Abstract
Recent studies have shown that live attenuated influenza vaccines (LAIVs) expressing avian influenza virus hemagglutinins (HAs) prime for strong protective antibody responses to an inactivated influenza vaccine (IIV) containing the HA. To better understand this priming effect, we compared H7 HA head and stalk domain–specific B-cell responses in H7N7 LAIV–primed subjects and non–H7-primed controls after a single dose of H7N7 IIV. As previously reported, H7N7 LAIV–primed subjects but not control subjects generated strong hemagglutination-inhibiting and neutralizing antibody responses to the H7N7 IIV. Here, we found that the quantity, epitope diversity, and affinity of H7 head–specific antibodies increased rapidly in only H7N7 LAIV-primed subjects after receipt of the IIV. However, all cohorts generated a vigorous, high-affinity, stalk-specific antibody response. Consistent increases in circulating memory B-cell frequencies after receipt of the IIV reflected the specificity of high-affinity antibody production. Our findings emphasize the value of LAIVs as a vehicle for prepandemic vaccination.
Keywords: Avian influenza, H7 hemagglutinin, live attenuated, vaccine, antibody affinity, anti-stalk antibodies, memory B cells
A key feature of pandemic influenza A viruses is a hemagglutinin (HA) that is novel to most of the human population. Novel HAs carried by pandemic viruses are typically derived from avian influenza viruses, which represent a source of at least 16 different HA subtypes (H1–H16). Avian influenza viruses expressing the H5 and H7 HA subtypes have raised pandemic concerns because of their ability to spread directly from birds to humans and cause severe disease [1, 2].
Vaccination strategies that generate protective levels of antibodies (Abs) against novel HA subtypes are key for pandemic preparedness. This reflects the major protective role of anti-HA Abs [3]. However, a challenge is the poor immunogenicity of novel avian HAs in adults. The hemagglutination inhibition (HI) and microneutralization (MN) Ab titers attained in prime-boost studies of unadjuvanted, inactivated H5 and H7 subvirion vaccines, even at high HA doses, have been at best modest [4–8]. Responses to live attenuated influenza vaccines (LAIVs) expressing H5 or H7 have also been poor [9–12].
Despite weak Ab induction by H5 or H7 LAIV alone, recent studies have shown that these vaccines effectively prime for a protective Ab response to a single dose of inactivated influenza vaccine (IIV) containing a matched or related HA. Talaat et al [13] evaluated the response to an H5 IIV in subjects who had previously received an LAIV expressing a matched or variant H5. In contrast to H5-naive subjects, most LAIV-primed subjects generated significant HI and MN responses to the IIV. Babu et al [12] demonstrated strong HI and MN responses to an H7 IIV in most subjects who had been primed with an LAIV expressing a closely related H7, whereas no response was detected in non–H7-primed subjects. The effect of LAIV priming in these studies likely reflects HA-specific immune memory, but mechanisms remain unclear.
The current study was undertaken to better understand the impact of H7N7 LAIV priming on the H7-specific B-cell response to an H7N7 IIV. To that end, we investigated whether LAIV priming modulated the relative contributions of H7 head and stalk–specific B-cell responses to the IIV. We also related LAIV priming to H7 head and stalk–specific memory B-cell (MBC) frequencies before and after receipt of the IIV. Abs that target the conserved HA stalk domain can have broad neutralizing activity against different HA subtypes [14]. However, there is evidence that the anti-stalk Ab response is diminished in the presence of MBCs specific for the immunodominant HA head domain [15, 16]. Our results demonstrate that H7N7 LAIV primes for a strong, diverse, and high-affinity H7 head–specific Ab response to the IIV, likely through MBC induction during the priming process. We also show that the response to the IIV in H7N7 LAIV–primed subjects, as in non–H7-primed subjects, includes strong production of high-affinity antistalk Abs.
MATERIALS AND METHODS
Ethics Statement
This study was conducted under a protocol approved by the University of Rochester Research Subjects Review Board. Informed written consent was obtained from each participant. Subject samples were de-identified. Serum analysis at Food and Drug Administration Center for Biologics Evaluation and Research was conducted under Research Involving Human Subjects exemption 03-118B.
Study Design
This study used samples that had previously been collected in an analysis of responses to a single dose of subvirion H7N7 IIV in 3 cohorts: (1) subjects who had previously received 2 doses of an H7N7 LAIV derived from the H7N7 A/Netherlands/219/2003 (NL/03) virus (H7N7 LAIV–primed cohort), (2) subjects who had previously received 2 doses of an H2N3 LAIV derived from H2N3 A/swine/Missouri/4296424/2006 (H2N3 LAIV–primed cohort), and (3) subjects who were H7 naive and received only the IIV (unprimed cohort). Subject selection, LAIV generation, and vaccine administration were described in an initial report of standard serological responses [12]. LAIVs were administered in a 2-dose regimen 28 days apart, followed 18 months later by a 45-µg dose of H7N7 IIV given intramuscularly. The IIV [6] contained the H7 of A/mallard/Netherlands/2/2000 (NL/00), which is antigenically similar to the H7 of NL/03 [17]. The current study analyzed sera and peripheral blood mononuclear cells (PBMCs) collected at the time of IIV administration (day 0) and on days 7, 14, and 28. PBMCs were frozen until required. Serum HI and MN titers were determined against the IIV virus [12].
Generation of H7 HA1 and HA2 Domains
The HA0 gene segments of H7N7 NL/03 and H7N9 A/Shanghai/1/2013 (SH/13) were chemically synthesized. DNAs encoding HA1 (1–320) and HA2 (331–540) of NL/03 and SH/13 were cloned, expressed, and purified as previously described [18]. HA1 proteins were properly folded and contained a high percentage of functional oligomers [18].
