Significance
Cation/proton antiporters (CPAs) are essential to life. The sodium/proton exchanger (NHE) branch of the CPA family has been studied exhaustively and is an important drug target; however, much less is known about the recently discovered NHA branch, represented by two genes in both humans and flies. Here we show that sodium/hydrogen antiporter (NHA) function is essential to life, and that both NHAs protect against salt stress. Their transport mechanisms are radically different, however, suggesting that function cannot be inferred from structural similarity. Although NHA2 was found to be a Na+/H+ exchanger as expected, NHA1 was seen to act as an electroneutral H+/Cl- cotransporter. This is an important finding for future studies of these transporters.
Keywords: Drosophila, physiology, transport, exchanger, genetics
Abstract
The cation/proton antiporter (CPA) family includes the well-known sodium/proton exchanger (NHE; SLC9A) family of Na+/H+ exchangers, and the more recently discovered and less well understood CPA2s (SLC9B), found widely in living organisms. In Drosophila, as in humans, they are represented by two genes, Nha1 (Slc9b1) and Nha2 (Slc9b2), which are enriched and functionally significant in renal tubules. The importance of their role in organismal survival has not been investigated in animals, however. Here we show that single RNAi knockdowns of either Nha1 or Nha2 reduce survival and in combination are lethal. Knockdown of either gene alone results in up-regulation of the other, suggesting functional complementation of the two genes. Under salt stress, knockdown of either gene decreases survival, demonstrating a key role for the CPA2 family in ion homeostasis. This is specific to Na+ stress; survival on K+ intoxication is not affected by sodium/hydrogen antiporter (NHA) knockdown. A direct functional assay in Xenopus oocytes shows that Nha2 acts as a Na+/H+ exchanger. In contrast, Nha1 expressed in Xenopus oocytes shows strong Cl− conductance and acts as a H+-Cl− cotransporter. The activity of Nha1 is inhibited by chloride-binding competitors 4,4′-diiso-thiocyano-2,2′-disulfonic acid stilbene and 4,4′-dibenzamido-2,2′-stilbenedisulphonate. Salt stress induces a massive up-regulation of NHA gene expression not in the major osmoregulatory tissues of the alimentary canal, but in the crop, cuticle, and associated tissues. Thus, it is necessary to revise the classical view of the coordination of different tissues in the coordination of the response to osmoregulatory stress.
The NHA Gene Family
Ionic homeostasis is essential for life and requires a significant fraction of an organism’s total energy budget. Primary ion-motive ATPases provide electrochemical ion gradients to drive an array of channels, cotransporters, and antiporters. The cation/proton antiporter (CPA) family is ubiquitous and best known for the NHE, or CPA1, branch exemplified by the classical Na+/H+ exchanger NHE1 (1, 2), which is a target for the potassium-sparing diuretic amiloride in the treatment of hypertension and congestive heart failure. More recently discovered are the sodium/hydrogen antiporters (NHAs), a subbranch of the CPA2 family, which is much less well understood (3, 4). Originally studied in bacteria, yeast, and plants (5, 6), this subbranch in humans includes two NHA genes in tandem: NHA1 (SLC9B1), which is testis-specific, and NHA2 (SLC9B2), which is ubiquitous (7, 8).
In osteoclasts, NHA2 colocalizes with the V-ATPase a3 subunit and the lysosomal marker LAMP2 (9), and resides in the plasma membrane of Madin–Darby canine kidney (MDCK) cells (7). In the pancreas, NHA2 is necessary for insulin secretion but localizes not to insulin-containing vesicles, but rather to transferrin-positive endocytic vesicles (4, 10). NHA2 also has been linked to hypertension (8). Thus, NHAs are multifunctional proteins expressed in a wide range of subcellular domains; however, a mechanistic understanding of the roles of NHAs in animals is lacking in comparison with their exhaustively studied NHE relatives, and a simple animal model is clearly needed.
NHAs in Drosophila
Drosophila melanogaster offers potent advantages as such a simple model system. Along with a sequenced genome and uniquely potent genetic tools, functional transport readouts can be provided by the simple renal system (11, 12). As in humans and the malaria vector mosquito Anopheles gambiae (13, 14), the fruit fly Drosophila has two NHA genes, Nha1 and Nha2 (5). The NHA family is relatively divergent, however, and asserting orthology between human and insect NHAs is difficult (5); in terms of sequence identity, insect NHA1 is slightly more similar to human NHA2, and vice versa. Both Drosophila NHAs are widely expressed but at particularly high levels in epithelia, suggesting a role in organismal ion homeostasis (15, 16). Given that insect epithelia are energized by an apical plasma membrane H+ V-ATPase (17, 18), the NHAs have been hypothesized to act as the partner exchangers, as predicted by Wieczorek and coworkers (17, 19), that colocalize with V-ATPase and use the proton electrochemical gradient to achieve transepithelial transport of sodium and potassium, similar to the position in mammalian MDCK cells (20).
Consistent with this, overexpression of GFP-tagged Nha1 or Nha2 labels the same apical membrane as the V-ATPase in the Malpighian (renal) tubule, a model epithelium in which transport and control mechanisms are conveniently studied (21, 22), and RNAi against one of the exchangers affects fluid secretion (15). Thus, Drosophila provides an ideal system in which to investigate the roles of NHAs in multicellular animals.
In this paper, we show that individual knockdowns of either Nha1 or Nha2 are deleterious, and that dual knockdowns are lethal, and thus that NHA activity is essential for survival. We further show that NHAs are essential for the response to salt stress, specifically protecting against Na+ rather than K+, and that this is achieved by up-regulating the expression of both genes. The two NHAs have different transport properties, however; whereas NHA2 behaves as an electroneutral Na+/H+ exchanger, NHA1 is best modeled as a H+-Cl− cotransporter.
Results and Discussion
NHAs Are Highly Enriched in Epithelia.
The online atlas of gene expression, FlyAtlas.org, shows that both Nha1 and Nha2 are widely expressed, but particularly in the epithelia of the alimentary canal (15, 23, 24). To validate this pattern of gene expression, we performed quantitative real-time PCR, which demonstrated consistent enrichment levels at least as high as those seen in the Affymetrix-derived FlyAtlas (Fig. S1). Thus, in terms of investment in mRNA, NHAs must play major roles in epithelial function. Interestingly, a broader meta-analysis of the major transporting epithelia of Drosophila (salivary glands, midgut tubules, and hindgut), showed that one of Nhe1, Nha1, or Nha2 was always very highly coexpressed with V-ATPase, implying that both branches of the CPA family can play epithelial roles in Drosophila (16).
