Abstract
Efforts to understand the ecological regulation of species diversity via bottom-up approaches have failed to yield a consensus theory. Theories based on the alternative of top-down regulation have fared better. Paine’s discovery of keystone predation demonstrated that the regulation of diversity via top-down forcing could be simple, strong, and direct, yet ecologists have persistently failed to perceive generality in Paine’s result. Removing top predators destabilizes many systems and drives transitions to radically distinct alternative states. These transitions typically involve community reorganization and loss of diversity, implying that top-down forcing is crucial to diversity maintenance. Contrary to the expectations of bottom-up theories, many terrestrial herbivores and mesopredators are capable of sustained order-of-magnitude population increases following release from predation, negating the assumption that populations of primary consumers are resource limited and at or near carrying capacity. Predation sensu lato (to include Janzen–Connell mortality agents) has been shown to promote diversity in a wide range of ecosystems, including rocky intertidal shelves, coral reefs, the nearshore ocean, streams, lakes, temperate and tropical forests, and arctic tundra. The compelling variety of these ecosystems suggests that top-down forcing plays a universal role in regulating diversity. This conclusion is further supported by studies showing that the reduction or absence of predation leads to diversity loss and, in the more dramatic cases, to catastrophic regime change. Here, I expand on the thesis that diversity is maintained by the interaction between predation and competition, such that strong top-down forcing reduces competition, allowing coexistence.
Keywords: carrying capacity, interspecific competition, predation, species diversity, trophic cascades
Understanding the regulation of species diversity has been a central goal of ecology since the time of Darwin and Wallace. Myriad theories have been proposed, but none has yet achieved general acceptance. If one considers theories that have enjoyed some prominence in the literature, they are an extraordinarily diverse lot (Table 1). Several are nonmechanistic, that is, they operate through no identified biological process. Other models of coexistence are built upon the potential for differential exploitation of heterogeneity in space and time. Some of the most prominent efforts to understand diversity are derived from the original Lotka–Volterra (L-V) model and have been framed in the bottom-up context of resource limitation and carrying capacity. Finally, there are models that emphasize top-down processes and interactions between top-down and bottom-up processes. Bottom-up forcing determines the energy flow available to a community or ecosystem, whereas top-down forces determine how that energy flow is distributed among trophic levels (25). Any theory of species diversity that rests solely on bottom-up or top-down forces is therefore in conflict with this undisputed reality.
Table 1.
Some theories of species diversity
| Theory | Author, date | Ref(s). |
| Nonmechanistic | ||
| Broken stick | MacArthur, 1957 | 1 |
| Log normal | Preston, 1962 | 2 |
| Log series | Whittaker, 1965 | 3 |
| Neutral theory | Hubbell, 1979, 2001 | 4, 5 |
| Heterogeneity | ||
| Intermediate disturbance | Connell, 1978 | 6 |
| Lottery competition | Chesson and Warner, 1981 | 7 |
| Spatial heterogeneity | Tilman and Pacala, 1993 | 8 |
| Dispersal limitation | Tilman, 1994 | 9 |
| Winner by forfeit | Hurtt and Pacala, 1995 | 10 |
| Individual variation | Clark, 2010 | 11 |
| Bottom-up | ||
| Resource competition | Lotka, 1925 | 12 |
| Resource competition | Volterra, 1926 | 13 |
| Limiting similarity | Hutchinson, 1959 | 14 |
| Habitat complexity | MacArthur and MacArthur, 1961 | 15 |
| Immigration vs. extinction | MacArthur and Wilson, 1967 | 16 |
| Resource gradients | Tilman, 1982 | 17 |
| Productivity | Brown, 2014 | 18 |
| Top-down | ||
| Keystone predation | Paine, 1966 | 19 |
| Escape in space | Janzen, 1970 | 20 |
| Escape in space | Connell, 1971 | 21 |
| Predation/competition | Menge and Sutherland, 1976 | 22 |
| Pathogens | Givnish, 1999 | 23 |
| Predation/competition | Chesson and Kuang, 2008 | 24 |
Nevertheless, the influence of bottom-up thinking has been pervasive in ecology for nearly a century and has colored our views of many issues, including the regulation of species diversity. The control of ecosystem structure and function via primary productivity seems simple, direct, and so obvious that other explanations appear unnecessary. However, after countless proposals put forward over many decades, a satisfying answer to the simple question of “Why are there so many species?” has remained elusive.
The formalization of bottom-up thinking began with the seminal work of Lotka (12) and Volterra (13), who independently formulated the concept of population regulation via resource limitation as embodied in the concept of carrying capacity. Although the concept of carrying capacity is so intuitive as to seem self-evident, it has the fatal weakness of defying empirical substantiation in wild populations of plants or animals. Carrying capacity has thus evolved into a scientific ghost—something everyone thinks is important but that no one has ever seen.
Lotka, a prodigious generator of ideas, was, with Volterra, also the originator of competition theory, but carrying capacity remained a central feature of the theory as devised by both authors, requiring that interactions between competitors be indirect, that is, mediated through resources, rather than directly through what is euphemistically called “interference.” In addition, Lotka (26) was the first to understand the complexity of predator–prey interactions, showing that the interaction could lead, depending on the choice of parameter values, to a stable state, damped oscillations, or to a stable limit cycle—ideas that are today presented in every introductory ecology text. In an elegant but less well-known work (27), he defined the geometry of prey capture by a pursuit predator.
The concept of carrying capacity focused attention on limiting resources and led to an early emphasis on primary productivity and energy transfer up the trophic ladder (28–30). Given this background, it was easy to reason by analogy that all higher trophic levels would also be resource limited, an idea that persists to this day among some ecologists.
In the discussion that follows, I shall develop the thesis that it is the interaction between competition and predation that regulates species diversity in ecological space/time. The role of evolution in generating diversity is not under consideration here. I shall begin by examining the features and assumptions of the basic bottom-up (resource centric) model as espoused by Lotka, Volterra, MacArthur, and others. Next will follow a section on predation and diversity, which leads into a discussion of the competition–predation trade-off. A simple graphical model summarizes current evidence. Birds and trees will be emphasized as well-studied representatives of producer and consumer trophic levels.
