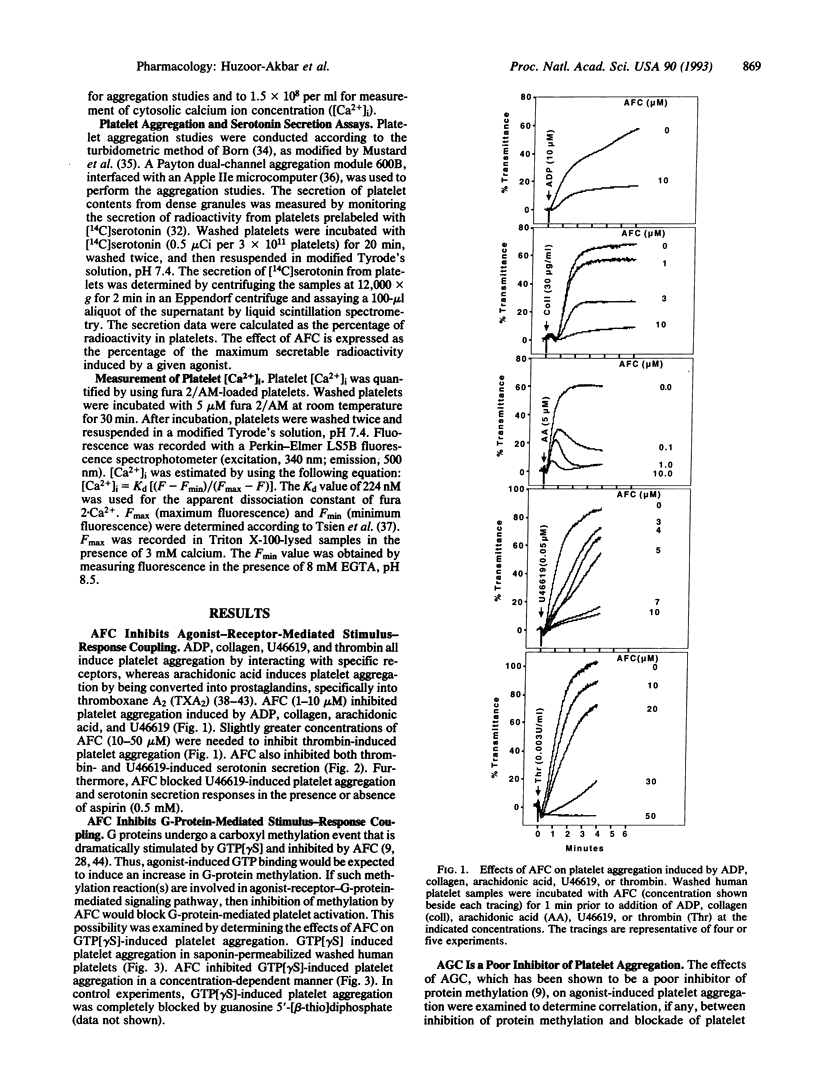
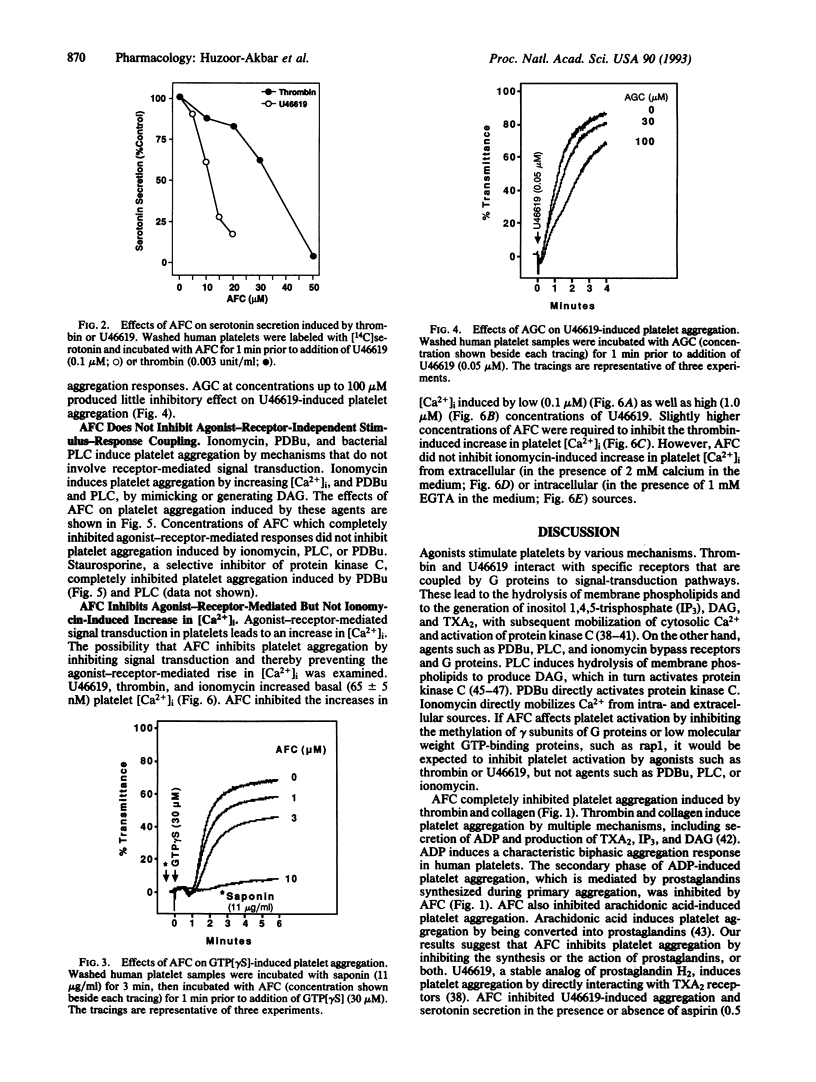
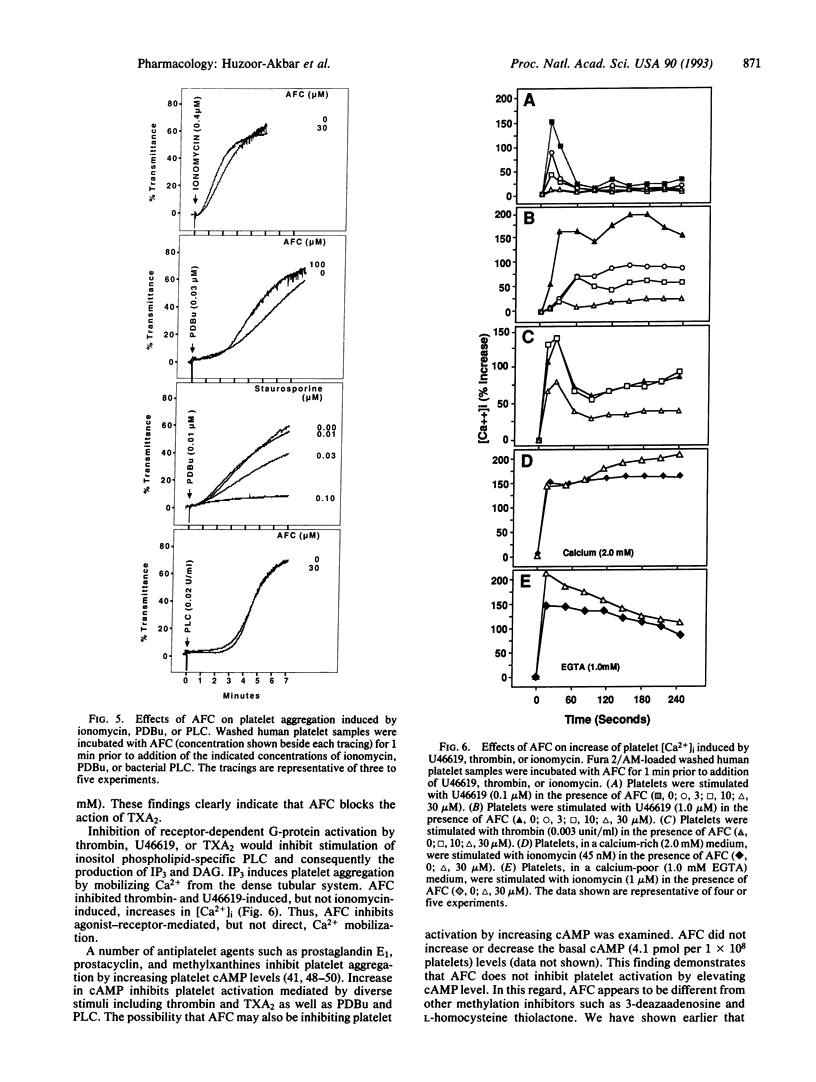
Abstract
Signal transduction components, including the Ras superfamily of low molecular weight GTP-binding proteins and the gamma subunits of heterotrimeric G proteins, are reversibly carboxyl methylated at C-terminal prenylcysteine residues. We have previously shown that the prenylcysteine analog N-acetyl-S-trans,trans-farnesyl-L-cysteine (AFC) inhibits carboxyl methylation of these proteins in human platelets. Here we show that concentrations of AFC that inhibit Ras carboxyl methylation (10-50 microM) also block responses to agonists such as ADP, collagen, arachidonic acid, U46619 (a stable analog of prostaglandin H2), thrombin, and guanosine 5'-[gamma-thio]triphosphate. AFC does not inhibit aggregation induced by effectors such as ionomycin, phorbol 12,13-dibutyrate, and bacterial phospholipase C that bypass G proteins to activate platelets at the level of cytosolic Ca2+ concentration and protein kinase C. These findings indicate that AFC inhibits agonist-receptor-mediated signal transduction in human platelets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar H., Navran S. S., Chang J., Miller D. D., Feller D. R. Investigation of the effects of phospholipase C on human platelets: evidence that aggregation induced by phospholipase C is independent of prostaglandin generation, released ADP and is modulated by cyclic AMP. Thromb Res. 1982 Aug 15;27(4):405–417. doi: 10.1016/0049-3848(82)90058-5. [DOI] [PubMed] [Google Scholar]
- Anwer K., Wallace D., Romstedt K., Huzoor-Akbar Human platelet activation by bacterial phospholipase C: mechanism of inhibition by flurazepam. Thromb Res. 1988 Jan 15;49(2):225–239. doi: 10.1016/0049-3848(88)90216-2. [DOI] [PubMed] [Google Scholar]
- Araki S., Kaibuchi K., Sasaki T., Hata Y., Takai Y. Role of the C-terminal region of smg p25A in its interaction with membranes and the GDP/GTP exchange protein. Mol Cell Biol. 1991 Mar;11(3):1438–1447. doi: 10.1128/mcb.11.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Aksamit R. R. Guanine nucleotide-dependent carboxyl methylation of mammalian membrane proteins. J Biol Chem. 1988 Nov 5;263(31):15864–15867. [PubMed] [Google Scholar]
- Bokoch G. M., Quilliam L. A. Guanine nucleotide binding properties of rap1 purified from human neutrophils. Biochem J. 1990 Apr 15;267(2):407–411. doi: 10.1042/bj2670407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. L., Holmsen H., Adelstein R. S. Thrombin-stimulated myosin phosphorylation in intact platelets and its possible involvement secretion. Thromb Haemost. 1977 Dec 15;38(4):984–989. [PubMed] [Google Scholar]
