Abstract
Pancreatic cancer is an aggressive and deadly malignancy responsible for the death of over 37,000 Americans each year. Gemcitabine-based therapy is the standard treatment for pancreatic cancer but has limited efficacy due to chemoresistance. In this study, we evaluated the in vitro and in vivo effects of gemcitabine combined with the selective nuclear export (CRM1) inhibitor KPT-330 on pancreatic cancer growth. Human pancreatic cancer MiaPaCa-2 and metastatic pancreatic cancer L3.6pl cell lines were treated with different concentrations of KPT-330 and gemcitabine alone or in combination, and anchorage–dependent/independent growth was recorded. In addition, L3.6pl cells with luciferase were injected orthotopically into pancreas of athymic nude mice, which were treated with 1) vehicle (PBS 1 mL/kg IP, 2/week and povidone/pluronic F68 1 mL/kg PO, 3/week), 2) KPT-330 (20 mg/kg PO, 3/week), 3) gemcitabine (100 mg/kg IP, 2/week), or 4) KPT-330 (10 mg/kg) + gemcitabine (50 mg/kg) for 4 weeks. KPT-330 and gemcitabine alone dose-dependently inhibited anchorage-dependent growth in vitro and tumor volume in vivo compared to vehicle treatment. However, the combination inhibited growth synergistically. In combination, KPT-330 and gemcitabine acted synergistically to enhance pancreatic cancer cell death greater than each single-agent therapy. Mechanistically, KPT-330 and gemcitabine promoted apoptosis, induced p27, depleted survivin, and inhibited accumulation of DNA repair proteins. Together, our data suggest that KPT-330 potentiates the antitumor activity of gemcitabine in human pancreatic cancer through inhibition of tumor growth, depletion of the anti-apoptotic proteins, and induction of apoptosis.
Keywords: gemcitabine, selective nuclear export inhibitor, KPT-330, synergy, pancreatic cancer, CRM1, XPO1, survivin
Introduction
Advanced pancreatic ductal adenocarcinoma is a highly aggressive and lethal malignancy. It is the fourth leading cause of cancer-related mortality in the United States with a median survival of <1 year (1). More than 80% of patients present with unresectable or metastatic disease, which is typically resistant to conventional chemotherapy and radiotherapy. Gemcitabine, a nucleoside analogue, remains the mainstay of pancreatic cancer treatment, although it only demonstrates a median survival of 5.7 months and 20% 1-year survival (2, 3). Recent clinical trials using gemcitabine-based combinations with erlotinib and nab-paclitaxel (Abraxane) have shown modest but significant improvements in patient survival (4, 5). Combination chemotherapy with 5-fluorouracil, oxaliplatin, and irinotecan (FOLFIRINOX) has demonstrated superior efficacy over single-agent gemcitabine (median survival: 11.1 vs. 6.8 months); however, this treatment option is limited by tolerability and is therefore reserved for only selected patients (6). Therefore, new drugs or drug combinations that target key regulatory pathways are needed to reduce mortality and increase survival of patients with pancreatic cancer.
Chromosomal region maintenance-1 (CRM1), also referred as exportin-1 (XPO1), is one of the major transporters of proteins and mRNAs out of the nucleus (7). CRM1 is the sole nuclear exporter of various tumor suppressor, cell cycle, and growth regulatory proteins, including p21, p27, p53, p73, FOXO, NF-κB, Rb, and NPM, and is upregulated in several cancer types (8–11). Nuclear exclusion of tumor suppressor proteins (TSPs) by CRM1 renders cancer cells resistant to apoptosis (11). In many commonly used anticancer drugs, including gemcitabine, 5-fluorouracil, and platinum-based drugs, TSPs are activated through their nuclear retention. However, in tumors, including pancreatic cancer tumors, elevated CRM1 expression results in mislocalization of TSPs through enhanced nuclear export, attenuating their tumor suppressor function and contributing to treatment failure. Furthermore, elevated CRM1 expression is correlated with poor overall survival rates in various tumors including pancreatic cancer (7, 12–15). Therefore, targeted inhibition of CRM1 with selective nuclear export inhibitor compounds could provide therapeutic benefit by enhancing nuclear localization of TSPs and inducing tumor-specific apoptosis (9). Here, we tested the effect of the KPT-330 in combination with gemcitabine on pancreatic cancer cell and metastatic tumor growth.
MATERIALS AND METHODS
Reagents and animals
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. (Z)-3-(3-(3,5-bis (trifluoromethyl) phenyl)-1H-1,2,4-triazol-1-yl)-N’-(pyrazin-2-yl) acrylohydrazide (KPT-330) was provided by Karyopharm Therapeutics Inc (Natick, MA). Gemcitabine-HCl was purchased from Eli Lilly and Company. Female athymic nude mice were obtained from the National Cancer Institute (Frederick, MD). The animal protocol used in the study was approved by our Institutional Animal Care and Use Committee.
Cell culture and growth
MiaPaCa-2 pancreatic cancer cells and L3.6pl metastatic pancreatic cancer cells were grown in monolayers with DMEM medium, supplemented with 10% FBS, 2 mM L-glutamine, penicillin (50 IU/mL), and streptomycin (50 mg/mL). The cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% O2. MiaPaCa-2 cells were purchased from ATCC, Manassas, VA in January 2015, and L3.6pl cells were provided by Dr. Jose Riveno, University of Florida, Gainesville, Florida in 2013. Immortalized human pancreatic normal epithelial (HPNE) cells were obtained from Dr. Paul Campbell, University of North Carolina in 2013 (16). These cell lines are well characterized and tested at regular intervals using RT-PCR and mycoplasma kit.
