Abstract
The cognitive model of depression posits that cognitive therapy’s (CT) effect on depressive symptoms is mediated by changes in cognitive content (e.g., automatic negative thoughts dysfunctional attitudes, failure attributions). We tested improvement and normalization of cognitive content among outpatients (N = 523) with recurrent major depressive disorder treated with acute-phase CT (Jarrett & Thase, 2010; Jarrett et al., 2013). We also tested whether improvement in cognitive content accounted for subsequent changes in depressive symptoms and vice versa. Five measures of content improved substantively from pre- to post-CT (median d = 0.96), and the proportions of patients scoring in “healthy” ranges increased (median 45% to 82%). Evidence for cognitive mediation of symptom reduction was limited (median r = .06), as was evidence for symptom mediation of cognitive content improvement (median r = .07). We discuss measurement and design issues relevant to detection of mediators and consider alternative theories of change.
Keywords: cognitive therapy, major depressive disorder, cognitive content, mediation
The role of change in relevant cognitive processes in cognitive therapy (CT; Beck, Rush, Shaw, & Emery, 1979) for major depressive disorder (MDD) is surprisingly unclear. After decades of research, researchers know clearly that acute-phase CT produces large decreases in depressive symptoms (e.g., 1–3 SD from pre- to post-CT) and moderately high response rates (e.g., roughly 50–70% of patients who complete CT no longer meet criteria for MDD), similar to other depression-specific treatments (e.g., interpersonal psychotherapy, antidepressant medication) and superior to non-active control conditions (e.g., placebo; Vittengl & Jarrett, 2014). However, the extent to which changes in depressive cognitive content (e.g., dysfunctional attitudes, hopelessness, global and stable attributions for failures) are causal forces in depressive symptom reductions is uncertain (e.g., Kazdin, 2009; Longmore & Worrell, 2007). In this article, we examine the extent to which cognitive content improves and is normalized, as well as the degree and direction of relations of changes in cognitive content with depressive symptoms in a large sample (N = 523) of patients with recurrent MDD treated with acute-phase CT (Jarrett & Thase, 2010).
In the cognitive theory of depression, negative affect (e.g., sadness, fear, anger) is assumed to be subjective and to result from cognition, specifically the evaluation of external and internal stimuli or events (Clark, Beck, & Alford, 1999). In this framework, cognitive structures that facilitate evaluation of stimuli include schema (relatively stable, internal structures used to organize new information; e.g., assumptions such as “I must always succeed” and core beliefs such as “I am helpless”), modes (clusters of inter-related schema organized to handle demands; e.g., clusters of affective, cognitive-structural, and behavioral schema pertaining to personal loss or deprivation), and personality (broad, stable affective tendencies, and beliefs about the self and others that organize schema to respond to everyday life). These cognitive structures may be activated by stimuli matching their content (e.g., losing a job may activate schema with the assumption “I must always succeed”). Activated schema then dominate information processing so that subsequent thought, behavior, and cognition become consistent with the activated schema (e.g., having the thought “I am a failure and always will be” and engaging in avoidance behavior). Outputs of activated schema may include negative automatic thoughts (quick, specific, involuntary, biased judgments of reality, including the self) and cognitive errors (e.g., overgeneralization of negative conclusions, dichotomous thinking), as well as behaviors and emotions diagnostic of depression (Clark et al., 1999).
More recently, Clark and Beck (2010) expanded the cognitive theory of depression to incorporate neurophysiological processes. They proposed that environmental triggers may interact with genetic and personality diatheses to activate negative schemas, including increased bottom-up (automatic) processing, reactivity in the amygdala and hippocampus (and perhaps prefrontal cortex metabolism), and alteration of the ventromedial prefrontal cortex. Negative schema activation then evokes top-down (effortful, reflective) information processing that may involve inhibition (often leading to poor coping, avoidance, and depressive symptoms) or activation (often leading to effective coping and euthymia) of cognitive control. Cognitive control involves inhibition versus activation of brain areas including the anterior cingulate cortex, medial and lateral prefrontal cortex, and the orbitofrontal cortex. Cognitive control, coping behavior, and depressive symptoms may feed back to increase or decrease negative schema activation. Effective cognitive therapy is proposed to correct biased information processing by dampening negative schema activation directly and/or by strengthening top-down (effortful, reflective) processing (Clark & Beck, 2010). That is, changes in cognition within neurophysiological substrates are hypothesized to mediate CT’s effects on depression.
Patient-report measures of cognitive content relevant to depression attempt to tap schema and assess their products (Dunkley, Blankstein, & Segal, 2010). For example, the Dysfunctional Attitudes Scale (DAS; Weissmann, 1979) may measure semantic content of depressive schema, and the Attributional Style Questionnaire (ASQ; Peterson et al., 1982; Dykema, Bergbower, Doctora, & Peterson, 1996) and Beck Hopelessness Scale (BHS; Beck, Weissman, Lester, & Trexler, 1974) may measure products of activated schema. In contrast, the Self-Control Schedule (SCS; Rosenbaum, 1980) aims to measure learned resourcefulness (e.g., positive coping behaviors) in response to challenging internal and external events. Researchers have used these and similar measures in attempts to clarify whether CT’s effects on depression are mediated by change in cognition.
Although conceptual definitions of mediation are broadly consistent among researchers, operational definitions appear to vary widely and result in disparate conclusions about cognitive mediation in CT. Conceptually, mediators are possible mechanisms by which treatments produce their effects on outcome variables (e.g., see Kazdin, 2007; Kraemer, Wilson, Fairburn, & Agras, 2002; MacKinnon, Fairchild, & Fritz, 2007). Mediators are present when a treatment (e.g., CT) first changes an intervening variable (e.g., cognitive content), and the intervening variable (mediator) subsequently accounts for changes in the outcome (e.g., depressive symptoms). Additional experimentation is then required to establish conclusively that identified mediators are causal forces (mechanisms) rather than proxies for causal forces (i.e., all mechanisms are mediators but not all mediators are mechanisms).
Rigorous methods to test mediation include a large-sample randomized clinical trial (e.g., CT vs. comparison treatment) of carefully diagnosed patients receiving CT from therapists with demonstrated competence, at least three assessment time points (e.g., pre-, mid-, and post-treatment) for both potential mediators and outcome variables, and measures with well-known psychometric properties (cf. Kazdin, 2007; Kraemer et al., 2002; MacKinnon et al., 2007). This rigorous design would provide adequate statistical power to establish clearly that CT changes cognition relative to control, and that the changes in cognition account for CT’s subsequent effects on depressive symptoms relative to control. We know of no study of acute-phase CT that meets this design standard fully. Instead, researchers have made limited progress identifying mediators in datasets with varying strengths and departures from this ideal design. Perhaps not surprisingly, their findings also have varied.
