Abstract
Temperature responsive hydrogels based on ionic polymers exhibit swelling transitions in aqueous solutions as a function of shifting pH and ionic strength, in addition to temperature. Applying these hydrogels to useful applications, particularly for biomedical purposes such as drug delivery and regenerative medicine, is critically dependent on understanding the hydrogel solution responses as a function of all three parameters together. In this work, interpenetrating polymer network (IPN) hydrogels of polyacrylamide and poly(acrylic acid) were formulated over a broad range of synthesis variables using a fractional factorial design, and were examined for equilibrium temperature responsive swelling in a variety of solution conditions. Due to the acidic nature of these IPN hydrogels, usable upper critical solution temperature (UCST) responses for this system occur in mildly acidic environments. Responses were characterized in terms of maximum equilibrium swelling and temperature-triggered swelling using turbidity and gravimetric measurements. Additionally, synthesis parameters critical to achieving optimal overall swelling, temperature-triggered swelling, and sigmoidal temperature transitions for this IPN system were analyzed based on the fractional factorial design used to formulate these hydrogels.
Introduction
Thermally-sensitive hydrogels, which exhibit temperature-dependent volumetric swelling changes in aqueous environments, can be used as drug release platforms actuated by temperature changes.1–6 Temperature shifts in a hydrogel network may originate from a surrounding aqueous environment, or due to a heat source located physically within the polymer matrix.4,5,7–10
Two distinct types of thermally-driven swelling transitions are observed with temperature-responsive hydrogels (excluding swelling caused by degradation or separation of thermally-labile bonds11), those which result in polymer network expansion and solvent intake with increasing temperature, and those which result in polymer network retraction and solvent expulsion with increasing temperature. Usually transitions are non-linear, with an inflection occurring near a critical point with respect to equilibrium temperature.
Sigmoidal curve shape is observed under conditions where a critical temperature transition occurs. In the case where the hydrogel exhibits a sudden volume collapse, the change is commonly referred to as a lower critical solution temperature (LCST) transition, where the solvent and polymer undergo complex coacervation as temperature is decreased below an LCST. Poly(N-isopropyl acrylamide) (NIPAAm) as well as gels based on cellulose ethers and vinyl-methyl-ethers exhibit LCST responses and are frequently incorporated into temperature responsive hydrogels and composite systems.4,5,7,9,12–18
With hydrogels that exhibit volumetric dilation (simple coacervation) with increasing temperature, a substantially sigmoidal volumetric expansion with increasing temperature is regarded as an upper critical solution temperature (UCST) transition. This behavior is observed with far greater rarity than the LCST swelling response, and acrylic acid is commonly incorporated into hydrogels and hydrogel composites that exhibit UCST transitions in aqueous solutions.1,6,8,19–25 More recently however, UCST transitions have been observed in polymers not based on acrylic acid derivatives.26–29 Very promising UCST responses were noted with poly(N-acryloylglycinamide) (PNAGA) homopolymers.27,28 PNAGA hydrogels have shown reversible thermosresponsive transitions caused by hydrogen bonding interactions in aqueous solvents, which include both pure water and solutions containing dissolved electrolytes. At significantly low crosslinker levels, PNAGA hydrogels exhibit sigmoidal transitions, similar to LCST systems based on NIPAAm and the UCST IPN hydrogels examined here. Interestingly, PNAGA polymers were not observed to undergo UCST transitions previously due to trace polyelectrolyte inclusion into polymer chains originating from either acrylate contaminated monomers, use of ionic initiators, chain transfer agents, and / or amide hydrolysis.28 Poly(acryloylasparaginamide) formed by reverse addition fragmentation chain transfer (RAFT) polymerization has exhibited sharp UCST transitions in aqueous media. Additionally, copolymers of ploy(allylurea-co-allylamine), crosslinked on pendant groups with glutaraldehyde have exhibited UCST transitions in physiologically-relevant solution conditions as well.29 For a comprehensive analysis of UCST thermoresponsive polymers, the reader is referred to an excellent review by Seuring and Agarwal.30
For drug release applications, either LCST or UCST based hydrogels can be utilized. LCST gels ‘squeeze’ drugs out at elevated temperatures like a compressed sponge, while UCST gels ‘free’ drugs at elevated temperatures much like an expanding cage, and both types of systems have been used in solute release applications.4–7,12,13
While non-ionic temperature responsive gels such as poly(N-isopropyl acrylamide) or poly(N-acryloylglycinamide) are largely unaffected by ionic solution conditions, equilibrium swelling transitions in UCST hydrogels that require acrylic acid or other ionic components must be regarded as occurring in response to temperature, pH, and ionic strength together. In drug delivery applications, operating ranges of temperature, pH, and ionic strength for any hydrogel responsive to these stimuli need to be designed for the relevant physiological conditions, which includes all solution conditions experienced while in transit to the intended anatomical target. In human blood plasma, the normal environmental conditions relevant to hydrogel swelling are temperature of 37 °C; pH of 7.4; tonicity to NaCl (and similarly ionic strength) of 0.16 mol/L; and osmolarity of 290 mOsmol/L.31 For utility in areas of the human body, such as within intracellular compartments, the solution conditions vary significantly.32,33 A sigmoidal response near the triggering point for a hydrogel used in drug delivery applications ensures the polymer network undergoes the bulk of its switching volume transition over a range sufficiently narrow for physiological practicality.
Interpenetrating polymer networks (IPNs) of poly(acrylic acid) and polyacrylamide have been shown to have a dramatic equilibrium volume transition over a relatively narrow temperature band.6 The cause for this behavior is traditionally thought to be due to hydrogen bonding complexes between acrylic acid and acrylamide subunits in the hydrogel during the collapsed state, ensuring that the aqueous penetrant is “locked-out” of the polymer’s intramolecular environment.6,34–36 In this description, rapid swelling (in the equilibrium temperature domain) at a critical point is based on temperature elevation overwhelming hydrogen bonding complexes, which are established as adjacent dimers of amides and protonated carboxylic acids along neighboring polymer chains. This creates a solvent front that rapidly ‘unzips’ these sequential molecular units of hydrogen-bonded structures on adjacent chains. With random copolymers of poly(acrylic acid) (PAA) and polyacrylamide (PAAm), the temperature responses are typically exponential or linear, however sigmoidal transition behavior has been notably demonstrated for copolymers of P(AA-co-AAm).20,21 The difference in behavior between random PAAm / PAA copolymers and PAAm / PAA IPNs is reportedly due to random placement of monomer units along the polymer backbone in the copolymer, which prevents the sequential H-bonding arrangement found in the IPN that is crucial for a sigmoidal response.6
In this contribution, we report on the synthesis and swelling characteristics of polyacrylamide/poly(acrylic acid) IPN hydrogels synthesized via thermally initiated free radical solution polymerization. We fully characterize the swelling transitions as a response to temperature, pH, and ionic strength solution conditions.
IPN hydrogels were prepared by reacting and crosslinking a poly(acrylic acid) network within a swollen polyacrylamide network. This synthesis strategy forms two intermeshed and individually crosslinked polymer network gels which are physically-entangled, but free of covalent connections between the two sequentially-formed networks. For the purposes of this work, multiple synthesis parameters were examined for both the initial acrylamide network, and the polymerization of the intermeshed poly(acrylic acid) network which completes IPN formation. Parameter tuning was carried out using a fractional factorial design to isolate the critical synthesis parameters and levels necessary in achieving optimal swelling responses.