Fig. S1.
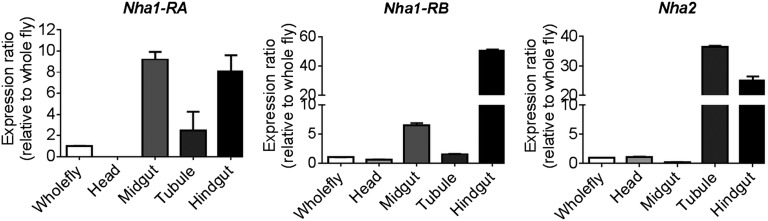
NHA expression is predominantly in epithelia of the alimentary canal. Quantitative real-time PCR data for the two major transcripts of Nha1 and Nha2, relative to expression in the whole fly. Where error bars are not visible, they are too small to plot.
A reverse genetic understanding of the roles of NHAs requires mutants of both loci. However, classical null alleles may be lethal, and cannot be targeted to specific tissues. In Drosophila, the GAL4/UAS binary system allows targeting of RNAi to cell populations of choice. Accordingly, we purchased available lines from the Vienna Drosophila Research Centre and also created our own using the prize vector, and screened them for knockdown efficiency using quantitative real-time PCR. We identified an insertional mutant of Nha1, along with RNAi alleles of Nha1 and Nha2, that each showed 60–80% knockdown when RNAi was expressed in whole flies with the actin-GAL4 driver line (Fig. 1). Although these hypomorphic alleles are not true nulls, their viability allows physiological experimentation on NHA mutant animals.
Fig. 1.
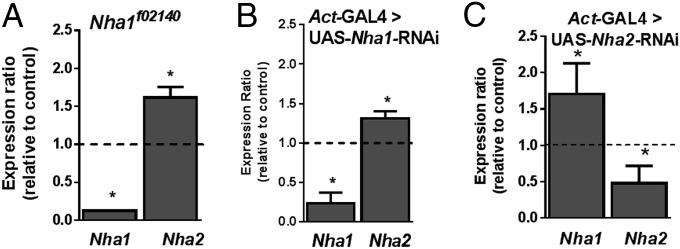
Knockdown of either NHA elicits up-regulation of the other, suggesting functional complementation. (A) Knockdown of Nha1 with an insertional allele, Nha1f02140. (B) Knockdown of Nha1 with RNAi, driven by actin-GAL4 at 22 °C. (C) Knockdown of Nha2 with RNAi, driven by actin-GAL4 at 22 °C. Significant differences (two-tailed Student’s t test; n = 4) are marked with an asterisk.
NHAs Show Compensatory Expression.
A problem in reverse genetic analysis of any multigene family is that knockdown of any one member may be rescued either by functional redundancy with other family members or by up-regulation of expression of other family members. To test whether such compensatory gene regulation occurred, we measured gene expression of the other gene in the Nha1 and Nha2 knockdowns (Fig. 1). We found that such compensatory regulation indeed occurs, suggesting coordinated control of expression of the NHA gene family.
NHAs Are Essential for Life.
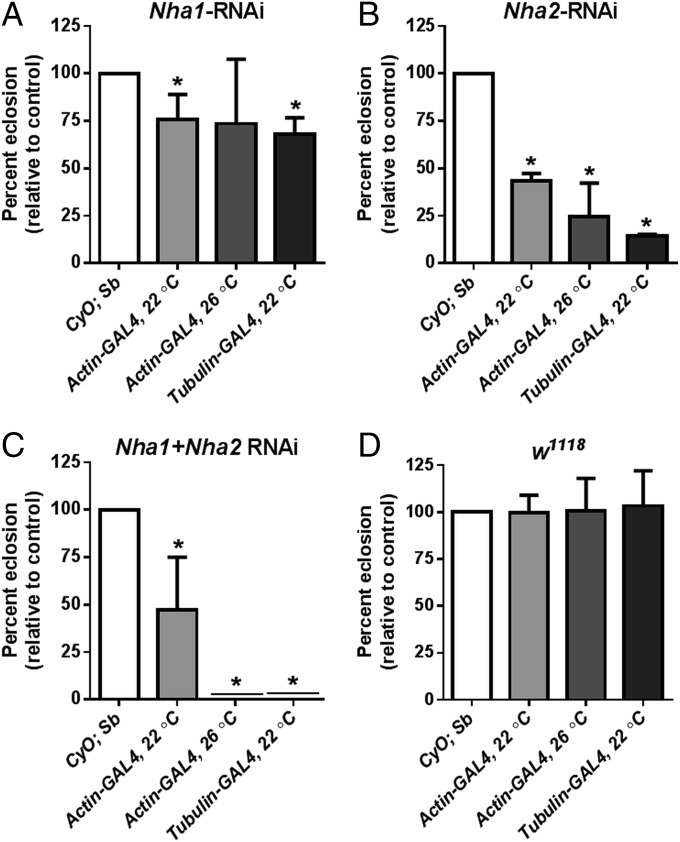
What is the impact of global RNAi-mediated NHA knockdown? We assessed this with both single and double knockdowns, using the actin-GAL4 and stronger tubulin-GAL4 drivers, the latter in the presence of UAS-dicer2, a double-stranded RNA processing enzyme that increases the efficiency of RNAi in Drosophila. In addition, we performed the experiments both at 22 °C and 26 °C, because higher temperatures drive the GAL4/UAS system more strongly. The results show that NHA activity is essential for survival (Fig. 2). When driven with the actin-GAL4 driver, the Nha1 or Nha2 knockdowns individually show reduced viability (with Nha2 knockdown having a stronger effect), but both knockdowns combined are semilethal at 22 °C, with only a few flies escaping to adulthood. At 26 °C, the double knockdown demonstrates complete lethality. With the strong tubulin-GAL4 driver in the presence of UAS-dicer2, the double knockdown is completely lethal, even at 22 °C (Fig. 2B).
Fig. 2.
NHA activity is essential for survival. When driven at 22 °C with actin-GAL4, NHA knockdowns display significantly reduced viability (A, B, and C, light-gray bars). At 26 °C, the effects are stronger, and the double knockdown demonstrates complete lethality (medium-gray bars). At 22 °C, when driven by the strong tubulin-GAL4 driver in the presence of UAS-dicer2, the NHA2 knockdown is almost lethal, and the double knockdown is completely lethal (dark-gray bars). By contrast, control crosses to w1118 (D) showed no lethality at either 22 °C or 26 °C. Emergence of each genotype was calculated by comparing the number of GAL4→UAS-RNAi adult flies that emerged (eclosed) for each cross, to the expected number of eclosures (white bars). Where bars or errors are not visible, they are too small to plot. Significant differences (χ2 test; n = 4) are marked with an asterisk.