Consistent with cognitive mediation, it is well established that CT decreases depressive cognitive content and that pre/post-CT changes in cognitive content correlate moderately with pre/post-CT changes in depressive symptoms. Further “sudden gains” (relatively quick, large drops in depressive symptoms) during CT are sometimes preceded by changes in cognitive content (e.g., see reviews by Garratt, Ingram, Rand, & Sawalani, 2007; Webb, Auerbach, & DeRubeis, 2012). These findings are necessary but not sufficient to establish cognitive mediation of CT’s effects on depressive symptoms.
Inconsistent with mediation, research suggests that depressive symptoms improve more than cognitive content, especially early in CT (e.g., Furlong & Oei, 2002; Jarrett, Vittengl, Doyle, & Clark, 2007), and cognitive content change does not account fully for pre/post-CT symptom change (e.g., Christopher et al., 2009; Jarrett et al., 2007). Further, sudden gains also occur pre-treatment (e.g., Busch et al., 2006) and with pill placebo treatment (e.g., Vittengl, Clark, & Jarrett, 2005), and symptom change in CT does not require cognitive-content change, leading Kazdin (2007) to conclude “whatever may be the basis of changes with CT, it does not seem to be the cognitions as originally proposed” (p. 8). Similarly, CT appears to have active ingredients other than techniques that focus on cognitive change because behavioral activation strategies used early in CT to increase patients’ engagement with sources of reinforcement and to improve functioning (Beck et al., 1979) have alone been shown to be an effective treatment for depression (Dimidjian et al., 2006; Jacobson et al., 1996). Moreover, non-cognitive treatments (e.g., pharmacotherapy) often (but not always) improve cognitive content as much as does CT (Longmore & Worrell, 2007).
Finer grained analyses have also yielded mixed results regarding cognitive mediation in CT. For example, Quilty, McBride, and Bagby (2008) examined changes in dysfunctional attitudes and depressive symptoms from pre- to post-treatment among 130 patients with MDD randomized to CT (Greenberger & Padesky, 1995), interpersonal psychotherapy (Weissman, Markowitz, & Klerman, 2000), or pharmacotherapy (several antidepressant medications used). In support of the hypothesis that changes in cognition mediate CT’s effects on depressive symptoms, CT produced larger decreases in dysfunctional attitudes than did interpersonal psychotherapy, and changes in dysfunctional attitudes predicted change in depressive symptoms. When contrasting CT with pharmacotherapy, however, Quilty et al. found that the reverse causal model fit better— pharmacotherapy produced larger changes in depressive symptoms than did CT and the change in depressive symptoms predicted decreases in dysfunctional attitudes. Whether cognition mediates CT’s effect on depressive symptoms appears sensitive to the question, “CT’s effect compared to what?” Quilty et al.’s conclusions were limited further by testing only simultaneous change (i.e., between two assessments, pre- and post-treatment) in cognition and symptoms, thus, they could not establish that cognitive content improvements drove later symptom reduction.
In a large sample of outpatient adults (N = 521) seeking treatment for depression and/or anxiety (92% received a diagnosis of dysthymic disorder or MDD), Burns and Spangler (2001) measured symptoms (depression and anxiety), as well as dysfunctional attitudes pre- and post-CT (37% of patients also received pharmacotherapy). A series of causal models revealed cross-sectional correlations between symptoms and attitudes, and between longitudinal changes in symptoms and changes in attitudes. However, evidence suggesting that changes in attitudes drove changes in symptom or vice versa was limited. Instead, results fit an unknown “common cause” producing simultaneous change in both symptoms and cognitive content.
Finally, Forman et al. (2012) analyzed data from 174 outpatients seeking treatment for depression and/or anxiety in a university clinic. Patients were randomized to CT or to a newer variant of CT, acceptance and commitment therapy (ACT). Patients completed a brief self-report measure of symptoms and potential mediators unique to this study at each treatment session. Symptoms decreased substantially in both treatments, but relations of changes in potential mediators and symptoms varied partly between CT and ACT. There was some evidence that increased rationality of patients’ thoughts predicted symptom reduction in CT (which emphasizes testing and changing the potential bias in negative thoughts) but not in ACT (which emphasizes noticing and accepting thoughts). In contrast, increased acceptance predicted reduced symptoms in ACT. The interesting results of this study are difficult to generalize because of the unique, brief measures and because only 35% of patients had a depressive disorder.
The aim of the current analyses was to add to the knowledge base about change in cognitive content and its potential mediation of CT’s effects on depressive symptoms. We used a dataset meeting most (large sample, three assessment time points, well-established cognitive content and symptom measures, patients with carefully diagnosed MDD treated by competent therapists) but not all (uncontrolled trial of acute-phase CT) design features for strong mediation tests. Based on findings from past research and cognitive theory, we tested three hypotheses: (1) cognitive content would improve substantively during acute-phase CT, including increases in the proportions of patients in “healthy” ranges of the cognitive content measures based on normative data; (2) improvement in cognitive content from pre- to post-CT would correlate with, and largely be accounted for by, reductions in depressive symptoms; and (3) improvements in cognitive content would predict subsequent reductions in depressive symptoms. We also tested reverse causality (i.e., whether changes in cognitive content were produced by reduction in depressive symptoms) in a series of structural equation models (SEMs).
Method
Data were drawn from a two-site randomized clinical trial comparing continuation-phase CT, fluoxetine, or pill placebo among acute-phase CT responders (Jarrett & Thase, 2010; Jarrett et al., 2013). Following we summarize relevant methods from the acute phase of this trial. Jarrett and Thase (2010) provide detail about continuation and follow-up phases not analyzed here.
Participants
Potential participants were recruited via clinical referrals and newspaper, bulletin board, and Internet announcements. Clinic staff screened potential participants by telephone or in-person and scheduled them for initial and follow-up diagnostic evaluations. Among 1359 who began evaluation, participants were 523 outpatients who (a) provided written informed consent; (b) met DSM-IV criteria for recurrent MDD (American Psychiatric Association, 2000) via the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1996); (c) previously had remitted between depressive episodes, ≥ 1 depressive episode with complete inter-episode recovery, or antecedent dysthymic disorder; and (d) scored ≥ 14 on the 17-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960).1,2 The trial excluded patients who (a) had severe or poorly controlled concurrent medical disorders that could cause depression, (b) had psychotic or organic mental disorders, bipolar disorder, active substance dependence, or primary obsessive-compulsive or eating disorders, (c) could not complete questionnaires in English, (d) were an active suicide risk, (e) were <18 or >70 years old, (f) had not responded previously to ≥ 8 weeks of CT or 6 weeks of fluoxetine, or (g) were pregnant or planned to become pregnant during the first 11 months after intake. Included patients (N = 523) were M = 42.4 (SD = 12.1) years old and had completed M = 15.1 (SD = 2.9) years of education; 67.5% were women; 80.9% were white, 10.3% black, and 8.8% other races/ethnicities. Participants’ age of MDD onset was 21.2 (SD = 10.8) years and their current major depressive episode had lasted M =25.0 (SD = 45.1) months.