Materials and Methods
Reagents and Chemicals used in Hydrogel Polymerization
Preparation of polyacrylamide hydrogels, as well as interpenetrating polymer networks (IPNs) of polyacrylamide and poly(acrylic acid), were carried out with commercially available monomers, initiators, and reagents. The monomers used in this work were acrylamide (AAm) (electrophoresis grade 99.9%, Fisher Bioreagents) and acrylic acid (AA) (99%, stabilized with 180 – 200 ppm monomethyl ether hydroquinone, Aldrich), with N’N’-methylene-bisacrylamide (MBAAm) (99%, Sigma Aldrich) incorporated as a crosslinker. Initiators used for polymerization were ammonium persulfate (APS) (98%, ACS grade, Aldrich), benzoyl peroxide (BPO) (Luperox® A98, 98%, reagent grade, Sigma Aldrich (Arkema)), and tert-butylperoxy 2-ethyl-hexyl carbonate (TBEC) (Luperox® TBEC, 95%, Aldrich). Solvents for solution polymerization included both water and dimethyl sulfoxide (DMSO) (certified ACS, Fisher Chemical), with sodium chloride (NaCl) (certified ACS, Fisher Chemical) added to adjust ionic strength. Water used in all experiments was deionized (DI) with a Milli-Q Plus Ultrapure Water System (Millipore) equipped with a 0.22 µm in-line outlet filter. Glass plates specified as siliconized were treated with Sigmacote® (Sigma Life Sciences).
All glass plates were soaked in a base bath of (w/w) 86% ethanol / 14% sodium hydroxide solution (50% w/w) for 24 hours, followed by neutralizing in hydrochloric acid (HCl), rinsing in water, and soaking in a 0.2 M nitric acid solution for several minutes. All plates were rinsed and dried again prior to use. Plates specified as siliconized were exposed to Sigmacote for several minutes, and heated to 100 °C for 30 minutes to increase the durability of the hydrophobic coating.
Polyacrylamide Hydrogel Preparation
Crosslinked polyacrylamide (PAAm) hydrogels were prepared by thermally-initiated solution polymerization. Acrylamide and N’N’-methylene-bisacrylamide (MBAAm) were combined by weight, protected from light, and dissolved into 14 mL of the polymerization solvent (water) by stirring. A separate vial with the specified amount of initiator was combined with 1 mL of solvent (water), prepared separately to prevent premature initiation. Specifications for reactions are described below and are included in Table 1. Following complete dissolution, monomer and initiator solutions were septa sealed and purged on a nitrogen manifold for 30 minutes and 5 minutes respectively to displace dissolved O2. The septa sealed bottles containing the purged reactants were brought into an atmospherically-controlled glove box (Labmaster 130, Mbraun) via a custom-modified antechamber which allowed for positive-pressure nitrogen purging. Polymer and initiator solutions were combined and mixed for approximately 10 – 20 seconds. The combined pre-polymerization solution was rapidly pipetted between 6” glass plates (separated by 0.5 mm PTFE spacers) and tightly clamped together.
Table 1. Fractional Factorial Design Parameters for PAAm Hydrogels.
Parameters examined for initial PAAm network synthesis included: whether or not glass plates were siliconized; the initiator to total monomer molar ratio; the percentage of total monomer that was crosslinker; and acrylamide concentration used in the prepolymer solution.
| Level | Siliconized | Initiator / Monomer mol Ratio |
MBAAmmol % |
AAm Conc. (g/mL) |
|
| Low (−1) | No | 0.0015 | 0.35 | 0.20 | |
| Center (0) | N/A | 0.0025 | 0.50 | 0.27 | |
| High (+1) | Yes | 0.0035 | 0.65 | 0.33 | |
| Formulation | Siliconized | Initiator / Monomer mol Ratio |
MBAAmmol % |
AAm Conc. (g/mL) |
Max RSM in DI Water |
| 1 | 1 | 1 | −1 | −1 | 13.15 +/− 0.18 |
| 2 | −1 | 1 | −1 | 1 | 9.44 +/− 0.15 |
| 3 | −1 | 1 | 1 | −1 | 10.85 +/− 0.14 |
| 4 | −1 | 0 | 0 | 0 | 10.54 +/− 0.58 |
| 5 | 1 | 0 | 0 | 0 | 10.29 +/− 0.16 |
| 6 | 1 | −1 | 1 | −1 | 12.06 +/− 1.21 |
| 7 | −1 | −1 | −1 | −1 | 14.14 +/− 0.24 |
| 8 | 1 | 1 | 1 | 1 | 7.85 +/− 0.06 |
| 9 | 1 | −1 | −1 | 1 | 9.29 +/− 0.34 |
| 10 | −1 | −1 | 1 | 1 | 7.66 +/− 0.45 |
Solution-filled glass plate assemblies were sealed in a reactor vessel and removed from the controlled environment. Atmospheric O2 was monitored and maintained below 20 ppm throughout the filling of plate assemblies, and during sealing within the reaction vessel. Once removed from the glove box, the reaction vessel was placed in a covered hot water bath at 80 °C for 24 hours to form the crosslinked PAAm hydrogels.
Following synthesis, the plates were removed from the reaction vessel and separated to allow gentle remove of the crosslinked polymer films. Polyacrylamide films (polymerized in water) were generally transparent in appearance, and physically robust enough to allow intact removal by pulling the hydrogel film from the plate. The upper inch of the hydrogel film was cut away and discarded. PAAm films were purified by soaking in a large volume of ultrapure water for 6 days, with daily water changes. DMSO-polymerized samples were stepped down from 100% DMSO to 100% water (v/v) in pre-mixed, 25% increments over 5 days, followed by 6 days of washing in water. Following purification, discs were cut from the film with a 20 mm cork borer, and placed in a sealed polypropylene storage box to allow for controlled drying. In this arrangement, Kimwipes® were suspended above the polymer discs with the lid closed, and the Kimwipe® was changed at regular intervals to facilitate even, slow drying. Slow and controlled drying prevented excessive curling and cracking of the discs. Once the discs were sufficiently hardened, they were heated to 50 °C for 12 hours. Dry discs (polymerized in water) were generally clear and glassy, and showed minimal curvature.
Polyacrylamide / Poly(acrylic acid) Interpenetrating Polymer Network Preparation
Interpenetrating polymer network (IPN) hydrogel discs of polyacrylamide PAAm and poly(acrylic acid) (PAA) were synthesized by sequential free radical solution polymerization. PAAm discs, prepared, washed, and dried as described above, were soaked in a solution of acrylic acid for 24 hours at 8 °C. The acrylic acid solutions were composed of monomer (AA), crosslinker (MBAAm), and initiator (APS), with formulations specified below and shown in Table 2. All acrylic acid loading solutions were prepared in sufficient excess solvent (water) to ensure dried PAAm discs occupied less than about 5% of the total monomer solution. Equilibrium swelling studies were conducted with representative PAAm discs at 8 °C prior to monomer loading to ensure discs had achieved full swelling equilibrium in the monomer solutions prior to polymerization. Swelling durations longer than 24 hours were avoided to reduce the likelihood of undesired reaction initiation prior to removal of the AA-soaked PAAm discs from the AA solution.
Table 2. Fractional Factorial Design Parameters for IPN Hydrogels.