NHAs Are Necessary for Survival Under Salt (NaCl but Not KCl) Stress.
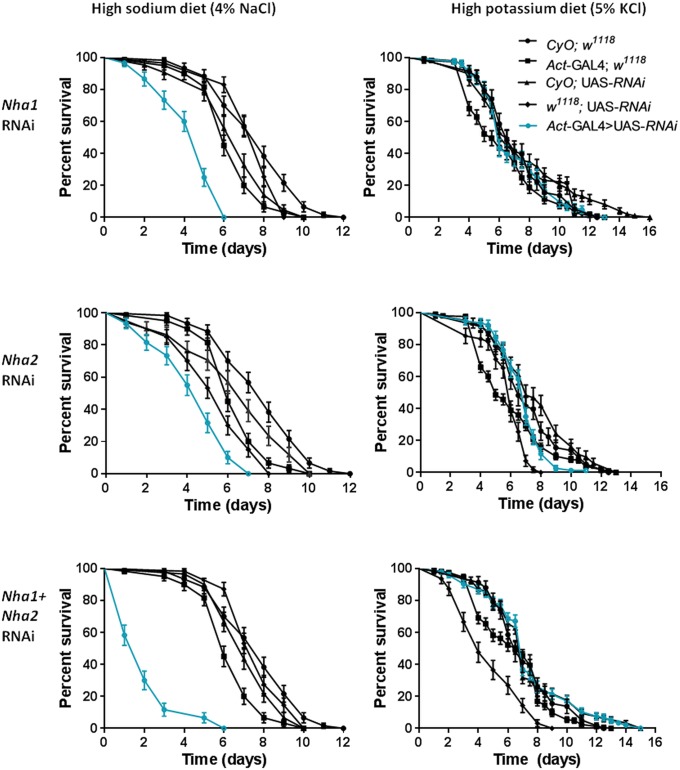
Having established that NHAs are essential, it is important to identify the key physiological processes in which they are involved. An obvious candidate process, given their structural identity as alkali metal ion exchangers and their enriched expression in epithelia, would be ionic homeostasis. Accordingly, we exposed the flies to salt stress. We and others have previously shown that a diet supplemented with 4% wt/vol NaCl is lethal to wild type (WT) Drosophila over several days (25). When WT flies were compared with either Nha1 or Nha2 knockdowns, or Nha1/Nha2 double knockdown escapers, it was obvious that NHA knockdowns reduced survival time on a NaCl diet (Fig. 3). Therefore, NHAs play a key role in acute defense against salt stress. Is this lethality a response to Na+, to Cl−, or to both? When WT and knockdown flies were fed the (higher) levels of KCl required for lethality, NHA knockdown did not affect survival, demonstrating that the pathology of salt stress is due mainly to the Na+ ion, and, further, that NHAs are not directly involved in K+ handling in the fly.
Fig. 3.
NHA knockdown increases sensitivity to NaCl loading, but not to KCl loading. Ubiquitous knockdown of Nha1 or Nha2 modulates survival under sodium stress, but not under potassium stress. Shown is survival of Nha1-RNAi (Top), Nha2-RNAi (Middle), and double knockdown (Bottom) flies on a high-sodium diet (Left) and a potassium diet (Right). actin-GAL4/CyO flies were crossed to flies homozygous for the relevant UAS-RNAi knockdown line, and the survival of the desired actin-GAL4 →UAS-RNAi progeny (blue lines) compared with the nondriven sibling CyO;UAS-RNAi controls (black). Further controls shown are outcrosses of the parental lines to w1118 flies, to produce transgene dosages comparable with the experimental flies. Data are expressed as number of surviving flies up to 16 d ± SEM, n > 30 flies per vial, repeated three times for each genotype.
Nha1 and Nha2 Have Distinct Ionic Specificities.
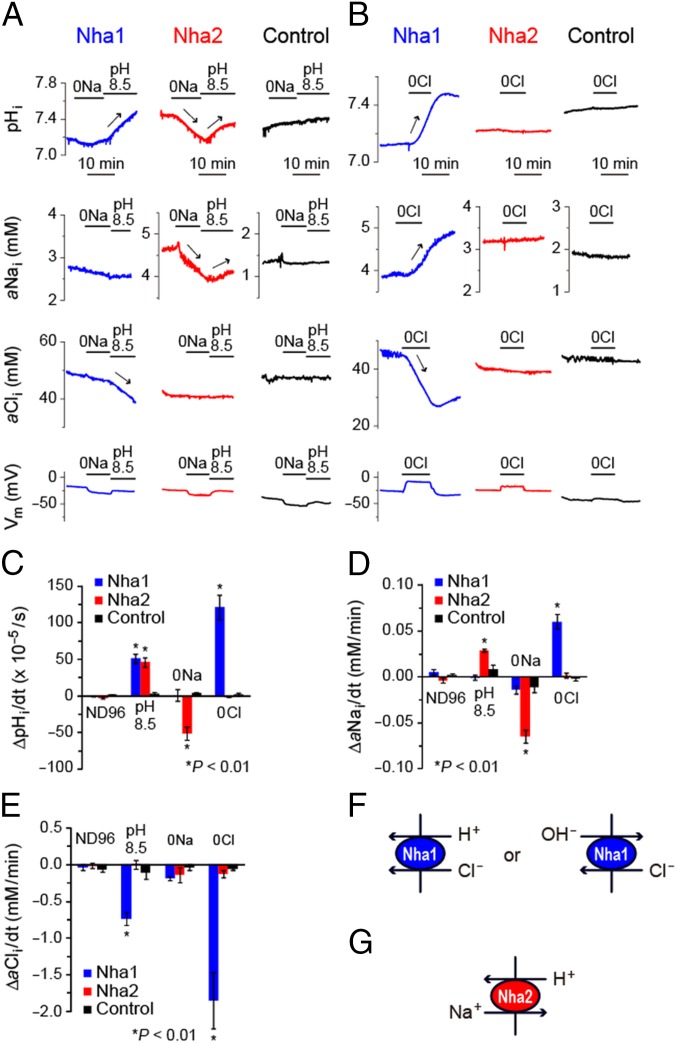
Given that all functionally characterized members of the CPA family are Na+/H+ exchangers (3, 5), that all members show structural similarity, and that we have shown compensatory overexpression in response to knockdown of either gene in Drosophila (Fig. 1), it seems likely that both Nha1 and Nha2 proteins would act as canonical Na+/H+ exchangers. Surprisingly, however, this is not the case (Fig. 4 and Table 1). When expressed in Xenopus oocytes, the intracellular pH (pHi) of both Nha1 and Nha2 oocytes, responded to changes in extracellular pH (ΔpHo), but only the pHi of Nha2 oocytes responded to changes in bath [Na+] (Fig. 4 A and C and Table 1). Intracellular Na+ activity (aNai) of Nha2 oocytes, but not of Nha1 oocytes, responded to changes in bath pH and [Na+] (Fig. 4 A and D and Table 1). Membrane potentials (Vm) of Nha1 and Nha2 oocytes were insensitive to ΔpHo (Fig. 4A). All of these responses were not observed in water-injected control oocytes. Taken together, these results show that in Drosophila, Nha1 and Nha2 have distinct ion specificities; Nha2 behaves as a classic electroneutral Na+/H+ (i.e., Nha) (Fig. 4G), whereas Nha1 acts as a previously unidentified class of H+ transporter with no Nha activity.
