Abstract
Background
Little is known on long-term survival and causes of death among individuals born small or large for gestational age. This study investigates birth weight in relation to survival and causes of death over time.
Methods
A national cohort of 1.7 million live-born singletons in Denmark was followed during 1979–2011, using the Danish Civil Registration System, the Medical Birth Registry and the Cause of Death Registry. Cox proportional hazards were estimated for the impact of small (SGA) and large (LGA) gestation weight and mortality overall, by age group and birth cohort.
Results
Compared to normal weight children, SGA children were associated with increased risk of dying over time. Though most of the deaths occurred during the first year of life, the cumulative mortality risk was increased until 30 years of age. The hazard ratios [HR] for dying among SGA children ages <2 years were: 3.47 (95% CI, 3.30–3.64) and 1.06 (95% CI, 0.60–1.87) in 30 years and older. HR for dying among SGA adults (20–29 years) were: 1.20 (95% CI, 0.99–1.46) in years 1979–1982 and 1.61 (95% CI, 1.04–2.51) in years 1989–1994. The SGA born had increased risk of dying from infection, heart disease, respiratory disease, digestive disease, congenital malformation, perinatal conditions, and accidents, suicide, and homicide. Individuals born LGA were associated with decreased mortality risk, but with increased risk of dying from malignant neoplasm.
Conclusions
Survival has improved independently of birth weight the past 30 years. However, children born SGA remain at significantly increased risk of dying up till they turn 30 years of age. Individuals born LGA have lower mortality risk but only in the first two years of life.
Introduction
Several studies have investigated the association between birth weight and mortality and morbidity [1–4]. How birth weight is taken into account such as absolute birth weight, centile charts and other weight definitions has been a topic of debate and has also recently been evaluated [3]. Though birth weight is highly correlated with morbidity and mortality, it is not a true risk factor but rather the result of one or several causing events. The underlying mechanism of intrauterine growth is not fully understood but hypotheses gather around maternal metabolic vascular health during pregnancy and differences in placental implantation [5]. In the 1990s, David Barker formulated a hypothesis suggesting that events occurring during intrauterine life and in early infancy can influence the occurrence of diseases in adulthood. This theory proposes that undernutrition and other insult or adverse stimulus in utero and during infancy can permanently change the body's structure, physiology and metabolism, and the lasting or lifelong effects will depend on the period in the development or gestational week at which it occurs [6].
Over the past 40 years perinatal care has improved survival of preterm and low-birth-weight infants tremendously. For example between 1995 and 2006, survival of preterm infants, born between 22 and 25 weeks’ gestation, improved by as much as 13% [7]. Similar findings of improved survival have been reported among infants born with extremely low weight (501 to 1500 grams) [2]. Though the main focus has been on low birth weight, several studies have recently also reported U-shaped associations, with increased mortality risks related to high birth weight [1;4;8;9]. The success of improved survival among individuals born early or with extreme birth weight faces challenges such as increased morbidity in infancy and childhood. Long-term survival may also be compromised but few studies have had sufficient length of follow-up to address this issue.
In the present study we took advantage of the unique population-based registries in Denmark to study the entire population of live-born children over a period of 30 years. We investigated how far into adulthood a child’s birth weight influences survival and studied the underlying cause of death.
Material and Methods
All children born in Denmark are assigned a civil personal registration number allowing for identity-secure linkage of information between national registries. We used data from the Civil Registration System (CRS) [10] to identify all live-born singletons in Denmark between January 1, 1979 and December 31, 2011, with a Danish-born mother (N = 1 708 714). Deaths and dates of death were also identified in the CRS whereas information on the causes of death was extracted from the Cause of Death Registry [11]. Information on birth characteristics e.g. birth weight and gestational age at time of birth were retrieved from the Medical Birth Registry (MBR) [12].
Unless otherwise specified, relative weight was defined as small, large and normal weight for gestational age. Infants born below the 10th percentile birth weight among all infants within the same gestational week, with same gender and born within the same birth cohort were categorized as small for gestational age (SGA). Infants born above the 90th percentile birth weight among all infants within the same gestational week, with same gender and born within the same birth cohort were categorized as large for gestational age (LGA). Infants born within the 11–89% cut-off were categorized as ‘normal for gestational age’ and served as the reference group in most of the analyses.