Bottom-Up Theory and Interspecific Competition
The bottom-up approach to understanding species diversity is epitomized by the work of Robert MacArthur and his colleagues (31). The basic idea is that overlap in the exploitation of limiting resources will result either in the coexistence of competing populations or the spatial exclusion of one by the other, depending on the values of their respective carrying capacities and competition coefficients. It is assumed that each population draws its diet from a spectrum of resources. Examples are prey for predators, fruit size for frugivores, seed size and hardness for seed predators, and leaf nitrogen or tannin content for herbivores. MacArthur’s models incorporate the Gauseian maxim that no two species can occupy the same niche, complemented by the concept of limiting similarity (14, 32), which supposes that coexisting species possess sufficient trait differences to reduce overlap on shared major resource axes. MacArthur’s models (31, 33, 34) presume bell-shaped (normal) resource utilization functions on continuous resource axes and endeavor to quantify the minimum difference between means in the utilization curves of competing species sufficient to confer stable coexistence (35).
There have been many attempts to construct tests of this basic bottom-up theory, but these tests have not yielded convincing results for the compelling reason that the required parameters cannot readily be measured in the field. In principle, whether the interaction between two species leads to coexistence or exclusion depends on the relative value of terms, Ki and αi,j, where Ki is the carrying capacity of the ith species and αi,j is the effect of an individual of species j on the rate of increase of species i. (Carrying capacity is defined here as the population density of a species that can be supported by the available resource supply, absent sources of mortality other than resource limitation.) First, there is no accepted means of estimating the carrying capacity of populations in nature, and second, the respective α values are based on overlap in resource use, which, for any two species, can vary greatly from place to place, season to season and year to year. Moreover, and crucially for this discussion, resource overlap feeds back strongly on population growth only at population densities near Ki. The empirical reality is that neither K values nor α values can be measured in the field with adequate precision for parameterizing the model. Therefore, the ratios, K/α, are subject to such large errors of estimation that comparisons between species are meaningless.
A further obstacle to implementing the theory is that, in nature, any two species either coexist or occupy different habitats. Thus, even if the relevant K values and α values could be measured with precision, there remains the insurmountable dilemma that suspected competitors can only legitimately be compared where they coexist, so that the threshold value of K/α that results in exclusion can never be determined.
Bottom-up diversity theory rests on a series of assumptions and propositions that have more often been taken on faith than empirically verified. I shall now briefly review and comment on these features of the theory.
High Levels of Coexistence Can Only Occur Under Restricted Conditions.
Most solutions of the basic L-V and derivative models predict exclusion, not coexistence. A large theoretical literature has explored modifications of the L-V model in quest of variants that are more permissive of coexistence. In general, such models allow broad coexistence only under restricted conditions (35).
High diversities, amounting to hundreds or even thousands of species of vertebrates/invertebrates/plants are found in all humid tropical continental areas, a fact that seems to conflict with the idea that broad coexistence can occur only under restricted conditions.
Interspecific Competition Is Assumed to be Indirect and Symmetrical.
Interspecific competition is assumed to be indirect and symmetrical, indirect in the sense that interactions are mediated through a shared limiting resource, and symmetrical in the sense that, under some conditions, species A wins and, under others, B wins. The model predicts abrupt replacements on environmental gradients as relative values of K and α change with environmental conditions. Behavioral interactions are not involved.
Studies of species replacements on environmental gradients have been the classical approach to studying this issue. Early evidence was circumstantial (i.e., abutting, nonoverlapping ranges), but did not distinguish between hypotheses (36, 37). Accumulated evidence shows unequivocally that spatial segregation, at least in vertebrates, is maintained by asymmetric interactions enforced through direct aggression and dominance (38–42). Intraspecific and interspecific competition among vertebrates is often manifested as aggression, including dominance, displacement from food sources, and/or territoriality. Strong interspecific aggression is typically directed at a small number of target species in diverse communities (40). Aggression serves to ensure access to patchy resources and to skew the burden of resource scarcity toward subordinate species and low-ranking or nonbreeding conspecifics. Other contexts in which asymmetric interactions prevail in ecology are succession and patch dynamics.
The Niche Compression Hypothesis Predicts Low α- and High β-Diversity in Tropical Environments.
Predictions of the bottom-up competition model pertain to small numbers of species, rarely more than two or three, so say little about community-level diversity. MacArthur (43) proposed that the species-level theory could be scaled up to the community level via what he termed the “niche compression hypothesis” (NCH). The concept is most fully expounded in The Theory of Island Biogeography (16) in the context of comparing temperate and tropical diversity. Given an order-of-magnitude increase in species numbers in the tropics, MacArthur felt that competition would force ecologically related species into different habitats, because limiting similarity would impose hard limits on the compression of dietary niches. The emergent prediction was that the α-diversity of tropical habitats should not be substantially greater than of temperate habitats and that the “extra” species in tropical environments would have to be accommodated via finer habitat subdivision, or β-diversity (43, 44). Data adequate to testing the idea did not appear for many years. Robinson and Terborgh (45) found that approximately two-thirds of the ±300 land bird species found in a heterogeneous tropical landscape co-occur in the matrix habitat, terra firme (upland) forest, indicating high α-diversity and low β-diversity. This was further confirmed through the comprehensive mapping of avian territories in a 100-ha tract of Amazonian lowland forest (46). When the mapped territories of territorial species were superimposed, as many as 150 species were found to overlap at a point—the narrowest definition of α-diversity. In addition, this total did not include as many as 50 nonterritorial species present in the community. The combined total of around 200 territorial and nonterritorial species represents an α-diversity 5–10 times greater than that found in temperate bird communities.
These findings with birds are strongly supported by data on the tree community in the same heterogeneous landscape in Peru. Pitman et al. (47) showed that ≥75% of the tree species in this landscape occur in ≥1 major habitat (upland forest, floodplain forest, swamp, succession). In a parallel result, Wittmann et al. (48) investigated the habitat occurrences of 658 tree species found in Brazilian varzea (floodplain) habitat and found that only 10% were restricted to varzea. Thus, tropical birds and trees, at least in Amazonia, are habitat generalists, not specialists, as predicted by the NCH. These results definitively refute the NCH and support a diametrically opposite interpretation, namely, that interspecific competition in species-rich tropical habitats is weak, not strong, as assumed by MacArthur and many others.