Parameters examined in forming the secondary interpenetrating PAA network included: the concentration of acrylic acid relative to the concentration of hydrated acrylamide repeat units (C Value); the initiator to total monomer molar ratio; the percentage of total monomer that was crosslinker; and the ionic strength.
| Level | C Value | Initiator / Monomer mol Ratio |
MBAAmmol % |
Ionic Strength (mol/L) |
|
| Low (−1) | 0.5 | 0.003 | 0.25 | 0.0 | |
| Center (0) | 1.0 | 0.009 | 0.50 | 0.4 | |
| High (+1) | 1.5 | 0.015 | 0.75 | 0.8 | |
| Formulation | C Value | Initiator / Monomer mol Ratio |
MBAAmmol % |
Ionic Strength (mol/L) |
Max RSM in 0.5 mmol/LHCl* |
| 1 | 1 | −1 | −1 | 1 | 4.84 +/− 0.24 |
| 2 | 1 | −1 | 1 | −1 | 4.68 +/− 0.12 |
| 3 | 1 | 1 | −1 | −1 | 4.84 +/− 0.02 |
| 4 | −1 | 1 | −1 | 1 | 7.05 +/− 0.09 |
| 5 | −1 | 1 | 1 | −1 | 7.89 +/− 0.25 |
| 6 | −1 | −1 | −1 | −1 | 7.46 +/− 0.11 |
| 7 | 1 | 1 | 1 | 1 | 4.68 +/− 0.19 |
| 8 | 0 | 0 | 0 | 0 | 5.14 +/− 0.05 |
| 9 | −1 | −1 | 1 | 1 | 6.94 +/− 0.12 |
| 10 | 0 | 0 | 0 | −1 | 4.93 +/− 0.13 |
HCl solution is 0.5 mmol/L HCl and 1.0 mmol/LNaCl
Following soaking, AA solutions with discs were loaded into an oxygen deficient glove box as above. Solutions were needle purged with nitrogen for 8 minutes within the glove box, while excess nitrogen was flooded in to maintain atmospheric oxygen below 20 ppm. Discs were then carefully removed from the monomer solution and placed with even spacing on a siliconized glass plate. Once all discs were transferred to the bottom plate, a PTFE spacer and a top glass plate were added and clamped together to seal the discs gently between the two plates. The spacer was slightly larger (0.6 mm PTFE) than was used to prepare the initial PAAm networks to prevent significantly compressing AA-swollen PAAm discs during IPN synthesis. The plates were then sealed within the reactor vessel as described previously, and the entire reactor vessel was removed from the glove box and placed in a heated water bath for 5 days at 50 °C.
Following polymerization, the plates were removed from the reaction vessel, and separated to allow removal of the crosslinked IPN discs. The discs ranged in appearance from slightly opaque to nearly transparent, and were more robust than the PAAm hydrogels used in the initial network of the IPNs. The discs were purified by soaking in a large volume of ultrapure water for 5 days, with daily water changes. Some formulations had a light coating of PAA outside the IPN network. This coating was removed simply by gentle scraping with a spatula during wash steps. Discs were dried as described above PAAm hydrogels.
Gravimetric Swelling Experiments
Swelling of PAAm and PAAm / PAA networks was characterized gravimetrically in aqueous solutions. PAAm networks were studied in three different aqueous solvents over several days at 50 °C. Kinetic swelling data was captured over the first 144 minutes of swelling, with remaining time points spread out over 5 days. The time scale, temperature, and solvent conditions were selected to study possible changes in initial PAAm networks that potentially arise during the thermally-initiated polymerization of PAA to form the IPN. Solution conditions examined were pH 1.0 with ionic strength of 0.1 (formic acid buffer); pH 2.2 with ionic strength of 0.1 (phosphate buffer); and pH 2.2 with ionic strength of 0.8 (phosphate buffer). Solution values were chosen to approximate conditions experienced by the AAm network during the formation of the interpenetrated AA network (pH 2.2 with ionic strength varied between 0 and 0.8 in these experiments). Since AA content was varied over a large range during synthesis, a lower pH formic acid buffer solution was examined as well to verify polyacrylamide stability in a more acidic environment for the duration (up to 5 days) of acidic exposure at 50 °C.
Determining the response behavior for the polyacrylamide / poly(acrylic acid) interpenetrating polymer networks was done using similar methods to those for pure polyacrylamide networks. The penetrating solvent conditions selected were based on mapping temperature responses in IPNs, as well as turbidity measurements of linear PAAm and PAA polymers over a range of pH and ionic strength values as discussed below. The aqueous solutions used were deionized water; 1.5 mmol/L NaCl; and 0.5 mmol/L HCl with 1.0 mmol/L NaCl. Both solutions containing NaCl had an ionic strength of 1.5 mmol/L.
All swelling studies were conducted in sealed jars, modified to allow solvent to penetrate the disc from both the top and bottom surfaces of the disc simultaneously. Polymer swelling behavior experiments were conducted starting from fully dried PAAm and PAAm/PAA IPN discs. All swelling measurements were performed in triplicate, with uncertainty indicating standard deviation of measurement data.
Turbidity Measurements
Turbidity determination on equimolar solutions of linear polyacrylamide and poly(acrylic acid) was made by combining solutions of HCl or NaOH, with NaCl in a pH series starting from 8 mmol/L NaOH, going through DI water, and then down to 8 mmol/L HCl. NaCl was varied from DI water to 20 mmol/L, orthogonally to the pH series. Polymer concentration was 0.5 mg/mL for each polymer type, with a total of 1 mg/mL in 2× and mixed polymer solutions, for all combinations of acid, base, and/or salt. Samples were made in triplicate in 200 microliter volumes, and read at 220, 300, and 340 nm on a Synergy HT multimode microplate spectrometer. (BioTek Instruments, Inc.)
Temperature-dependent turbidity measurements were carried out by equilibrating a tape-sealed 96 well plate in an oven until the desired temperature stabilized. The average equilibration time was approximately one hour, and the plate reader was preheated to match the plate temperature during measurement.
IPN Swelling Response Mapping
Representative interpenetrating polymer networks of PAAm and PAA were examined at several pH and ionic strength values to isolate promising regions for swelling responses. The overall areas chosen to examine were based on temperature-dependent complexation results from turbidity measurements as discussed. Additionally, more extreme solution conditions were examined to verify boundaries for swelling responses. In these experiments, ionic strength was varied between 0 and 0.5 mol/L, with most attention toward the lower ionic strength values. Solution pH was varied between 1.6 and 12.7, with more focus on pH values in the acidic range. Characteristic discs were measured at 20 °C and 50 °C, while solution conditions were varied over a gradient of ionic strength and pH. Ionic strength was adjusted with NaCl. HCl and sodium hydroxide (NaOH) were used to adjust low and high pH respectively for each temperature point. The responses examined in the experiment were the ratio of the swelling response at 50 °C to 20 °C as a means to quantify temperature response, and the ultimate relative swelling mass (RSM) at 50 °C.
Formulations for PAAm and IPN hydrogels
Synthesis parameters for PAAm hydrogels were established using a resolution IV, 2-level fractional factorial design (24-1) with center points. The synthesis parameters are shown in Table 1. Initiator to monomer mole ratio represents the number of moles of initiator to the number of moles of total monomer (AAm and MBAAm both being monomers). The mole percent crosslinker is defined as molar fraction percentage of total monomer that is crosslinker.
The experimental design for PAAm/PAA IPN hydrogels was carried out similarly to that used for the PAAm hydrogels, also using a 2-level fractional factorial design (24-1). The factors and their associated levels are shown in Table 2.
Since the final polymer hydrogel is an interpenetrating polymer network, in which the second network (AA) is crosslinked within and throughout an already crosslinked PAAm hydrogel, the concentration of the AAc network is set relative to the concentration of the polyacrylamide in the initial swollen PAAm network. Referencing to the swollen PAAm network is appropriate since the AAc is diffused into and reacted in the presence of an initial PAAm hydrogel in the swollen state. Acrylic acid and acrylamide were equilibrated on a molar basis for the center point of the fractional factorial design. Determination of acrylamide concentration in swollen networks was done with Equation 1. In this equation, k represents the molar concentration of acrylamide in the swollen PAAm hydrogel, expressed as moles AAm / g H2O. The wt% AAm term is the weight fraction of total monomer that is AAm, and is usually very close to 1. MWAAm is the molecular weight of the acrylamide repeat unit (71.08 g/mol). RSM is the relative swelling mass of the polyacrylamide network under equilibrium conditions in an acrylic acid prepolymer solution, and is a measure of a disc’s swollen weight to its dry weight. Equation 1 is based on the assumption that in the swollen polyacrylamide network, the total amount of monomer is fully hydrated by the imbibed water.
| Equation 1 |
For the fractional factorial design, the AAm concentration, k, was multiplied by a scaling factor to adjust acrylic acid concentration relative to the solvated acrylamide concentration within the swollen network as a means to vary factor levels. The scaling factor, referred to here as the C-value, ranged from 0.5 to 1.5, with 1.0 (matched concentration) being the center point value for the acrylic acid concentration.