The relative weight categories were calculated within birth cohort year (1979–1982, 1983–1988, 1989–1994, 1995–2000, 2001–2006, and 2007–2011), gender and gestational age strata. Gestational age at birth was categorised as follows: 19–28 weeks, 29–31 weeks, 32–33 weeks, 34–35 weeks, 36 weeks, 37 weeks, 38–42 weeks, and 43–45 weeks, using mother’s information on first day of last menstruation and sonogram measurements. Only singletons with known gestational age between 19 and 45 gestational weeks and known birth weight between 500 and 7000 grams were included in the study. We compressed coding in the cause of death registry into ten groups using the International Classification of Diseases (ICD) 10th Edition. The ten groups were; infection (ICD-10 code: A00-A09, A15-A99, B00-B99); malignant neoplasm (ICD-10 code: C00-D09); heart disease (ICD-10 code: I00-I25, I27, I30-I52); respiratory disease (ICD-10 code: J00-J99); digestive disease (ICD-10 code: K00-K93); congenital malformation, deformations and chromosomal abnormalities (ICD-10 code: Q00-Q99); condition originating in the perinatal period (ICD-10 code: P00-P96); accident, suicide, and homicide (ICD-10 code: V01-Y89); other; and unknown.
Statistical analyses
Cox proportional hazards regression was used to estimate hazard ratios [HRs] and 95% confidence intervals [CIs] for the association between small and large gestation weight and mortality over time, using attained age (1-day intervals) as the underlying time scale in all analyses. The baseline hazard function was stratified on gender and birth year. The assumption of proportional hazards was explored by use of cumulative residuals as described by Lin et al [13]. In case of non-proportional hazards, a figure of the HR as a continuous smooth function of age was estimated with natural cubic spline.
Individuals were considered at risk as long as they were registered as living in Denmark. Individuals who emigrated and later immigrated were censored at emigration and reincluded in the cohort at date of immigration to Denmark. To evaluate mortality by years of age, we defined age groups as follows: <2 years, 2–5 years, 6–13 years, 14–19 years, 20–29 years, and 30 years and older. The association between age and relative weight was estimated in each age group after excluding earlier deaths. We also considered the percentile of the relative birth weight as a continuous variable. The effect of this percentile was estimated with a cubic spline plot with knots specified at the 5th, 10th, 20th, 40th, 60th and 80th percentile, with the 50th percentile as reference. Cause-specific probabilities of death were estimated non-parametrically with the Aalen-Johansen estimator [14].
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
Ethical Considerations
The study was register-based and complied with the regulations and instructions set up by the Danish Data Protection Agency (Danish Protection Board approval no. 2008-54-0472). We only used anonymized data, we only present data in aggregate and anonymous form, and we neither contacted any study participants nor required any active participation from them.
Results
Overall, 877 303 men and 831 411 women contributed at least one day of observation time between January 1, 1979, and December 31, 2011. Subjects were followed for 27 million person-years and 14 167 deaths occurred in the study period (5 474 in women, 8 693 in men).
The median birth weight changed over calendar time with individuals born between 1979 and 1982 having a median birth weight of 3410 grams and individuals born between 2007 and 2011 a median birth weight of 3550 grams (Table 1). Though birth weight has increased over the past 40 years, the increase has levelled out in most recent years.
Table 1. Infant Baseline Characteristics 1979–2011.