Acute-phase CT
Patients withdrew from psychotropic medications before, and were not prescribed medications during, the acute-phase CT protocol. Cognitive therapists (n = 16) had completed at least 1 year of CT training, submitted videotaped sessions for review, participated in weekly group supervision/feedback sessions, and demonstrated competence by maintaining Cognitive Therapy Scale (Young & Beck, 1980) scores ≥ 40.3 The acute-phase CT protocol lasted 12 weeks, with up to 2 additional weeks allowed for rescheduling. Patients received 2 CT sessions per week for 4 weeks, after which patients with ≥ 40% reduction in HRSD scores received 8 additional weekly sessions (16 total sessions), whereas patients with < 40% reduction in HRSD scores received 4 additional weeks of twice-weekly sessions before beginning weekly sessions (20 total sessions).4 More CT sessions were provided to patients with less early improvement to increase their chance of response and eligibility for the continuation phase (not analyzed here; see Jarrett & Thase, 2010). Among 523 consenting, 410 patients completed the acute-phase protocol by attending ≥ 14 (of 16) or ≥ 18 (of 20) CT sessions, and 292 responded by having no major depressive episode and HRSD ≤ 12 at the end of acute-phase CT (Jarrett et al., 2013).
Measures
Depression symptom severity
The 17-item HRSD, 30-item Inventory of Depressive Symptomatology—Self Report (IDS-SR; Rush et al., 1986), and the 21-item Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) were used to measure symptom severity. Clinicians administered the HRSD-17 and patients completed the BDI and IDS-SR at diagnostic evaluation, weekly during acute-phase treatment, within 7 days of the last acute-phase CT session, and any time a patient exited the protocol. Clinicians also administered the HRSD-17 at a follow-up interview to diagnostic evaluation. Higher scores on the HRSD-17, IDS-SR, and BDI indicate more severe depressive symptoms, and these measures’ reliability and validity for assessing depression symptom severity is well established (e.g., Vittengl, Clark, Kraft, & Jarrett, 2005). Because these measures mark the same symptom construct (Vittengl et al., 2005; Vittengl et al., 2013), we aggregated them to provide a robust symptom index by standardizing the measures based on the distribution of scores at intake and averaging them. Alpha internal consistency reliability computed for the composite was high at the assessments used in the current analyses: pre-acute-phase CT (i.e., diagnostic evaluation; .81), mid-CT (i.e., week 7 in CT; .94), post-CT (i.e., within 7 days of the last acute-phase CT session; .95).
Dysfunctional Attitudes Scale (DAS)
On the 40-item DAS (Form A; Weissman, 1979), patients rate statements about their self-concept, happiness, perfectionism, and thoughts and feelings relevant to depression using 7-point scales ranging from “agree very much” to “disagree very much.” Higher total scores reflect more dysfunctional attitudes with greater severity. The DAS differentiates persons diagnosed with depression versus non-depressed controls (Otto et al., 2007; Nelson, Stern, & Cicchetti, 1992). Alpha internal consistency reliability for the DAS total score was high at our pre- (.93), mid- (.94), and post-acute-phase CT (.94) assessments. We also submitted DAS self-criticism (perfectionism) and need for approval subscales to mediation analyses because these attitude dimensions may relate differently to symptom change and treatment response (e.g., Imber et al., 1990; Quilty et al., 2008).
Attributional Style Questionnaire (ASQ)
On the revised, shortened ASQ (Dykema, Bergbower, Doctora & Peterson, 1996), patients generate causes for 12 hypothetical negative events and rate the extent to which the events’ causes are stable (vs. unstable) and global (vs. specific) from −3 to +3. Higher total scores reflect more depressive (i.e., stable and global) attributions. In support of the measure’s validity, higher ASQ scores have correlated with both acute and chronic depression (Riso et al., 2003) and poorer rehabilitation in cardiac patients (Bennett & Elliott, 2005). The stable and global scales, respectively, demonstrated acceptable alpha internal consistency pre- (.81, .79), mid- (.85, .81), and post- (.86, .84) acute-phase CT.
Beck Hopelessness Scale (BHS)
The BHS measures negative expectancies about the future (Beck & Steer, 1988; Beck, Weissman, Lester, & Trexler, 1974). The self-report scale includes 20 true/false items, and higher total scores indicate greater hopelessness. BHS scores correlate positively with greater depression severity (Beck, Kovacs, & Weissman, 1975) and suicidality (Beck, Steer, Kovacs, & Garrison, 1985). The BHS had high internal consistency pre- (.89), mid- (.90), and post-acute-phase CT (.92) in the current dataset.
Self-Control Schedule (SCS)
The SCS is 36-item self-report measure of learned resourcefulness assessing use of self-control methods to solve behavioral problems (Rosenbaum, 1980). Scale items tap use of cognitive strategies, problem-solving strategies, delay of gratification, and belief in one’s ability to regulate internal events. Respondents rate items on 6-point scales ranging from +3 (very characteristic of me) to −3 (very uncharacteristic of me). Higher total scores correlate with higher confidence (Akgun, 2004), lower scores on the BDI (Slessareva & Muraven, 2004), and response to CT among patients with more severe depressive symptoms (Burns, Rude, Simons, Bates, & Thase, 1994). The SCS showed good internal consistency at pre- (.85), mid- (.87), and post-acute-phase CT (.89).
Treatment of Missing Data
Missing values on study measures occurred for several reasons over the decade (plus)-long, multisite clinical trial, including attrition from the study and patient fatigue. The available sample size for the measures, descriptive statistics, and inter-correlations appear in the Appendix. A total of 19.3% of observations were missing among the 523 patients, 6 measures, and 3 assessment periods. Rather than exclude patients with missing values and potentially bias hypothesis tests, we used a multiple-imputation procedure to include all available data, maximize statistical power, and increase our results’ generalizability. Following published guidelines for missing data (e.g., Allison, 2003; Schafer & Graham, 2002), we generated 10 data sets with missing values imputed via the Markov chain Monte Carlo method in PROC MI, computed standard analyses (e.g., ANOVA, regression, SEM) on each dataset, and pooled the results via PROC MIANALYZE in SAS software version 9.3 (SAS Institute, Inc., Cary, NC).
