The study of evolutionary processes is necessarily retrospective, but past pathways can lull us into imagining that future changes will generally follow the same trajectory. The arms races between pathogens and drugs provide a particularly useful window to view these events in relatively short time frames. In PNAS, Pelleau et al. (1) provide an excellent demonstration of these selective changes. Their paper details an unexpected mechanism by which Plasmodium falciparum malaria parasites responded to the pressure of antimalarial medicines in French Guiana. Their analysis combines long-term surveillance of the parasites in the field with genomic analysis and precise editing of the gene involved to demonstrate interesting twists that organisms use to evade existential threats.
Pelleau et al.’s (1) study focuses on the response of P. falciparum to chloroquine, a synthetic antimalarial introduced just after World War II (2). Chloroquine was intensively used throughout malaria-endemic regions and chloroquine-resistant populations emerged and spread during the 1970s and 1980s in South America (3), Asia, and Africa (4). The major determinant of chloroquine resistance is the pfcrt gene that encodes a 424-amino acid integral membrane protein, PfCRT, a transporter present in the parasites’ digestive vacuole membrane (see ref. 2). All of the resistant parasites carried a pfcrt allele encoding a distinctive set of amino acids in positions 72–76 and all shared a crucial change from lysine to threonine at amino acid position 76 (5, 6). Direct comparison of DNA sequences flanking the pfcrt gene demonstrated that emergence of chloroquine-resistance alleles was rare, but parasites that carried one specific allele had spread very widely from origins in Asia to Africa (6). Old World P. falciparum parasites shared a resistant PfCRT with amino acid sequence CVIET (cysteine, valine, isoleucine, glutamic acid, and threonine) at positions 72–76 (6). South American parasites evolved resistance independently, but carried the crucial K76T change; the 72–76 residues were variations on SVMNT (serine, valine, methionine, asparagine, threonine) (3). The K76T mutation was adopted as a molecular marker of chloroquine resistance to distinguish between chloroquine-sensitive (K76) and chloroquine-resistant parasites (76T) (7).
This molecular marker was widely used to map the spread of chloroquine resistance in endemic regions. In 1993, Malawi replaced chloroquine with an unrelated antimalarial, Fansidar. Retrospective molecular analysis of Malawian parasites documented a precipitous decline in the prevalence of the Old World pfcrt allele, CVIET, from 85% in 1992 to 13% in 2000, and eventually close to zero (8). The change was not caused by reversion of 76T to K76, but by replacement of parasites that carried the CVIET allele with parasites encoding a “wild type” chloroquine-sensitive CVMNK (9). Replacement of mutant parasites by the wild-type conformed to a popular assumption: drug-resistant parasites are less fit than susceptible ones and susceptible parasites will return after drug pressure is removed. In French Guiana, a far more interesting evolutionary process has occurred. Over the last two decades as chloroquine use decreased, a novel mutation emerged in the original drug-resistant population. Chloroquine-sensitive descendants that carry the original SVMNT genotype plus a single new mutant amino acid at position C350R in the pfcrt gene now predominate.
The Institut Pasteur de la Guyane, led by Lise Musset and Eric Legrand, first uncovered the phenotype change from chloroquine resistance to susceptibility. The Institut Pasteur has operated in French Guiana since 1940 and the team capitalized on the long-term sample archive of the Institut, using an in vitro laboratory assay (10) to assess chloroquine susceptibility of more than 1,000 isolates collected between 1994 and 2012. Comparing these data to results measured from isolates collected between 1983 and 1987 (11) revealed that highly resistant parasites prevailed until about 1999. However, when chloroquine treatment was no longer recommended for P. falciparum malaria the level of resistance fell sharply, and plummeted after introduction of artemisinin combination therapies in 2002 (12, 13). Pelleau et al. (1) assessed the genotypes of more than 1,000 of these chloroquine-susceptible isolates: all parasites still retained the ancestral SVMNT “chloroquine-resistant” genotype!