| Total Cohort Population (percentile) | |||||||
|---|---|---|---|---|---|---|---|
| Birth cohort | Characteristics | 5th | 10th | 50th | 90th | 95th | Total N |
| 1979–1982 | Birth Weight * | 2500 | 2750 | 3410 | 4060 | 4250 | 171 782 |
| Gestational Age ** | 37 | 38 | 40 | 41 | 42 | ||
| 1983–1988 | Birth Weight * | 2500 | 2770 | 3450 | 4100 | 4300 | 295 159 |
| Gestational Age ** | 37 | 38 | 40 | 41 | 42 | ||
| 1989–1994 | Birth Weight * | 2580 | 2820 | 3500 | 4150 | 4350 | 342 287 |
| Gestational Age ** | 37 | 38 | 40 | 41 | 42 | ||
| 1995–2000 | Birth Weight * | 2620 | 2890 | 3560 | 4226 | 4430 | 334 109 |
| Gestational Age ** | 37 | 38 | 40 | 42 | 42 | ||
| 2001–2006 | Birth Weight * | 2640 | 2900 | 3560 | 4225 | 4430 | 314 106 |
| Gestational Age ** | 37 | 38 | 40 | 42 | 42 | ||
| 2007–2011 | Birth Weight * | 2640 | 2880 | 3550 | 4195 | 4390 | 251 271 |
| Gestational Age ** | 37 | 38 | 40 | 42 | 42 | ||
*grams
**weeks
In the figures of cumulated residuals (not show) we observed violations of the proportional hazard assumption in the <2 years old, but no signs of model violations after the age of 2 years. Among SGA individuals, an up to 4.5 fold increased HR was found in the first days of life followed by a strong decline to a relative stable level of about 2.0–2.5 in the age span from 90 days to 2 years, S1 Fig. Among LGA individuals, we observed decreased mortality (although fluctuating) in age <1.5 years, followed by a slightly (non-significant) increased risk in 1.5 to 2 years of age.
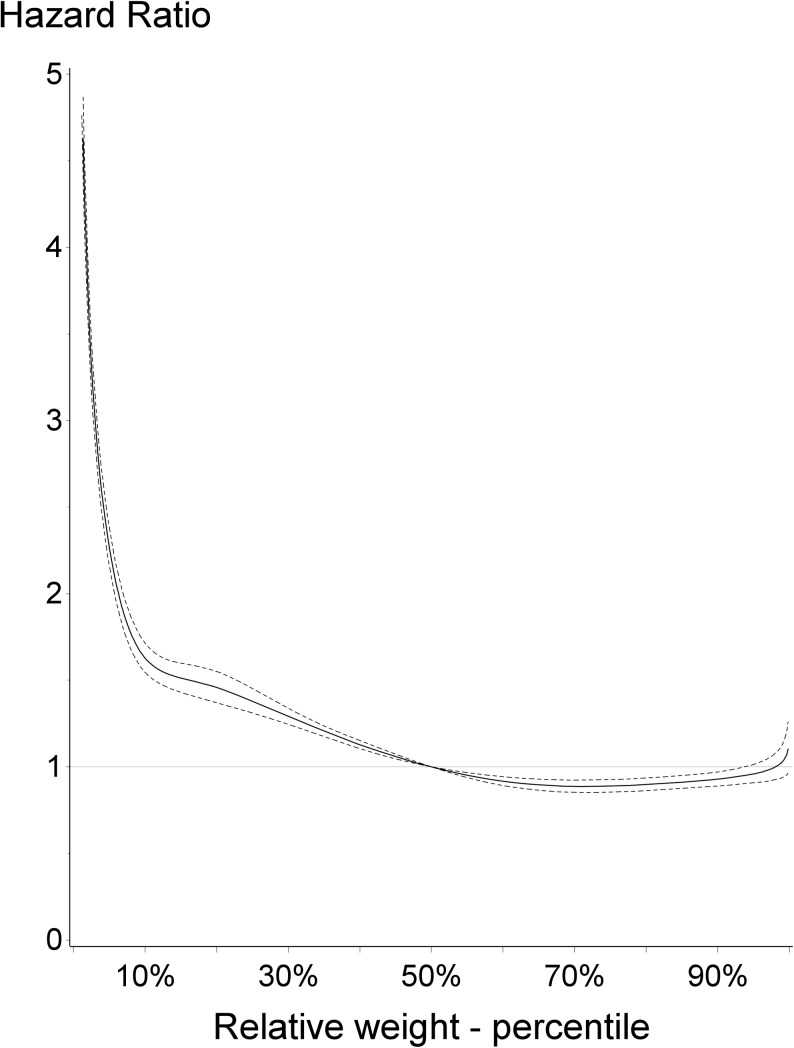
In Fig 1, the overall mortality risk by relative birth weight is presented for the entire study period. Subjects born below the 50th percentile were associated with increased risk of dying, compared with individuals born with normal weight. Conversely, individuals born within the higher percentiles, above the 50th percentile, were at modest decreased risk of dying, compared with individuals born with normal weight for gestation.
Fig 1. Overall mortality risk by relative birth weight, time period 1979–2011.