Fig. 4.
Nha1 and Nha2 act as a H+-Cl− cotransporter and an Na+/H+ exchanger, respectively, in Xenopus oocytes. (A and B) Representative traces of pHi, aNai, aCli, and Vm of oocytes in response to changes in bath pH, [Na+], and [Cl−]. Blue, red, and black traces indicate the results for Nha1, Nha2, and water-injected (control) oocytes, respectively. Significant increases and decreases are indicated by arrows. (C–E) Quantitative comparison of changes in pHi, aNai, and aCli of oocytes. (F and G) Schematic representations of ion transports mediated by Nha1 and Nha2. Significant changes are marked with an asterisk (Student’s t test; n = 3–30).
Table 1.
pHi, aNai, aCli, and Vm measurements in Nha1, Nha2, and control oocytes
| Condition | Units | Nha1 | Nha2 | Control | |||
| Average ± SEM | n | Average ± SEM | n | Average ± SEM | n | ||
| Resting pHi (96Na, 103.6Cl, pH 7.5) | 7.16 ± 0.02*** | 31 | 7.19 ± 0.06 | 6 | 7.33 ± 0.04 | 24 | |
| ΔpHi/dt (96Na, 103.6Cl, pH 7.5) | × 105 pH units/s | −0.4 ± 0.6 | 30 | −2.5 ± 1.6 | 18 | −1.7 ± 0.7 | 6 |
| ΔpHi/dt (96Na, 103.6Cl, pH 8.5) | × 105 pH units/s | 51.0 ± 6.1** | 3 | 46.0 ± 6.6** | 3 | 3.3 ± 1.3 | 3 |
| ΔpHi/dt (0Na, 103.6Cl, pH 7.5) | × 105 pH units/s | 1.0 ± 7.9 | 3 | −51.0 ± 9.1** | 3 | −3.7 ± 1.7 | 3 |
| ΔpHi/dt (96Na, 0Cl, pH 7.5) | × 105 pH units/s | 120 ± 16*** | 37 | −2.0 ± 0.0 | 7 | 3.0 ± 1.8 | 10 |
| ΔpHi/dt (0Na, 0Cl, pH 7.5) | × 105 pH units/s | 167 ± 17*** | 14 | −9.8 ± 2.2 | 4 | ||
| Resting aNai (96Na, 103.6Cl, pH 7.5) | mM | 3.7 ± 0.3*** | 6 | 4.4 ± 0.6*** | 11 | 1.8 ± 0.1 | 7 |
| ΔaNai/dt (96Na, 103.6Cl, pH 7.5) | mM/min | 0.005 ± 0.003 | 12 | −0.004 ± 0.003 | 10 | 0.002 ± 0.002 | 6 |
| ΔaNai/dt (96Na, 103.6Cl, pH 8.5) | mM/min | −0.001 ± 0.003 | 3 | 0.029 ± 0.002** | 3 | 0.009 ± 0.005 | 4 |
| ΔaNai/dt (0Na, 103.6Cl, pH 7.5) | mM/min | −0.014 ± 0.004 | 3 | −0.055 ± 0.006*** | 8 | −0.018 ± 0.003 | 7 |
| ΔaNai/dt (96Na, 0Cl, pH 7.5) | mM/min | 0.060 ± 0.008*** | 8 | 0.002 ± 0.003 | 3 | −0.002 ± 0.002 | 6 |
| ΔaNai/dt (0Na, 0Cl, pH 7.5) | mM/min | −0.001 ± 0.002 | 5 | −0.009 ± 0.001 | 3 | ||
| Resting aCli (96Na, 103.6Cl, pH 7.5) | mM | 51.0 ± 3.5* | 15 | 42.5 ± 2.1 | 13 | 41.7 ± 1.7 | 8 |
| ΔaCli/dt (96Na, 103.6Cl, pH 7.5) | mM/min | −0.04 ± 0.04 | 6 | −0.01 ± 0.04 | 7 | −0.07 ± 0.03 | 7 |
| ΔaCli/dt (96Na, 103.6Cl, pH 8.5) | mM/min | −0.74 ± 0.09** | 4 | 0.00 ± 0.06 | 3 | −0.11 ± 0.08 | 3 |
| ΔaCli/dt (0Na, 103.6Cl, pH 7.5) | mM/min | −0.18 ± 0.03 | 3 | −0.14 ± 0.10 | 3 | −0.04 ± 0.04 | 3 |
| ΔaCli/dt (96Na, 0Cl, pH 7.5) | mM/min | −1.85 ± 0.39*** | 7 | −0.12 ± 0.05 | 7 | −0.06 ± 0.02 | 4 |
| Resting Vm (96Na, 103.6Cl, pH 7.5) | mV | −37.0 ± 1.9*** | 52 | −35.1 ± 2.2*** | 48 | −49.7 ± 1.8 | 22 |
| ΔVm (96Na, 103.6Cl, pH 8.5) | mV | −2.2 ± 1.4 | 9 | 0.9 ± 0.6 | 9 | −4.0 ± 1.6 | 10 |
| ΔVm (0Na, 103.6Cl, pH 7.5) | mV | −18.5 ± 1.5*** | 9 | −12.7 ± 1.7 | 9 | −7.2 ± 2.3 | 10 |
| ΔVm (96Na, 0Cl, pH 7.5) | mV | 24.8 ± 2.7*** | 30 | 4.1 ± 1.6 | 13 | −0.3 ± 1.6 | 9 |
n, number of experiments. Statistical significance was calculated by Student's t test. ***P < 0.001; **P < 0.01; *P < 0.05.