Identification of Healthy Participants
We estimated the proportion of patients in the “healthy” range of cognitive content based on available norms. We used a cutoff of 1.28 SD from the normative mean in the direction of more depressive cognition as the limit for health (i.e., in a normal distribution, about 90% of the normative population would be identified as healthy and 10% unhealthy). This conservative cutoff identifies fewer healthy participants than the traditional benchmark of 2 SD from the mean (2% unhealthy in the general population). Although partly arbitrary, the conservative cutoff used in the current analyses replicates a previous study of cognitive content (Jarrett et al., 2007) and reflects the high prevalence of MDD (e.g., about 13–17% over the lifetime and 5–7% over the past year in U.S. national samples; Kessler, Chiu, et al., 2005; Kessler, Berglund, et al., 2005; Hasin, Goodwin, Stinson, & Grant, 2005).
We obtained normative data from several sources. Dozois, Covin, and Brinker (2003) compiled general adult norms for the DAS total score (M = 115.0, SD = 26.7) and BHS (M = 3.06, SD = 3.11) by pooling data from a number of published reports, primarily community control and normal comparison groups. Similarly for the SCS, we averaged published data from several reports using Kendall and Sheldrick’s (2000) methods to weight studies’ means and SDs by sample sizes to estimate population values. Studies contributing SCS normative data were Ingledew, Hardy, and Cooper (1992); Katz and Singh (1986); Flett, Blankstein, Bator, and Pliner (1989); Goff (2010); MacLachlan (1985); Mezo and Heiby (2004); Redden, Tucker, and Young (1983); Richards (1985); and Rosenbaum (1980; only data from the English-language SCS included), which yielded pooled M = 26.0, SD = 22.3. Mindful of patients’ difficulty in completing the original ASQ (Peterson et al., 1982), Dykema, Bergbower, Doctora, and Peterson (1996) created the short form with better internal consistency and strong validity used in this study. Because few normative data are available for this measure, we elected not to compute health estimates.
Results
In support of our first hypothesis, patients’ cognitive content improved substantially during acute-phase CT (see Table 1). Dysfunctional attitudes, hopelessness, and stable and global attributions for failures decreased, and learned resourcefulness (positive coping) increased. Improvement effect sizes from pre- to post-acute-phase CT were large (ds 0.77–0.99).
Table 1.
Changes in Cognitive Content during Acute-Phase Cognitive Therapy
| Measure | Pre-CT | Mid-CT | Post-CT | Pre to Post Decrease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Healthy | M | SD | Healthy | M | SD | Healthy | d | r | dres | |
| BHS | 12.02 | 5.16 | 22% | 7.65 | 5.32 | 53% | 6.15 | 5.36 | 63% | 0.97* | .64* | −0.25* |
| DAS | 150.51 | 35.23 | 48% | 131.32 | 34.65 | 70% | 116.53 | 33.67 | 84% | 0.96* | .50* | −0.01 |
| ASQ-S | 1.16 | 0.86 | --- | 0.46 | 1.00 | --- | 0.30 | 1.05 | --- | 0.79* | .33* | 0.17 |
| ASQ-G | 1.16 | 0.98 | --- | 0.57 | 1.05 | --- | 0.26 | 1.17 | --- | 0.77* | .33* | 0.15 |
| SCS | −5.86 | 26.57 | 45% | 7.45 | 26.32 | 65% | 21.62 | 26.89 | 82% | −0.99* | −.40* | −0.22* |
Note. N = 523 in 10 datasets completed via multiple imputation. BHS = Beck Hopelessness Scale. DAS = Dysfunctional Attitudes Scale. ASQ = Attributional Style Questionnaire failure scales; S = stable and G = global. SCS = Self-Control Schedule. Healthy = proportion of patients with scores <1.28 SD above (≈ 90th percentile) the general adult population mean. --- = proportion healthy not estimated due to lack of appropriate normative data. d = standardized mean difference (effect size) in cognitive content means derived from t-tests. r = correlation of changes in cognitive content with concurrent changes in depressive symptoms. dres = residual change in cognitive content controlling correlation with change in depressive symptoms.
p < .05, two-tailed.
As described above, we estimated the proportion of patients in the “healthy” range on the BHS, DAS, and SCS. Before acute-phase CT, less than half of participants showed healthy-range levels of hopelessness (22%), dysfunctional attitudes (48%), and self-control (45%) (see Table 1). Healthy proportions of patients increased significantly (ps < .01) by mid-CT, and again from mid- to post-CT (ps < .01), with final proportions well above half (but far from all) of patients in the healthy range of hopelessness (63%), dysfunctional attitudes (84%), and learned resourcefulness (82%). Viewed in terms of pre- to post-CT change, many patients improved from unhealthy to healthy scores on the hopelessness (45%), dysfunctional attitudes (38%), and learned resourcefulness (40%) measures; and very few deteriorated from healthy to unhealthy status in hopelessness (4%), dysfunctional attitudes (3%), and learned resourcefulness (3%).
Pre- to post-CT improvement in cognitive content and depressive symptoms were moderately to moderately highly correlated (rs .33–.64), indicating that symptom and cognitive change are related but not synonymous (see Table 1). In addition, controlling symptom change in general linear models, residual change in dysfunctional attitudes and stable and global failure attributions was reduced to non-significant levels. Stated another way, with no change in depressive symptoms, no change in dysfunctional attitudes or failure attributions was expected. Learned resourcefulness still showed a statistically significant but small improvement (d = −0.22) when controlling depressive symptoms, whereas hopelessness increased slightly (d = −0.25) if patients’ depressive symptoms did not improve at all during acute-phase CT. In contrast, decreases in depressive symptoms remained large (d = 1.09, p < .01) when controlling change in all five cognitive content measures together.
To test possible causal relations between changes in depressive symptoms and cognitive content, we computed a series of cross-lagged SEMs (see Figure) in which we controlled concurrent relations between symptoms and cognitive content, as well as retest correlations within measures. Of greatest interest are prediction of changes in cognitive content from depressive symptoms (paths a1 and a2), and especially hypothesized changes in depressive symptoms from cognitive content (paths b1 and b2). We computed separate models for each of the five cognitive content measures (BHS, DAS, ASQ-S, ASQ-G, and SCS), as well as the measures’ common factor.
Figure 1.