Hazard ratio and 95% confidence interval presented with 50th percentile as the reference group.
Among individuals born with extremely low birth weight, i.e. 500–1499 grams in weeks 19–28, the risk of dying dropped by more than 50% between 1979–1989 and 2000–2011, (S2 and S4 Figs). Among those born weighing 1500–1999 grams in week 36 and onwards, the cumulative mortality risk did not change much over the past 30 years (S2–S4 Figs).
As given in Table 2, individuals small for gestational age were at increased risk of dying throughout young adulthood; HR in ages <2 years: 3.47; 2–5 years 1.70; 6–13 years 1.42; 14–19 years 1.34 and in 20–29 years 1.30. Individuals large for gestational age had a significantly lower mortality risk in the age group <2 years (HR 0.78), whereas no significant association was found in older age groups. Aggregated data for reproducing Table 2, is available as supporting information, S1 Aggregated Data.
Table 2. Mortality and Relative Birth Weight by Age Group 1979–2011.
| Age group (years) | Relative Birth Weight | Person-Years of Follow-Up (1000s) | No. of Deaths | HR (95% CI) |
|---|---|---|---|---|
| <2 | SGA | 318 | 2349 | 3.47 (3.30–3.64) |
| Normal | 2611 | 5732 | 1 [Reference] | |
| LGA | 363 | 601 | 0.78 (0.72–0.85) | |
| 2–5 | SGA | 588 | 196 | 1.70 (1.45–1.99) |
| Normal | 4706 | 866 | 1 [Reference] | |
| LGA | 651 | 108 | 0.96 (0.79–1.17) | |
| 6–13 | SGA | 993 | 163 | 1.42 (1.20–1.68) |
| Normal | 7422 | 813 | 1 [Reference] | |
| LGA | 974 | 113 | 1.14 (0.93–1.38) | |
| 14–19 | SGA | 556 | 230 | 1.34 (1.16–1.54) |
| Normal | 3773 | 1133 | 1 [Reference] | |
| LGA | 432 | 122 | 0.98 (0.82–1.19) | |
| 20–29 | SGA | 458 | 264 | 1.30 (1.14–1.49) |
| Normal | 2823 | 1249 | 1 [Reference] | |
| LGA | 284 | 132 | 1.05 (0.88–1.26) | |
| ≥30 | SGA | 23 | 14 | 1.06 (0.60–1.88) |
| Normal | 139 | 77 | 1 [Reference] | |
| LGA | 13 | 5 | 0.70 (0.28–1.72) |
Abbreviation: SGA, small for gestational age; LGA, large for gestational age; HR, Hazard ratio; CI, Confidence interval
When looking at mortality risk by birth cohort in strata of age groups and with one overall reference group (Table 3), survival has improved over time in normal individuals born in years 2007–2011: HR 0.22 (95% CI, 0.19–0.25) compared to individuals born in years 1979–1982. Similarly, in absolute terms, compared to same reference group, survival improved among SGA individuals within the same time span; HR 2.68 (95% CI, 2.37–3.02) in years 1979–1982 to HR 1.28 (95% CI 1.09–1.50) in years 2007–2011. Thereupon, the relative increased mortality among SGA individuals compared to normal born in the period 2007–2011 was almost 6-fold. Increased mortality was also observed among adults (20–29 years), from 1.2-fold increased risk in years 1979–1982 to 1.6-fold increased risk in years 1989–1994.
Table 3. Mortality and Relative Birth Weight by Age and Birth Cohort Among Individuals Born Between 1979 and 2011.