Cell proliferation MTT assay
Cells were seeded in 96-well plates at a density of 3,000 cells/well and allowed to attach overnight. Cells were then incubated for 72 hours with various concentrations of gemcitabine and KPT-330 (0.1 to 10 µM) or dimethyl sulfoxide (DMSO) (5%)/phosphate-buffered saline (PBS) as vehicle control. Medium was aspirated and replaced with 20 µL of 1 mg/mL MTT and incubated for 2–4 hours at 37°C in a humidified atmosphere of 5% CO2. Medium was aspirated, 200 µL of DMSO were added to each well, samples were incubated for 5 minutes with shaking, and absorbance was read at 540 nm.
Isobologram analysis
Gemcitabine and KPT-330 were combined at different IC50 values to plot the isobolograms using fraction effects and combination index through ClacuSyn software (Biosoft, Cambridge, UK).
Trypan blue dye exclusion assay
The treated cells were trypsinized and washed with PBS, and cell suspension (50 µL) was mixed with 50 µL of Trypan blue dye and incubated for 2 minutes at room temperature. A 10-µL volume was loaded onto a hemacytometer, and cells were scored as live or dead based on Trypan blue dye exclusion.
Anchorage-independent growth assay
Standard soft agar colony formation assays were performed using MiaPaca-2 and L3.6pl cells. The cells were seeded at a density of 5,000/well in 12-well plates in 0.3% agar over a 0.6% bottom agar layer. Colonies were fed with growth media and gemcitabine (5 µM) and KPT-330 (1 µM), and growth of colony formation was observed for 14 days. Colonies were photographed after overnight incubation with 1 mg/mL MTT in the wells. The colonies were counted under a stereomicroscope and compared with control. All experiments were performed at least twice, each in triplicate.
Western blot analysis
Proteins were extracted from cells and pancreatic tumor tissues using RIPA lysis buffer containing protease inhibitors (Thermo Scientific, Rockford, IL). Extracted proteins (40 µg) were resolved on 12.5% SDS-polyacrylamide gel (SDS-PAGE) running gel and a 5% stacking gel. Proteins were then electrotransferred onto nitrocellulose membranes. After membranes were blocked in 5% nonfat powdered milk for 1 hour, they were washed and treated with antibodies to CRM1, p27, cleaved PARP, Bax, survivin, and β-actin (1:1000) overnight at 4°C (Santa Cruz Biotechnology, Santa Cruz, CA; Cell Signaling, Danvers, MA). After blots were washed, they were incubated with horseradish peroxidase-conjugated secondary antibody IgG (1:5000) for 1 hour at room temperature. Washed blots were then treated with SuperSignal West Pico chemiluminescent substrate (Pierce) for positive antibody reaction. Membranes were exposed to X-ray film (KODAK) for visualization and densitometric quantization of protein bands using AlphaEaseFC software (Alpha Innotech).
For DNA damage repair protein assessment, MiaPaCa-2 cells were seeded in 6-well plates (750,000 cells/well). The next day, cells were treated with KPT-330 (50–1000 nM) or vehicle (DMSO) for 24 and 48 hours. For the combination studies, cells were treated with KPT-330 (1 µM), gemcitabine (5 µM), combination, or vehicle (DMSO) for 24 hours. Cells were then lysed with RIPA buffer, and whole protein lysates were probed on immunoblots with Chk1, Rad51, MLH1 (Cell Signaling), PMS2, and beta-actin (Santa Cruz Biotechnology) antibodies and analyzed with Odyssey Reader (Licor).
Animals and drug treatments
Female athymic nude mice (n = 20) were injected with luciferase-tagged L3.6pl human metastatic pancreatic cancer cells orthotopically into the pancreas. Mice were divided into four treatment groups of 5 animals each: group 1 was treated with vehicle (PBS 1 mL/kg IP, 2/week and povidone/pluronic F68 1 mL/kg PO, 3/week); group 2 was treated with KPT-330 (20 mg/kg PO, 3/week); group 3 was treated with gemcitabine (100 mg/kg IP, 2/week); and group 4 was treated with KPT-330 (10 mg/kg PO, 3/week) + gemcitabine (50 mg/kg IP, 2/week) for 4 weeks. The treatment was initiated 1 week after orthotopic injection of cell lines. The body weights were recorded every other day, and tumor volumes were recorded every week using luciferin injection and recording of bioluminescence (Xenogen IVIS 200). The tumor weights were recorded after 4 weeks of treatment, at which time animals were euthanized and blood was collected in heparin vials. The entire pancreas was harvested and fixed in buffered formalin for further analyses. Other pancreatic tissues were snap frozen in liquid nitrogen and kept at −80°C for biochemical analysis. Liver metastasis score was measures as bioluminescence units by IVIS 200 (Xenogen).
Histologic evaluation
Formalin-fixed, paraffin-embedded tissues were sectioned (4 µm) and stained with hematoxylin-eosin. Immunohistochemistry was performed using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) per the manufacturer’s protocol with proprietary reagents. Briefly, slides were deparaffinized on the automated system with EZ Prep solution. Sections were heated for antigen retrieval. For immunohistochemistry, tissue sections were incubated with antibodies specific to Ki-67, CRM1, p27, cleaved caspase-3, and survivin at 1:4000 dilutions for 60 minutes. Detection was performed using the Ventana OmniMap kit.
Immunofluorescence
MiaPaCa-2 cells were seeded on coverslips in 6-well plates at a density of 500,000 cells/well. The next day, cells were treated with gemcitabine (5 µM) or DMSO for 30 hours, followed by exposure to KPT-330 (1 µM) for the next 6 hours. Cells were fixed with methanol and stained with gamma H2A.X (Cell Signaling) antibody. Nuclei were stained with DAPI.
Immunohistochemical assessment
All stained tissues were examined by one independent observer (DC). Cleaved caspase-3, p27, survivin, and Ki-67-stained tissues were assessed for signal in neoplastic areas. Percent expression was recorded for each area (cytosolic or nuclear) and then averaged for each mouse. For CRM1, the percentage of positive cells (1 = 1–33%; 2 = 34–66%; 3 = 67–100%) was recorded followed by the intensity (0–3 for negative, mild, moderate, and strong, respectively) of the stain.