Results and Discussion
Turbidity Results
To validate measuring turbidity of linear poly(acrylic acid) and polyacrylamide to determine optimal solution conditions for temperature responses, PAA and PAAm were added individually, and blended together in DI water and 20 mmol/L salt solutions. Significant complexation was observed in a 20 mmol/L NaCl solution due to the polyelectrolyte effect. At 220 nm, both polymers absorb incident light significantly, which inherently confounds turbidity interpretation. However at 300 and 340 nm, little absorbance occurs, and measured intensity is due to scattered light from polymer complexes and precipitates dispersed in the solution. As expected in scattering, a slight decrease in measured intensity was noted as wavelength increased.
For temperature studied, linear polyacrylamide and linear poly(acrylic acid) were combined in solutions of HCl, NaOH, and NaCl and were examined at various temperatures between 25 and 50 °C. Polymer concentrations correlate to 7 mmol/L for both carboxylic acids and amides. NaCl concentration was varied from 0 to 20 mmol/L. The molar concentration of strong acid and base was examined between 0 and 8 mmol/L.
The temperature results for DI and NaOH samples did not indicate complexation at any temperature examined and are not included here. Figure 1 shows temperature-dependent complexation in acidic solutions with linear PAAm / PAA formulations set at equimolar ratios. In general, higher salt levels resulted in increased turbidity, as expected due to charge screening and changes in AA conformation caused by the polyelectrolyte effect. At 2 mmol/L HCl, the turbidity is consistently high due to extensive protonation of the carboxylic acid, which both reduces ionic repulsion due to charge neutrality, but also allows a higher fraction of carboxylic acids to participate in hydrogen bonding. In the solutions with the highest turbidity levels, precipitation was noted in the plate wells, indicating complex coacervation between the polymers.
Figure 1.
Temperature Responses in Linear Complexes of PAAm and PAA Indicated by Turbidity. Temperature-driven decomplexation between linear segments of PAAm and PAA is observed in 1 mmol/L HCl (pH 2.4) (35 – 40 °C), and 0.5 mmol/L HCl (pH 2.7) (25 – 30 °C), for solutions of 10 mmol/L NaCl or less.
A temperature response between 25 and 30 °C was observed at 0.5 mmol/L HCl, in both DI water and 0.5 mmol/L NaCl. This corresponds to a pH of 2.7 and an ionic strength of 2 – 2.5 mmol/L. Additionally, a temperature response was observed between 35 and 40 °C, in 1 mmol/L HCl, in both DI water and 0.5 mmol/L NaCl. This solution region corresponds to a pH of 2.4, and an ionic strength 4 – 4.5 mmol/L.
Polyacrylamide / Poly(acrylic acid) IPN Solvent Mapping
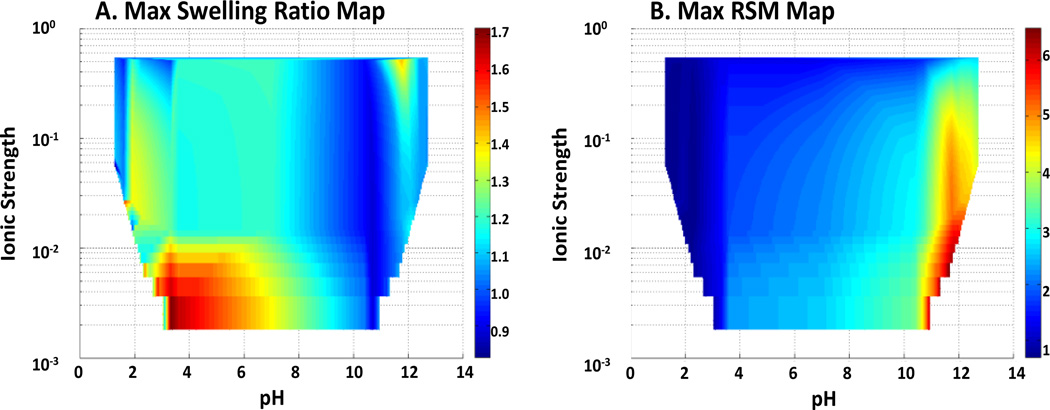
Turbidity measurements for temperature-dependent complexation on linear polymers allowed for rapid mapping of solution condition regions for temperature responses in IPN hydrogels. Color intensity maps for responses were produced from sample data using a 3D mesh algorithm in MATLAB® (Figure 2). The hydrogel films studied in this work were PAA / PAAm IPNs in a 50/50 feed ratio (C-value of 1.0), synthesized under UV initiation, with all reactants levels set to the values of formulation 10 in Table 2. As shown in Figure 2A, the most significant temperature response is observed between pH 3 and pH 6, and with ionic strength below 10 mmol/L. At extreme pH values, poly(acrylic acid) is either substantially protonated and neutrally-charged (acidic conditions), and ionic repulsion does not overcome the formation of strong hydrogen bonding between acrylic acid units; or the polymer is substantially deprotonated and charged (basic conditions) that the IPN is completely swollen, regardless of temperature. In either extreme, the effects of temperature are overwhelmed by the ionic considerations of the network. Figure 2B shows the range of pH and ionic strength values where maximal swelling occurs. Above around pH 10, the polymer discs are extremely swollen, and completely lack temperature sensitivity. Temperature responses were only observed in low ionic strength solutions, with solution pH near the pKa for the anionic monomer. The plots taper up on the bottom corners since dissociation of added acids and bases adds to solution ionic strength.
Figure 2.
Swelling Response Maps for IPN Hydrogels with Respect to Solution Conditions. A) Solution conditions for maximum temperature response (Ratio of RSM at 50 to RSM at 20 °C). B) Solution conditions for highest overall RSM swelling response at 50 °C, which is referenced to a dried hydrogel disc.
Polyacrylamide Hydrogel Swelling Responses
Polyacrylamide discs formulated as described in Table 1, and were studied by measuring aqueous solvent uptake as a function of time in various pH and ionic strength conditions. Responses analyzed in these experiments include the relative swelling mass for PAAm as a function of time in DI water and solutions conditions mimicking PAA for in situ network formation. Since PAAm is not ionic, it was expected that network swelling would be largely independent of solution pH and ionic strength, except under conditions where acrylamide hydrolysis occurs as discussed below.