| Birth cohort (years) | |||||||
|---|---|---|---|---|---|---|---|
| 1979–1982 | 1983–1988 | 1989–1994 | 1995–2000 | 2001–2006 | 2007–2011 | ||
| Age group (years) | Relative Birth Weight | HR (95% CI) | |||||
| <2 | SGA | 2.68 (2.37–3.02) | 2.38 (2.14–2.64) | 2.15 (1.93–2.40) | 1.94 (1.73–2.18) | 1.96 (1.74–2.21) | 1.28 (1.09–1.50) |
| Normal | 1 [Reference] | 0.93 (0.85–1.00) | 0.71 (0.65–0.77) | 0.46 (0.42–0.50) | 0.31 (0.28–0.34) | 0.22 (0.19–0.25) | |
| LGA | 0.71 (0.55–0.91) | 0.73 (0.61–0.88) | 0.52 (0.44–0.62) | 0.40 (0.34–0.48) | 0.22 (0.18–0.28) | 0.21 (0.16–0.29) | |
| 2–5 | SGA | 1.38 (0.95–1.99) | 1.60 (1.19–2.14) | 0.99 (0.70–1.41) | 0.91 (0.61–1.36) | 1.02 (0.67–1.54) | 0.67 (0.25–1.82) |
| Normal | 1 [Reference] | 0.94 (0.77–1.16) | 0.66 (0.53–0.81) | 0.53 (0.42–0.67) | 0.35 (0.27–0.45) | 0.27 (0.16–0.44) | |
| LGA | 0.70 (0.37–1.32) | 0.85 (0.55–1.32) | 0.75 (0.51–1.11) | 0.52 (0.34–0.79) | 0.29 (0.17–0.51) | 0.50 (0.20–1.21) | |
| 6–13 | SGA | 1.25 (0.87–1.78) | 0.99 (0.71–1.37) | 1.10 (0.80–1.50) | 0.73 (0.48–1.12) | 0.71 (0.31–1.60) | |
| Normal | 1 [Reference] | 0.90 (0.75–1.09) | 0.55 (0.45–0.68) | 0.46 (0.37–0.57) | 0.35 (0.24–0.51) | ||
| LGA | 0.90 (0.53–1.53) | 1.27 (0.90–1.79) | 0.74 (0.51–1.07) | 0.38 (0.24–0.61) | 0.26 (0.10–0.70) | ||
| 14–19 | SGA | 1.13 (0.85–1.49) | 1.11 (0.88–1.40) | 1.12 (0.87–1.44) | NA | ||
| Normal | 1 [Reference] | 0.82 (0.71–0.94) | 0.68 (0.58–0.79) | 0.49 (0.32–0.75) | |||
| LGA | 1.07 (0.75–1.55) | 0.90 (0.67–1.20) | 0.57 (0.41–0.80) | 0.13 (0.02–0.92) | |||
| 20–29 | SGA | 1.20 (0.99–1.46) | 1.20 (0.99–1.47) | 1.61 (1.04–2.51) | |||
| Normal | 1 [Reference] | 0.90 (0.80–1.02) | 0.73 (0.56–0.95) | ||||
| LGA | 1.00 (0.77–1.32) | 1.05 (0.82–1.35) | 0.45 (0.19–1.09) | ||||
| >30 | SGA | 1.06 (0.60–1.87) | |||||
| Normal | 1 [Reference] | ||||||
| LGA | 0.69 (0.28–1.72) | ||||||
Abbreviation: SGA, small for gestational age; LGA, large for gestational age; HR, Hazard ratio; CI, Confidence interval; NA, not applicable
The cumulative mortality by any cause of death was estimated using the Aalen-Johansen estimator between years 1979 and 2011. Risk of death stratified by relative weight (SGA, Normal, LGA) and by disease group is shown in more detail, S5 Fig. Briefly, before 1 year of age SGA individuals were at 1.5% increased risk of dying, while the mortality risks among normal and LGA were much lower, 0.41% and 0.42% respectively. Besides the first year of life being the highest risk of dying, risks were still increasing among SGA individuals up to age 30 years, 2.8%. Among normal and LGA individuals the corresponding risks were 1.4% and 1.3% respectively.
Individuals born SGA were at increased risk of dying, in all disease categories but malignant neoplasms, Table 4. The strongest risks were observed for congenital malformations (HR, 3.63; 95% CI, 3.40–3.87) and perinatal conditions (HR, 3.44; 95% CI, 3.20–3.70). Statistically significant increased risks were also observed for dying from respiratory disease, heart diseases and digestive diseases, Table 4. Individuals born LGA were at decreased risk of dying from congenital malformations and perinatal conditions (HR, 0.90; 95% CI, 0.79–1.02 vs. HR, 0.64; 95% CI, 0.55–0.74). Noteworthy, increased risk of dying from malignant neoplasms was observed among LGA individuals, HR, 1.21; 95% CI, 1.01–1.46.