Characterization of Nha1 as a H+-Cl− Cotransporter or a Cl−/OH− Exchanger.
Analysis for intracellular Cl− activity (aCli) revealed the function of Nha1 as a Cl− transporter. The aCli of Nha1 oocytes, but not of Nha2 oocytes, responded to changes in bath pH and [Cl−] (Fig. 4 A, B, and E and Table 1). In addition, the pHi of Nha1 oocytes, but not of Nha2 oocytes, increased in Cl−-free solution (Fig. 4 B and C and Table 1). The pHi increase of Nha1 oocytes was observed in NaCl-free solution as well (Fig. 5D). These results indicate that Drosophila Nha1 functions as a Na+-independent H+-Cl− cotransporter or a Cl−/OH− exchanger (Fig. 4F). In Cl−-free solution, the Vm of Nha1 oocytes, but not of Nha2 oocytes, were depolarized (Fig. 4B and Table 1). When Vm were clamped, the membrane current of Nha1 oocytes did not significantly respond to changes in bath [Cl−], whereas the pHi largely increased and decreased by the removal and readdition, respectively, of bath Cl− (Fig. 5C). These results suggest that the H+-Cl− cotransport or Cl−/OH− exchange activity of Nha1 is electroneutral, and that the depolarization of Nha1 in Cl−-free solution is a secondary phenomenon possibly mediated by endogenous membrane activity of Xenopus oocytes.
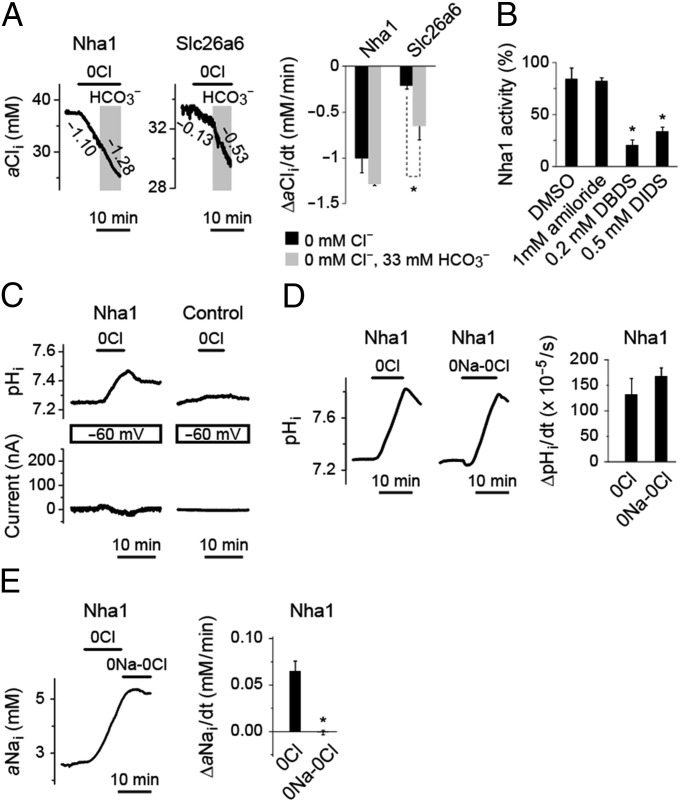
Fig. 5.
Functional characterization of Nha1 as an electroneutral H+-Cl− cotransporter or Cl−/OH− exchanger. (A) aCli of Xenopus oocytes expressing Drosophila Nha1 in response to changes in bath [Cl−] and [HCO3−]. Oocytes expressing mouse Slc26a6, a known anion exchanger with Cl−/OH− and Cl−/HCO3− exchange activity, serve as a comparison. Representative traces of aCli (Left) and quantitative comparison of changes in aCli (Right) are shown. Significant changes are marked with an asterisk (Student’s t test; n = 3–5). Numbers nest to the traces indicate the rate of changes in aCli (mM/min). (B) Inhibition of Nha1 activity by amiloride, DBDS, and DIDS. Rates of pHi increase in Cl−-free bath solution were compared in the presence and absence of inhibitors. Significant changes are marked with an asterisk (Student’s t test; n = 3–4). (C) Electroneutral nature of H+-Cl− cotransport or Cl−/OH− exchange activity of Nha1. Representative traces of pHi and membrane current (I) of voltage-clamped Nha1 and control oocytes are shown. (D) Na+ independence of H+-Cl− cotransport or Cl−/OH− exchange activity of Nha1. Representative traces and quantitative comparison of changes in pHi of Nha1 oocytes in Cl−-free and NaCl-free solutions are shown (P = 0.31, Student’s t test; n = 11–14). (E) A representative trace and quantitative comparison of changes in aNai of Nha1 oocytes in Cl−-free and NaCl-free solutions. Significant changes are marked with an asterisk (Student’s t test; n = 5).
The Cl− transport activity of Nha1 was not altered in the presence or absence of HCO3− (Fig. 5A). A similar analysis performed on mouse Slc26a6, a well-known anion exchanger with both Cl−/HCO3− and Cl−/OH− exchange activities (26), revealed significantly enhanced Cl− transport activity (Fig. 5A). These results indicate that Nha1 is not a Cl−/HCO3− exchanger. The Nha1 activity was sensitive to inhibitors of anion transport, 4,4′-dibenzamido-2,2′-stilbenedisulphonate (DBDS) and 4,4′-diiso-thiocyano-2,2′-disulfonic acid stilbene (DIDS), but not to amiloride, an inhibitor of several Na+ exchangers and channels (Fig. 5B). The aNai of Nha1 oocytes was increased in Cl−-free solution (Fig. 4 B and D and Table 1), but not in NaCl-free solution (Fig. 5E). It is not clear whether this aNai change is mediated by an alternative mode of Nha1 activity [e.g., Na+/(H+,Cl−) exchange], which our experimental conditions cannot reveal, or by endogenous membrane activity of Xenopus oocyte.