Apoptosis by TUNEL assay
TUNEL apoptotic staining was carried out in slides of tumor tissue sections using In Situ Cell Death Detection Kit (Fluorescein) from Roche as per the manufacturer’s instructions.
Statistical analysis
Data are expressed as means ± SEM, analyzed statistically using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests using SAS statistical software for comparison between different treatment groups. Significance was set at P < 0.05.
RESULTS
In vitro effects of KPT-330 alone and in combination with gemcitabine on proliferation of human pancreatic cancer cells
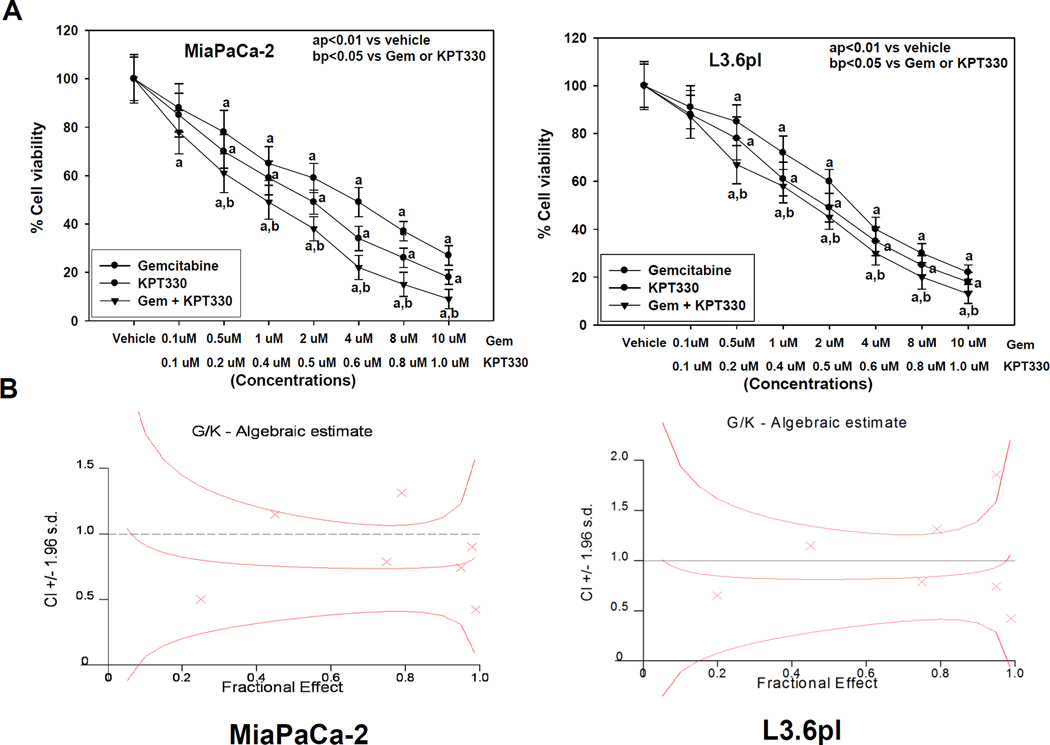
To assess whether KPT-330 alone and in combination with gemcitabine affected the growth of pancreatic cancer cells, we evaluated cell viability after 72 hours of drug treatment using an MTT assay. MiaPaCa-2 human pancreatic cancer and the highly metastatic pancreatic cancer L3.6pl cells were treated with vehicle, KPT-330, gemcitabine, or KPT-330 + gemcitabine at concentrations of 0–10 µM (Fig. 1A). The doses of 0.1–1.0 µM KPT-330 and 0.1–10.0 µM gemcitabine inhibited cell proliferation in a concentration-dependent manner. KPT-330 significantly augmented gemcitabine inhibition of cell proliferation in both cell lines. However, the proliferation of normal HPNE cells was unaltered after treatment with KPT330 (0.1 to 1.0 µM) concentration (Supplemental Figure S1). Furthermore, to confirm synergy, we determined combination index (CI) values for the two drugs. Our results show that gemcitabine + KPT-330 treatment had a strong synergistic effect on cell viability of MiaPaCa-2 cells (CI = 0.76, Fig. 1B) and weak synergistic effect on cell viability of L3.6pl cells (CI = 0.92, Fig. 1B).
Fig. 1.
A, Effect of gemcitabine (Gem) and KPT-330 alone and in combination on pancreatic cancer cell proliferation (MTT assay). Gemcitabine and KPT-330 significantly (aP < 0.001,aP<0.01) decreased proliferation of MiaPaCa-2 and L3.6pl cells versus vehicle, with the combination being more effective in inhibiting cell proliferation than either drug alone (bP < 0.05). Points, means; bars, SE (n = 3–5). All statistical analyses were performed using ANOVA with Duncan test. B, Isobologram with combination index (CI) showing synergistic effect of gemcitabine plus KPT-330 (1:1; G/K) in MiaPaCa-2 and L3.6pl cells.
We further investigated the effects of gemcitabine and KPT-330 alone and in combination on anchorage-independent growth of both MiaPaCa-2 and L3.6pl cells using soft agar colony formation assay (Fig. 2A). In MiaPaCa-2 cells, gemcitabine (5 µM) alone inhibited colony formation by 48%, and KPT-330 (1 µM) alone inhibited colony formation by 52%. Gemcitabine (5 µM) + KPT-330 (1 µM) resulted in 95% inhibition of anchorage-independent growth in MiaPaCa-2 cells (Fig. 2B). On the other hand, in metastatic L3.6pl cells, gemcitabine (5 µM) alone inhibited colony formation by 51%, and KPT-330 (1 µM) alone inhibited colony formation by 42%. Gemcitabine (5 µM) + KPT-330 (1 µM) resulted in 85% inhibition of anchorage-independent growth, suggesting that L3.6pl cells are less susceptible to malignant transformation with the drug combination than MiaPaCa-2 cells (Fig. 2B).