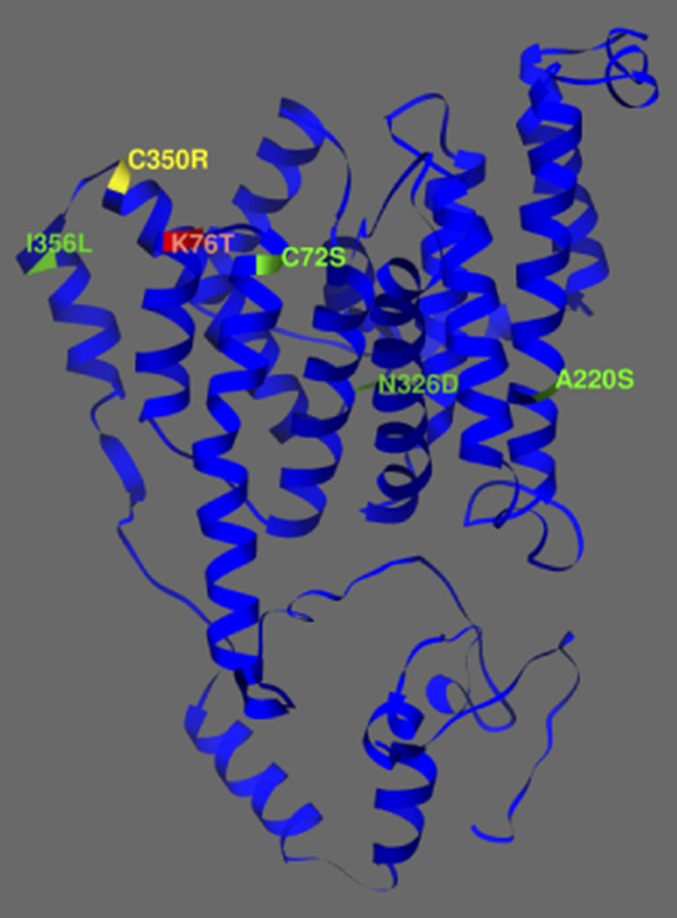
To probe this discordance between the genotype and phenotype of the parasites, Sarah Volkman and Dyann Wirth (Harvard University), and Dan Neafsey (the Broad Institute), determined the full genome sequence of more than 50 recently collected Guianan parasites. A single nucleotide polymorphism encoding the nonsynonymous mutation C350R was the only highly significant variable distinguishing chloroquine sensitive parasites with the SVMNT genotype (14, 15) (Fig. 1).
Fig. 1.
Predicted 3D structure of Plasmodium falciparum PfCRT. The novel C350R mutation (yellow) is proximal to both the molecular marker K76T (red) and the edge of the parasite digestive vacuole; the PfCRT background mutations C72S, A220S, N326D, I356L are also in predicted membrane spanning helices (green residues) (14, 15).
The last piece of the puzzle was delivered by David Fidock and Stan Gabryszewski (Columbia University) (1). Using direct gene editing, these researchers demonstrate that chloroquine-resistant parasites carrying the SVMNT signature became chloroquine-sensitive with the sole addition of the 350R mutation. The reverse transformation, changing 350R back to C350, restored the chloroquine-resistant phenotype.
PfCRT is an intrinsic membrane protein and its major role in mediating responses to antimalarials has been intensively studied (16). Fig. 1 shows the four other changes within the predicted membrane-spanning domains of the pfcrt gene that were in the gene when the parasites were chloroquine-resistant. In this study, the authors demonstrate that parasites carrying the compensatory 350R change had significantly lower resistance to five currently used antimalarials (1). One exception was piperaquine; mutants carrying the 350R change were more resistant to this drug, suggesting a PfCRT-mediated response. Fig. 1 shows the proximity of residue 350 to the resistance motif in positions 72–76 providing new clues to the transport functions of PfCRT. Pelleau et al. (1) suggest that adoption by French Guiana in the mid-2000s of a piperaquine-containing combination antimalarial may have provided a selective advantage to the emerging SVMNT+350R parasites.
Pelleau et al.’s (1) work also provides important insights relevant to public health. The evolution of resistance to antimalarials has been a major public health challenge in the past (17) and remains so currently (18). P. falciparum and its compact 23-Mb genome (19) has served as a model system facilitating the development of genetic and genomic tools for clinical, molecular, and epidemiological investigations of antimalarial resistance (see for example, refs. 20–22). These tools have been elegantly applied here (1) and demonstrate the potential for further understanding contemporary patterns of drug resistance in other eukaryotic pathogens.
Pelleau et al.’s (1) study suggests that the compensatory addition of the 350R amino acid to the SVMNT chloroquine-resistant parasites caused a loss of their resistance to chloroquine but a gain of some resistance to piperaquine. Relatively low malaria transmission leading to limited genetic diversity of the parasite populations, coupled with profligate misuse of antimalarials, characterize the selective environment of the Guiana Shield region in which these parasites evolved (23). Similar elements are implicated in selection of antimalarial-resistant parasites in Southeast Asia (24), and this information may aid in better understanding of the piperaquine failure observed there recently (25). Identification of the common selective forces at work may aid in identifying similar regions where new foci of resistance may emerge and expand.