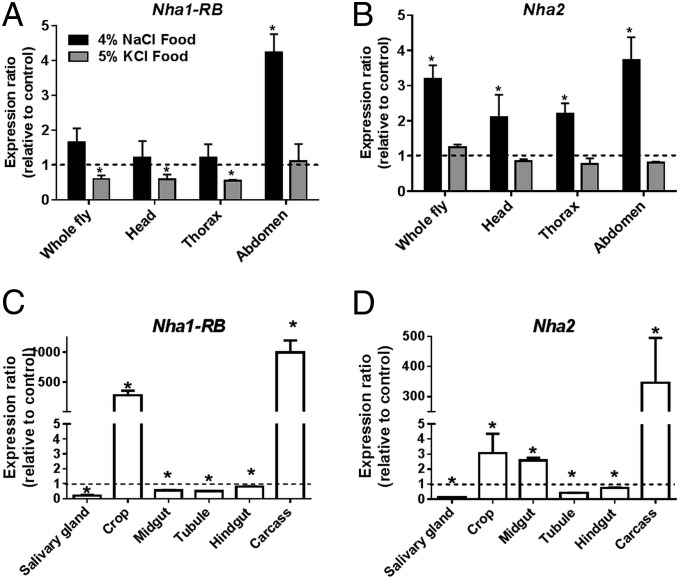
Salt Stress Induces Tissue-Specific Changes in NHA Expression.
If NHAs defend the organism against salt, then it is reasonable to expect that salt loading will lead to increased expression of NHAs in key osmoregulatory tissues. Accordingly, we assessed NHA gene expression in salt-stressed flies by quantitative real-time PCR. The expression of both Nha1 and Nha2 increased in whole flies, in response to sodium exposure, but not to potassium exposure (Fig. 6 A and B), but, surprisingly, expression decreased in the classical osmoregulatory tissues of the alimentary canal (Fig. 6 C and D), apart from a small rise in Nha2 expression in the midgut. In contrast, strong up-regulation of both Nha1 and Nha2, albeit from very low resting levels (FlyAtlas.org), was seen in the cuticle-dominated crop and abdominal carcass (Fig. 6). The cuticular epithelium is known to play an active role in ion transport, forming the “moulting fluid” that helps digest the old cuticle of moulting insects (27), and so is not merely a simple cuticle-secreting factory, and some ion transport competence must be inferred. It is also possible that more cell types within the cuticle are particularly sensitive to hemolymph Na+ levels, and protect themselves against Na+ intoxication through up-regulation of NHAs.
Fig. 6.
Salt stress induces up-regulation of NHA expression, but not in the classical osmoregulatory tissues. (A and B) After 48 h of salt loading, Nha1-RB (A) and Nha2 (B) were up-regulated in the abdomen by NaCl, but not by KCl. (C and D) NaCl loading decreased the expression of Nha1-RB (C) and Nha2 (D) in the major tissues of the alimentary canal. In contrast, the abdominal body wall (“carcass”) and crop showed strong up-regulation of Nha1 and Nha2. Significant changes in expression are marked with an asterisk (Student’s t test; n = 4).
Although the foregoing results demonstrate that NHAs are important players in organismal epithelial transport, homeostasis, and survival, they also suggest that, in Drosophila at least, NHAs can be called on facultatively to defend individual tissues against failures in hemolymph homeostasis. This devolved model may provide extra robustness of the whole system. It will be interesting to identify exactly how tissues invoke a massive up-regulation of NHAs when under Na+ challenge, and whether this confers any adaptive advantage.
Conclusions
This work demonstrates that Nha function is essential for survival, and that the Drosophila NHAs specifically protect against excess Na+. The applicability of these data to mammals will require the generation of not just Nha2 knockout mice (available now), but also Nha1 knockout and double knockout mice. Meanwhile, the relative speed and low cost of Drosophila can provide a strong indicator of essentiality.
Nha1 is the first member of the SLC9 family shown not to act as a Na+/H+ exchanger. This need not be surprising; there are examples of differing specificity in otherwise uniform families. For example, the Slc26 family can act as Cl/HCO3− exchangers, Cl channels, or an anion sensor (prestin) (28); the ClC family of Cl channels includes nCl−/H+ exchangers (29); the members of the Slc5 family show Na+-coupled transport of glucose, iodide, choline, or monocarboxylates (30); and the Slc4 family of HCO3− transporters includes the borate transporter Slc4a11 (31). Although we cannot exclude the possibility that Nha1 is capable of handling Na+, we were unable to devise conditions under which this could be shown. The dominant transport modality appears to be an H+/Cl− cotransport.
It is also significant that the close coupling of a V-ATPase to an exchanger to elicit a net transmembrane efflux of Na+ or K+ [the Wieczorek model (17)] is only partly explained by the Nhas. Nha1 does not function as an exchanger, and Nha2 is a Na+/H+ exchanger, but not a K+/H+ exchanger, and in the mosquito may not colocalize with V-ATPase (32). Thus, there may be no single partner K+/H+ exchanger to explain the net K+ transepithelial transport that characterizes most insect epithelia; indeed, transcriptomic studies show that different CPAs are coexpressed with V-ATPase in different insect epithelia (16). The search will need to be extended throughout the CPA gene family, several of which show coenrichment with V-ATPase in insect transporting epithelia (16).
Significantly, although both Drosophila Nha1/Slc9b1 and Nha2/Slc9b2 clearly sit within the CPA2 family, the insect exchangers diverge relatively basally to the human exchangers (5), to the extent that it would be imprudent to assert direct homology between them. Nonetheless, our results do imply that the functions of each member of the CPA2 family need to be established experimentally, rather than inferred from the prototype.
Materials and Methods
Drosophila Methods.
Drosophila were reared on a standard diet (33) in vials, at 22 °C and 45–55% relative humidity with a 12-h:12-h photoperiod. Where required, they were anesthetized by brief exposure to CO2. Crosses were maintained at 22 °C, or at 26 °C when noted when it was desired to drive expression of the GAL4/UAS system as strongly as possible.
Generation of RNAi Alleles.
Commercially available UAS RNAi stocks were ordered from the Vienna Drosophila Research Centre (34). Because only a minority of RNAi stocks display a phenotype when driven, we also generated our own RNAi stocks. In brief, short regions throughout the gene were selected and cloned in inverse hairpin orientation into the prize vector (35). Inserts were verified by sequencing and sent for commercial germ line transformation by BestGene. Multiple insertions for each construct were validated for knockdown by quantitative real-time PCR, and the best of each was selected for study.
Drosophila Stocks.