Fig. 2.
A, Effect of gemcitabine and KPT-330 alone and in combination on pancreatic cancer malignant transformation in MiaPaCa-2 and L3.6pl cells. V, vehicle. B, Gemcitabine and KPT-330 alone significantly inhibited colony formation (*P < 0.01, aP<0.02), with combination resulting in almost complete inhibition of the malignant transformation versus vehicle (**P<0.001, bP<0.001) or versus gemcitabine or KPT-330 alone (#P < 0.001, cP<0.01). Bars (means) ± SE (n = 3). All statistical analyses were performed using ANOVA with Duncan test.
Effects of KPT-330 alone and in combination with gemcitabine on pancreatic cancer cell death in vitro
To verify whether growth inhibition by KPT-330 and gemcitabine in MiaPaca-2 and L3.6pl cells is due to induction of cell death, cells were exposed to the combination therapy for 48 hours, harvested, and counted using Trypan blue assay. Figure 3A shows that, relative to single-agent gemcitabine, a combination with KPT-330 elicited a significantly (P < 0.001) higher percentage of cell death in pancreatic cancer cell lines, suggesting that viable cell loss is due to induction of the cell death pathway. We confirmed apoptotic cell death by Western blot analysis of cleaved PARP and Bax in MiaPaCa-2 and L3.6pl cells. Treatment of MiaPaCa-2 and L3.6pl cells with gemcitabine and KPT-330 as single agents induced apoptosis, whereas the combination induced a more profound induction of apoptosis (Fig. 3B and 3C). Our data confirm induction of apoptosis by gemcitabine, KPT-330, and their combination in MiaPaCa-2 and L3.6pl cells.
Fig. 3.
A, Effect of gemcitabine and KPT-330 alone and in combination on pancreatic cancer survival (Trypan blue) in MiaPaCa-2 and L3.6pl cells. Both gemcitabine and KPT-330 significantly induced cell death compared to vehicle (aP < 0.001). However, the combination resulted in greater effect on cell death than vehicle alone (aP < 0.001) or gemcitabine or KPT-330 alone (bP < 0.05). Bars (means) ± SE (n = 3–5). All statistical analyses were performed using ANOVA with Duncan test. B, Western blot of CRM1, cleaved PARP, p27, Bax, and survivin and densitometry of the protein bands in MiaPaCa-2 cells. KPT-330 but not gemcitabine almost completely and significantly (aP<0.001) depleted CRM1 protein expression in MiaPaCa-2 cells compared to vehicle, with the combination depleting CRM1 expression significantly (bP<0.05). Gemcitabine and KPT-330 alone significantly (bP<0.05 and aP<0.001) induced p27 and Bax expression, whereas the combination eliciting greater induction than either drug alone. Gemcitabine and KPT-330 alone significantly (aP<0.001 and bP<0.05) depleted survivin expression, whereas the combination had greater depletion than either drug alone. Gemcitabine and KPT-330 alone and in combination induced apoptosis (cleaved PARP) in these cells. Points, means; bars, SE (n = 3). C, Western blot of CRM1, cleaved PARP, p27, Bax, and survivin and densitometry of the protein bands in L3.6pl cells. KPT-330 but not gemcitabine significantly (aP<0.001) depleted CRM1 protein expression in L3.6pl cells versus vehicle, with combination also significantly depleting CRM1 expression (aP<0.001). Gemcitabine and KPT-330 alone and in combination significantly (bP<0.05 and aP<0.001) induced p27 and Bax expression in these cells. Gemcitabine and KPT-330 alone significantly (aP<0.001) depleted survivin expression, with the combination having greater depletion than either drug alone. Gemcitabine and KPT-330 alone and in combination induced apoptosis (cleaved PARP) in these cells. Points, means; bars, SE (n = 3). All statistical analyses were performed using ANOVA with Duncan test.
Effects of KPT-330 alone and in combination with gemcitabine on cellular targets in pancreatic cancer cells in vitro
To verify whether KPT-330 works by targeting the nuclear export protein CRM1 and whether the combination of KPT-330 and gemcitabine abrogates cell cycle progression, Western analyses was performed to measure the protein expression of CRM1, the cell cycle inhibitor p27, and the anti-apoptotic protein survivin in MiaPaca-2 and L3.6pl cells 48 hours after treatment. As expected, KPT-330 dramatically depleted CRM1 protein expression in MiaPaCa-2 cells and CRM1 protein expression in L3.6pl cells compared to vehicle (Fig. 3B and 3C). However, gemcitabine alone did not alter CRM1 protein levels in either cell line. KPT-330 and gemcitabine increased the cell cycle inhibitor p27 protein expression in both cell lines, but to a more profound degree when the two drugs were combined (Fig. 3B and 3C). The depletion of survivin by KPT-330 and gemcitabine alone and in combination was noted in both cell lines (Fig. 3B and 3C). Interestingly, survivin depletion was more prominent with KPT-330 treatment than with gemcitabine in both cell lines. The combination of the two drugs resulted in depletion of more survivin protein than either drug alone.
Treatment of MiaPaCa-2 cells with increasing concentrations of KPT-330 for 24 and 48 hours reduced the expression of DNA damage repair proteins including RAD51, Chk1, PMS2, and MLH1 in a dose-dependent manner (Fig. 4A). Whereas treatment with gemcitabine alone induced DNA damage and activated Chk1 protein (phosphorylated Chk1; Fig. 4B), KPT-330 inhibited this activation. The inhibition of DNA damage repair resulted in increased cell apoptosis, visualized by increased caspase-3 cleavage (Fig. 4B). KPT-330 alone did not induce DNA damage, but it inhibited the repair of DNA damage, which was induced by gemcitabine as evidenced by higher gamma-H2AX phosphorylation (Fig. 4C). These findings further solidify the case for KPT-330 as a nuclear export inhibitor and revealed a biological explanation for the synergism seen in the combination of KPT-330 with gemcitabine in human pancreatic cancer.