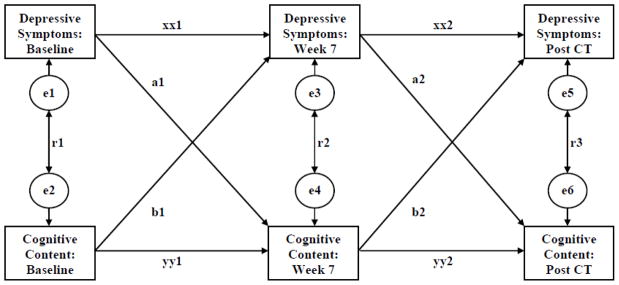
Cross-lagged Model of Relations between Depressive Symptoms and Cognitive Content
We aggregated the cognitive content measures via their common factor because the measures converged both cross-sectionally and longitudinally. In particular, principal-axis factor analyses of the five cognitive content measures showed that their first factor accounted for 100% of their common variance pre-, mid-, and post-CT, and the BHS (mean = .60), DAS (mean = .59), ASQ-S (mean = .70), ASQ-G (mean = .66), and SCS (mean = −.56) each loaded moderately on it. Patients’ longitudinal changes on the five cognitive content measures also converged both earlier and later in treatment. In factor analyses of partial correlations among the cognitive content measures (a) mid-CT controlling early-CT values, and (b) late-CT controlling mid-CT values, the first factor accounted for 100% of the shared variance and all cognitive content measures loaded substantively on it (|loading| mean = .57, range .49–.66). Thus, we computed factor scores pre-, mid-, and post-CT to reflect broadly generalizable depressive cognitive content not tied to a specific measure.
As Table 2 shows, all six SEMs fit the data adequately, as judged by goodness of fit (.98–.99), comparative fit (.98–1.00), and non-normed fit (.93–.99) index values ≥ .90 (Kline, 2005). The models showed small-to-moderate concurrent correlations of cognitive content with depressive symptoms (absolute values of standardized betas .12–.49), and moderate retest reliabilities for both symptoms (.42–.67) and cognitive content (.44–.72).
Table 2.
Relations among Depressive Symptoms and Cognitive Content during Acute-Phase Cognitive Therapy (CT) for Depression
| Symptoms predict cognitive content | Cognitive content predicts symptoms | Symptom retest reliabilities | Cognitive content retest reliabilities | Concurrent correlations between symptoms and cognitive content | Model fit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | a1 | a2 | b1 | b2 | xx1 | xx2 | yy1 | yy2 | r1 | r2 | r3 | GFI | CFI | NNFI |
| BHS | .00 | .15* | .05 | .15* | .46* | .59* | .48* | .59* | .48* | .45* | .33* | .99 | .99 | .98 |
| DAS | −.09 | .03 | .14* | .05 | .49* | .67* | .53* | .71* | .41* | .31* | .20* | .98 | .98 | .94 |
| DAS-SC | −.07 | .02 | .12* | .04 | .49* | .67* | .53* | .71* | .40* | .28* | .19* | .98 | .98 | .92 |
| DAS-AP | −.08 | .04 | .09* | .05 | .48* | .67* | .57* | .72* | .27* | .25* | .16* | .98 | .98 | .93 |
| ASQ-S | .09* | .13* | .06 | .08* | .42* | .63* | .44* | .62* | .32* | .29* | .12* | .99 | .99 | .98 |
| ASQ-G | .06 | .13* | −.02 | .08 | .43* | .62* | .50* | .57* | .34* | .34* | .13* | .99 | .98 | .93 |
| SCS | −.05 | −.08 | −.04 | −.06 | .44* | .65* | .55* | .70* | −.20* | −.27* | −.17* | .98 | .98 | .93 |
| Common factor | .06 | .18* | .06 | .06 | .43* | .57* | .51* | .72* | .49* | .44* | .25* | .99 | 1.00 | .99 |
N = 523 in 10 datasets completed via multiple imputation. Table entries are standardized path coefficients. See Figure for path diagram. BHS = Beck Hopelessness Scale. DAS = Dysfunctional Attitudes Scale total score and subscales for self-criticism (SC) and need for approval (AP). ASQ = Attributional Style Questionnaire failure scales; S = stable and G = global. SCS = Self-Control Schedule. Common factor = first common factor score among the five cognitive content measures. GFI = goodness of fit index. CFI = comparative fit index. NNFI = non-normed fit index. Bold type indicates paths predicted by cognitive theory.
p < .05, two-tailed.
Prediction of cognitive content from depressive symptoms, and vice versa, forward in time is of greater theoretical interest. However, many cross-lagged relations were not statistically significant. Patients with higher pre-CT depressive symptoms experienced less improvement in stable failure attributions (but not hopelessness, dysfunctional attitudes captured by the DAS total and subscale scores, global failure attributions, self-control, or the common factor) by mid-CT (see path a1 in Figure and Table 2). Prediction of cognitive content later in CT was a bit stronger—patients with higher depressive symptoms mid-CT showed less improvement in hopelessness, stable and global failure attributions, and the cognitive-content common factor (but not dysfunctional attitudes or self-control) from mid- to post-CT (see path a2 in Figure and Table 2). Overall, however, the a coefficients for prediction of later cognitive content from earlier symptoms were small (median standardized beta = .07, range −.09–.18, when reversing the sign of the SCS values and including only the total DAS scores).
The cognitive model of depression posits that cognitive content will predict subsequent depressive symptoms. Among our pre-CT measures of cognitive content, only more dysfunctional attitudes (on the DAS total and self-criticism and approval subscale scores) predicted less improvement in symptoms by mid-CT (see path b1 in Figure and Table 2). In addition, patients with greater hopelessness and more stable failure attributions (but not the other cognitive-content measures) at mid-CT experienced less improvement in symptoms from mid- to post-CT (see path b2 in Figure and Table 2). All b path coefficients were small (median standardized beta = .06, range −.02–.15, when reversing the sign of the SCS values and including only the total DAS scores).
Discussion
The current analyses extend the literature regarding improvements in cognitive content during acute-phase CT for MDD and cognition mediation of acute-phase CT’s effects on depressive symptoms. We utilized a large sample of adults with recurrent MDD and a design well-suited for these goals. Although our analyses of acute-phase CT did not include a control condition, past research (e.g., Garratt et al., 2007; Webb et al., 2012) suggests that CT was at least partly responsible for the current patients’ substantive improvements in cognitive content. Whether our analyses support changes in cognitive content as mediators of improvement in depressive symptoms depends on the operational definition of mediation. We found stronger support for simpler (concurrent change in cognitive content and depressive symptoms) than for more sophisticated (earlier cognitive change accounting for later symptom change) definitions.