- Farrell F. X., Ohmstede C. A., Reep B. R., Lapetina E. G. cDNA sequence of a new ras-related gene (rap2b) isolated from human platelets with sequence homology to rap2. Nucleic Acids Res. 1990 Jul 25;18(14):4281–4281. doi: 10.1093/nar/18.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Gerrard J. M., Carroll R. C. Stimulation of platelet protein phosphorylation by arachidonic acid and endoperoxide analogs. Prostaglandins. 1981 Jul;22(1):81–94. doi: 10.1016/0090-6980(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Maturation of isoprenylated proteins in Saccharomyces cerevisiae. Multiple activities catalyze the cleavage of the three carboxyl-terminal amino acids from farnesylated substrates in vitro. J Biol Chem. 1992 May 25;267(15):10457–10464. [PubMed] [Google Scholar]
- Huzoor-Akbar, Anwer K. Thrombin-induced abnormal platelet activation in spontaneously hypertensive rats is linked with phosphoinositides turnover and phosphorylation of 47,000 and 20,000 dalton proteins. Thromb Res. 1988 Jan 1;49(1):5–21. doi: 10.1016/0049-3848(88)90355-6. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Patel S., Kokrady S. S., Witiak D. T., Newman H. A., Feller D. R. Effects of clofibrate and 6-substituted chroman analogs on human platelet function: mechanism of inhibitory action. Biochem Pharmacol. 1981 Jul 15;30(14):2013–2020. doi: 10.1016/0006-2952(81)90213-6. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Romstedt K. 3-Deazaadenosine and L-homocysteine inhibit human platelet activation induced by arachidonic acid, U46619 and phospholipase C. Thromb Res. 1984 Nov 15;36(4):369–376. doi: 10.1016/0049-3848(84)90329-3. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Romstedt K., Manhire B. Computerized aggregation instruments: a highly efficient and versatile system for acquisition, quantitation, presentation and management of platelet aggregation data. Thromb Res. 1983 Nov 1;32(3):335–341. doi: 10.1016/0049-3848(83)90169-x. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Winegar D. A., Lapetina E. G. Carboxyl methylation of platelet rap1 proteins is stimulated by guanosine 5'-(3-O-thio)triphosphate. J Biol Chem. 1991 Mar 5;266(7):4387–4391. [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Takai Y. Enhancement of collagen-induced phosphoinositide turnover by thromboxane A2 analogue through Ca2+ mobilization in human platelets. FEBS Lett. 1985 Nov 11;192(1):104–108. doi: 10.1016/0014-5793(85)80052-1. [DOI] [PubMed] [Google Scholar]