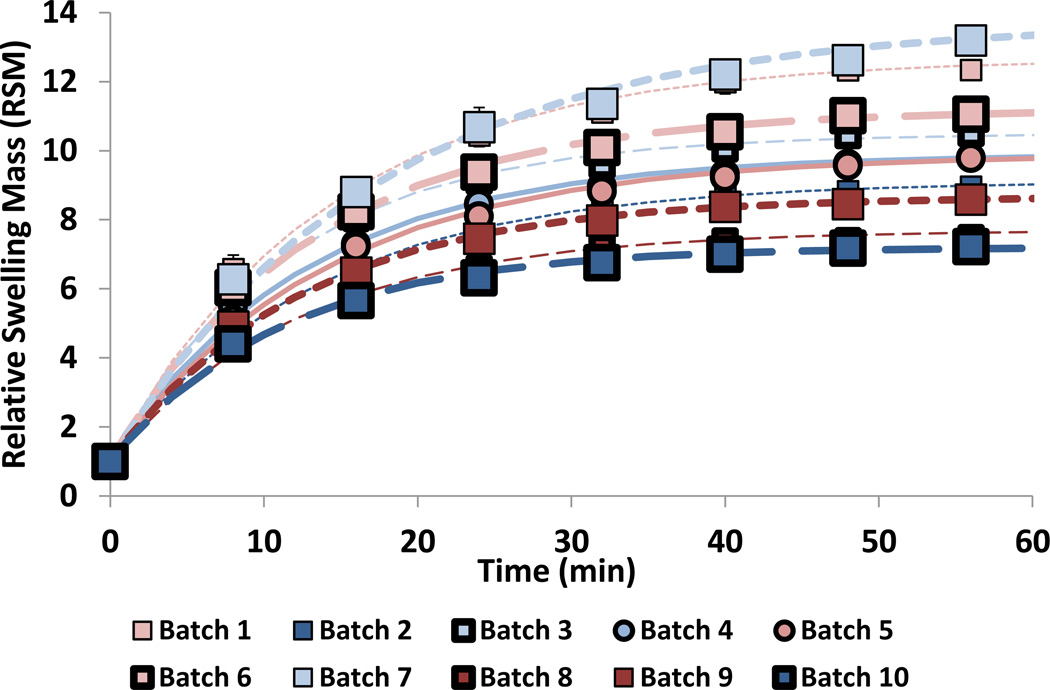
Dried, glassy PAAm hydrogel discs consistently reached their full equilibrium swelling within the first 144 minutes of swelling in the three separate aqueous solvents at 50 °C (Figure 3). There were no differences observed in swelling behavior within any single formulation for any of the aqueous solvents examined. PAAm kinetic responses for hydrogel discs were fit to the exponential growth function shown by Equation 2.
| Equation 2 |
In this equation, RSMo represent the starting relative swelling mass, and was forced to 1 in the curve fits since the first value at time t = 0 is with a fully dried disc. RSMmax is the ultimate swelling volume at equilibrium, neglecting long term changes in the network due to possible amide hydrolysis or polymer backbone degradation. The term, t, is the elapsed time in minutes. The time constant term, τ, for swelling responses was determined across formulations, and indicated the kinetic swelling behavior for polyacrylamide discs was controlled by geometry, and is formulation-independent as expected. This suggests that all PAAm hydrogels tested were similarly dry and glassy to start, and that no structural variations sufficient to have a significant impact on swelling kinetics were noted between the formulations.
Figure 3.
Kinetic Swelling Responses of PAAm Hydrogels at 50 °C to Determine Ideal IPN Initial Network. Error bars represent standard deviation of repeated measurements (n = 4 discs).
Polyacrylamide swelling at 50 °C, over five days (not shown) for all formulations and in all aqueous solvent combinations (pH 1, I = 0.1 mol/L; pH 2.2, I = 0.1 mol/L; and pH 2.2, I = 0.8 mol/L) showed excellent stability. Exposure duration of five days was chosen to match the time of IPN formation, where the AA network is reacted into the existing PAAm network over 5 days at 50 °C with an acidic solution pH. In virtually all formulations, only the RSM in pH 1 solution varied during the course of the study. The small decrease in RSM is fairly consistent among all heated batches examined during the 5 days. This change is likely caused by hydrolysis of PAAm, converting a small percentage of amides to carboxylic acids, but is only shown to occur at a full pH unit lower than is provided by acrylic acid during synthesis. Importantly, this indicates that the PAAm network is stable during the course of AA polymerization to form the IPN.
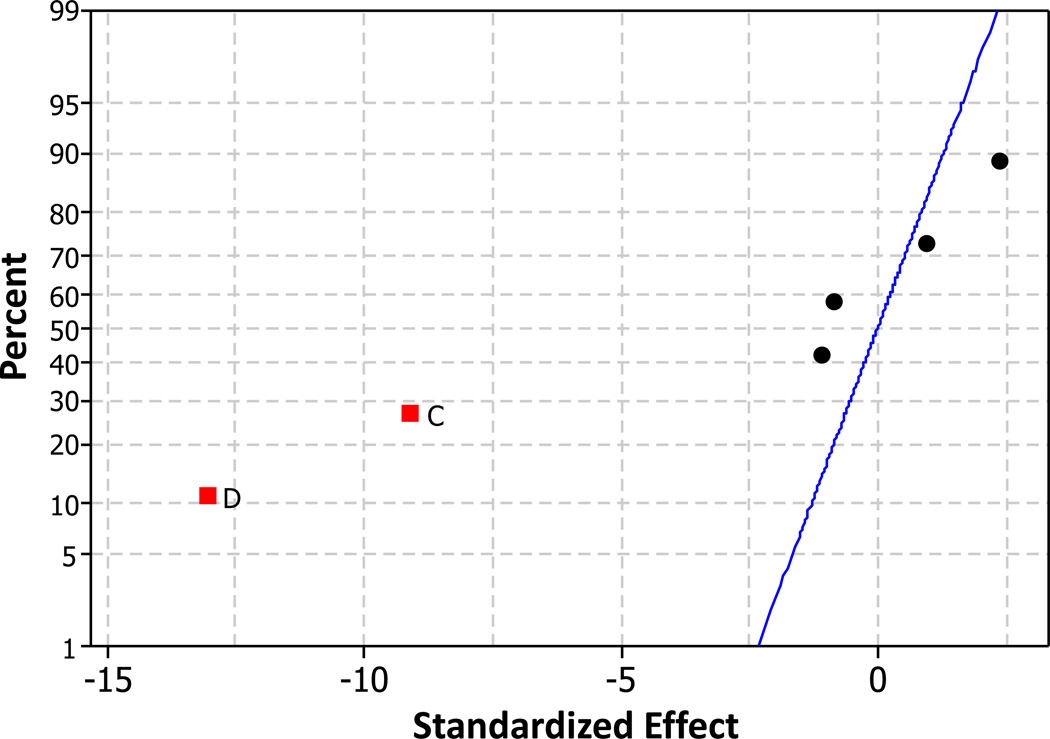
A normal effects plot for the maximum swelling response in PAAm hydrogels is shown in Figure 4. The results show that higher levels of both monomer and crosslinker reduce the overall swelling response in PAAm. A minor effect may also be observed with initiator, but it isn’t shown to be statistically significant with sufficient confidence, so the effect if present is very minor. No significant higher order interactions in driving PAAm equilibrium responses was observed in analyzing the fractional factorial design. Based on these results, Formulation 1 was chosen as the initial network for IPN formation.
Figure 4.
Normal Effects Plot of Standardized Effects for PAAm Hydrogels. The response variable is max relative swelling mass (RSM). The effects shown are: A: Siliconized, B: Initiator Concentration, C: Relative Crosslinker Concentration, and D: Acrylamide Concentration. Significant effects are shown as labeled squares. Position of the points indicates effect polarity. Non-significant effects are shown as unlabeled points. Confidence for effect significance is 0.95 (α = 0.05).
Selected PAAm formulations were also polymerized in DMSO, using two different initiators, benzoyl peroxide (BPO) and Luperox® TBEC (TBEC) (not shown). BPO was attempted on many batches, however most were too weak and fragile to carry out swelling measurements, and a few did not even last through purification intact. Batches 2 and batch 5 were polymerized with both BPO and TBEC in DMSO. The results indicated that PAAm swelling was highest in BPO–initiated hydrogels; second highest in TBEC-initiated hydrogels; and lowest in APS-initiated hydrogels for both batches 2 and 5. Higher swelling responses of initial networks also correlated to the much higher fragility of the gels. Further study with these networks was abandoned due to the extreme difficulty in maintaining these gels intact.
PAAm / PAA IPN Hydrogel Temperature Responses
Based on the results of the pH / ionic strength IPN swelling map shown in Figure 2, PAAm / PAA IPN hydrogels formulated according to Table 2 were examined in three aqueous solvents to analyze a fractional factorial design for IPN hydrogel networks. Temperature-responsive swelling was examined in DI water, NaCl solution, and 0.5 mmol/L HCl solution. The ionic strength of both aqueous solutions containing electrolytes was 1.5 mmol/L, adjusted with sodium chloride. Parameters examined in all three swelling solutions included maximum relative swelling mass (RSM); the term, b, from Equation 3, indicating the steepness of the volume change with respect to temperature at the inflection point; and the temperature swelling ratio.