We found that mean scores on all five cognitive content measures improved substantially from pre- to post-CT (ds 0.77–0.99). We also found that the proportions of patients scoring in the “healthy” range (<90th percentile of depressive cognitive content of non-clinical samples) increased significantly on the BHS, DAS, and SCS from pre- (22–48%) to post- (63–84%) CT, similar to Jarrett et al. (2007). It is interesting to note that pre-CT depressive cognitive content could not account for a substantial minority of patients’ depression (i.e., 22–48% of the sample had “healthy-range” cognitive content, as measured by the BHS, DAS, and SCS, whereas 100% had MDD and elevated HRSD scores pre-treatment). Further, the large average improvement in cognitive-content scores did not result in cognitive “health” for 16–37% of patients by post-CT. Because residual depressive symptoms and high hopelessness have been linked to recurrence and suicide, respectively (e.g., Dahlsgaard, Beck, & Brown, 1998; Brown et al., 2000; Fava, Fabbri, & Sonino, 2002), the current results underline the importance of measuring both symptoms and cognitive content during treatment of MDD.
Some researchers have viewed simple correlations between changes in cognitive content and depressive symptoms from pre- to post-CT as evidence of mediation (e.g., Garratt et al., 2007). By this purely statistical operational definition, our data offer clear support for mediation. We found that reductions in depressive symptoms correlated moderately to moderately highly (rs .33–.64) with improvements in cognitive content during CT. However, operationalizing mediation as changes in cognitive content accounting for subsequent changes in depressive symptoms (cf. Kazdin, 2007), our data offer only limited support based on cross-lagged causal models. We found that higher pre-CT dysfunctional attitudes (but not any other of our cognitive-content measures or their common factor) predicted more depressive symptoms mid-CT, and greater mid-CT hopelessness and stable failure attributions (but not any other of our cognitive-content measures or their common factor) predicted greater symptoms post-CT. Thus, support for mediation of symptom reduction by cognitive content was inconsistent and weak (3 of 12 b paths significant; median standardized beta = .06).
Our results were just as consistent with reverse causality—that is, changes in depressive symptoms may drive improvements in cognitive content to a limited extent. Higher pre-CT depressive symptoms predicted greater stable failure attributions mid-CT, and greater mid-CT depressive symptoms predicted hopelessness, stable and global failure attributions, and the cognitive content measures’ common factor post-CT. But again, the few significant paths were weak (5 of 12 of a paths significant; median standardized beta = .07). Our finding that reverse causality fit the data about as well as predictions from cognitive theory replicates a study of group CT for depression (Oei, Bullbeck, & Campbell, 2006). In addition, we found that the residual change in cognitive-content measures was small (ds ≤ |0.25|) when controlling change in depressive symptoms, whereas depressive symptoms improved substantially (d = 1.09), even after accounting for change in cognitive content, replicating Jarrett et al. (2007).
Given the current results and past researchers’ similar difficulties in clearly establishing cognitive mediation of CT’s effects on depressive symptoms, future researchers might consider alternative theories and measurement strategies. Regarding theory, we speculate similarly to Burns and Spangler (2001) that some unknown “common cause,” common causes, or yet to be identified proxy variable/s may link symptom change and cognition change in CT for depression. For example, DeRubeis and Feeley (1990) found that therapists’ adherence to concrete and technical aspects of CT during sessions (e.g., setting and following session agendas, labeling cognitive errors and practicing rational responses with patients, assigning and reviewing homework) was more strongly correlated with subsequent change in depressive symptoms than was therapists’ abstract discussion with patients. Further, patients’ engagement in CT homework (e.g., Burns & Spangler, 2000) and learning of CT skills (e.g., Jarrett et al., 2011) are important predictors of success in CT, and behavioral activation strategies alone are an effective treatment for depression (e.g., Dimidjian et al., 2006). Consequently, future research might test the degree to which an active, authoritative advocate for change (i.e., the competent cognitive therapist, in-person or embodied in workbooks or software) produces connected changes in multiple domains of patients’ functioning (e.g., symptoms, cognitive content, social-interpersonal). Leading patients to engage new (or to reactivate lost) behaviors and thoughts linked with well-being, regardless of the specific sequence or exact content of the behaviors and thoughts, possibly is an important part of CT’s overlapping effects on both depressive symptoms and cognitive content (cf. Longmore & Worrell, 2007).
Current measurement strategies might also limit detection of mediation and require improvement. First, depressive symptom and cognitive content measures overlap. Diagnostic criteria for MDD (APA, 2000, 2013) and frequently used depressive symptom measures (e.g., BDI, HRSD, IDS) all include cognitive content items (e.g., guilt, pessimism). Alternative depression criteria and measures omitting cognitive items would allow a cleaner test of mediation but might also change the concept of “depression” itself and reduce the immediate clinical utility of results. Second, researchers may need to consider high-frequency feedback and mediation between changes in mood and cognition (cf. Lynch, Hempel, & Clark, in press). If mediation occurs more quickly than measures taken weeks apart (as in the current and most other clinical datasets) can capture, then concurrent change of symptoms and cognition may be all that is detectable without more frequent measurement and increased response burdens for patients and therapists. Third, methods of assessing patients’ cognitive content other than patient verbal reports, such reaction-time assessment of attention and memory biases (e.g., Lim & Kim, 2005) or fMRI measures of brain reactivity to emotional stimuli (e.g., Siegle, Carter, & Thase, 2006; Smoski et al., 2009), may provide complementary information useful in identifying real-time cognitive mediators of symptom reduction. Consequently, operational and temporal separation of depressive symptoms and cognitive content to establish mediation may be a challenging but profitable means to understand CT’s mechanisms, improve its outcomes, and move toward development of curative approaches for depression.
Supplementary Material
Acknowledgments
This report was supported by Grants Number K24 MH001571, R01 MH58397, R01 MH69619 (to Robin B. Jarrett, Ph.D.) and R01 MH58356 and R01 MH69618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. We also appreciate the careful review by members of the trial’s Data Safety and Monitoring Board. We are indebted to our research teams and our colleagues at The University of Texas Southwestern Medical Center at Dallas, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania (Dr. Thase’s current affiliation). We appreciate the participation of colleagues, previously named, and study participants without whom such research could not have been completed.
Footnotes
Due to a scoring error, 2 patients erroneously entered CT with HRSD = 13 at one of two diagnostic visits. During CT, one of these patients responded and one dropped out. As recommended by the Data Safety and Monitoring Board (DSMB), the two patients are analyzed here as they were treated during data collection.
In the current study, HRSD scores demonstrated high inter-rater reliability (ICC = .91 among 28 patients rated by 4–14 clinicians each) and major depressive episode diagnoses demonstrated moderate inter-rater reliability (ICC = .61 among 39 patients rated by 4 to 21 clinicians each) in multilevel model analyses.
A repeated-measures ANOVA predicting the 6 measures collected at 3 time points (see Appendix for list of measures) did not reveal a significant main effect of therapist, or significant interactions of therapist with time and measure, ps > .20.