- Kim S., Mizoguchi A., Kikuchi A., Takai Y. Tissue and subcellular distributions of the smg-21/rap1/Krev-1 proteins which are partly distinct from those of c-ras p21s. Mol Cell Biol. 1990 Jun;10(6):2645–2652. doi: 10.1128/mcb.10.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G. The signal transduction induced by thrombin in human platelets. FEBS Lett. 1990 Aug 1;268(2):400–404. doi: 10.1016/0014-5793(90)81293-w. [DOI] [PubMed] [Google Scholar]
- Lazarowski E. R., Lapetina E. G. Activation of platelet phospholipase C by fluoride is inhibited by elevation of cyclic AMP. Biochem Biophys Res Commun. 1989 Jan 31;158(2):440–444. doi: 10.1016/s0006-291x(89)80067-1. [DOI] [PubMed] [Google Scholar]
- Leonard S., Beck L., Sinensky M. Inhibition of isoprenoid biosynthesis and the post-translational modification of pro-p21. J Biol Chem. 1990 Mar 25;265(9):5157–5160. [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M., Repko E. M., Erdman R. A. Post-translational modification of low molecular mass GTP-binding proteins by isoprenoid. J Biol Chem. 1990 Feb 5;265(4):2148–2155. [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kawata M., Kondo J., Teranishi Y., Takai Y. Molecular cloning of smg p21B and identification of smg p21 purified from bovine brain and human platelets as smg p21B. Biochem Biophys Res Commun. 1990 Jan 30;166(2):1010–1016. doi: 10.1016/0006-291x(90)90911-6. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L., Ohmstede C. A., Lapetina E. G. Properties of the exchange rate of guanine nucleotides to the novel rap-2B protein. Biochem Biophys Res Commun. 1990 Aug 31;171(1):319–324. doi: 10.1016/0006-291x(90)91395-9. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Kikuchi A., Yamamoto K., Kawata M., Kondo J., Takai Y. Identification of a platelet Mr 22,000 GTP-binding protein as the novel smg-21 gene product having the same putative effector domain as the ras gene products. Biochem Biophys Res Commun. 1988 Dec 15;157(2):670–676. doi: 10.1016/s0006-291x(88)80302-4. [DOI] [PubMed] [Google Scholar]
- Ohmstede C. A., Farrell F. X., Reep B. R., Clemetson K. J., Lapetina E. G. RAP2B: a RAS-related GTP-binding protein from platelets. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6527–6531. doi: 10.1073/pnas.87.17.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Chardin P., Lerosey I., Olofsson B., Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the 'effector' region. Oncogene. 1988 Aug;3(2):201–204. [PubMed] [Google Scholar]