Fig. 4.
A, Western blot showing effect of KPT-330 on DNA damage repair proteins RAD51, Chk1, PMS2, MLH1, and cleaved caspase-3 after 24- and 48-hour incubation in MiaPaCa-2 cells. KPT-330 reduced steady-state levels of DNA damage repair proteins in a dose- and time-dependent manner. B, Western blot showing effect of gemcitabine and KPT-330 alone and in combination on DNA damage repair proteins in MiaPaCa-2 cells after 24 hours. KPT-330 abrogated activation through phosphorylation of Chk1 protein after gemcitabine treatment. In addition, the combination enhanced apoptosis, marked by increased caspase-3 cleavage. C, Immunofluorescence of gamma-H2AX phosphorylation in MiaPaCa-2 cells by confocal microscopy (green fluorescence) was higher with KPT-330 + gemcitabine.
Effects of KPT-330 alone and in combination with gemcitabine on pancreatic tumor volume, proliferation index, and tumor weight and liver metastasis in mice
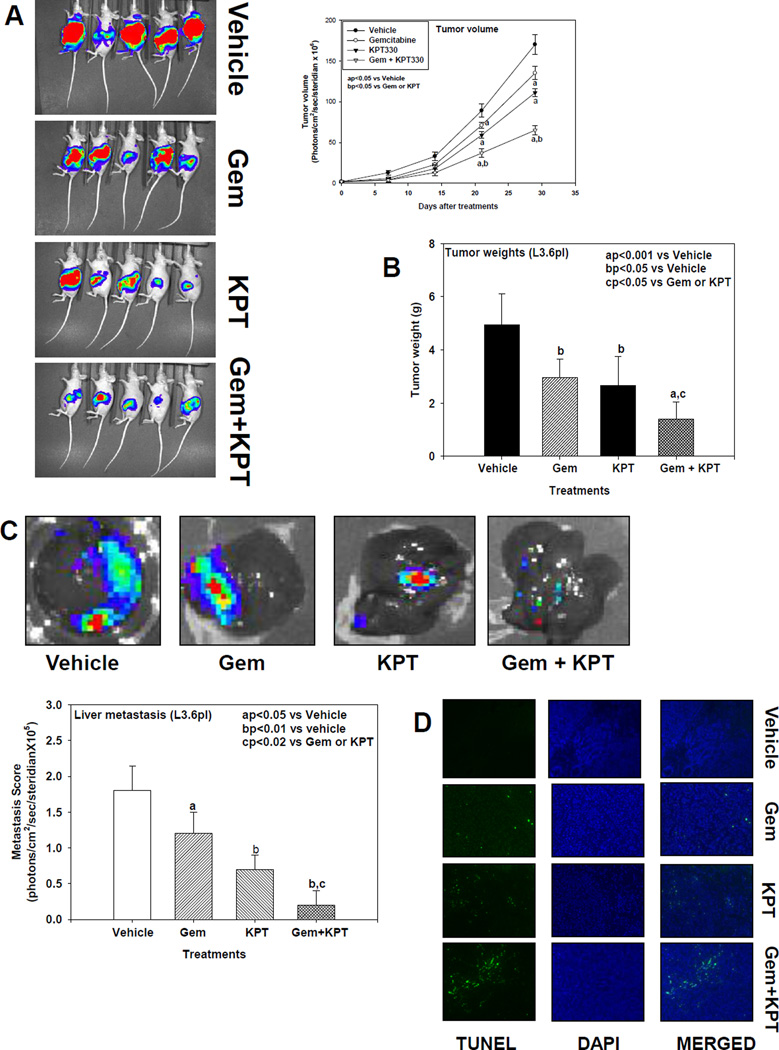
To further expand on our in vitro observations, we tested the antitumor efficacy of these drugs alone and in combination using an orthotopic mouse model of pancreatic cancer. As shown in Fig. 5A, single-agent gemcitabine was less effective (P<0.05) than KPT-330 (P<0.02) at reducing tumor growth, but their combination significantly decreased tumor volume compared to vehicle (P<0.001) or each agent alone. Similarly, as shown in Fig. 5B, tumor weights were significantly decreased after KPT-330 or gemcitabine treatment (P<0.05). When combined, tumor weight was significantly decreased compared to vehicle (P<0.001) or single-agent therapy. We also observed a mark reduction in Ki-67 immunostaining in the gemcitabine (P<0.05), KPT-330 (P<0.001), and gemcitabine + KPT-330 (P<0.001) groups, respectively, compared with the vehicle group (Fig. 6A), confirming the in vitro inhibition of cell proliferation. As shown in Fig. 5C, gemcitabine was less effective (P<0.05) than KPT-330 (P<0.01) when administered alone in reducing the liver metastasis compared to vehicle, but the combination of the two drugs significantly decreased the liver metastasis compared to vehicle (P<0.01) or compared to either drug alone (P<0.02).
Fig. 5.
A, Effect of gemcitabine and KPT-330 alone and in combination on orthotopic pancreatic L3.6pl tumor growth in mice. Gemcitabine or KPT-330 significantly reduced tumor volume (aP<0.05) compared to vehicle, with the combination resulting in more significantly reduced tumor volume compared to vehicle (aP<0.001) or either drug alone (P<0.01 and P<0.05). Results are means and SE (n=5). B, Mean pancreatic tumor weight changes in mice after drug treatment. Gemcitabine and KPT-330 significantly reduced tumor weight (bP<0.05) compared to vehicle, with their combination eliciting a more significant reduction versus vehicle (aP<0.001) or either drug alone (cP<0.05). Bars indicate SE (n=5). All statistical analyses were performed using ANOVA with Duncan test. C, Liver metastasis changes in mice after drug treatment. Gemcitabine and KPT-330 significantly reduced liver metastasis (aP<0.05 and bP<0.01, respectively) compared to vehicle. However, the combination of the 2 drugs more significantly reduced liver metastasis compared to vehicle (aP<0.01) or compared to gemcitabine or KPT-330 alone (cP<0.02). Bars indicate SE (n=5). All statistical analyses were performed using ANOVA with Duncan test. D, Tumor apoptosis (TUNEL) immunofluorescence staining changes in mice after drug treatment. Gemcitabine and KPT-330 induced apoptosis in tumor compared to vehicle. However the combination of the 2 drugs induced more apoptosis than either drug alone in tumor tissues of mice.