Table 4. Mortality by Relative Gestational Weight and Cause of Death 1979–2011.
| Cause of Death | Relative Birth Weight* | No. of Deaths | HR (95% CI) |
|---|---|---|---|
| Infection | SGA | 133 | 1.52 (1.22–1.90) |
| Normal | 329 | 1 [Reference] | |
| LGA | 24 | 0.74 (0.48–1.12) | |
| Malignant Neoplasm | SGA | 408 | 0.99 (0.88–1.12) |
| Normal | 1277 | 1 [Reference] | |
| LGA | 128 | 1.21 (1.01–1.46) | |
| Heart Disease | SGA | 113 | 1.56 (1.21–2.02) |
| Normal | 189 | 1 [Reference] | |
| LGA | 21 | 1.27 (0.80–2.00) | |
| Respiratory Disease | SGA | 178 | 1.84 (1.51–2.25) |
| Normal | 327 | 1 [Reference] | |
| LGA | 32 | 1.05 (0.73–1.52) | |
| Digestive Disease | SGA | 80 | 1.56 (1.15–2.11) |
| Normal | 141 | 1 [Reference] | |
| LGA | 14 | 1.18 (0.68–2.05) | |
| Congenital malformation | SGA | 1839 | 3.63 (3.40–3.87) |
| Normal | 2488 | 1 [Reference] | |
| LGA | 260 | 0.90 (0.79–1.02) | |
| Perinatal Condition | SGA | 1729 | 3.44 (3.20–3.70) |
| Normal | 2443 | 1 [Reference] | |
| LGA | 197 | 0.64 (0.55–0.74) | |
| Accident, Suicide, Homicide | SGA | 1787 | 1.21 (1.14–1.29) |
| Normal | 3711 | 1 [Reference] | |
| LGA | 307 | 1.00 (0.89–1.12) | |
| Other | SGA | 1245 | 1.72 (1.60–1.84) |
| Normal | 2864 | 1 [Reference] | |
| LGA | 230 | 0.79 (0.69–0.90) | |
| Unknown | SGA | 77 | 1.42 (1.06–1.89) |
| Normal | 202 | 1 [Reference] | |
| LGA | 14 | 0.62 (0.36–1.07) |
Abbreviation: SGA, small for gestational age; LGA, large for gestational age; HR, Hazard ratio; CI, Confidence interval
*Person-Years of Follow-Up (1000s): SGA, 7546; Normal, 29137; LGA, 2929
When we modelled the effect of relative weight as a function of age stratified by cause of death, SGA born individuals were at strongest increased risk of dying from infection; heart disease; respiratory disease; perinatal conditions; congenital malformation; accidents, suicide, and homicide during the first years of life. LGA-born individuals had a decreased risk of dying from congenital malformations and perinatal condition during the first years of life. Further, in the LGA-born individuals, the risk of dying from malignant neoplasms was highest between age 6 and 13 years, (HR, 1.56; 95% CI, 1.13–2.16). Increased risk of dying from digestive disease (HR, 6.80; 95% CI, 2.11–21.91), (2–5 years old) and heart disease (HR, 2.37; 95% CI, 1.04–5.44), (14–19 years old) was also observed, S1 Table.
Discussion
Our study observed high mortality among individuals born small for gestational age between years 1979 and 2011. With a total of 1.7 million live-born children followed for 27 million person-years during a 30-year period, our study had unique power to describe the long-term survival among individuals born small and large for gestational age. This also allowed us to investigate how mortality decreased over calendar time independently of birth weight. In the USA reports have shown that prenatal and neonatal care improved during the 90’s [2]. Noteworthy, the change in perinatal and neonatal care can be explained by increased use of caesarean section, steroid treatment, and respiratory aid. The North American study reported declining mortality among infants born with a birth weight below 1500 grams until 1995, but no further decline at end of follow-up in 1999. In the present study, we observed a steady decline in mortality over time among children in all relative weight groups, all the way up to the end of follow-up in 2011.