The GAL4-UAS binary system allows the expression of arbitrary genetic payloads under control of a yeast UAS enhancer in any cell type for which a GAL4 driver line is available, simply by crossing the requisite GAL4 and UAS lines and studying the progeny (36, 37). This system provides a valuable toolbox for integrative physiology and functional genomics (11, 38).
Stocks were either generated or purchased as above or purchased from the Bloomington Drosophila Stock Center. The following stocks were used: Canton S (WT); w1118 (background for all P-element insertions used); Nha1f02140, a piggy-Bac insertional, homozygous-viable allele of Nha1 (Bloomington Drosophila Stock Center); Nha1-RNAi (UAS stock generated in our laboratory); Nha2-RNAi (UAS stock generated in our laboratory); and double RNAi (line homozygous for both Nha1-RNAi and Nha2-RNAi UAS lines).
The GAL4 drivers used were actin-GAL4/CyO (ubiquitous expression under strong promoter, a chromosome II insertion balanced over Curly of Oster) and tubulin-GAL4; UAS-dicer2 (ubiquitous expression from a very strong promoter, with coexpression of Dicer2, a protein that enhances the efficiency of RNA interference in Drosophila) (39).
Emergence Counts of Drosophila Crosses.
GAL4-driven UAS-RNAi crosses were set up in vials in quadruplicate and reared in standard conditions at the noted temperature (22 °C or 26 °C). Progeny were collected and sorted each day. The number of flies of each genotype was counted daily until all flies had emerged. The number of observed flies of each genotype was compared with the expected number of flies for each genotype, and χ2 analysis was performed. Each driver was also crossed to w1118 and maintained at 22 °C or 26 °C, to ensure the absence of a survival phenotype associated with the GAL4 transgene alone.
Quantitative Real-Time PCR.
Whole Drosophila, or acutely dissected tissues (23), were homogenized in TRIzol, and total RNA was extracted. Quantitative real-time PCR was performed in a One-Step real-time PCR machine with TaqMan primers, and expression was quantified using the ΔΔCt method, with β-tubulin serving as a reference gene (23).
Survival Assays.
Survival assays were performed as described previously (25). In brief, 7-d-old adult female flies were taken from their vials, kept in empty vials for 4 h to ensure prompt subsequent feeding, and then transferred in groups of 20–30 to vials containing a normal diet supplemented with different concentrations of NaCl or KCl. To allow the study of living double-knockdown flies, crosses were performed with the weaker actin-GAL4 driver at 22 °C. Survival was assessed regularly over the next few days, and survivorship (Kaplan–Meier survival analysis) data were collected.
Xenopus Oocyte Methods.
The ORFs of the major transcripts of Nha1 (Nha1-RA) and Nha2 (Nha2-RA) were inserted into the pGEMHE Xenopus laevis expression vector. The plasmid was linearized with NotI, and cRNAs were transcribed in vitro using the T7 mMessage mMachine kit (Ambion). X. laevis oocytes were dissociated with collagenase and injected with 50 nL of water or a solution containing cRNA at 0.5 μg/μL (25 ng per oocyte), as described previously (40). Oocytes were incubated at 16 °C in OR3 medium and studied at 4–7 d after injection.
The pHi, aNai, and aCli of oocytes were measured using H+, Na+, or Cl− ion-selective microelectrodes prepared with H+ ionophore I-mixture B ion-selective resin (Fluka), Na+ ionophore mixture A (Fluka), or Cl− ionophore I mixture A (Fluka), respectively, as described previously (40–42). The Vm was measured as the difference between a KCl microelectrode and an extracellular calomel electrode. The oocyte was held on a nylon mesh in a chamber and perfused with ND96 saline solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM Hepes; pH 7.5). Vm and pHi or aNai were constantly recorded at 0.4 Hz, and the bath solution was replaced with test solutions prepared as follows. Na+-free (0Na) solution was prepared by substituting NaCl with choline chloride. Cl−-free (0Cl) solution was prepared by substituting gluconate salts for all Cl salts. NaCl-free (0Na-0Cl) solution was prepared by substituting Na+ with choline and Cl− with gluconate. For CO2/HCO3− equilibrated solutions, 33 mM NaCl was replaced with 33 mM NaHCO3 (33 mM sodium gluconate in non-CO2/HCO3− solution), and the HCO3− solution was bubbled with 5% CO2/95% O2 during the experiments. The osmolarity and pH of these media were adjusted to ∼200 mOsm and 7.5, respectively. The pH of high-pH ND96 (pH 8.5) solution was adjusted with NaOH solution.
The effects of inhibitors were analyzed as follows. Amiloride, DIDS, and DBDS were dissolved in DMSO to prepare 200 mM, 40 mM and 100 mM stock solutions, respectively. The oocytes were perfused with ND96 and then perfused with 0Cl-ND96. After the maximum ΔaCli/min was recorded (∼2 min after the buffer change), the oocytes were incubated in 0Cl-ND96 containing 1 mM amiloride, 0.2 mM DBDS, or 0.5 mM DIDS for ∼5 min. Final concentrations of DMSO with or without the inhibitors were adjusted to 0.5%. The inhibitory rate was calculated by comparing ΔaCli/min in 0Cl-ND96 the presence or absence of inhibitor.
pHi measurements during Vm clamping were performed as follows. Two KCl electrodes and one pH electrode were inserted into an oocyte, followed by clamping to a holding potential (Vh) of −60 mV via agar KCl bridges. Current and pHi were monitored constantly and recorded at 0.5 Hz as described previously (43). At steady state, the bath solution was changed from ND96 to 0Cl-ND96 solution.
Statistics.
Where appropriate, the significance of differences was assessed with Student’s t test or the χ2 test (two-tailed). Significant differences in survival were assessed by testing Kaplan–Meier data with the log-rank test. All testing was done using GraphPad Prism software. Throughout, the critical level was taken as P = 0.05.
Acknowledgments
We thank Dr. Masayuki Komada for use of the laboratory facilities. This work was funded by a grant from the UK Biotechnology and Biological Sciences Research Council, Pfizer Animal Health (now Zoetis, Inc.), and the National Institutes of Health (Grants DK92408 and DK100227). A.K. was supported by the Tokyo Institute of Technology’s Program for Promoting the Enhancement of Research Universities and Japan Society for the Promotion of Science KAKENHI Grants 25650114 and 26292113.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508031112/-/DCSupplemental.