Under the Bottom-Up Model, Strong Competitive Interactions Occur Only at Densities Near K (Carrying Capacity).
The central role of K in bottom-up theory presents an awkward dilemma in that, empirically, K is a phantom quantity. It can be determined for a given species under controlled conditions in a laboratory, but it is rarely, if ever, determined for a wild population. To illustrate why, just think of what would be entailed in revealing the magnitude of K in a wild context: it would be necessary to impose rigorous control over competitors, predators, parasites, pathogens, and hiccups in the weather (49). Absent such controls, it has simply been assumed that populations in nature are at or near K. This was always what I assumed myself until I became acquainted with an archipelago of predator-free islands in Lago Guri, a vast, 4,300-km2 hydroelectric impoundment in Venezuela.
Flooding of the Caroní Valley in 1986 created hundreds of land bridge islands, isolated fragments of a formerly intact tropical dry forest landscape. Because predators typically need more space to maintain their populations than their prey, area contraction during flooding selectively eliminated predators of vertebrates from all but the larger islands (50). Small islands in the 1- to 10-ha size classes, many of them several kilometers from the mainland, were left without predators of vertebrates but a diversity of primary and secondary consumers in three guilds (predators of invertebrates, granivores, and generalist herbivores). Table 2 shows that the members of these guilds increased in density by roughly an order-of-magnitude in the absence of predators, dramatically illustrating the enigmatic phenomenon of “density overcompensation” (57). The term refers to a situation commonly observed on small islands where the combined density of species representing a particular guild or taxon exceeds that observed on the adjacent mainland, notwithstanding reduced insular species numbers. The combined density of generalist herbivores, for example, on small Lago Guri islands was more than 10 times greater than in the same habitat on the nearby mainland (54). These densities were sustained for 16 y until the project ended, so they were not simply an ephemeral aberration of island isolation (50).
Table 2.
Hyperabundance of three guilds of consumers on predator-free islets in Lago Guri, Venezuela
| Guild, species | Census method | Population density, island/mainland | Ref. |
| Insectivore | |||
| Ameiva ameiva (lizard) | No./hour of search | to 17 | 51 |
| Dendrobates leucomelas (frog) | No./hour of search | to 20 | 51 |
| Tarantula sp. | No./hour of search | to 6.3 | 51 |
| Birds | Spot map, point count | to 6 | 52 |
| Granivore | |||
| Rodents (six spp.) | No./100 trap nights | to ≥30 | 53 |
| Generalist herbivore | |||
| Alouatta seniculus (howler monkey) | Transect, direct count | to ≥10 | 54 |
| Geochelone carbonaria (tortoise) | Mark, recapture | 7 | 55 |
| Iguana iguana (lizard) | Dung counts | ≥10 | 51 |
| Atta spp. (leaf-cutter ants) | Transect, direct count | to 100 | 56 |
Seeing these results, one cannot avoid the conclusion that K for generalist herbivores in Venezuelan dry forest is far greater than suggested by the densities of the same species in mainland habitats supporting predators. These results also speak to the issue of interspecific competition as it might operate in the three guilds of species. Release from competition cannot explain density overcompensation. One species might increase at the expense of another or all others, but all guild members would not be expected to increase in concert. That can only happen if, as already concluded, K is a much greater density than one measures in mainland habitats. Again, the increase of all members of these diverse guilds implies that interspecific competition in the “normal” circumstances of the mainland environment must be weak.
Predation.
Predation is not incorporated into the basic L-V model of interspecific competition, but has been incorporated in modern elaborations of the theory, typically in the context of density dependence and demographic proximity to K. The dramatic consequences of releasing vertebrates and some invertebrates from predation on Lago Guri islands brings me to my main point, which is that predation, not competition, regulates the densities of most species (other than top carnivores and some others, as noted below) to levels far below K. It follows that, if populations subsist at densities far below K, competition between them must be weak to negligible. Parenthetically, one must make an exception here for species with evolved social mechanisms or morphological adaptations for thwarting predation—e.g., many primates, flocking birds, migratory herd-forming ungulates, toxic and/or aposematic species, etc. (58, 59).
Top-Down Regulation of Diversity: A Counterpoint to Bottom-Up
I now come to the crucial question of whether predation can regulate diversity. The answer is definitively yes, as Paine (19) showed with the removal of the starfish Pisaster from the rocky intertidal zone of Tatoosh Island in Washington State, United States. Predator removal unleashed a dominant competitor, the mussel, Mytilus californicus, to increase in abundance and monopolize available space, displacing a number of chitons, limpets, and barnacles with a concomitant sharp reduction in diversity. In regulating the abundances of several prey species through its preference for the dominant competitor, termed “keystone” predation, the effect of Pisaster on the diversity of prey was simple, strong, and direct, yet ecologists have persistently failed to perceive generality in Paine’s result and others like it.
Keystone predation is a special case of the much broader concept of the trophic cascade (60, 61). Research on trophic cascades has gained momentum in recent years but we have yet to connect the dots between trophic cascades and a deeper understanding of how species diversity is regulated in nature. Whereas bottom-up theories of resource limitation and limiting similarity have been refractive to definitive tests (62), the influence of top-down control can be tested experimentally (see below).
A signal feature of trophic cascades is that adding or removing one trophic level (usually the topmost) can trigger a state change in the entire system (25, 63). Often one state is dramatically more diverse than its alternative, as in the Pisaster case. Another well-known and widely accepted example is that of the indirect control of kelp forests by sea otters (64). In the absence of sea otters, high-diversity kelp forests disappear and are replaced by low-diversity “sea urchin barrens” (65). Equally definitive experiments have confirmed the operation of strong trophic cascades in streams (66, 67) and lakes (68), in each case with consequences for diversity.
The most direct way to test for the existence of a “Paine effect” (intensification of competition with resultant decrease in prey diversity in response to reduction in predation) in communities is to conduct appropriate predator removal experiments. However, experiments analogous to Paine’s starfish removal from 16-m2 sections of intertidal habitat are much more challenging to implement on land where top predators like wolves (Canis lupus) and cougars (Felis concolor) require hundreds of square kilometers of habitat to maintain populations. Top predators of the open ocean are even less tractable as experimental subjects. Thus, few experiments have been conducted at appropriate scales.