Affinity Measurement by Surface Plasmon Resonance (SPR)
Steady-state equilibrium binding of serum Ab after H7N7 IIV administration was monitored at 25°C, using a Bio-Rad ProteOn SPR biosensor [19, 20]. The recombinant HA globular head domain (rHA1-His6) or stalk domain (rHA2-His6) of NL/03 or SH/13 was coupled to a GLC sensor chip. The spatial density of antigen on the chip surface was adjusted to measure only monovalent Ab binding. Ab off-rate constants, which describe the fraction of antigen-Ab complexes that decay per second, were determined directly from sample interactions with rHA1 or rHA2 proteins, using SPR in the dissociation phase [19, 20].
H7 Gene-Fragment Phage Display Library (GFPDL) Construction and Panning With Sera
The HA0 gene segment of H7N7 NL/03 was used for construction of a GFPDL [21, 22]. Equal volumes of pooled polyclonal human sera from each cohort were used for each round of GFPDL panning. GFPDL selection was carried out in solution (with protein A/G) [20, 21, 23].
Enzyme-Linked Immunospot (ELISpot) Assay for Ab-Secreting Cells
Ab-secreting cells (ASCs) were enumerated by an ELISpot assay performed largely as previously described [24]. PBMCs were analyzed directly (day 7 after IIV receipt) or after in vitro stimulation (days 0, 14, and 28 after IIV receipt). The following reagents were used to coat plates for antigen-specific ASC enumeration: baculovirus-expressed trimeric HA ectodomains [25] from the H7N7 influenza strain NL/00, the H7N3 strain A/chicken/British Columbia/CN-6/2004 (BC/04), and the H7N9 strain A/Anhui/1/2013 (AN/13); a trimeric HA globular head protein from the H7N9 strain SH/13 [26]; chimeric HAs [27] consisting of an H4 head domain and H7 stalk domain (cH4/7) [26] or an H9 head domain and H1 stalk domain (cH9/1) [28]; and baculovirus-expressed full-length HAs from the H7N7 strain NL/03 (BEI Resources Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health), the H1N1 strain A/California/04/2009 (CA/09; BEI Resources Repository), and the H3N2 strain A/Wisconsin/67/2005 (WI/05; Protein Sciences, Meriden, Connecticut). The chimeric HAs primarily identified Ab binding to the HA stalk domain, since they carried exotic head domains that are novel to most humans [26, 28]. Wells were coated with goat antihuman immunoglobulin G (IgG; Life Technologies, Carlsbad, California) to measure total IgG ASCs. Negative control wells were coated with bovine serum albumin.
MBC Analysis
Measurement of antigen-specific MBC frequencies was based on previously described methods [29, 30]. PBMCs obtained on days 0, 14, and 28 after IIV administration were rested overnight and then stimulated for 6 days in complete medium containing 1 µg/mL R848 (Sigma Aldrich, St. Louis, Missouri), 10 ng/mL interleukin 2 (Life Technologies), and 55 µM 2-mercaptoethanol (Life Technologies). Antigen-specific and total IgG ASCs generated from stimulated MBC precursors were enumerated by an ELISpot assay. Antigen-specific IgG MBCs are shown as a percentage of total IgG+ MBCs, calculated as the percentage of antigen-specific ASCs among the total number of IgG ASCs. The limit of sensitivity for MBC frequency determination was set at 2 antigen-specific IgG ASCs/5 × 105 stimulated PBMCs.
Statistical Analysis