Four (1 early and 3 late) responders were misclassified as late and early responders, respectively. As recommended by the DSMB, they are analyzed here as they were treated during data collection.
Drs. Vittengl and Clark have no financial interest or conflict of interest in the research. Dr. Thase has no conflicts of interest pertaining to this paper, although he does report the following relationships with companies that develop treatment for depression or provide education pertaining to those treatments: Dr. Thase has provided scientific consultation to Alkermes, Astra-Zeneca, Bristol-Myers Squibb Company, Dey Pharma, L.P., Eli Lilly & Company, Forest Pharmaceuticals, Inc., Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, H. Lundbeck A/S, MedAvante, Inc., Merck and Co. Inc., Neuronetics, Inc., Novartis, Otsuka, Ortho-McNeil Pharmaceuticals, PamLab, L.L.C., Pfizer (formerly Wyeth-Ayerst Laboratories), Schering-Plough (formerly Organon, Inc.), Shire US Inc., Sunovion Pharmaceuticals, Inc., Takeda (Lundbeck), and Transcept Pharmaceuticals. Dr. Thase receives grant funding from the Agency for Healthcare Research and Quality, Eli Lilly & Company, GlaxoSmithKline (ended 7/2010), National Institute of Mental Health, Otsuka Pharmaceuticals, and Sepracor, Inc. He has equity holdings in MedAvante, Inc. and receives royalty income from American Psychiatric Foundation, Inc., Guilford Publications, Herald House, Oxford University Press, and W.W. Norton & Company. His wife is employed as the Group Scientific Director for (Embryon – formerly Advogent; which does business with BMS and Pfizer/Wyeth). Dr. Jarrett’s medical center collects the payments from the cognitive therapy she provides to patients. Dr. Jarrett is a paid consultant to the NIMH.
Contributor Information
Jeffrey R. Vittengl, Truman State University
Lee Anna Clark, University of Notre Dame.
Michael E. Thase, University of Pennsylvania
Robin B. Jarrett, The University of Texas Southwestern Medical Center
References
- Allison PD. Missing data techniques for structural equation modeling. Journal of Abnormal Psychology. 2003;112:545–557. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Hopelessness Scale. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA. Relationship between hopelessness and ultimate suicide: A replication with psychiatric outpatients. American Journal of Psychiatry. 1990;147:190–195. doi: 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison BS. Hopelessness and eventual suicide: A 10-year prospective study of patients hospitalized with suicidal ideation. American Journal of Psychiatry. 1985;142:559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. Journal of Consulting & Clinical Psychology. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: A 20-year prospective study. Journal of Consulting And Clinical Psychology. 2000;68:371–377. doi: 10.1037/0022-006X.68.3.371. [DOI] [PubMed] [Google Scholar]
- Burns DD, Spangler DL. Does psychotherapy homework lead to improvements in depression in cognitive–behavioral therapy or does improvement lead to increased homework compliance? Journal of Consulting and Clinical Psychology. 2000;68:46–56. doi: 10.1037/0022-006X.68.1.46. [DOI] [PubMed] [Google Scholar]
- Burns DD, Spangler DL. Do changes in dysfunctional attitudes mediate changes in depression and anxiety in cognitive behavioral therapy? Behavior Therapy. 2001;32:337–369. doi: 10.1016/S0005-7894(01)80008-3. [DOI] [Google Scholar]
- Busch AM, Kanter JW, Landes SJ, Kohlenberg RJ. Sudden gains and outcome: A broader temporal analysis of cognitive therapy for depression. Behavior Therapy. 2006;37:61–68. doi: 10.1016/j.beth.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Christopher MS, Jacob KL, Neuhaus EC, Neary TJ, Fiola LA. Cognitive and behavioral changes related to symptom improvement among patients with a mood disorder receiving intensive cognitive-behavioral therapy. Journal of Psychiatric Practice. 2009;15:95–102. doi: 10.1097/01.pra.0000348362.11548.5f. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends in Cognitive Sciences. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory of depression. New York, NY: Wiley; 1999. [Google Scholar]
- Dahlsgaard KK, Beck AT, Brown GK. Inadequate response to therapy as a predictor of suicide. Suicide and Life-Threatening Behavior. 1998;28:197–204. [PubMed] [Google Scholar]
- DeRubeis RJ, Feeley M. Determinants of change in cognitive therapy for depression. Cognitive Therapy and Research. 1990;14:469–482. doi: 10.1007/BF01172968. [DOI] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dozois DA, Covin R, Brinker JK. Normative data on cognitive measures of depression. Journal of Consulting and Clinical Psychology. 2003;71:71–80. doi: 10.1037/0022-006X.71.1.71. [DOI] [PubMed] [Google Scholar]
- Dunkley DM, Blankstein KR, Segal ZV. Cognitive assessment: Issues and methods. In: Dobson KS, editor. Handbook of cognitive-behavioral therapies. 3. New York, NY US: Guilford Press; 2010. pp. 133–171. [Google Scholar]
- Dunn TW, Vittengl JR, Clark LA, Carmody T, Thase ME, Jarrett RB. Change in psychosocial functioning and depressive symptoms during acute-phase cognitive therapy for depression. Psychological Medicine. 2012;42:317–326. doi: 10.1017/S0033291711001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykema J, Bergbower K, Doctora JD, Peterson C. An Attributional Style Questionnaire for general use. Journal of Psychoeducational Assessment. 1996;14:100–108. doi: 10.1177/073428299601400201. [DOI] [Google Scholar]
- Dykema J, Bergbower K, Doctora JD, Peterson C. An Attributional Style Questionnaire for general use. Journal of Psychoeducational Assessment. 1996;14:100–108. [Google Scholar]
- Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: An emerging therapeutic target. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:1019–1027. doi: 10.1016/S0278-5846(02)00226-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Flett GL, Blankstein KR, Bator C, Pliner P. Affect intensity and self-control of emotional behaviour. Personality and Individual Differences. 1989;10:1–5. doi: 10.1016/0191-8869(89)90169-4. [DOI] [Google Scholar]
- Forman EM, Chapman JE, Herbert JD, Goetter EM, Yuen EK, Moitra E. Using session-by-session measurement to compare mechanisms of action for acceptance and commitment therapy and cognitive therapy. Behavior Therapy. 2012;43:341–354. doi: 10.1016/j.beth.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Furlong M, Oei TS. Changes to automatic thoughts and dysfuntional attitudes in group CBT for depression. Behavioural and Cognitive Psychotherapy. 2002;30:351–360. doi: 10.1017/S1352465802003107. [DOI] [Google Scholar]
- Garratt G, Ingram RE, Rand KL, Sawalani G. Cognitive processes in cognitive therapy: Evaluation of the mechanisms of change in the treatment of depression. Clinical Psychology: Science and Practice. 2007;14:224–239. doi: 10.1111/j.1468-2850.2007.00081.x. [DOI] [Google Scholar]