Fig. 6.
A, Effect of gemcitabine and KPT-330 alone and in combination on pancreatic tumor cell proliferation index (Ki-67 immunostaining) in mice. Both gemcitabine and KPT-330 significantly inhibited tumor cell growth compared to vehicle (bP<0.05 and aP<0.001). However, the combination resulted in greater inhibition of tumor cell growth versus vehicle (aP < 0.001) or either drug alone (cP < 0.02). Bars (means) ± SE (n=5). B, KPT-330 more significantly increased p27 nuclear immunostaining (bP<0.001) than gemcitabine (aP<0.01) compared to vehicle, with the combination having a greater significant effect versus vehicle (bP<0.001) or either drug alone (cP<0.02). Bars (means) ± SE (n=5). C, KPT-330 more significantly decreased survivin immunostaining (aP<0.001) than gemcitabine (bP<0.05) compared to vehicle, with the combination having a greater significant effect versus vehicle (aP<0.001) or either drug alone (cP < 0.02). Bars (means) ± SE (n=5). D, Gemcitabine more significantly increased cleaved caspase-3 immunostaining (aP<0.001) than KPT-330 (bP<0.01) compared to vehicle, with the combination having a greater significant effect versus vehicle (aP<0.001) or either drug alone (cP < 0.01). Bars (means) ± SE (n=5). All statistical analyses were performed using ANOVA with Duncan test. E, Western blot of CRM1, p27, Bax, and survivin expression in orthotopic pancreatic L3.6pl tumor tissues of mice and densitometry of protein bands. KPT-330 but not gemcitabine significantly (aP<0.001) depleted CRM1 protein expression compared to vehicle. However, the combination resulted in a more depletion of CRM1 in tumor tissues than either drug alone. Gemcitabine and KPT-330 alone significantly (bP<0.05 and aP<0.001) induced p27 and Bax expression, whereas the combination elicited greater induction than either drug alone. Gemcitabine and KPT-330 alone significantly (bP<0.05) depleted survivin expression, whereas the combination had greater depletion than either drug alone. Bars (means) ± SE (n=3). All statistical analyses were performed using ANOVA with Duncan test.
Effects of KPT-330 alone and in combination with gemcitabine on CRM1, cell cycle inhibitor, and pro- and anti-apoptotic protein expression in pancreatic tumor tissue
We investigated the effects of gemcitabine and KPT-330 alone and in combination on CRM1 protein expression in pancreatic tumor samples by immunohistochemical staining and Western blot analyses. As expected, CRM1 protein expression was significantly depleted after treatment with KPT-330 alone and in combination with gemcitabine, but was not changed with gemcitabine treatment alone (Fig. 6E). As shown in Fig. 6B and 6E, KPT-330 more significantly induced p27 nuclear staining (P<0.001) than gemcitabine (P<0.01) compared to vehicle control in pancreatic tumor tissues, suggesting a potential mechanism for cell cycle arrest. Interestingly, p27 induction was greater with the combination than with either drug alone (P<0.02). In addition, our results demonstrate an enhanced depletion of the anti-apoptotic protein survivin in pancreatic tumor tissues treated with KPT-330 alone or in combination with gemcitabine compared to vehicle control or gemcitabine alone (Fig. 6C and 6E). Treatment with gemcitabine or KPT-330 enhanced protein expression of the proapoptotic protein Bax compared to control (Fig. 6E), with the combination resulting in more Bax expression than either drug alone. We also observed a significant increase in cleaved caspase-3 immunostaining in pancreatic tumor tissues in mice treated with gemcitabine, KPT-330, and gemcitabine + KPT-330 compared to control (Fig. 6D) with the combination producing a larger amount of apoptosis (P<0.001). We also confirmed the apoptosis induced by gemcitabine and KPT330 in tumor tissues by TUNEL assay (Fig. 5D). The combination induced more apoptosis than induced by either drug alone.
DISCUSSION
Effective chemotherapies for advanced pancreatic cancer are scarce, and improving long-term survival is a priority. Therefore, any new modality to replace or support current treatments for pancreatic cancer would be highly desirable. In the present study, we evaluated the preclinical efficacy of the selective nuclear export inhibitor KPT-330 alone and in combination with gemcitabine, a commonly used chemotherapeutic agent against pancreatic cancer, both in vitro and in vivo. We found that CRM1 inhibition could become a therapeutic intervention in combination with standard gemcitabine chemotherapy for pancreatic cancer. Furthermore, our in vitro and in vivo data showed that KPT-330 works, in part, through forced nuclear retention of p27, induction of the proapoptotic protein Bax, and depletion of anti-apoptotic protein survivin. KPT-330 also inhibits gemcitabine-induced DNA repair proteins.