In a notable exception, Henke and Bryant (69) removed coyotes from replicate 5,000-ha blocks in western Texas, United States, and followed the impact on the rodent community for 2 y. Within 9 mo of coyote removal, rodent species richness and diversity declined in both shrubland and grassland habitats. An initial rodent assemblage of 12 species collapsed in the absence of coyotes to a single species, Dipodomys ordi, the largest member of the guild, suggesting behavioral dominance. Mesopredators [skunk (Mephitis mephitis), badger (Taxidea taxus), fox (Urocyon cinereogaster), bobcat (Felis rufus)], scarce to absent before coyote removal, increased manyfold.
Isolation of predator-free forest fragments in a reservoir in Thailand had similar consequences, as an initially diverse rodent assemblage collapsed to a single species, Rattus tiomanicus, after 25 y (70). Prima facie evidence does not distinguish whether the rodent community collapsed as a consequence of resource competition, interference competition, or predation, but the probable answer lies in the scores of well-documented cases of predation-driven extinctions of small vertebrates (birds, reptiles, small mammals) on islands following the invasion of Rattus spp. (71).
Density-independent predation is another feature of many models that departs from reality. Density-independent predation may be a workable approximation in low-diversity communities, such as those of the Arctic, in which important predators are prey specialists, e.g., lynx (Lynx rufus), snowy owl (Nyctea scandiacus). However, in the hyperdiverse ecosystems of the tropics, whether savanna or forest, most predators take a wide range of prey and each prey is sought by many predators (72). In tropical forests where the principal predators hunt by stealth and ambush, prey are taken as encountered in a perfectly density-dependent fashion (73). Prey dynamics in these systems should thus display the reciprocal behavior characteristic of apparent competition (74, 75).
The presence of species at intermediate levels of food webs is predicted to weaken the signal of predator removal, but the reality can be more complicated. When released from predation, omnivores can attain higher population densities than carnivores of equivalent size through consuming plant matter. Mesopredator release can thus have catastrophic impacts on smaller animals, including birds, lizards, frogs, and large arthropods (76, 77). Mesopredators that prey on a mix of smaller predators and herbivores can have either positive or negative indirect effects on plants, depending on the prey preferences of the smaller predators (78). Thus, the trophic cascades caused by single species can have complex effects (79), whereas the consequences of community-level cascades are more regular and predictable (25).
Herbivore release is a predictable response to relaxed top-down regulation (80). Lago Guri islets provide a compelling example. Under the intense herbivory of hyperabundant howler monkeys, iguanas, and leaf-cutter ants, sapling mortality exceeded recruitment in nearly every woody plant species in the community, presaging a drastic reduction in diversity (81). Hyperabundant deer (Odocoileus hemonius, Odocoileus virginiana) have been documented to drive equally drastic transformations of vegetation in temperate North America (82, 83). Experimental removal of dominant kangaroo rats (Dipodomys spp.) from exclosures led to the conversion of shrubland to grassland in the Chihuahuan Desert, United States (84). In addition, in Arctic Eurasia, small rodents, principally voles, are capable of transforming tundra vegetation when experimentally transferred to predator-free islands (85). Similarly, salt marshes (Spartina alterniflora) in the southeastern United States are reduced to barrens by removing predators of the major herbivore, the snail, Littoraria irrorata (86). Food-limited herbivores have thus been found to drive alternative states in Arctic, temperate, and tropical ecosystems, lending support to the likelihood that many ecosystems are vulnerable to catastrophic regime shifts in the absence of predators.
Vertebrate herbivores like deer and rodents operate on large spatial scales relative to the dimensions of individual plants, but invertebrates and other small organisms (e.g., nematodes) often operate in a spatially restricted manner. Janzen (20) and Connell (21) realized this long ago and proposed the mechanism of escape in space as a top-down alternative to resource competition in high-diversity plant communities. A recent resurgence of interest in the Janzen-Connell (J-C) mechanism is lending it strong experimental support in both temperate (87, 88) and tropical forests (89–93). The J-C mechanism produces strong negative density dependence at the seed and seedling stages that favors less common species over common ones. Both host-generalist and host-restricted mortality agents kill propagules in a density-dependent fashion, but only host-restricted agents cause the distance-dependent mortality that underlies the diversity-promoting action of the J-C mechanism (91). Responsible mortality agents include arthropods and fungi and perhaps still unidentified soil organisms, but generally not vertebrates (90, 92, 93). The J-C mechanism has recently been found to promote diversity in coral reefs (94). How much wider its distribution in nature may be remains to be determined.
In sum, predation sensu lato (to include J-C mortality agents) has been unequivocally shown to promote diversity in a wide range of ecosystems, including rocky intertidal shelves, coral reefs, the nearshore ocean, salt marshes, streams, lakes, temperate and tropical forests, and Arctic tundra. Given the compelling variety of these ecosystems, one can hardly doubt that top-down forcing plays a universal role in regulating diversity. This conclusion is further supported by studies showing that the reduction or absence of predation leads to diversity loss and, in the more dramatic cases, to catastrophic regime change.
If predation enhances diversity, there must be a limit to the trend, for intensifying predation indefinitely can lead to the elimination of prey populations and diversity loss. Predator-induced diversity loss has most dramatically been associated with the introduction of “superpredators,” here defined as novel top predators intentionally or inadvertently introduced to naïve ecosystems (Table 3). Introduction of superpredators can have devastating consequences through the swift elimination of many native species. Impacts of the Nile perch (Lates niloticus) on Lake Victoria cichlids and the brown tree snake (Boiga irregularis) on the native birds of Guam are textbook cases. The human-induced extermination of megafauna around the world is another (102, 103). Recently and surprisingly, the fortuitous establishment of a superpredator (the Burmese python, Python molurus) in Florida, United States, has had an enormous impact, reducing populations of some prey species in Everglades National Park by 98% (101).
Table 3.