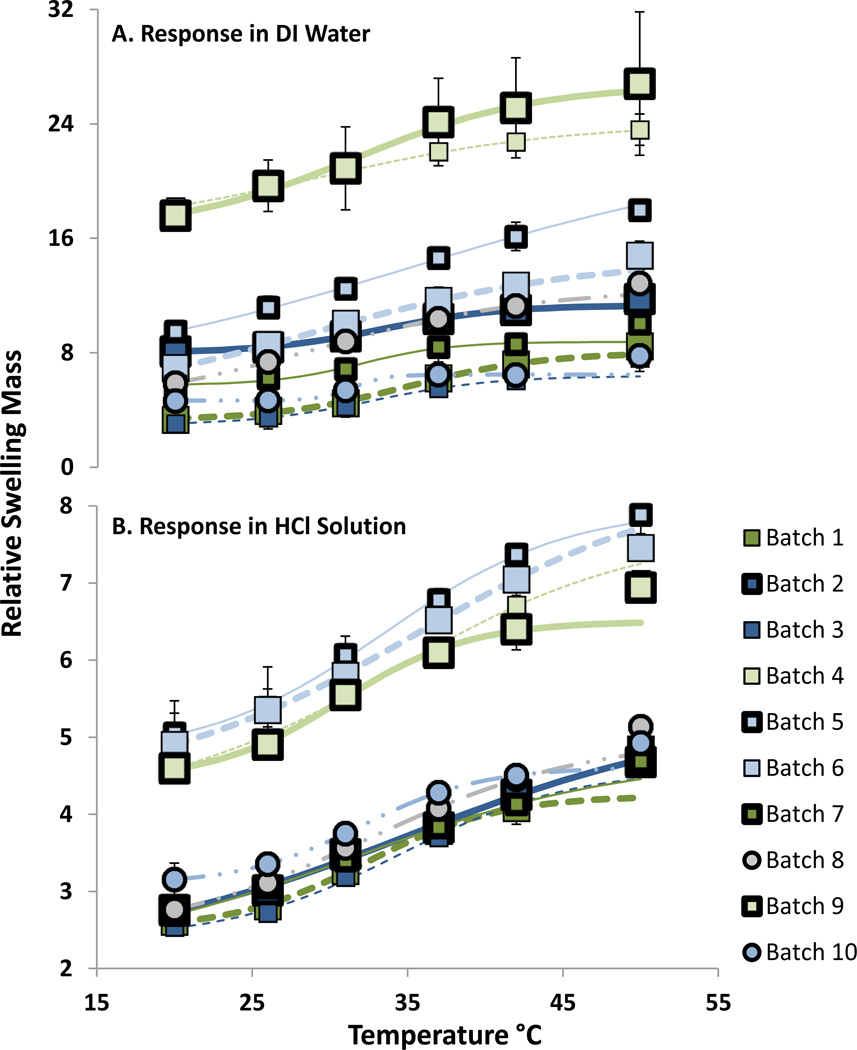
As described in above, a sudden transition in the equilibrium swelling volume around a specific temperature is indicative of a UCST transition for the polymer network and solvent. IPN hydrogels in this work were studied as a function of temperature from 20 °C to 50 °C, for each formulation. The results of these swelling experiments in DI water and 0.5 mmol/L HCl solution are shown in Figure 5. The swelling responses shown in Figure 5 were fit to the sigmoid function shown in Equation 3.
| Equation 3 |
This equation is a simplified case of the generalized logistic function.37 In this equation, the RSMo* term is an approximation on the initial swelling at 20 °C for the IPN. The RSMmax* term, is the carrying capacity for the function, and in this instance approximates the maximum RSM at high temperature. These two terms were not fixed to the actual RSMo and RSMmax during the regression since better curve fits were obtained by leaving them as free variables. Additionally, RSMmax isn’t known since there is no expectation of a steady maximum swelling value within the temperature range examined, particularly in consideration of possible network changes due to amide hydrolysis that may occur in acidic or basic conditions at elevated temperatures over extended time periods. The primary terms of interest in the regression are To, and b. To is the inflection temperature, and can be thought of as the critical transition temperature in a UCST or LCST hydrogel. The average temperature for the inflection point was 31.9 +/− 2.4 °C in water, and 33.2 +/− 1.7 °C in acidic solutions. The term b dictates the ‘steepness’ (or growth rate) of the change with respect to temperature near the inflection point, and can be thought of as a quantitative descriptor of the degree to which a particular formulation exhibits a sigmoidal temperature response. Temperature responses that approach a more sigmoidal behavior have greater potential applicability in biomedical applications since their response occurs over a narrower temperature range. The temperature response results for the formulations of PAAm / PAA IPNs examined show that the highest swelling response typically occurred in salt (1.5 mmol/L NaCl), and DI water solutions; however temperature transitions best approximated sigmoidal behavior in weak acid solutions. Since the overall swelling magnitude was dramatically reduced in weakly acidic batches, some formulations were examined in less acidic (HCl = 0.25 mmol/L, with equivalent ionic strength) (data not shown) to determine if a sigmoidal behavior could be retained, but with larger swelling degrees. The results of this experiment indicated that, for all formulations tested, overall swelling response did increase, however the shape of the transition with respect to temperature changed to linear or exponential.
Figure 5.
Equilibrium Swelling Temperature Responses of PAAm / PAA IPN Hydrogels in A) DI Water, and B) 0.5 mmol/L HCl. Error bars represent standard deviation of repeated measurements (n = 3 discs).
In most formulations, the RSM was slightly higher in a weak salt solution than in DI water. This is explainable since as the network is swollen with counterions, electrostatic repulsions between and within AA chains are reduced, resulting in a less rigid network where slightly charged chains can more easily approach one another in solution.38 This tends to expel aqueous solvent. However, the increase in ions also reduces the pKa of the carboxylic acids along the AA chains, which liberates additional protons.39,40 The increase in free protons within the network increases osmotic pressure relative to the hydrogel exterior, and encourages greater solvent uptake. Opposing secondary effects would also be relevant, specifically the decrease in electric field repulsion caused by charge screening with counterions allowing for increased hydrogen bonding for AAm along the network. However, the excess of protons liberated as a result of the pKa change will offset this effect to some extent. Finally, a net increase of ion concentration on both sides of the gel will reduce the osmotic pressure gradient, but only slightly. The observation that with some formulations, a low concentration of salt actually increases gel swelling at the same temperature indicates that the trade-off exists between the effect of the pKa shift and the effect of charge screening. Formulation specific factors dictate which effect is greater in driving the swelling behavior.