We are not aware of any other study that has reported on the association between being born small or large for gestational age and mortality risk up to 30 years after birth. Previous studies on this topic have primarily explored the association of birth weight and gestational age separately. As an example a study based on the Swedish medical birth registry reported increased mortality in infancy, early childhood and young adulthood among individuals born at 37–38 weeks of gestation [15]. In the present study we took this a step further and combined gestational age with weight. It is well known that SGA infants are at higher mortality risk than non-SGA infants or infants born within the normal weight span [16;17]. We found this increased mortality risk to be present in all strata of year of birth and age. The mortality risks among SGA individuals were 4.5-folded the first days of life, with a strong reduced risk the following 90 days, and further decreased up to adulthood. The increased mortality in early age may be expected; but the elevated mortality up to adulthood however, was somewhat surprising.
Mortality risks decreased over calendar time, but were still strong within each birth cohort among individuals born small for gestational age. Interestingly, though advanced neonatal care and improved survival of SGA individuals likely result in different long-term outcome for individuals born in the 1970’s and 2011, we observed similar increased risks in comparing SGA individuals to normal individuals with the highest difference in the later time period. A possible explanation to this could be that care of those born extremely early and/or extremely small have improved, consequently more extreme individuals survive their first day in life and are therefore more frequently included in the later cohorts. Thereby, one can argue that relative weight is a rough estimate, suggesting that the definition of SGA should be reconsidered.
In general, SGA born individuals require more intervention, longer stay at hospital with neonatal care and are at risk for certain conditions compared to individuals born normal weight for gestational age. Preterm SGA and term SGA born individuals face different medical conditions. Previous report discovered that term born SGA individuals are likely related to sociodemographic status while preterm are related to chronic hypertension and preeclampsia [18]. Also discovered in the same study, term SGA individuals are more likely to face prenatal deaths while preterm neonatal death. In the present study we do not differ between preterm and term SGA individuals. With regard to that our study is restricted to only live born individuals, it is possible that our findings corresponds to SGA individuals born preterm rather than term.
In a previous study the authors observed a 2-fold increased risk of prenatal and neonatal death among term and post–term individuals in the 15th birth weight percentile, compared with the previously used 10th percentile [9]. In our study we observed a 2-fold increased mortality risk below the 7.5th percentile. We took into account birth cohort, gender and gestational age when setting the cut-offs for relative birth weight, and this difference in study design and study population could explain the dissimilarities comparing our results with earlier published results.
Some studies have reported an U-shaped association between birth weight and all-cause mortality [1]. Overall we observed a decrease in mortality risk within each birth cohort among individuals born large for gestational age, with the strongest effect in infants and toddlers. This contrasts with earlier studies observing an increased risk or no significant risk difference between LGA and normal individuals [9;16]. It is however possible that our study including individuals below 40 years of age, is too young to show metabolic and cardiovascular consequences of LGA. An earlier Norwegian study investigated survival among post-term infants. The authors found post-term infants to be at increased risk of dying before one year of age. Further, among boys, increased risks were observed also in late childhood [8]. We have no immediate explanation for these differences in our findings. However when investigating cause-specific mortality, we observed that malignant neoplasms was associated with increased mortality risk among LGA individuals; this partly confirms earlier findings of increased cancer mortality among adults per kg increased birth weight [4].
The major strengths of the present study are the prospective study design, the long follow-up, and the large sample size. The registry-based design allowed us to assemble a nationwide cohort with independent ascertainment of exposure and outcome and complete follow-up of all infants with accurate longitudinal information. In Denmark health care is free of charge and encouraged for all pregnant women irrespective of income and immigrant status. We used the Danish CRS [10;19] and thereby covered complete information on maternal identity among women born in Denmark in April 1935 or later and all their children born in Denmark in 1960 or later. Full information on immigrations, emigrations and permanent residence by municipality and full address in Denmark was covered from 1977 and onwards [19]. There are a few drawbacks with the Danish MBR [12]. In the years 1973–1978 the weights were reported rounded by 500 gram [20]; to avoid inducing non-differential misclassification we excluded information from these years. The use of digital scales replaced the mechanical scales in 1996 and thereafter weight was no longer presented by 50- and 100-gram values. The possible impact on the calendar time analysis was reduced by restricting the classification of the overall relative weight to the years 1979 to 2011. Further, during 1973–79 gestational age was coded in intervals with regard to the number of weeks counted as preterm. In the following years gestational age was registered as weeks or days. To unify the coding we therefore used early registered gestational age categories for the entire calendar time in our classification of gestational age. Further we had no possibility to distinguish between gestational age measures (last menstrual period or ultrasound dates), but believe this has had little impact on our study. A few problems regarding data in the Cause of Death Registry [11] have been observed. In most of our analyses we used all-cause of death where possible misclassification or discontinuity in registrations would have no impact on the results.