Some examples of superpredators
| Superpredator | Where introduced | Impact | Ref. |
| Peacock bass | Lake Gatun, Panama | Extirpation of native fish | 95 |
| Nile perch | Lake Victoria, Africa | Extirpation of native fish | 96 |
| Fox, feral cat | Australia | Extirpation of native mammals | 97 |
| Stoat, rats | New Zealand | Extirpation of native birds | 98 |
| Humans, rats | Pacific Islands | Extirpation of native birds | 99 |
| Brown tree snake | Guam | Extirpation of native birds | 100 |
| Burmese python | Florida, United States | Extirpation of native mammals | 101 |
| Humans | Global | Extinction of megafauna | 102 |
The Competition–Predation Trade-off: A Sliding Scale
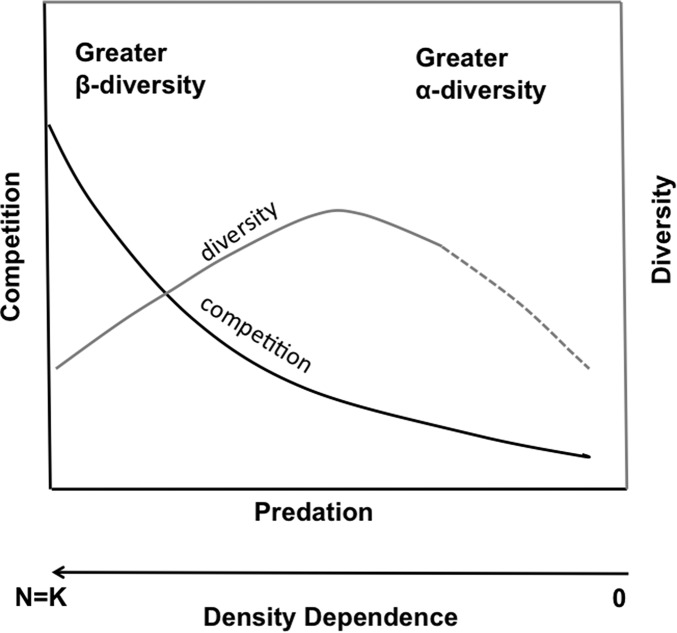
The superpredator phenomenon suggests that communities of all kinds are genetically adapted and/or behaviorally adjusted to the historical predation regime, whether it be relatively strong or weak (104, 105). Learned or innate antipredator tactics that are adequate to limit mortality from preexisting predators are inadequate to thwart a superpredator. Thus, it can be argued that somewhere between the competition-driven bottom-up world of zero predation and the doomsday ushered in by a superpredator, there is a level of predation that maximizes diversity (Fig. 1). The balance between competition and predation determines the α-diversity of the matrix habitat in a landscape in relation to the regional species pool. This is the crucial element of a trophic theory of diversity.
Fig. 1.
Trade-off between competition and predation as regulators of species diversity. Under zero predation, competition is postulated to be strong, favoring β- over α-diversity via competitive exclusion. Intense (“normal”) predation damps competitive interactions, increasing the fraction of the regional species pool that coexists as α-diversity up to an optimum level of predation. Introduction of superpredators can result in supraoptimal predation, decimation of prey populations, and diminished diversity (dashed line). Interspecific and intraspecific density dependence is strong where competition is high and predation low (at left) and decreases as the strength of predation increases (toward the right). Density-dependent predation increases from left to right. Reciprocal effects of predators on competing prey species (“apparent competition”) is common in nature but not fully captured by the model illustrated.
Under zero predation (including lethal parasitism and disease), bottom-up conditions prevail and consumer communities should be structured by competition for resources (106). Low diversity should be the norm, and species should mostly represent distinct functional groups (e.g., low genus to species ratios). Increasing predation is expected to relax competition and create conditions permissive of greater diversity up to a postulated maximum representing the balance between predators and the antipredator tactics of prey. The introduction of a superpredator can upset the balance, resulting in overexploitation of prey, local extinctions, and reduced diversity.
In hyperdiverse ecosystems, such as tropical forests and coral reefs, highly diverse predator guilds impose strong density-dependent top-down forcing, reducing prey densities and lowering resistance to entry by members of the regional species pool with resulting high α-diversity. One sees evidence of this in high species-to-genus ratios in such communities (107). Conversely, communities of either plants or primary consumers that are exposed to little or no predation are expected to experience strong competition resulting in spatial (rarely temporal) exclusion that enhances β-diversity at the expense of α-diversity. Concomitantly, density dependence is expected to be high in bottom-up–regulated systems exposed to low predation, and low in top-down–regulated systems.
The existence of trade-offs between predation and competition has been investigated in terrestrial ecosystems with generally positive results with respect to detecting effects of both, including significant interactions between them (108, 109). Predation pressure in nature must vary between communities, but the factors that lie behind this variation are largely unexplored.
Within communities, the risk of predation varies widely among prey species, such that species and individuals of lesser body mass are at greater risk (72). Both predators and prey take advantage of landscape heterogeneity to benefit their respective goals, often at a cost to prey in foraging efficiency (59, 110). Prey calibrate the scale between energy maximization and time minimization in relation to the trade-off between perceived risk and energy acquisition. Intraspecific and/or interspecific competition exacerbates the relative scarcity of food and compels greater risk taking. Even invertebrate herbivores alter their habitat use in the presence of predators (111). Thus, the relative strengths of predation and competition are conditioned on local circumstances and therefore bound to vary within as well as between landscapes. Moreover, behavioral interactions between predators and prey and between potential/actual competitors are frequently conditional, adding a dimension of temporal heterogeneity to the complexity of the interactions (112).
Reduced within-guild redundancy in predator guilds at higher latitudes coupled with strong between-year climate variability driven by the North Atlantic Oscillation and other cycles (113) can allow prey to escape from top-down regulation, leading to outbreaks of lemmings, voles, snowshoe hares, and other small mammals and invertebrates (114, 115). Lower latitudes generally support a greater diversity of predators capable of opportunistic prey switching. Outbreaks are consequently rare and cycles damped.