References
- 1.Sardet C, Franchi A, Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 2.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev. 1997;77(1):51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na⁺/H⁺ exchangers. Mol Aspects Med. 2013;34(2-3):236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466(1):61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 5.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288(2):C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hunte C, et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435(7046):1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 7.Fuster DG, et al. Characterization of the sodium/hydrogen exchanger NHA2. J Am Soc Nephrol. 2008;19(8):1547–1556. doi: 10.1681/ASN.2007111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang M, Feng M, Muend S, Rao R. A human Na+/H+ antiporter sharing evolutionary origins with bacterial NhaA may be a candidate gene for essential hypertension. Proc Natl Acad Sci USA. 2007;104(47):18677–18681. doi: 10.1073/pnas.0707120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofstetter W, Siegrist M, Simonin A, Bonny O, Fuster DG. Sodium/hydrogen exchanger NHA2 in osteoclasts: Subcellular localization and role in vitro and in vivo. Bone. 2010;47(2):331–340. doi: 10.1016/j.bone.2010.04.605. [DOI] [PubMed] [Google Scholar]
- 10.Deisl C, et al. Sodium/hydrogen exchanger NHA2 is critical for insulin secretion in β-cells. Proc Natl Acad Sci USA. 2013;110(24):10004–10009. doi: 10.1073/pnas.1220009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dow JAT. Integrative physiology, functional genomics and the phenotype gap: A guide for comparative physiologists. J Exp Biol. 2007;210(Pt 9):1632–1640. doi: 10.1242/jeb.002691. [DOI] [PubMed] [Google Scholar]
- 12.Dow JAT. 2012. Drosophila as an experimental organism for functional genomics. eLS doi: 10.1002/9780470015902.a0000561.
- 13.Rheault MR, et al. Molecular cloning, phylogeny and localization of AgNHA1: The first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J Exp Biol. 2007;210(Pt 21):3848–3861. doi: 10.1242/jeb.007872. [DOI] [PubMed] [Google Scholar]
- 14.Xiang M, et al. Society for Experimental Biology Annual Main Meeting, 2011. Society for Experimental Biology; Glasgow, UK: 2011. Characterization of AgNHA2, the second isoform of the cation/proton antiporter from Anopheles gambiae; p. 64. [Google Scholar]
- 15.Day JP, et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci. 2008;121(Pt 15):2612–2619. doi: 10.1242/jcs.033084. [DOI] [PubMed] [Google Scholar]
- 16.Chintapalli VR, Wang J, Herzyk P, Davies SA, Dow JAT. Data-mining the FlyAtlas online resource to identify core functional motifs across transporting epithelia. BMC Genomics. 2013;14:518. doi: 10.1186/1471-2164-14-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieczorek H, Putzenlechner M, Zeiske W, Klein U. A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J Biol Chem. 1991;266(23):15340–15347. [PubMed] [Google Scholar]
- 18.Wieczorek H, Harvey WR. Energization of animal plasma membranes by the proton-motive force. Physiol Zool. 1995;68(4):15–23. [Google Scholar]
- 19.Azuma M, Harvey WR, Wieczorek H. Stoichiometry of K+/H+ antiport helps to explain extracellular pH 11 in a model epithelium. FEBS Lett. 1995;361(2-3):153–156. doi: 10.1016/0014-5793(95)00146-z. [DOI] [PubMed] [Google Scholar]
- 20.Kondapalli KC, Kallay LM, Muszelik M, Rao R. Unconventional chemiosmotic coupling of NHA2, a mammalian Na+/H+ antiporter, to a plasma membrane H+ gradient. J Biol Chem. 2012;287(43):36239–36250. doi: 10.1074/jbc.M112.403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halberg KA, Terhzaz S, Cabrero P, Davies SA, Dow JA. Tracing the evolutionary origins of insect renal function. Nat Commun. 2015;6:6800. doi: 10.1038/ncomms7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrero P, et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci USA. 2014;111(39):14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39(6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 24.Robinson SW, Herzyk P, Dow JAT, Leader DP. FlyAtlas: Database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013;41(Database issue) D1:D744–D750. doi: 10.1093/nar/gks1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stergiopoulos K, Cabrero P, Davies SA, Dow JAT. Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiol Genomics. 2009;37(1):1–11. doi: 10.1152/physiolgenomics.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: Functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283(4):F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 27.Jungreis AM, Harvey WR. Role of active potassium transport by integumentary epithelium in secretion of larval-pupal moulting fluid during silkmoth development. J Exp Biol. 1975;62(2):357–366. doi: 10.1242/jeb.62.2.357. [DOI] [PubMed] [Google Scholar]
- 28.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Aspects Med. 2013;34(2-3):494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol. 2012;2(3):1701–1744. doi: 10.1002/cphy.c110038. [DOI] [PubMed] [Google Scholar]
- 30.Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013;34(2-3):183–196. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med. 2013;34(2-3):159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang MA, Linser PJ, Price DA, Harvey WR. Localization of two Na+- or K+-H+ antiporters, AgNHA1 and AgNHA2, in Anopheles gambiae larval Malpighian tubules and the functional expression of AgNHA2 in yeast. J Insect Physiol. 2012;58(4):570–579. doi: 10.1016/j.jinsphys.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Ashburner M. Drosophila: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 34.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, Inagaki S, Yasuda K, Kageyama Y. Rapid construction of Drosophila RNAi transgenes using pRISE, a P-element–mediated transformation vector exploiting an in vitro recombination system. Genes Genet Syst. 2006;81(2):129–134. doi: 10.1266/ggs.81.129. [DOI] [PubMed] [Google Scholar]
- 36.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 37.Duffy JB. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis. 2002;34(1-2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 38.Dow JT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev. 2003;83(3):687–729. doi: 10.1152/physrev.00035.2002. [DOI] [PubMed] [Google Scholar]
- 39.Lee YS, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117(1):69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 40.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol. 1998;274(2 Pt 2):F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Kato A, Hirata T, Hirose S, Romero MF. Na+/H+ and Na+/NH4+ activities of zebrafish NHE3b expressed in Xenopus oocytes. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R315–R327. doi: 10.1152/ajpregu.00363.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato A, et al. Identification of renal transporters involved in sulfate excretion in marine teleost fish. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1647–R1659. doi: 10.1152/ajpregu.00228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang MH, et al. Euryhaline pufferfish NBCe1 differs from nonmarine species NBCe1 physiology. Am J Physiol Cell Physiol. 2012;302(8):C1083–C1095. doi: 10.1152/ajpcell.00233.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]