- Pérez-Sala D., Tan E. W., Cañada F. J., Rando R. R. Methylation and demethylation reactions of guanine nucleotide-binding proteins of retinal rod outer segments. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3043–3046. doi: 10.1073/pnas.88.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E., Horne W. C. Ionomycin can elevate intraplatelet Ca2+ and activate phospholipase A without activating phospholipase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):393–397. doi: 10.1016/0006-291x(84)90426-1. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Siess W., Winegar D. A., Lapetina E. G. Rap1-B is phosphorylated by protein kinase A in intact human platelets. Biochem Biophys Res Commun. 1990 Jul 31;170(2):944–950. doi: 10.1016/0006-291x(90)92182-y. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Butrynski J. E., Gautam N., Unson C. G., Spiegel A. M. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J Biol Chem. 1991 Mar 25;266(9):5363–5366. [PubMed] [Google Scholar]
- Spiegel A. M. G proteins in cellular control. Curr Opin Cell Biol. 1992 Apr;4(2):203–211. doi: 10.1016/0955-0674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990 Sep 25;265(27):16248–16254. [PubMed] [Google Scholar]
- Stimmel J. B., Deschenes R. J., Volker C., Stock J., Clarke S. Evidence for an S-farnesylcysteine methyl ester at the carboxyl terminus of the Saccharomyces cerevisiae RAS2 protein. Biochemistry. 1990 Oct 16;29(41):9651–9659. doi: 10.1021/bi00493a021. [DOI] [PubMed] [Google Scholar]
- Takahara K., Murray R., FitzGerald G. A., Fitzgerald D. J. The response to thromboxane A2 analogues in human platelets. Discrimination of two binding sites linked to distinct effector systems. J Biol Chem. 1990 Apr 25;265(12):6836–6844. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker C., Lane P., Kwee C., Johnson M., Stock J. A single activity carboxyl methylates both farnesyl and geranylgeranyl cysteine residues. FEBS Lett. 1991 Dec 16;295(1-3):189–194. doi: 10.1016/0014-5793(91)81415-5. [DOI] [PubMed] [Google Scholar]
- Volker C., Miller R. A., McCleary W. R., Rao A., Poenie M., Backer J. M., Stock J. B. Effects of farnesylcysteine analogs on protein carboxyl methylation and signal transduction. J Biol Chem. 1991 Nov 15;266(32):21515–21522. [PubMed] [Google Scholar]