Plant communities that experience no herbivory, as on some islands, must be structured purely by competition and characteristically support low diversity (106, 116). At the other extreme, where herbivores are present and predators absent, excessive herbivory can transform vegetation from a competition-structured to a herbivore-structured state, also lacking in diversity, composed of resistant and/or tolerant plant species, as seen on experimentally manipulated islands in both tropical and arctic settings (81, 85). Again, this suggests with respect to plant diversity that there is an optimum at intermediate levels of herbivory [reminiscent of Connell’s (6) intermediate disturbance hypothesis] at which diversity is maximum (117). Communities near this optimum would include a mix of species possessing a wide range of plant defenses (106). However, much remains to be learned about how uneven herbivore pressure, as postulated for the “landscape of fear” (118), influences local variation in plant diversity and species composition.
Discussion and Conclusions
Now, more than 50 y after Hutchinson’s Santa Rosalia paper (14), ecologists have not reached consensus on the manner and degree to which interspecific competition structures plant and animal communities. I suggest that our collective failure to understand how competition regulates diversity follows from our early seduction as students by the simple and compelling logic of the L-V model. Pervasive bottom-up thinking has blinded us to alternatives.
Bottom-up models rest on resource partitioning in space/time through competition or differential habitat selection, whereas top-down models are built on trophic interactions involving predators (including herbivore predation on plants) and pathogens. Both competition and predation are recognized as essentially universal processes in natural communities (119), and when predator behavior includes prey switching and density-dependent predation, coexistence is favored (120, 121). Thus, one must wonder why both bottom-up and top-down processes have not been included in all theories of diversity.
I have examined the central features of resource-based diversity theory and found that several are contradicted by empirical evidence. High levels of diversity are widespread throughout the continental tropics, suggesting that the conditions for coexistence are relaxed, not restricted. Broad coexistence, such as is found in hyperdiverse tropical ecosystems, requires equalization of fitness, so that no species can increase at the expense of others, and stabilization (negative density dependence), so that a species can increase when reduced below its average abundance (122). Density-dependent predation is the mechanism that provides both equalization and stabilization when coupled with the existence of prey refugia in a heterogeneous environment, or escape in space, in the case of plants (87, 123, 124).
Classical models assume that interspecific competition is indirect and symmetrical, whereas available evidence suggests the contrary, i.e., that competitors interact directly through aggression and that the interactions are characteristically asymmetrical. The NCH predicts that speciose tropical faunas and floras should exhibit high β-diversity and only moderate α-diversity, whereas empirical data from the Amazon reveal the opposite for both birds and trees: high α- and low β-diversity. Finally, resource competition depends on strong density dependence at population levels near K, whereas the densities of many species in the wild appear to be as low as 0.1 K.
By including top-down as well as bottom-up forcing in the model, these contradictions disappear. The notion that predation regulates diversity is an old one in ecology, dating to the 1960s, if not before (125). In fact, a number of authors explicitly proposed decades ago that competition and predation interact to regulate diversity (19, 126–129). Subsequent neglect of predation as a vital structuring force in nature was a casualty of the huge popularity of the Hutchison–MacArthur bottom-up tradition abetted by the imposing logistical difficulties inherent in the implementation of predator removal experiments. Widespread predator persecution and fortuitous experiments like the construction of Lago Guri, along with a few controlled manipulations, have now given us a convincing body of evidence confirming that predator removal inexorably leads to powerful cascading effects that include loss of biodiversity and transitions to alternative ecosystem states.
To conclude that competition structures hyperdiverse plant and animal communities, one would have to postulate that hundreds of species all had more or less equivalent competitive rank stably maintained over generations, and that these features of equality and stability of outcomes were virtually ubiquitous around the tropics in all manner of communities and ecosystems. This is more or less what Hubbell (4, 5) proposed with neutral theory, but that has been challenged on a number of grounds and thoroughly refuted (130–135).
Both bottom-up and top-down processes operate interactively in intact natural ecosystems. About this there is no argument. The thesis presented here is that the interaction of bottom-down and top-down processes regulates species diversity in ecological space/time, as depicted in Fig. 1.
By considering the ecological regulation of diversity as an interactive process involving multiple trophic levels, many conclusions reached are opposite to those that emerge from classical competition theories. Notably, high α-diversity, assumed by bottom-up models to imply the existence of strong competition, is instead the consequence of relaxed competitive interactions. β-Diversity is a better indicator of competition at the landscape scale. Islands, long thought to be havens of diminished competition for birds and other animals, are here viewed instead as environments of low predation pressure and weakened trophic cascades. Consequently, interspecific competition in island faunas is predicted to be greater than on corresponding mainlands. Finally, diversity gradients accompany gradients in latitude, seasonality, rainfall, and elevation. Does the predation/competition trade-off also vary in a systematic fashion along these environmental gradients? Answering the question stands as an important goal for future research.
Acknowledgments
I owe the ideas imparted here to 50 y of experience in the primary forests of South America and to my many students, collaborators, and assistants. Servicio Nacional de Áreas Naturales Protegidas (Peru) and La Comisión Nacional de Investigación Científica y Tecnológica (Venezuela) are thanked for authorizing our research year after year. I am grateful to James Estes, Robert Holt, Robert May, Robert Paine, Oswald Schmitz, and two anonymous reviewers for reading the manuscript and providing much valued input. Financial support of the MacArthur Foundation, Andrew Mellon Foundation, and National Science Foundation is gratefully acknowledged.
Footnotes
The author declares no conflict of interest.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century. See the companion article “Analytical Note on Certain Rhythmic Relations in Organic Systems” on page 410 in issue 7 of volume 6 and see Anniversary Profile on page 9493 in issue 31 of volume 112.
This article is a PNAS Direct Submission.