This compensatory mutation pathway would not necessarily be viewed as a surprise in other pathogenic organisms (26), and this possibility
Pelleau et al. suggest that adoption by French Guiana in the mid-2000s of a piperaquine-containing combination antimalarial may have provided a selective advantage to the emerging SVMNT+350R parasites.
has been explored in laboratory studies of malaria as well. However, this comprehensive study (1) based on changes in parasite populations in the field, coupled with the history of drug use and social changes that impact drug selection, sets a foundation and a standard. In the future, when surveillance studies reveal that the prevalence of molecular markers appears discordant with antimalarial response, compensatory mutations will be considered.
Finally, Pelleau et al.’s (1) work illustrates the power of collaborations among groups with complementary skills. The field studies of the parasites alone would have been interesting, but could have remained a curiosity without the detailed genomic characterization of the emergent chloroquine-sensitive population pinpointing a potential mechanism. Demonstrating that the newly identified C350R mutation was sufficient to explain the seemingly discordant chloroquine response confirmed that the new genotype was indeed responsible for the phenotype measured in the laboratory and also in isolates from the jungles of French Guiana.
In addition, the archive of the Institut Pasteur de la Guyane provided the foundation for this very modern study. Long-term samples and data archives are required in any study attempting to understand the pace and trajectory of genetic changes underpinning a current situation. Such archives also preserve the local knowledge embedded in the metadata and, as illustrated here, can be the key to understanding the forces influencing the observed changes. Highlighting the key role of long-term field studies coupled with laboratory and genomic analyses is a major contribution both to malaria research and to evolutionary study of drug resistance in the field.
Footnotes
The authors declare no conflict of interest.
See companion article on page 11672.
References
- 1.Pelleau S, et al. Adaptive evolution of malaria parasites in French Guiana: Reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci USA. 2015;112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184(6):770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 3.Cortese JF, Caraballo A, Contreras CE, Plowe CV. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J Infect Dis. 2002;186(7):999–1006. doi: 10.1086/342946. [DOI] [PubMed] [Google Scholar]
- 4.Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987;3(8):241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- 5.Fidock DA, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6(4):861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wootton JC, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418(6895):320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 7.Djimdé A, Doumbo OK, Steketee RW, Plowe CV. Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet. 2001;358(9285):890–891. doi: 10.1016/S0140-6736(01)06040-8. [DOI] [PubMed] [Google Scholar]
- 8.Nkhoma S, Molyneux M, Ward S. Molecular surveillance for drug-resistant Plasmodium falciparum malaria in Malawi. Acta Trop. 2007;102(2):138–142. doi: 10.1016/j.actatropica.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Kublin JG, et al. Reemergence of choloroquine-sensetive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187(12):1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedet JP, Germanetto P, Cordoliani G, Bonnevie O, Le Bras J. In vitro activity of various antimalarials (chloroquine, amodiaquine, quinine and mefloquine) against 32 isolates of Plasmodium falciparum in French Guiana. Bull Soc Pathol Exot. 1988;81(1):88–93. French. [PubMed] [Google Scholar]
- 12.Legrand E, Volney B, Meynard JB, Mercereau-Puijalon O, Esterre P. In vitro monitoring of Plasmodium falciparum drug resistance in French Guiana: A synopsis of continuous assessment from 1994 to 2005. Antimicrob Agents Chemother. 2008;52(1):288–298. doi: 10.1128/AAC.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legrand E, et al. Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes and Plasmodium falciparum in vitro drug susceptibility to 4-aminoquinolines after drug policy change in French Guiana. Antimicrob Agents Chemother. 2012;56(3):1382–1389. doi: 10.1128/AAC.05280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, et al. The I-TASSER Suite: Protein structure and function prediction. Nat Methods. 2015;12(1):7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersen EF, et al. UCSF Chimera A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 16.Roepe PD. PfCRT-mediated drug transport in malarial parasites. Biochemistry. 2011;50(2):163–171. doi: 10.1021/bi101638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters W. Chemotherapy and Drug Resistance in Malaria. 2nd Ed Academic; London: 1987. [Google Scholar]
- 18.WHO 2014 World Malaria Report, 2014. Available at www.who.int/malaria/publications/world_malaria_report_2014/en/. Accessed August 1, 2015.
- 19.Gardner MJ, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straimer J, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347(6220):428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miotto O, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47(3):226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musset L, et al. Malaria on the Guiana Shield: A review of the situation in French Guiana. Mem Inst Oswaldo Cruz. 2014;109(5):525–533. doi: 10.1590/0074-0276140031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miotto O, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45(6):648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leang R, et al. Evidence of falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: Dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59(8):4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin BR, Perrot V, Walker N. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics. 2000;154(3):985–997. doi: 10.1093/genetics/154.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]