Differences were examined by paired t tests (for within-cohort comparisons) and by 2-sample t tests (for between-cohort comparisons), using data on a log scale. The corresponding nonparametric sign test and Wilcoxon rank sum test were used for confirmation of findings. Correlations were tested by Pearson correlation analysis and confirmed by Spearman correlation analysis. P values of <.05 were considered statistically significant.
RESULTS
HI and MN Responses
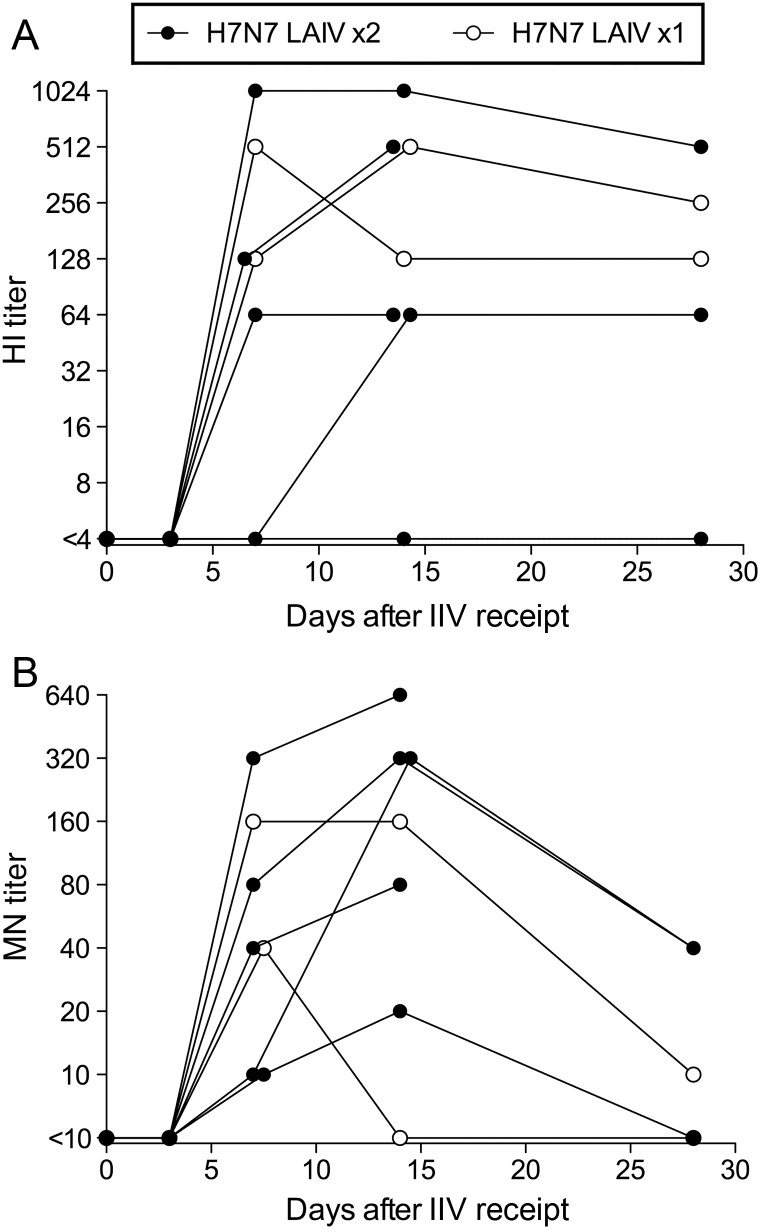
Babu et al [12] reported the shedding and immunogenicity of H7N7 LAIV and the HI and MN Ab responses to H7N7 IIV in the H7N7 LAIV–primed and unprimed cohorts. One or 2 doses of H7N7 LAIV did not generate a detectable H7-specific HI or MN response [12]. H7N7 LAIV–primed subjects in the current analysis represent a subset of subjects from the previous study [12] who were selected on the basis of sample availability. Of these, 6 of 7 responded to the H7N7 IIV, with HI and MN titers of ≥40, including 2 subjects who received only 1 of 2 scheduled H7N7 LAIV doses (Figure 1A and 1B). In contrast, no subject in the H2N3 LAIV–primed and unprimed cohorts had detectable HI or MN responses following IIV receipt ([12] and data not shown). There was no relationship between HI and MN responses to H7N7 IIV and H7N7 LAIV growth during priming. H7N7 LAIV shedding was detected in only 3 of 7 subjects after the first dose and in no subjects after the second dose.
Figure 1.
Hemagglutination inhibition (HI) and microneutralization (MN) antibody (Ab) titers after H7N7 inactivated influenza vaccination in H7N7 live attenuated influenza vaccine (LAIV)–primed subjects. Serum HI and MN titers against H7N7 NL/03 were determined at intervals after the inactivated vaccine. Each line connects data points for an individual subject (n = 7). Data points for subjects who received 2 LAIV doses are shown as filled circles; open circles identify data points for the 2 subjects who received only 1 H7N7 LAIV dose. Two subjects did not provide serum on day 28. Abbreviation: IIV, inactivated influenza vaccine.
Analysis of Serum Ab Binding to H7 HA1 and HA2
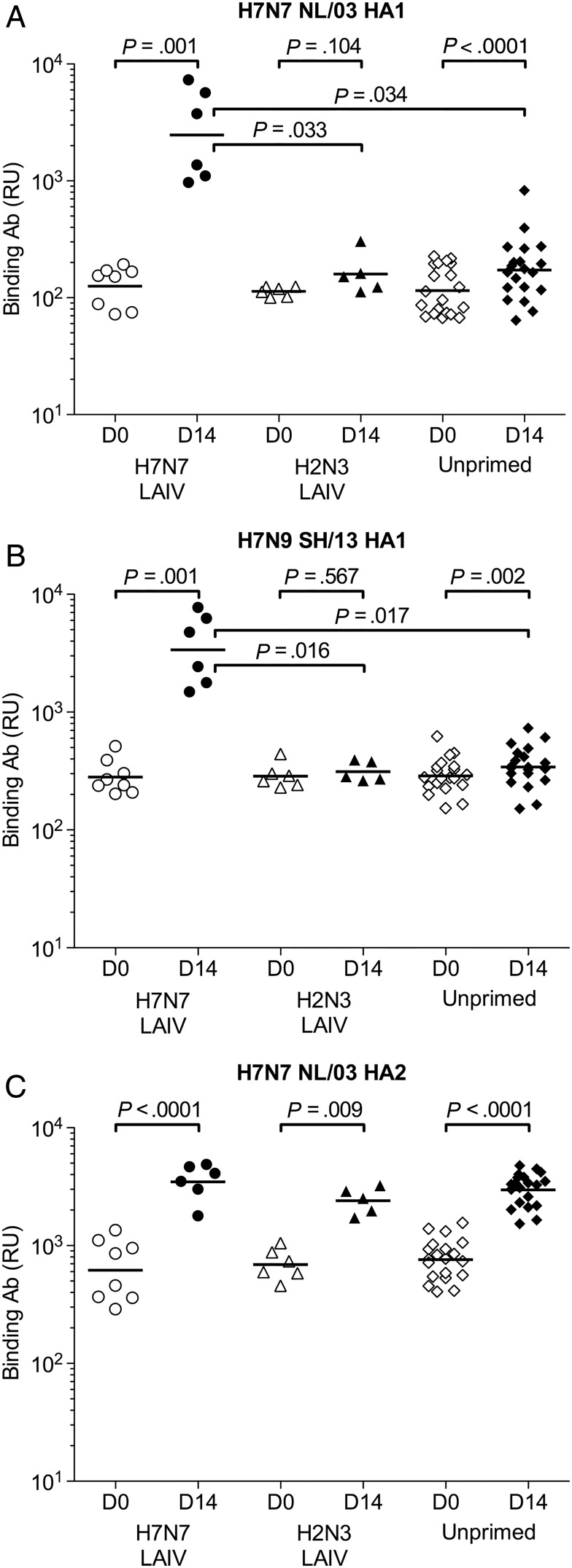
Polyclonal serum Ab binding to the HA1 (head) and HA2 (stalk) subunits of H7 was evaluated by SPR. Individual subject samples from days 0 and 14 were tested for binding to homologous HA1 and HA2 from H7N7 NL/03 and to a variant HA1 from H7N9 SH/13 (Supplementary Figure 1). Binding to the recombinant proteins was present on day 0, likely reflecting antigenic cross-reactivity between H7 and the HAs of seasonal influenza viruses (Supplementary Figure 1). Ab binding to NL/03 HA1 was equally low in all cohorts on day 0 and provided no evidence of prior H7-specific B-cell responses in H7N7 LAIV–primed subjects (Figure 2A). Binding to the homologous HA1 increased substantially in the H7N7 LAIV–primed cohort from days 0 to 14. This increase was also significant in the unprimed cohort but was much smaller than in the H7N7 LAIV–primed cohort. On day 14, total anti-HA1 Ab binding was significantly higher in the H7N7 LAIV–primed cohort than in the other cohorts. Binding to the variant HA1 closely resembled the result for the homologous HA1, demonstrating extensive cross-reactivity by Abs induced by the IIV in the H7N7 LAIV–primed cohort (Figure 2B). HA2 binding significantly increased in all cohorts after the IIV (Figure 2C).