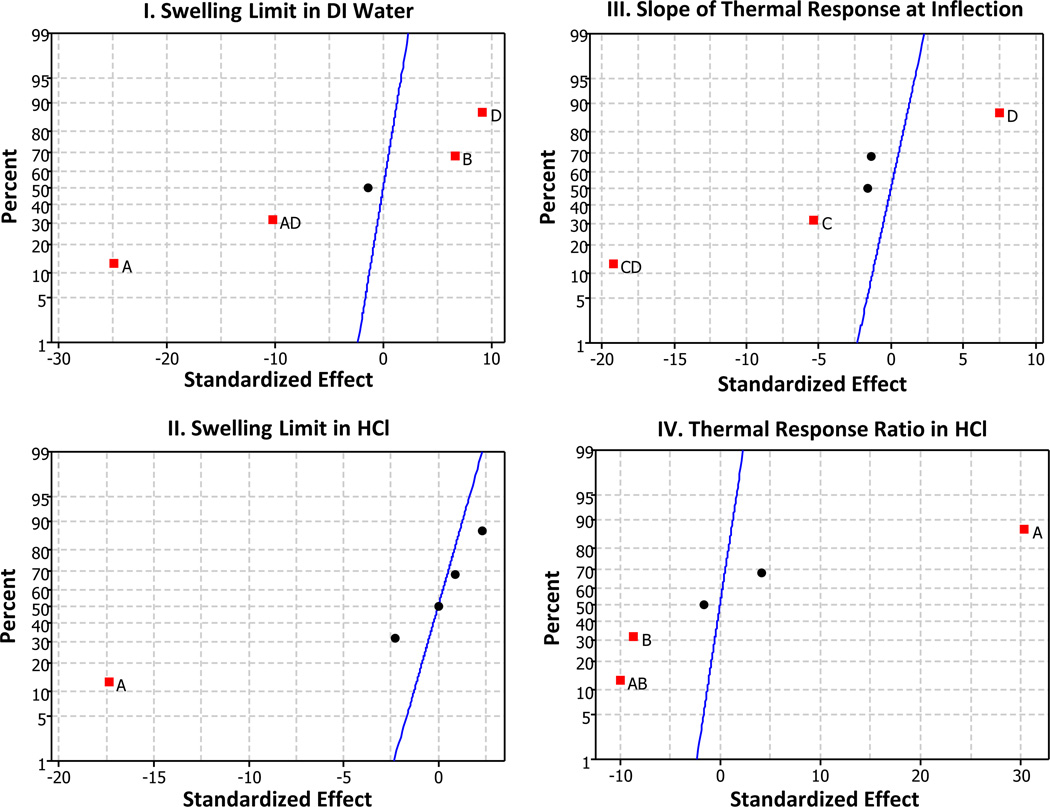
The response variables for the fractional factorial design of IPN formulations summarized in Table 2 are shown in Figure 6. The normal plot of standardized effects in Figure 6-I and Figure 6-II indicates that an effects regression model on the selected parameters show C-value, ionic strength, and crosslinker concentration as all impacting the overall relative swelling mass, particularly in DI water. There is an interaction between terms AD (C-value and ionic strength) as well that is indicated to be statistically significant in DI water swelling. It should be noted however the observed AD interaction could actually be either a BC interaction or an AD interaction due to confounding.41 The AD interaction is more likely since the BC interaction involves a term which has virtually no impact on RSM. The main effects plot shows the C-value (relative concentration of AA) is inversely proportional to the relative swelling mass in DI water. Both ionic strength and crosslinker concentration vary proportionally with maximum RSM response. The interaction term between ionic strength and C-value demonstrates that ionic strength has much less effect on the RSM at higher AA concentrations. Thus maximizing the ultimate RSM in DI water for the IPN system would be achieved by maintaining C-value low and ionic strength high during polymerization. The results of the IPN analysis for RSM in acidic solution show that only C–value is statistically significant in having a strong influence on swelling magnitude. This is expected since the overall magnitude of the response is lower, and thus any factors that change the total swelling will be diminished and therefore more difficult to distinguish.
Figure 6.
Normal Plots of Standardized Effects for IPN Responses: The effects shown for each response variable are: A: C Value, B: Relative Crosslinker Concentration, C: Initiator Concentration, and D: Ionic Strength. Significant effects are shown as labeled squares. Non-significant effects are shown as unlabeled points. Position of the points indicates effect polarity. Effects to the left of the line indicate a negative effect on the response, while points to the right indicate a positive effect on the response. Confidence for effect significance is 0.95 (α = 0.05).
The tendency for the temperature response to demonstrate sigmoidal behavior is shown in Figure 6-III. The normal plot of effects illustrates the primary determining factors are initiator and ionic strength. The most significant factor is an interaction between CD (or possibly AB due to confounding). CD is more probable due to the main effects of C and D indicated in the normal plot of effects. The interpretation based on this assumption is that ionic strength increases the response variable, b, at lower initiator values, but has the opposite effect at higher initiator values. Since the main effects for C-value and crosslinker are low, the response in an acidic solution would be optimized by maximizing ionic strength and maintaining initiator concentration low.
Polymerization of poly(acrylic acid) in the presence of high salt concentration has been shown to affect cyclization within gel networks.42 In a pure PAA hydrogel network, increased cyclization tends to result in higher swelling magnitudes due to a reduction in effective crosslinking. In this work, IPNs polymerized in high ionic strength for the secondary network had higher overall swelling, but also appeared more sigmoidal in terms of their temperature response. The larger degree of swelling could be due to this lower effective crosslinking, though the overall swelling is still limited by the limits of the crosslinked initial PAAm network, provided the two networks are effectively interpenetrated. It has been shown that rapid polymerization of acrylic acid in seeded-IPN microspheres caused by high polymerization temperatures resulted in a loss of the temperature response and maximum swelling volumes due to lack of interpenetration between PAAm and PAA networks.43
Figure 6-IV shows the main effects on determining overall temperature swelling ratio, which indicates the magnitude of the temperature response. The main factor affecting swelling ratio is C-value, with a strong direct relationship. There is an interaction AB, which may be significant, as well as factors B and C, as shown in the normal plot of effects.
Potential for acid- or base-catalyzed hydrolysis caused by synthesis or use was examined using dispersions of linear polyacrylamide exposed to various acid, base, and temperature conditions. Hydrolysis of acrylamide repeat units to form carboxylic acids from amides was quantified using potentiometric titration. The results (not shown) indicate that hydrolysis beyond about 5% was isolated to comparatively extreme solution conditions. Specifically, base exposure of pH 13.5 at 50 °C for 2 hours resulted in 44% carboxylation, and acid exposure below pH 0.5 at 50 °C for 24 hours resulted in 34% carboxylation. In general, PAAm showed more resistance to acid-catalyzed hydrolysis than base-catalyzed hydrolysis, which is important since relevant temperature responses occur under mildly acidic conditions. Acidic attack on linear polyacrylamide with pH 2.1 at 50 °C for 7 days showed no significant hydrolysis. This finding further supports observations of relatively modest shifts in overall RSM (20% maximum) in PAAm discs that were solvated in pH 1 at 50°C for 5 days, and no noticeable shifts in overall RSM of PAAm discs under similar conditions at pH 2.2.
A recent analysis of the complexation between acrylamide and acrylic acid at the appropriate pH and ionic strength conditions for hydrogen bond complexation revealed that the complexation does not occur in a 1:1 molar ratio.34 In pure solutions of PAAm and PAA prior to mixing, PAA and PAAm are pre-complexed into multi-macroion cluster domains and hydrogen-bonded associates respectively, with the strength of interactions in complexed domains between polymer chains inversely proportional to ion presence in solution. Upon mixing of PAAm and PAA in conditions conducive to hydrogen bond formation, PAA and PAAm complexation occurred primarily around PAA clusters, making it more critical to complexation and leaving out excess PAAm. In a crosslinked IPN, the excess PAAm would result in both greater overall swelling and reduced temperature sensitivity. This behavior is observed in these experiments.
Conclusions
Taking all swelling results together, designing the response for the PAAm / PAA IPN is possible by adjustment of specific synthesis variables. Maximum PAAm swelling response is achieved by maintaining concentrations of acrylamide and crosslinker relatively low. However maximum swelling response alone is not necessarily useful in potential biomedical applications since there may be an associated loss of temperature sensitivity. Reducing poly(acrylic acid) content relative to polyacrylamide in the IPN does increase overall IPN swelling, but also reduces temperature sensitivity, probably due in part to limited macroion clusters available as complexation sites for intermolecular interactions with polyacrylamide. Since the concentration of acrylic acid is scaled from acrylamide concentration in the initial network (C-value), an IPN with good temperature sensitivity and higher swelling potential would be interpenetrated into a PAAm network which exhibits a large RSM. To maximize the temperature response, the acrylic acid content would be scaled up from the acrylamide content. Narrowing the range of the temperature response, or increasing the magnitude of b from the curve fit to the sigmoidal function shown in Equation 3, is accomplished by polymerization of the acrylic acid portion of the IPN network with a relatively lower initiator level and higher ionic strength. It should be noted however, that thorough purification of IPNs polymerized in high ionic strength solutions is necessary since residual salts will have a major impact on the swelling of these gels.
