med Hyg 1981; 30:1164–7. [DOI] [PubMed] [Google Scholar]
- 3.Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J Cell Biol 1981; 90:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier AG, Duraisingh MT, Reeder JC et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med 2003; 9:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994; 264:1941–4. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JK, Triglia T, Reed MB, Cowman AF. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Microbiol 2001; 41:47–58. [DOI] [PubMed] [Google Scholar]
- 7.Awandare GA, Spadafora C, Moch JK, Dutta S, Haynes JD, Stoute JA. Plasmodium falciparum field isolates use complement receptor 1 (CR1) as a receptor for invasion of erythrocytes. Mol Biochem Parasitol 2011; 177:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spadafora C, Awandare GA, Kopydlowski KM et al. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog 2010; 6:e1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosnier C, Bustamante LY, Bartholdson SJ et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011; 480:534–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas AD, Williams AR, Illingworth JJ et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Comm 2011; 2:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bei AK, Duraisingh MT. Functional analysis of erythrocyte determinants of Plasmodium infection. Int J Parasitol 2012; 42:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog 2005; 1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson KE, McCallum FJ, Reiling L et al. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest 2008; 118:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum J, Pinder M, Conway DJ. Erythrocyte invasion phenotypes of Plasmodium falciparum in The Gambia. Infect Immun 2003; 71:1856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deans AM, Nery S, Conway DJ, Kai O, Marsh K, Rowe JA. Invasion pathways and malaria severity in Kenyan Plasmodium falciparum clinical isolates. Infect Immun 2007; 75:3014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo CA, de Frazao K, Rodriguez M, Reid M, Zalis M, Lustigman S. Invasion profiles of Brazilian field isolates of Plasmodium falciparum: phenotypic and genotypic analyses. Infect Immun 2004; 72:5886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoyeh JN, Pillai CR, Chitnis CE. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect Immun 1999; 67:5784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Escobar N, Amambua-Ngwa A, Walther M, Okebe J, Ebonyi A, Conway DJ. Erythrocyte invasion and merozoite ligand gene expression in severe and mild Plasmodium falciparum malaria. J Infect Dis 2010; 201:444–52. [DOI] [PubMed] [Google Scholar]
- 19.Jennings CV, Ahouidi AD, Zilversmit M et al. Molecular analysis of erythrocyte invasion in Plasmodium falciparum isolates from Senegal. Infect Immun 2007; 75:3531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Perez M, Villasis E, Machado RL et al. Plasmodium falciparum field isolates from South America use an atypical red blood cell invasion pathway associated with invasion ligand polymorphisms. PLoS One 2012; 7:e47913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadley TJ, Klotz FW, Pasvol G et al. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest 1987; 80:1190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owusu-Agyei S, Asante KP, Adjuik M et al. Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar J 2009; 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasasa S, Asoala V, Gosoniu L et al. Spatio-temporal malaria transmission patterns in Navrongo demographic surveillance site, northern Ghana. Malar J 2013; 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinkenberg E, McCall P, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J 2008; 7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll K, Kaneko A, Scherf A, Wahlgren M. Methods in malaria research. 6th ed Manassas, VA: Malaria Research and Reference Reagent Resource Center (MR4), 2013. [Google Scholar]
- 26.Theron M, Hesketh RL, Subramanian S, Rayner JC. An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites. Cytometry A 2010; 77:1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nery S, Deans AM, Mosobo M, Marsh K, Rowe JA, Conway DJ. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. Mol Biochem Parasitol 2006; 149:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tham WH, Wilson DW, Lopaticki S et al. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A 2010; 107:17327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams AR, Douglas AD, Miura K et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog 2012; 8:e1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran TM, Ongoiba A, Coursen J et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis 2014; 209:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu CY, Healer J, Thompson JK et al. Association of antibodies to Plasmodium falciparum reticulocyte binding protein homolog 5 with protection from clinical malaria. Front Microbiol 2014; 5:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.