Figure 2.
Serum antibody (Ab) binding to H7 HA1 and HA2 proteins in H7N7 live attenuated influenza vaccine (LAIV)–primed, H2N3 LAIV–primed, and unprimed cohorts before and after H7N7 inactivated influenza vaccination. Total Ab binding to properly folded functional H7N7 NL/03 HA1-His6 (A), H7N9 SH/13 HA1-His6 (B), and H7N7 NL/03 HA2 (C) was measured by surface plasmon resonance. Maximum resonance unit (RU) values for Ab binding are shown for individual H7N7 LAIV–primed (circles), H2N3 LAIV–primed (triangles), and unprimed (diamonds) subjects on days 0 (open symbols) and 14 (filled symbols). Differences were examined by paired t tests (for within-cohort comparisons) and 2-sample t tests (for between-cohort comparisons), using data on a log scale. P values are shown for all within-cohort comparisons and for between-cohort comparisons that were significantly different (P < .05). Abbreviation: HA, hemagglutinin.
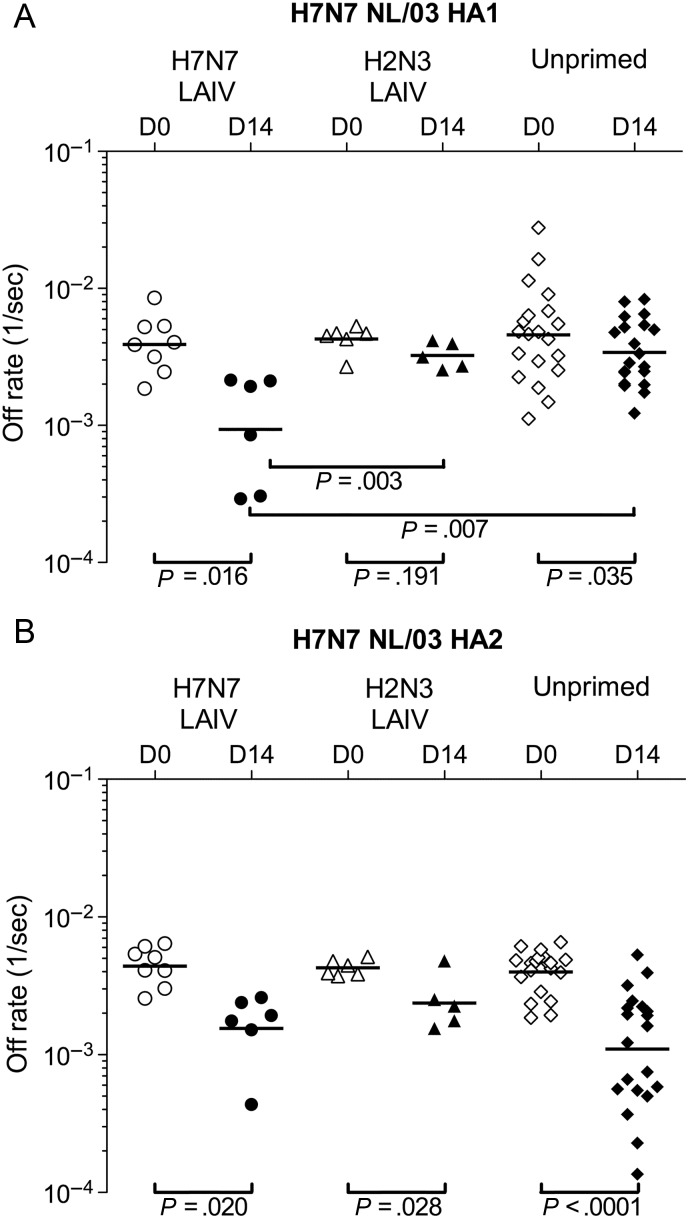
SPR determinations of dissociation rates for serum Abs bound to antigen are independent of Ab concentration and provide a measure of the net affinity of polyclonal Ab binding. The off rates for Abs bound to H7 HA1 decreased significantly from days 0 to 14 in the H7N7 LAIV–primed cohort and the unprimed cohort, indicating increased Ab affinity maturation (Figure 3A). However, Ab affinity for HA1 on day 14 was significantly stronger in the H7N7 LAIV–primed cohort than in the other cohorts. Ab affinity for H7 HA2 increased significantly in all cohorts from days 0 to 14, indicating that Ab affinity had not been maximized by exposure to the HA2 of seasonal influenza viruses (Figure 3B). Production of high-affinity Abs specific for HA1 in the H7N7 LAIV–primed cohort (in particular) and for HA2 in all cohorts after the IIV suggests activation of preexisting MBCs. Both the quantity and net affinity of HA1-binding Abs (but not HA2-binding Abs) on day 14 correlated with HI and MN titers (Supplementary Figure 2 and data not shown).