References
- 1.MacArthur RH. On the relative abundance of bird species. Proc Natl Acad Sci USA. 1957;43(3):293–295. doi: 10.1073/pnas.43.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston FW. The canonical distribution of commonness and rarity: Part I. Ecology. 1962;43(2):185–215. [Google Scholar]
- 3.Whittaker RH. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science. 1965;147(3655):250–260. doi: 10.1126/science.147.3655.250. [DOI] [PubMed] [Google Scholar]
- 4.Hubbell SP. Tree dispersion, abundance, and diversity in a tropical dry forest. Science. 1979;203(4387):1299–1309. doi: 10.1126/science.203.4387.1299. [DOI] [PubMed] [Google Scholar]
- 5.Hubbell SH. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Univ Press; Princeton: 2001. [Google Scholar]
- 6.Connell JH. Diversity in tropical rain forests and coral reefs. Science. 1978;199(4335):1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 7.Chesson PL, Warner RW. Environmental variability promotes coexistence in lottery competitive systems. Am Nat. 1981;117:923–943. [Google Scholar]
- 8.Tilman D, Pacala S. In: The maintenance of species richness in plant communities. Species Diversity in Ecological Communities. Ricklefs RE, Schluter D, editors. Univ of Chicago Press; Chicago: 1993. pp. 13–25. [Google Scholar]
- 9.Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. [Google Scholar]
- 10.Hurtt GC, Pacala SW. The consequences of recruitment limitation: Reconciling chance, history and competitive differences between plants. J Theor Biol. 1995;176:1–12. [Google Scholar]
- 11.Clark JS. Individuals and the variation needed for high species diversity in forest trees. Science. 2010;327(5969):1129–1132. doi: 10.1126/science.1183506. [DOI] [PubMed] [Google Scholar]
- 12.Lotka AJ. Elements of Physical Biology. Williams and Wilkins; Baltimore: 1925. [Google Scholar]
- 13.Volterra V. Variazione e fluttuazioni de numero d’individui in specie animali conviventi. Mem Acad Lincei. 1926;2:31–113. [Google Scholar]
- 14.Hutchinson GE. Homage to Santa Rosalia, or why are there so many kinds of animals. Am Nat. 1959;93:145–159. [Google Scholar]
- 15.MacArthur RH, MacArthur J. On bird species diversity. Ecology. 1961;42:594–598. [Google Scholar]
- 16.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton Univ Press; Princeton: 1967. [Google Scholar]
- 17.Tilman D. Resource Competition and Community Structure. Princeton Univ Press; Princeton: 1982. [PubMed] [Google Scholar]
- 18.Brown JH. Why are there so many species in the tropics? J Biogeogr. 2014;41(1):8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 20.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 21.Connell JH. In: On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dynamics of Populations. den Boer PJ, Gradwell GR, editors. Centre for Agricultural Publications and Documentation; Wageningen, The Netherlands: 1971. pp. 298–310. [Google Scholar]
- 22.Menge BA, Sutherland JP. Species diversity gradients: Synthesis of the roles of predation, competition and temporal heterogeneity. Am Nat. 1976;110(973):351–369. [Google Scholar]
- 23.Givnish TJ. On the causes of gradients in tropical tree diversity. J Ecol. 1999;87:193–210. [Google Scholar]
- 24.Chesson P, Kuang JJ. The interaction between predation and competition. Nature. 2008;456(7219):235–238. doi: 10.1038/nature07248. [DOI] [PubMed] [Google Scholar]
- 25.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 26.Lotka AJ. Analytical note on certain rhythmic relations in organic systems. Proc Natl Acad Sci USA. 1920;6(7):410–415. doi: 10.1073/pnas.6.7.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotka AJ. Contribution to the mathematical theory of capture: Conditions for capture. Proc Natl Acad Sci USA. 1932;18(2):172–178. doi: 10.1073/pnas.18.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindemann RL. The trophic-dynamic aspect of ecology. Ecology. 1944;23:399–418. [Google Scholar]
- 29.Odum HT. Trophic structure and productivity of Silver Springs, Florida. Ecol Monogr. 1957;27:55–112. [Google Scholar]
- 30.Odum EP, Smalley AE. Comparison of population energy flow of a herbivorous and a deposit-feeding invertebrate in a salt marsh ecosystem. Proc Natl Acad Sci USA. 1959;45(4):617–622. doi: 10.1073/pnas.45.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacArthur RH. Geographical Ecology: Patterns in the Distribution of Species. Harper and Row; New York: 1972. [Google Scholar]
- 32.MacArthur R, Levins R. The limiting similarity, convergence and divergence of coexisting species. Am Nat. 1967;101:377–385. [Google Scholar]
- 33.MacArthur R. Species packing and competitive equilibrium for many species. Theor Popul Biol. 1970;1(1):1–11. doi: 10.1016/0040-5809(70)90039-0. [DOI] [PubMed] [Google Scholar]
- 34.May RM, MacArthur RH. Niche overlap as a function of environmental variability. Proc Natl Acad Sci USA. 1972;69(5):1109–1113. doi: 10.1073/pnas.69.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roughgarden J. Species packing and the competition function with illustrations from coral reef fish. Theor Popul Biol. 1974;5(2):163–186. doi: 10.1016/0040-5809(74)90039-2. [DOI] [PubMed] [Google Scholar]
- 36.Diamond JM. Distributional ecology of New Guinea birds: Recent ecological and biogeographical theories can be tested on the bird communities of New Guinea. Science. 1973;179(4075):759–769. doi: 10.1126/science.179.4075.759. [DOI] [PubMed] [Google Scholar]
- 37.Terborgh J. Distribution on environmental gradients: Theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Perú. Ecology. 1971;52:23–40. [Google Scholar]
- 38.Jaeger RG. Competitive exclusion as a factor influencing the distributions of two species of terrestrial salamanders. Ecology. 1971;52:535–546. doi: 10.2307/1934151. [DOI] [PubMed] [Google Scholar]
- 39.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185(4145):27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 40.Robinson SK, Terborgh J. Interspecific aggression and habitat selection by Amazonian birds. J Anim Ecol. 1995;64:1–11. [Google Scholar]