- Goff A. Stressors, academic performance, and learned resourcefulness in baccalaureate nursing students. Dissertation Abstracts International Section A. 2010;71 doi: 10.2202/1548-923X.2114. [DOI] [PubMed] [Google Scholar]
- Greenberger D, Padesky CA. Mind over mood: A cognitive therapy treatment manual for clients. New York, NY: Guilford Press; 1995. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Kendall PC. Cognitive self-statements in depression: Development of an automatic thoughts questionnaire. Cognitive Therapy and Research. 1980;4:383–395. doi: 10.1007/BF01178214. [DOI] [Google Scholar]
- Ingledew DK, Hardy L, Cooper CL. On the reliability and validity of the Locus of Control scale of the Occupational Stress Indicator. Personality and Individual Differences. 1992;13:1183–1191. doi: 10.1016/0191-8869(92)90254-M. [DOI] [Google Scholar]
- Imber SD, Pilkonis PA, Sotsky SM, Elkin I, Watkins JT, Collins JF, Glass DR. Mode-specific effects among three treatments for depression. Journal of Consulting and Clinical Psychology. 1990;58:352–359. doi: 10.1037/0022-006X.58.3.352. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, Prince SE. A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64:295–304. doi: 10.1037/0022-006X.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher risk cognitive therapy responders: A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. Journal of American Medical Association, Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.1969. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA, Thase ME. Skills of Cognitive Therapy (SoCT): A new measure of patients’ comprehension and use. Psychological Assessment. 2011;23:578–586. doi: 10.1037/a0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Doyle K, Clark LA. Changes in cognitive content during and following cognitive therapy for recurrent depression: Substantial and enduring, but not predictive of change in depressive symptoms. Journal of Consulting and Clinical Psychology. 2007;75:432–466. doi: 10.1037/0022-006X.75.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett R, Thase M. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RC, Singh N. A comparison of current smokers and self-cured quitters on Rosenbaum’s Self Control Schedule. Addictive Behaviors. 1986;11:63–65. doi: 10.1016/0306-4603(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Understanding how and why psychotherapy leads to change. Psychotherapy Research. 2009;19:418–428. doi: 10.1080/10503300802448899. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Sheldrick RC. Normative data for normative comparisons. Journal of Consulting and Clinical Psychology. 2000;68:767–773. doi: 10.1037//0022-006X.68.5.767. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu W, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. Guilford Press; New York: 2005. [Google Scholar]
- Kraemer H, Wilson G, Fairburn CG, Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Archives Of General Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Lim S, Kim J. Cognitive processing of emotional information in depression, panic, and somatoform disorder. Journal of Abnormal Psychology. 2005;114:50–61. doi: 10.1037/0021-843X.114.1.50. [DOI] [PubMed] [Google Scholar]
- Longmore RJ, Worrell M. Do we need to challenge thoughts in cognitive behavior therapy? Clinical Psychology Review. 2007;27:173–187. doi: 10.1016/j.cpr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lynch TR, Hempel RJ, Clark LA. Flexibility and radical openness: Facilitating self-inquiry in overcontrolled personality disorder. In: Livesley WJ, Dimaggio G, Clarkin J, editors. Integrated Treatment for Personality Disorder. New York: Guilford Publications, Inc; in press. [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan I. Learned resourcefulness, depression and self esteem. IRCS Medical Science: Psychology & Psychiatry. 1985;13:816–817. [Google Scholar]
- Mezo PG, Heiby EM. A comparison of four measures of self-control skills. Assessment. 2004;11:238–250. doi: 10.1177/1073191104268199. [DOI] [PubMed] [Google Scholar]
- Oei TS, Bullbeck K, Campbell JM. Cognitive change process during group cognitive behaviour therapy for depression. Journal of Affective Disorders. 2006;92:231–241. doi: 10.1016/j.jad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Peterson C, Semmel A, von Baeyer C, Abramson LY, Metalsky GI, Seligman MP. The Attributional Style Questionnaire. Cognitive Therapy and Research. 1982;6:287–300. doi: 10.1007/BF01173577. [DOI] [Google Scholar]
- Quilty LC, McBride CC, Bagby RM. Evidence for the cognitive mediational model of cognitive behavioural therapy for depression. Psychological Medicine. 2008;38:1531–1541. doi: 10.1017/S0033291708003772. [DOI] [PubMed] [Google Scholar]
- Redden EM, Tucker RK, Young L. Psychometric properties of the Rosenbaum schedule for assessing self-control. The Psychological Record. 1983;33:77–86. [Google Scholar]
- Rosenbaum M. A schedule for assessing self-control behaviors: Preliminary findings. Behavior Therapy. 1980;11:109–121. doi: 10.1016/S0005-7894(80)80040-2. [DOI] [Google Scholar]
- Rosenbaum M. A schedule for assessing self-control behaviors: Preliminary findings. Behavior Therapy. 1980;11:109–121. [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory for Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Research. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Schafer J, Graham J. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/appi.ajp.163.4.735. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Jarrett RB. Major depressive disorder. In: Hofman SG, editor. The Wiley Handbook of Cognitive Behavioral Therapy (Volume 2: CBT for Specific Disorders. New York: Wiley; 2014. pp. xx–xx. [DOI] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Validity of sudden gains in acute phase treatment of depression. Journal of Consulting and Clinical Psychology. 2005;73:173–182. doi: 10.1037/0022-006X.73.1.173. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, Jarrett RB. Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute phase cognitive therapy. Psychological Medicine. 2005;35:693–704. doi: 10.1017/S0033291704004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Nomothetic and idiographic symptom change trajectories in acute-phase cognitive therapy for recurrent depression. Journal of Consulting and Clinical Psychology. 2013;81:615–626. doi: 10.1037/a0032879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, DeRubeis RJ. Processes of change in CBT of adolescent depression: Review and recommendations. Journal of Clinical Child and Adolescent Psychology. 2012;41:654–665. doi: 10.1080/15374416.2012.704842. [DOI] [PubMed] [Google Scholar]
- Weissman AN. The Dysfunctional Attitudes Scale: A validation study (Doctoral dissertation, University of Pennsylvania, 1979) Dissertation Abstracts International. 1979;40:1389B–1390B. [Google Scholar]
- Weissman MM, Markowitz JC, Klerman GL. Comprehensive guide to interpersonal psychotherapy. New York, NY US: Basic Books; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