For biomedical applications, the triggering point for thermoresponsive gels should be slightly higher than 37 °C, with pH around 7.4 for most physiological compartments. The actual transition temperature for the interaction between PAAm and PAA is a function of the pH relative to the pKa of the anionic portion of the hydrogel, and increases progressively as pH is reduced relative to the pKa.44 In addition to the pKa dependence on the ionic strength of the solution and its own ionization as discussed above, N-alkyl substitution of the anionic monomer component is a possible way to design to upshift the pH response region of the IPN to improve physiological relevance. For example, this can be accomplished by substitution of poly(propylacrylic acid) for poly(acrylic acid) in the PAAm / PAA IPN.
Acknowledgements
The authors gratefully acknowledge financial support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R21 EB012726-01A1). B.V.S acknowledges the US National Science Foundation for graduate research fellowship funding. The authors also thank the Department of Statistics and Data Science at The University of Texas for use of consulting resources in experimental design.
References
- 1.Aoki T, Kawashima M, Katono H, Sanui K, Ogata N, Okano T, Sakurai Y. Macromolecules. 1994;27:947–952. [Google Scholar]
- 2.Bae YH, Okanoand T, Kim SW. Pharmaceutical Research. 1991;8:624–628. doi: 10.1023/a:1015860824953. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Gu Y, Huand Y, Qian Z. PDA Journal of Pharmaceutical Science and Technology. 2007;61:303–313. [PubMed] [Google Scholar]
- 4.Sershen SR, Westcott SL, Halasand NJ, West JL. Journal of Biomedical Materials Research. 2000;51:293–298. doi: 10.1002/1097-4636(20000905)51:3<293::aid-jbm1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Zhangand J, Misra RDK. Acta Biomaterialia. 2007;3:838–850. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Katono H, Maruyama A, Sanui K, Ogata N, Okanoand T, Sakurai Y. Journal of Controlled Release. 1991;16:215–227. [Google Scholar]
- 7.Brazel CS. Pharmaceutical Research. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 8.Owens DE, Jian YC, Fang JE, Slaughter BV, Chenand YH, Peppas NA. Macromolecules. 2007;40:7306–7310. [Google Scholar]
- 9.Satarkarand NS, Hilt JZ. Acta Biomaterialia. 2008;4:11–16. [Google Scholar]
- 10.Satarkarand NS, Hilt JZ. Journal of Controlled Release. 2008;130:246–251. doi: 10.1016/j.jconrel.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins A, Satarkarand N, Hilt J. Pharmaceutical Research. 2009;26:667–673. doi: 10.1007/s11095-008-9804-z. [DOI] [PubMed] [Google Scholar]
- 12.Bae YH, Okano T, Hsuand R, Kim SW. Die Makromolekulare Chemie, Rapid Communications. 1987;8:481–485. [Google Scholar]
- 13.Bae YH, Okanoand T, Kim SW. Pharmaceutical Research. 1991;8:531–537. doi: 10.1023/a:1015871732706. [DOI] [PubMed] [Google Scholar]
- 14.Hoareand T, Pelton R. Langmuir. 2004;20:2123–2133. doi: 10.1021/la0351562. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nakamura S, Sakai K, Aoyagi T, Kikuchi A, Sakuraiand Y, Okano T. Macromolecules. 1998;31:6099–6105. [Google Scholar]
- 16.Kloudaand L, Mikos AG. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X-C, Chu L-Y, Chen W-M, Wangand S, Xie R. Langmuir. 2004;20:5247–5253. doi: 10.1021/la036230j. [DOI] [PubMed] [Google Scholar]
- 18.Gehrke SH. Responsive Gels: Volume Transitions II. Springer; 1993. pp. 81–144. [Google Scholar]
- 19.Bouillotand P, Vincent B. Colloid and Polymer Science. 2000;278:74–79. [Google Scholar]
- 20.Echeverria C, Lópezand D, Mijangos C. Macromolecules. 2009;42:9118–9123. [Google Scholar]
- 21.Echeverria C, Peppasand NA, Mijangos C. Soft Matter. 2012;8:337–346. [Google Scholar]
- 22.Tsutsui H, Moriyama M, Nakayama D, Ishiiand R, Akashi R. Macromolecules. 2006;39:2291–2297. [Google Scholar]
- 23.Wang QF, Li SM, Wang ZY, Liuand HZ, Li CJ. Journal of Applied Polymer Science. 2009;111:1417–1425. [Google Scholar]
- 24.Xiao XC, Chu LY, Chen WM, Wangand S, Li Y. Advanced Functional Materials. 2003;13:847–852. [Google Scholar]
- 25.Xiao X-C, Chu L-Y, Chenand W-M, Zhu J-H. Polymer. 2005;46:3199–3209. [Google Scholar]
- 26.Glatzel S, Laschewskyand A, Lutz J-F. Macromolecules. 2010;44:413–415. [Google Scholar]
- 27.Liu F, Seuringand J, Agarwal S. Macromolecular Chemistry and Physics. 2014;215:1466–1472. [Google Scholar]
- 28.Seuring J, Bayer FM, Huberand K, Agarwal S. Macromolecules. 2011;45:374–384. [Google Scholar]
- 29.Shimada N, Kidoakiand S, Maruyama A. RSC Advances. 2014;4:52346–52348. [Google Scholar]
- 30.Seuringand J, Agarwal S. Macromolecular Rapid Communications. 2012;33:1898–1920. doi: 10.1002/marc.201200433. [DOI] [PubMed] [Google Scholar]
- 31.Waymouth C. In vitro. 1970;6:109–127. doi: 10.1007/BF02616113. [DOI] [PubMed] [Google Scholar]
- 32.Marques MR, Loebenbergand R, Almukainzi M. Dissolution Technol. 2011;18:15–28. [Google Scholar]
- 33.Steenand JB, Turitzin SN. Respiration Physiology. 1968;5:234–242. doi: 10.1016/0034-5687(68)90061-3. [DOI] [PubMed] [Google Scholar]
- 34.Deng L, Wang C, Liand Z-C, Liang D. Macromolecules. 2010;43:3004–3010. [Google Scholar]
- 35.Eustace D, Sianoand D, Drake E. Journal of Applied Polymer Science. 1988;35:707–716. [Google Scholar]
- 36.Kleninaand O, Fain E. Polymer Science USSR. 1981;23:1439–1446. [Google Scholar]
- 37.Grosenbaugh L. Biometrics. 1965:708–714. [Google Scholar]
- 38.Hara M. Polyelectrolytes: Science and Technologies. CRC Press; 1993. [Google Scholar]
- 39.De Stefano C, Gianguzza A, Piazzeseand D, Sammartano S. Reactive and Functional Polymers. 2003;55:9–20. [Google Scholar]
- 40.Högfeldt E, Miyajima T, Marinskyand JA, Muhammed M. Acta Chem. Scand. 1989;43:496–499. [Google Scholar]
- 41.Montgomery DC. Design and analysis of experiments. New York: Wiley; 1984. [Google Scholar]
- 42.Elliott JE, Macdonald M, Nieand J, Bowman CN. Polymer. 2004;45:1503–1510. [Google Scholar]
- 43.Xiao X, Zhuo R, Xuand J, Chen L. European Polymer Journal. 2006;42:473–478. [Google Scholar]
- 44.Sudre G, Tran Y, Cretonand C, Hourdet D. Polymer. 2012;53:379–385. [Google Scholar]