Figure 3.
HA1-specific antibody (Ab) binding affinity following H7N7 inactivated influenza vaccination is enhanced by H7N7 live attenuated influenza vaccine (LAIV) priming. Off-rate constants are shown for polyclonal serum Ab binding to the H7 HA1 (A) and HA2 (B) proteins of influenza H7N7 NL/03. Sera were collected from H7N7 LAIV–primed (circles), H2N3 LAIV–primed (triangles), and unprimed (diamonds) cohorts at the time of inactivated vaccine administration (day 0; open symbols) and after 14 days (filled symbols). Ab off-rate constants, shown for individual subjects, were determined by surface plasmon resonance and provide a measure of net binding affinity. Differences were examined by paired t tests (for within-cohort comparisons) and 2-sample t tests (for between-cohort comparisons), using data on a log scale. P values are shown for all within-cohort comparisons and for between-cohort comparisons that were significantly different (P < .05). Abbreviation: HA, hemagglutinin.
Analysis of Epitope Profiles of H7-Specific Serum Abs
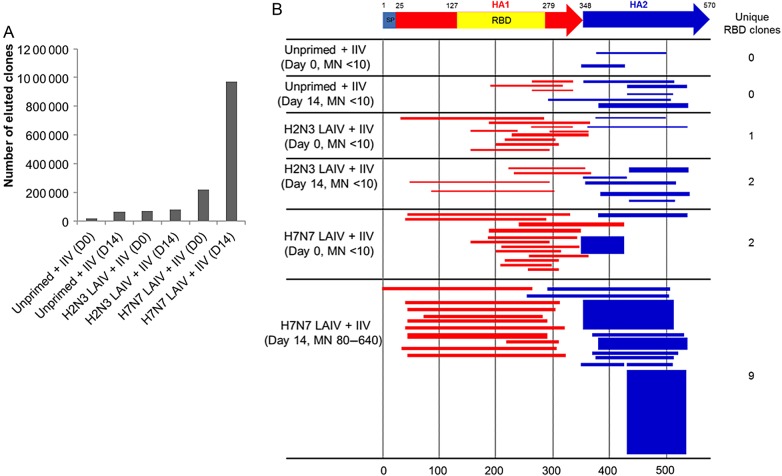
GFPDL analysis of serum Abs was used to relate H7N7 LAIV priming to the repertoire of H7-specific Abs generated by the IIV. Pooled sera collected on days 0 and 14 were used to pan a GFPDL library displaying H7 HA peptides. The number of bound phages increased substantially from days 0 to 14 in the H7N7 LAIV–primed cohort but changed minimally in the other cohorts (Figure 4A). In the H7N7 LAIV–primed cohort, day 0 serum Abs bound predominantly to epitopes in the C-terminus portion of HA1 and to epitopes in HA2 (Figure 4B). Importantly, HA1 epitopes bound by day 14 Abs in the H7N7 LAIV–primed cohort were mostly in multiple large unique sequences that encompassed the receptor-binding domain (RBD). These sequences are likely to contain conformational epitopes targeted by neutralizing Abs [21, 22]. The number of bound RBD-spanning sequences increased from 2 on day 0 to 9 on day 14 (Figure 4). Day 14 Abs in the H7N7 LAIV–primed cohort also bound a substantially greater number of phages that expressed HA2 epitopes. In the other cohorts, the number of bound HA2 epitope–expressing phages increased from day 0 to day 14, but binding to HA1 epitopes remained relatively weak. Overall, these findings indicate that H7N7 LAIV primed for a B-cell response to the IIV that was largely directed against RBD-associated epitopes and HA2 epitopes, whereas the response in the other cohorts was primarily against epitopes in HA2.
Figure 4.
Repertoire of H7-specific antibodies (Abs) in H7N7 live attenuated influenza vaccine (LAIV)–primed, H2N3 LAIV–primed, and unprimed cohorts before and after H7N7 inactivated influenza vaccination. A, Number of bound phages after H7 (NL/03) gene-fragment phage display library (GFPDL) affinity selection by pooled sera from each cohort on days 0 and 14. B, Schematic alignment of the peptides recognized by pooled sera on days 0 and 14. Peptides were identified by panning with an H7 (NL/03) GFPDL and were aligned to the H7 HA translated sequence. The amino acid designation is based on the HA protein sequence (Supplementary Figure 1). Bars indicate identified inserts in HA1 (red bars) and HA2 (blue bars). The thickness of each bar represents the frequency of repetitively isolated phage inserts (only clones with a frequency of ≥2 are shown). The HA1 receptor-binding domain (RBD) is depicted in yellow. The numbers of phage clones displaying unique sequences encompassing the entire RBD are shown to the right of the alignment. Abbreviations: HA, hemagglutinin; IIV, inactivated influenza vaccine; MN, microneutralization titer.