Currently available treatment modalities are ineffective in the majority of pancreatic cancer patients (8, 17–19). Extensive studies have demonstrated that the aggressiveness and underlying resistance of pancreatic cancer cells to therapeutic agents, such as gemcitabine, is attributed to inadequate activation of TSPs (8), as well as constitutive activation of the transcription factor NF-κB and its anti-apoptotic gene products, including survivin (20–26). In certain cases where TSPs are activated, their functions are inhibited by cellular mislocalization and continuous nuclear export by CRM1 (12, 14). CRM1 is one of the major nuclear export proteins and is overexpressed in most tumors, including pancreatic cancer (12).Therefore, targeted depletion of CRM1 is an important therapeutic strategy for pancreatic cancer. An earlier study using the specific CRM1 inhibitor, leptomycin B, showed efficacy but adverse off-target toxicities (15). To overcome toxicity, novel, highly specific small molecules were developed to antagonize the functions of CRM1 (27). KPT-330 binds to CRM1 in a slowly reversible fashion, which might contribute to its improved tolerability. Selective nuclear transport inhibitors block nuclear export of TSPs, thereby inducing growth inhibition and apoptosis in various cancer cells such as melanoma, blood, lung, prostate, hepatic, breast, and pancreatic, sparing normal cells (28–34). Earlier studies have shown the efficacy of CRM1 inhibition by selective nuclear export inhibitor compounds such as KPT-185 in human pancreatic cancer models and downstream mechanistic changes (34, 35). These studies demonstrated nuclear retention of ubiquitin ligase complex (Fbw7) and consequent Notch1 degradation, as well as nuclear retention of the tumor suppressor prostate apoptosis response 4 protein both in vitro and in xenograft models of human pancreatic cancer.
Loss of viability and induction of apoptotic cell death are two major mechanisms by which chemotherapeutic agents kill cancer cells. We conceptualized that KPT-330 might augment the growth inhibitory and cell death activity of gemcitabine in vivo using an orthotopic model of pancreatic cancer. Our data showed that KPT-330 significantly augmented gemcitabine inhibition of cell proliferation, with the combination of the two drugs resulting in a synergistic effect on cell viability. One of the mechanisms by which KPT-330 renders cell cycle arrest is through the induction and nuclear retention of cyclin-dependent kinase inhibitor p27kip1. Our data are in agreement with earlier in vitro studies on pancreatic cancer, which demonstrated nuclear retention of p27 and depletion of cyclin D1 after CRM1 inhibition (3, 5). Here, we demonstrated for the first time that the induction of p27 by KPT-330 treatment was further enhanced when combined with gemcitabine both in vitro as well as in vivo. This combination also caused a synergistic effect on anchorage-independent growth and malignant transformation of pancreatic cancer cells (36). Furthermore, our in vivo data demonstrated that L3.6pl tumor-bearing mice treated with KPT-330 + gemcitabine showed significantly reduced tumor growth (weight and volume of the tumor) compared to each therapeutic agent alone. Although KPT-330 has been shown to inhibit tumor growth in xenograft models of pancreatic cancer (3, 5), here we showed for the first time that it significantly potentiates the tumor inhibitory activity of gemcitabine in vivo. Our data further demonstrated that KPT-330 potentiates pancreatic cancer apoptotic cell death in vitro (cleaved PARP and elevated Bax protein expression) as well as in vivo (cleaved caspase-3 and elevated Bax protein expression) when combined with gemcitabine.
Emerging evidence indicates that the overexpression of anti-apoptotic proteins Bcl-XL, survivin, and XIAP are associated with poor prognosis, increased tumor resistance, and chemoresistance (26, 37–42). Recent studies demonstrate that XIAP and survivin overexpression in human pancreatic cancer is associated with poor prognosis and increased tumor recurrence (26, 38–40, 43). Our data support these observations, as KPT-330 significantly depleted survivin expression both in vitro and in vivo. Recently, knockdown of survivin using small interfering RNA has been shown to enhance pancreatic cancer chemosensitivity to gemcitabine (38). Our data showed that depletion of survivin was enhanced when KPT-330 was combined with gemcitabine, indicating the diminution of chemoresistance by KPT-330 in pancreatic tumors. Moreover, inhibition of cell survival anti-apoptotic proteins has been shown to induce caspases (−8, −9 and −3), leading to both extrinsic and intrinsic pathways of apoptosis (44). However, gemcitabine antitumor activity was related to inhibition of DNA synthesis and induction of apoptosis through the intrinsic pathway (45). Gemcitabine chemoresistance is directly related to anti-apoptotic proteins Bcl-XL and cFLIP (23). Our data clearly demonstrated that KPT-330 when combined with gemcitabine suppresses chemoresistance by depleting survivin and enhancing the antitumor activity of gemcitabine through p27 induction. The synergistic effects of KPT-330 on gemcitabine are also achieved through the down-regulation of DNA damage repair proteins. Once gemcitabine induces DNA damage, the cell cycle checkpoint Chk1 is activated by ATM/ATR and its active phosphorylated forms initiate a downstream cascade of events leading to cell cycle arrest and DNA damage repair (46). KPT-330 inhibits the recovery from gemcitabine-induced DNA damage by abrogating the expression of Chk1 in addition to inhibiting expression of other DNA damage-response proteins from the mismatch excision repair group: MSH2 and MLH1 and the homologous recombination repair group RAD51 (47). Such an inhibition delays DNA damage repair and leads to cell death.
Currently, gemcitabine is FDA approved as a single agent for pancreatic cancer patients. Our present study shows that KPT-330 has strong translational potential, warranting clinical investigations in human pancreatic cancer. Furthermore, we show that combining KPT-330 and gemcitabine has potent synergistic effects and could potentially increase the response rates and prolong the survival of pancreatic cancer patients. Presently, KPT-330 is being investigated in a phase I clinical trial in pancreatic cancer in combination with gemcitabine-based chemotherapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Financial support: The study was supported in part by Karyopharm Therapeutics Inc. The study was supported in part by National Cancer Institute Grant 1RO1 CA-129227-01A1 (M.P. Malafa).