Conclusion
In conclusion, our findings suggest that weight by gestational age at birth is an important predictor for mortality from birth until approximately at least 30 years of age. Multiple causes of death were associated with being born small for gestational age during this age span.
Supporting Information
(ZIP)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOC)
Abbreviations
- CI
confidence interval
- CRS
Civil Registration System
- HR
hazard ratio
- ICD
International Classification of Diseases
- LGA
large for gestation age
- MBR
Medical Birth Registry
- SGA
small for gestational age
Data Availability
All relevant data are within the paper and its Supporting Information files. Aggregated data for reproducing Table 2 is available as supporting information, S1 Aggregated Data.
Funding Statement
Dr. ECM Wennerström is supported by an Oak Foundation Fellowship (http://www.oakfnd.org), grant number OCAY-12-319. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baker JL, Olsen LW, Sorensen TI. Weight at birth and all-cause mortality in adulthood. Epidemiology 2008. March;19(2):197–203. 10.1097/EDE.0b013e31816339c6 [DOI] [PubMed] [Google Scholar]
- 2. Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002. July;110(1 Pt 1):143–51. [DOI] [PubMed] [Google Scholar]
- 3. Malin G, Morris R, Riley R, Teune M, Khan K. When is birthweight at term abnormally low? A systematic review and meta-analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG 2014. January 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011. June;40(3):647–61. 10.1093/ije/dyq267 [DOI] [PubMed] [Google Scholar]
- 5. Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 2000. May;71(5 Suppl):1344S–52S. [DOI] [PubMed] [Google Scholar]
- 6. Barker DJ. Fetal origins of coronary heart disease. BMJ 1995. July 15;311(6998):171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345:e7976 10.1136/bmj.e7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA 2008. March 26;299(12):1429–36. 10.1001/jama.299.12.1429 [DOI] [PubMed] [Google Scholar]
- 9. Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr 2010. April;99(4):550–5. 10.1111/j.1651-2227.2009.01674.x [DOI] [PubMed] [Google Scholar]
- 10. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011. July;39(7 Suppl):22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 11. Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011. July;39(7 Suppl):26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 12. Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998. June;45(3):320–3. [PubMed] [Google Scholar]
- 13. Lin DY, Wei L-J, Ying Z. Checking the Cox model with cumulative sums of martingale-based residual. 80, 557–572. 1993. Biometrika, Biometrika Trust. [Google Scholar]
- 14. Aalen O, Johansen S. An Empirical Transition Matrix for Non-homogeneous Markov Chains Based on Censored Observations. Scand J Statist 1978; 5:141–50. [Google Scholar]
- 15. Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology 2013. March;24(2):270–6. 10.1097/EDE.0b013e318280da0f [DOI] [PubMed] [Google Scholar]
- 16. Vashevnik S, Walker S, Permezel M. Stillbirths and neonatal deaths in appropriate, small and large birthweight for gestational age fetuses. Aust N Z J Obstet Gynaecol 2007. August;47(4):302–6. [DOI] [PubMed] [Google Scholar]
- 17. De Jesus LC, Pappas A, Shankaran S, Li L, Das A, Bell EF, et al. Outcomes of small for gestational age infants born at <27 weeks' gestation. J Pediatr 2013. July;163(1):55–60. 10.1016/j.jpeds.2012.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ota E, Ganchimeg T, Morisaki N, Vogel JP, Pileggi C, Ortiz-Panozo E, et al. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: secondary analyses of the WHO Multi-Country Survey on Maternal and Newborn Health. PLoS One 2014;9(8):e105155 10.1371/journal.pone.0105155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 2006. November;53(4):441–9. [PubMed] [Google Scholar]
- 20. Rogvi R, Mathiasen R, Greisen G. Defining smallness for gestational age in the early years of the Danish Medical Birth Registry. PLoS One 2011;6(1):e16668 10.1371/journal.pone.0016668 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Aggregated data for reproducing Table 2 is available as supporting information, S1 Aggregated Data.