Analysis of Ab-Secreting Plasmablasts (PBs)
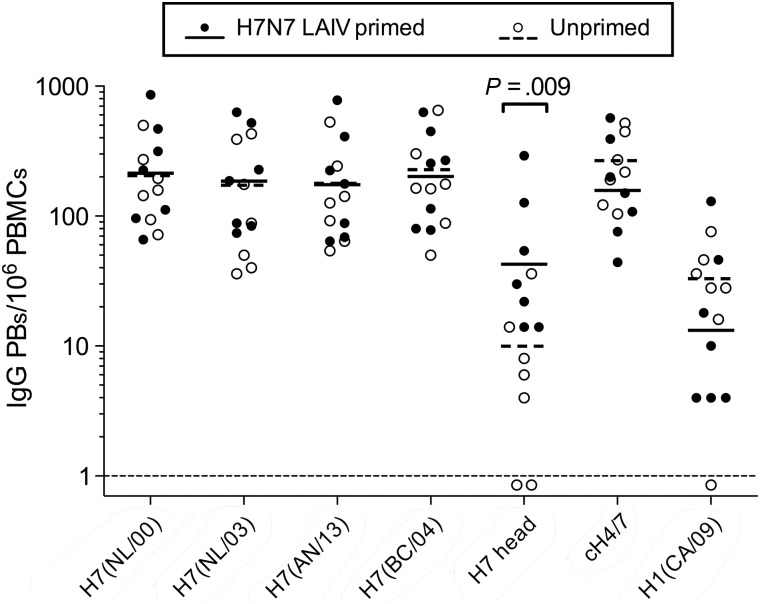
Strong Ab production following intramuscular influenza vaccination is associated with a brief wave of circulating Ab-secreting PBs that is thought to reflect MBC activation [15, 31]. PBMCs collected from the H7N7 LAIV–primed and unprimed cohorts on days 0 and 7 after IIV administration were analyzed for antigen-specific IgG PBs. Antigen-specific PBs were not detected on day 0. Both cohorts generated a strong H7 stalk–specific PB response to the IIV (Figure 5). H7 head–specific PBs were present in all subjects in the H7N7 LAIV–primed cohort but at lower frequencies than stalk-specific PBs. Some unprimed subjects had low numbers of H7 head–specific PBs, but most full-length H7-specific PBs in the unprimed cohort were specific for the stalk region. Low frequencies of PBs specific for the nonvaccine component H1 were present in both cohorts, suggesting some cross-reactive PB induction. Day 7 PB data were in agreement with the SPR analysis of HA1 and HA2 binding and the GFPDL epitope mapping performed using day 14 polyclonal sera (Figures 2 and 4, respectively).
Figure 5.
Circulating plasmablasts (PBs) induced by H7N7 inactivated influenza vaccination in H7N7 live attenuated influenza vaccine (LAIV)–primed and unprimed cohorts. Peripheral blood mononuclear cells (PBMCs) collected on day 7 after the inactivated vaccine were analyzed by an enzyme-linked immunospot assay for immunoglobulin G (IgG)–secreting PBs. PB specificity was assessed against H7 from NL/00, NL/03, AN/13, and BC/04; H7 head only from SH/13; chimeric cH4/7; and H1 from CA/09. Frequencies are shown for individual subjects (n = 7 for each cohort), together with the geometric mean titer (GMT). PBs specific for antigens in the panel were not detected in PBMCs obtained on day 0 from any subject (frequencies < 1/106 PBMCs). Differences between cohorts in specific PB frequencies were examined by 2-sample t tests, using data on a log scale. P values are only shown for comparisons that were significantly different (P < .05).
Analysis of Memory B Cells
Collectively, our analysis of serum Ab and PB responses to the IIV suggested activation of H7 head–specific MBCs in the H7N7 LAIV–primed cohort and H7 stalk–specific MBCs in all cohorts. To test this directly, we measured antigen-specific MBC frequencies in PBMCs on day 0. MBCs were also analyzed on days 14 and 28, when postvaccination frequencies were likely to be maximal [31].
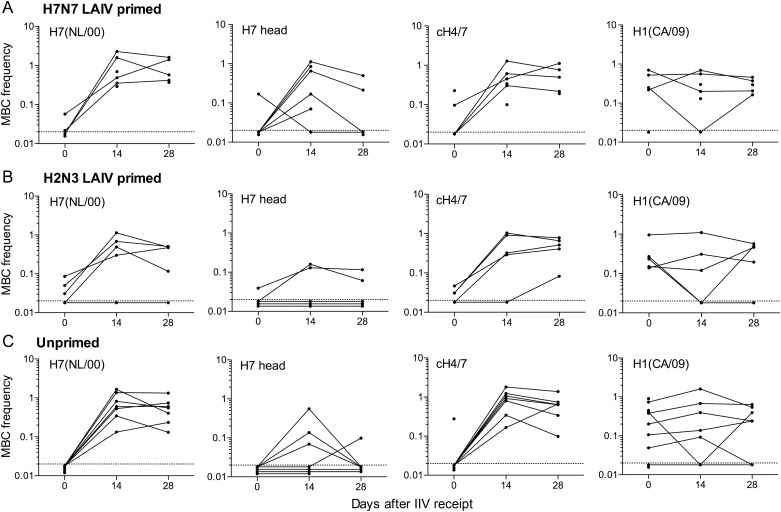
On day 0, most subjects in all cohorts had circulating MBCs specific for the full-length H1 and H3 HAs of recent seasonal influenza viruses and for the H1 stalk region (Figure 6 and Supplementary Figure 3). Generally, a smaller proportion of subjects in all cohorts had MBCs specific for the H7 stalk region. H7 head–specific MBCs were rarely detected in any cohort on day 0. In all cohorts, the frequencies of MBCs measured against full-length H7 and the H7 stalk were similar, indicating that few were specific for the head domain.
Figure 6.
Circulating memory B cells (MBCs) in H7N7 live attenuated influenza vaccine (LAIV)–primed (A), H2N3 LAIV–primed (B), and unprimed (C) cohorts before and after H7N7 inactivated influenza vaccination. Antigen-specific immunoglobulin G (IgG) MBC frequencies were determined in peripheral blood mononuclear cells (PBMCs) collected on days 0, 14, and 28. MBC analysis was based on in vitro stimulation to generate IgG antibody-secreting cells (ASCs), followed by enumeration of antigen-specific and total IgG ASCs. Antigen-specific MBC frequencies are shown as the percentage of total IgG MBCs. MBCs specific for H7 from NL/00, H7 head only from SH/13, chimeric cH4/7, and H1 from CA/09 are shown for each cohort. MBCs specific for H7 from NL/03, H3 from WI/05, and chimeric cH9/1 are shown in Supplementary Figure 3. PBMCs for all sampling times were not available for all subjects. To illustrate kinetic patterns, data points from consecutive samplings are connected for individual subjects. The numbers of data points per sampling time are 5–6 for the H7N7 LAIV–primed cohort, 5 for the H2N3 LAIV–primed cohort, and 7–9 for the unprimed cohort. Abbreviation: IIV, inactivated influenza vaccine.