Abbreviation List
- ANOVA
analysis of variance
- CI
combination index
- CRM1
chromosomal region maintenance-1
- DMSO
dimethyl sulfoxide
- PBS
phosphate-buffered saline
- TSP
tumor suppressor protein
Footnotes
Conflict of Interests: Trinayan Kashyap, Marsha Crochiere, Yosef Landesman, and Tami Rashal are employees of Karyopharm Therapeutics Inc.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Vickers MM, Powell ED, Asmis TR, Jonker DJ, Hilton JF, O’Callaghan CJ, et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer - results from NCIC CTG PA.3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur J Cancer. 2012;48:1434–1442. doi: 10.1016/j.ejca.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Arshad A, Al-Leswas D, Al-Taan O, Stephenson J, Metcalfe M, Steward WP, et al. Pooled Survival and response data from phase III randomized controlled trials for gemcitabine-based regimes in the treatment of advanced pancreatic cancer. Am J Clin Oncol. 2013;36:411–414. doi: 10.1097/COC.0b013e3182124216. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Drug Combo Effective against Pancreatic Cancer. Cancer Discovery. 2013;3:8. [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, et al. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep. 2009;21:229–235. [PubMed] [Google Scholar]
- 8.Gieseler F, Rudolph P, Kloeppel G, Foelsch UR. Resistance mechanisms of gastrointestinal cancers: why does conventional chemotherapy fail? Int J colorectal Dis. 2003;18:470–480. doi: 10.1007/s00384-003-0496-x. [DOI] [PubMed] [Google Scholar]
- 9.Mao L, Yang Y. Targeting the nuclear transport machinery by rational drug design. Current Pharm Des. 2013;19:2318–2325. doi: 10.2174/1381612811319120018. [DOI] [PubMed] [Google Scholar]
- 10.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 11.Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008;15:2648–2655. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- 12.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:315. [PubMed] [Google Scholar]
- 13.Noske A, Weichert W, Niesporek S, Roske A, Buckendahl AC, Koch I, et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112:1733–1743. doi: 10.1002/cncr.23354. [DOI] [PubMed] [Google Scholar]
- 14.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer. 1996;74:648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell PM, Lee KM, Ouellette MM, Kim HJ, Groehler AL, Khazak V, et al. Ras-driven transformation of human nestin-positive pancreatic epithelial cells. Methods Enzymol. 2008;439:451–465. doi: 10.1016/S0076-6879(07)00431-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 18.Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, et al. Overcoming drug resistance in pancreatic cancer. Expert Op Therap Targets. 2011;15:817–828. doi: 10.1517/14728222.2011.566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 21.Kong R, Sun B, Jiang H, Pan S, Chen H, Wang S, et al. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291:90–98. doi: 10.1016/j.canlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Kong R, Sun B, Wang SJ, Pan SH, Wang G, Chen H, et al. [An experimental study of gemcitabine inducing pancreatic cancer cell apoptosis potentiated by nuclear factor-kappa B P65 siRNA] Zhonghua Wai Ke Za Zhi. 2010;48:128–133. [PubMed] [Google Scholar]
- 23.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 2011;10:2363–2372. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalid O, Toledo Warshaviak D, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. J Comput Aided Mol Des. 2012;26:1217–1228. doi: 10.1007/s10822-012-9611-9. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Bill MA, Young GS, La Perle K, Landesman Y, Shacham S, et al. Novel small molecule XPO1/CRM1 inhibitors induce nuclear accumulation of TP53, phosphorylated mapk and apoptosis in human melanoma cells. PloS One. 2014;9:102983. doi: 10.1371/journal.pone.0102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura M, Ishizawa J, Ruvolo V, Dilip A, Quintas-Cardama A, McDonnell TJ, et al. Induction of p53-mediated transcription and apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma. Cancer Sci. 2014;105:795–801. doi: 10.1111/cas.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Hattori N, Chien W, Sun Q, Sudo M, GL EL, et al. KPT-330 has antitumour activity against non-small cell lung cancer. Br J Cancer. 2014;111:281–291. doi: 10.1038/bjc.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendonca J, Sharma A, Kim HS, Hammers H, Meeker A, De Marzo A, et al. Selective inhibitors of nuclear export (SINE) as novel therapeutics for prostate cancer. Oncotarget. 2014;5:6102–6112. doi: 10.18632/oncotarget.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y, Gery S, Sun H, Shacham S, Kauffman M, Koeffler HP. KPT-330 inhibitor of XPO1-mediated nuclear export has anti-proliferative activity in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74:487–495. doi: 10.1007/s00280-014-2495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y, Holloway MP, Nguyen K, McCauley D, Landesman Y, Kauffman MG, et al. XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin-dependent oncogenic switch in triple-negative breast cancer. Mol Cancer Ther. 2014;13:675–686. doi: 10.1158/1535-7163.MCT-13-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Azmi AS, Aboukameel A, Kauffman M, Shacham S, Abou-Samra AB, et al. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget. 2014;5:3444–3454. doi: 10.18632/oncotarget.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azmi AS, Aboukameel A, Bao B, Sarkar FH, Philip PA, Kauffman M, et al. Selective inhibitors of nuclear export block pancreatic cancer cell proliferation and reduce tumor growth in mice. Gastroenterology. 2013;144:447–456. doi: 10.1053/j.gastro.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Bai J, Sui J, Demirjian A, Vollmer CM, Jr, Marasco W, Callery MP. Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res. 2005;65:2344–2352. doi: 10.1158/0008-5472.CAN-04-3502. [DOI] [PubMed] [Google Scholar]
- 38.Liu WS, Yan HJ, Qin RY, Tian R, Wang M, Jiang JX, et al. siRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig Dis Sci. 2009;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 39.Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T, et al. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26:3265–3273. [PubMed] [Google Scholar]
- 40.Lee MA, Park GS, Lee HJ, Jung JH, Kang JH, Hong YS, et al. Survivin expression and its clinical significance in pancreatic cancer. BMC Cancer. 2005;5:127. doi: 10.1186/1471-2407-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang TM, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 43.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–2434. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 44.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 45.Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182–188. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 46.Capasso H, Palermo C, Wan S, Rao H, John UP, O’Connell MJ, et al. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J Cell Sci. 2002;115:4555–4564. doi: 10.1242/jcs.00133. [DOI] [PubMed] [Google Scholar]
- 47.Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105:370–388. doi: 10.1111/cas.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.