In all cohorts, there was a marked increase in the frequencies of H7 stalk–specific MBCs from days 0 to 14 (Figure 6). H7 head–specific MBC frequencies were also increased on day 14 in most subjects in the H7N7 LAIV–primed cohort and in a smaller proportion of subjects in the other cohorts. Full-length H7–specific MBC frequencies increased in all cohorts, reflecting the increased frequencies of head- and/or stalk-specific MBCs. A consistent increase in full-length H3–specific MBC frequencies in all cohorts from days 0 to 14 suggests conservation of at least some stalk epitopes in H7 and H3 (Supplementary Figure 3). H7 stalk–specific MBC frequencies in all cohorts remained substantially higher on day 28 than on day 0, but measurable H7 head–specific MBC frequencies in some subjects on day 14 had fallen to undetectable levels by day 28 (Figure 6). The frequencies of MBCs specific for the nonvaccine component H1 generally remained similar on days 0, 14, and 28 in all cohorts. Overall, the expansion of MBC populations largely reflected the specificity of high-affinity Ab production.
DISCUSSION
In the current study, we sought to better understand the impact of H7N7 LAIV priming on the H7-specific B-cell response to H7N7 IIV. Our analysis discriminated between responses to the H7 head and stalk domains. We show that H7N7 LAIV priming promotes a strong, diverse, and high-affinity Ab response to the H7 head domain, including epitopes in the RBD, after H7N7 IIV administration. Both the magnitude and net affinity of this response correlated with protective Ab levels. We also demonstrate strong H7 stalk–specific Ab responses to the IIV in H7N7 LAIV–primed and non–H7-primed subjects.
A key question is the nature of immune memory generated by H7N7 LAIV priming that influences the H7-specific B-cell response to the IIV. Our analysis indicates that the response to the IIV in H7N7 LAIV–primed subjects reflects more-extensive affinity maturation of H7 head–specific Abs than in non–H7-primed subjects. One possibility is that the IIV activated affinity-matured, H7 head–specific MBCs that were induced during H7N7 LAIV priming. This is suggested by the presence of circulating H7 head–specific PBs in all H7N7 LAIV–primed subjects on day 7 after the IIV (Figure 5). Circulating HA-specific PBs on day 7 after influenza vaccination are thought to be the products of activated MBCs, since they carry highly mutated immunoglobulin variable region genes [15, 31]. The activated MBCs may have differentiated directly into ASCs or entered germinal centers for additional rounds of affinity maturation before ASC formation [32]. Germinal center remodeling of the specificities of preexisting MBCs might account for the induction of day 7 PBs specific for the nonvaccine component H1 (Figure 5), without expansion of H1-specific MBC populations (Figure 6) [33]. We did not detect H7 head–specific MBCs in the circulation of most of the H7N7 LAIV–primed subjects at the time of H7 IIV administration (Figure 6). However, this does not necessarily indicate the absence of these cells, since the major MBC repositories are in secondary lymphoid tissues [34, 35]. Our analysis of H7 head–specific MBCs (Figure 6) suggests that small MBC populations generated by vaccination might be only transiently detected in the circulation.
The effect of H7N7 LAIV priming might also reflect the induction of H7-specific CD4+ T cells. Recently, Nayak et al [36] demonstrated that subjects primed with nonreplicating forms of H5 generated a long-maintained increase in H5-specific CD4+ T cells and responded to an H5 boost with a more robust expansion of H5-specific CD4+ T cells than did non–H5-primed subjects. Furthermore, the H5-specific CD4+ T-cell expansion correlated with MN Ab production. However, the extent to which MN Ab production reflected the availability of T-cell help or preexisting H5-specific MBCs is unclear. Healthy adults who have only been exposed to the HAs of seasonal influenza viruses have CD4+ T cells that cross-react with H5 and H7 HAs [36–38]. In our analysis, the vigorous stalk-specific Ab response to the H7N7 IIV by non–H7-primed subjects likely reflects a T-cell–dependent process involving CD4+ T cells and stalk-specific MBCs generated through encounters with seasonal influenza viruses. The implication is that H7 head–specific Ab production in non–H7-primed subjects is primarily limited by a deficiency in head-specific MBCs rather than by a shortage of T-cell help for H7-specific B-cell responses.
The H7N7 IIV used in the current study elicited very few significant HI and MN Ab responses when administered in a prime-boost regimen to H7-naive subjects, even at high doses [6]. Our analysis demonstrates that a single dose of this vaccine generates a rapid and vigorous H7 stalk–specific Ab response in H7N7 LAIV–primed and non–H7-primed subjects. Recent reports of human Ab responses to H5 and H7 IIVs described similar findings [26, 39]. It has been proposed that strong anti-stalk Ab responses are generated when stalk-specific MBCs respond in the absence of competition from MBCs specific for the immunodominant head region [15]. Recently, Ellebedy et al [16] demonstrated that the first dose of an H5 IIV elicited a stronger serum Ab response to the stalk than to the H5 head. However, a second dose of the same vaccine generated a vigorous antihead response but only a feeble antistalk response. This is consistent with the first dose expanding the head-specific MBC population so that it outcompeted the stalk-specific MBC population after the second dose. In our analysis, however, subjects primed to generate a strong anti–H7 head Ab response to the H7N7 IIV also generated a strong antistalk Ab response. Additional studies are required to determine whether this relates to the timing of the boost [5, 36, 40, 41] or to the character of HA-specific B-cell and T-cell memory generated when LAIV is used as the priming vehicle.
Our analysis extends previous studies demonstrating that LAIVs expressing avian HAs prime for protective HI and MN Ab responses to a single dose of the HA in the form of an unadjuvanted IIV [12, 13]. We show that H7N7 LAIV primes for a high-affinity H7 head-specific Ab response to H7N7 IIV without down-modulation of the antistalk Ab response. Collectively, our analysis indicates high-affinity H7 head–specific MBC induction by H7N7 LAIV, although we were unable to directly demonstrate these MBCs at the time of IIV administration. Notably, the 2 subjects who received only a single H7N7 LAIV dose could not be distinguished from the 2-dose recipients in their response to the H7N7 IIV. Thus, a single dose of LAIV expressing an avian HA might be sufficient as a highly effective, prepandemic priming strategy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Ariana Hirsh of F. K.'s laboratory, for excellent technical support; and Drs Vladimir Lugovtsev and Marina Zaitseva, for thorough review of the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH) Respiratory Pathogens Research Center (contract HHSN272201200005C); the Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases (NIAID), NIH; the NIH Centers for Excellence in Influenza Research and Surveillance (contracts HHSN272201400008C [F. K.] and HHSN266200700008C [L. M.-S.]); and the DIR, NIAID, NIH, (Cooperative Research and Development Agreement between the Laboratory of Infectious Diseases, NIAID, NIH, and MedImmune, and the Department of Health and Human Services Biomedical Advanced Research and Development Authority for the clinical trial of H7 vaccines [clinical trials registration NCT01534468]) (contract HHSN272200900026C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Watanabe T, Watanabe S, Maher EA, Neumann G, Kawaoka Y. Pandemic potential of avian influenza A (H7N9) viruses. Trends Microbiol 2014; 22:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol 2005; 3:591–600. [DOI] [PubMed] [Google Scholar]
- 3.Dormitzer PR, Galli G, Castellino F et al. Influenza vaccine immunology. Immunol Rev 2011; 239:167–77. [DOI] [PubMed] [Google Scholar]
- 4.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–51. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Frey SE, Graham I et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis 2011; 203:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One 2012; 7:e49704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RJ, Madhun AS, Hauge S et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889–97. [DOI] [PubMed] [Google Scholar]
- 8.Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Science Transl Med 2014; 6:234ra55. [DOI] [PubMed] [Google Scholar]
- 9.Talaat KR, Karron RA, Callahan KA et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine 2009; 27:3744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudenko L, Kiseleva I, Naykhin AN et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PLoS One 2014; 9:e87962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karron RA, Talaat K, Luke C et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 2009; 27:4953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu TM, Levine M, Fitzgerald T et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine 2014; 32:6798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talaat KR, Luke CJ, Khurana S et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014; 209:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 2013; 3:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li GM, Chiu C, Wrammert J et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellebedy AH, Krammer F, Li GM et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A 2014; 111:13133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhao SJ, Achenbach J, Swayne DE, Donis R, Cox N, Matsuoka Y. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine 2008; 26:1742–50. [DOI] [PubMed] [Google Scholar]
- 18.Khurana S, Coyle EM, Verma S et al. H5 N-terminal β sheet promotes oligomerization of H7-HA1 that induces better antibody affinity maturation and enhanced protection against H7N7 and H7N9 viruses compared to inactivated influenza vaccine. Vaccine 2014; 32:6421–32. [DOI] [PubMed] [Google Scholar]
- 19.Khurana S, Verma N, Talaat KR, Karron RA, Golding H. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis 2012; 205:610–20. [DOI] [PubMed] [Google Scholar]
- 20.Khurana S, Verma N, Yewdell JW et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Science Transl Med 2011; 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana S, Suguitan AL Jr, Rivera Y et al. Antigenic fingerprinting of H5N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med 2009; 6:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khurana S, Chearwae W, Castellino F et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Science Transl Med 2010; 2:15ra5. [DOI] [PubMed] [Google Scholar]
- 23.Khurana S, Loving CL, Manischewitz J et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Science Transl Med 2013; 5:200ra114. [DOI] [PubMed] [Google Scholar]
- 24.Sangster MY, Baer J, Santiago FW et al. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clin Vaccine Immunol 2013; 20:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 2012; 7:e43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krammer F, Jul-Larsen A, Margine I et al. An H7N1 influenza virus vaccine induces broadly reactive antibody responses against H7N9 in humans. Clin Vaccine Immunol 2014; 21:1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hai R, Krammer F, Tan GS et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 2012; 86:5774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pica N, Hai R, Krammer F et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 2012; 109:2573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J Immunol Methods 2013; 391:50–9. [DOI] [PubMed] [Google Scholar]
- 30.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol 2009; 39:1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrammert J, Smith K, Miller J et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog 2012; 8:e1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 2015; 16:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc Natl Acad Sci U S A 2008; 105:3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joo HM, He Y, Sundararajan A, Huan L, Sangster MY. Quantitative analysis of influenza virus-specific B cell memory generated by different routes of inactivated virus vaccination. Vaccine 2010; 28:2186–94. [DOI] [PubMed] [Google Scholar]
- 36.Nayak JL, Richards KA, Yang H, Treanor JJ, Sant AJ. Effect of influenza A(H5N1) vaccine prepandemic priming on CD4+ T-cell responses. J Infect Dis 2015; 211:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards KA, Nayak J, Chaves FA et al. Seasonal influenza can poise hosts for CD4 T cell immunity to H7N9 avian influenza. J Infect Dis 2015; 212:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol 2008; 180:1758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachbagauer R, Wohlbold TJ, Hirsh A et al. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol 2014; 88:13260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledgerwood JE, Zephir K, Hu Z et al. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 2013; 208:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khurana S, Wu J, Dimitrova M et al. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis 2013; 208:413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.