- 41.Jankowski JE, Robinson SK, Levey DJ. Squeezed at the top: Interspecific aggression may constrain elevational ranges in tropical birds. Ecology. 2010;91(7):1877–1884. doi: 10.1890/09-2063.1. [DOI] [PubMed] [Google Scholar]
- 42.Pasch B, Bolker BM, Phelps SM. Interspecific dominance via vocal interactions mediates altitudinal zonation in neotropical singing mice. Am Nat. 2013;182(5):E161–E173. doi: 10.1086/673263. [DOI] [PubMed] [Google Scholar]
- 43.MacArthur RH. Patterns of species diversity. Biol Rev Camb Philos Soc. 1965;40:510–533. [Google Scholar]
- 44.MacArthur RH, Recher H, Cody M. On the relation between habitat selection and species diversity. Am Nat. 1966;100:319–332. [Google Scholar]
- 45.Robinson SK, Terborgh J. In: Lowland tropical forest bird communities of a site in western Amazonia. Biogeography and Ecology of Forest Bird Communities. Keast A, editor. W. Junk; The Hague, The Netherlands: 1990. pp. 229–258. [Google Scholar]
- 46.Terborgh J, Robinson SK, Parker TA, III, Munn CA, Pierpont N. Structure and organization of an Amazonian forest bird community. Ecol Monogr. 1990;60:213–238. [Google Scholar]
- 47.Pitman NC, Terborgh J, Silman MR, Nuñez VP. Tree species distributions in an upper Amazonian forest. Ecology. 1999;80:2651–2661. [Google Scholar]
- 48.Wittmann F, et al. Habitat specificity, endemism and the neotropical distriution of Amazonian white-water floodplain trees. Ecography. 2013;36:590–707. [Google Scholar]
- 49.Holt RD. Trophic cascades in terrestrial ecosystems. Reflections on Polis et al. Trends Ecol Evol. 2000;15(11):444–445. doi: 10.1016/s0169-5347(00)01991-1. [DOI] [PubMed] [Google Scholar]
- 50.Terborgh J, Feeley K. In: Ecosystem decay in closed forest fragments. Tropical Forest Community Ecology. Carson WP, Schnitzer SA, editors. Wiley-Blackwell Publishing; Oxford: 2008. pp. 308–321. [Google Scholar]
- 51.Terborgh J, Lopez L, Tello J, Yu D, Bruni AR. In: Transitory states in relaxing ecosystems of land bridge islands. Tropical Forest Remnants. Laurance WF, Bierregaard RO Jr, editors. Univ of Chicago Press; Chicago: 1997. pp. 256–274. [Google Scholar]
- 52.Terborgh J, Lopez L, Tello SJ. Bird communities in transition: The Lago Guri Islands. Ecology. 1997;78:1494–1501. [Google Scholar]
- 53.Lambert TD, et al. Rodents on tropical land-bridge islands. J Zool (Lond) 2003;260:179–187. [Google Scholar]
- 54.Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294(5548):1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 55.Aponte C, Barreto GR, Terborgh J. Consequences of habitat fragmentation on age structure and life history in a tortoise population. Biotropica. 2003;35:550–555. [Google Scholar]
- 56.Rao M, Terborgh J, Nuñez P. Increased herbivory in forest isolates: Implications for plant community structure and composition. Conserv Biol. 2001;15:624–633. [Google Scholar]
- 57.MacArthur RH, Diamond J, Karr J. Density compensation in island faunas. Ecology. 1972;53:330–342. [Google Scholar]
- 58.Fryxell JM, Greever J, Sinclair ARE. Why are migratory ungulates so abundant? Am Nat. 1988;131:781–798. [Google Scholar]
- 59.Hopcraft JGC, Olff H, Sinclair ARE. Herbivores, resources and risks: Alternating regulation along primary environmental gradients in savannas. Trends Ecol Evol. 2010;25(2):119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Paine RT. Food webs: Linkage, interaction strength and community infrastructure. J Anim Ecol. 1980;49:666–685. [Google Scholar]
- 61.Power ME, et al. Challenges in the quest for keystones. BioScience. 1996;46:609–620. [Google Scholar]
- 62.Abrams P. The theory of limiting similarity. Annu Rev Ecol Syst. 1983;14:359–376. [Google Scholar]
- 63.Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol Evol. 2003;18:648–656. [Google Scholar]
- 64.Estes JA, Palmisano JF. Sea otters: Their role in structuring nearshore communities. Science. 1974;185(4156):1058–1060. doi: 10.1126/science.185.4156.1058. [DOI] [PubMed] [Google Scholar]
- 65.Stenek RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 66.Power ME, Matthews WJ, Stewart AJ. Grazing minnows, piscivorous bass and stream algae: Dynamics of a strong interaction. Ecology. 1985;66:1448–1456. [Google Scholar]
- 67.Power ME. Top-down and bottom-up forces in food webs: Do plants have primacy? Ecology. 1992;73(3):733–746. [Google Scholar]
- 68.Carpenter SR, Kitchell JF. The Trophic Cascade in Lakes. Cambridge Univ Press; Cambridge, UK: 1983. [Google Scholar]
- 69.Henke SE, Bryant FC. Effects of coyote removal on the faunal community in western Texas. J Wildl Manage. 1999;63(4):1066–1081. [Google Scholar]
- 70.Gibson L, et al. Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science. 2013;341(6153):1508–1510. doi: 10.1126/science.1240495. [DOI] [PubMed] [Google Scholar]
- 71.Towns DR, Atkinson IAE, Daugherty H. Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions. 2006;8:863–891. [Google Scholar]
- 72.Sinclair ARE, Mduma S, Brashares JS. Patterns of predation in a diverse predator-prey system. Nature. 2003;425(6955):288–290. doi: 10.1038/nature01934. [DOI] [PubMed] [Google Scholar]
- 73.Emmons LH. Comparative feeding ecology of felids in a Neotropical rainforest. Behav Ecol Sociobiol. 1987;20:271–283. [Google Scholar]
- 74.Holt RD. Predation, apparent competition, and the structure of prey communities. Theor Popul Biol. 1977;12(2):197–29. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 75.Terborgh J. Maintenance of diversity in tropical forests. Biotrop. 1992;24:283–292. [Google Scholar]
- 76.Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- 77.Brashares JS, et al. In: Ecological and conservation implications of mesopredator release. Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Terborgh J, Estes JA, editors. Island Press; Washington, DC: 2010. pp. 221–240. [Google Scholar]
- 78.Spiller DA, Schoener TW. A terrestrial field experiment showing the impact of eliminating top predators on foliage damage. Nature. 1990;347:469–472. [Google Scholar]
- 79.Polis GA, Sears AL, Huxel GR, Strong DR, Maron J. When is a trophic cascade a trophic cascade? Trends Ecol Evol. 2000;15(11):473–475. doi: 10.1016/s0169-5347(00)01971-6. [DOI] [PubMed] [Google Scholar]
- 80.Ripple WJ, Rooney TP, Beschta RL. In: Large predators, deer, and trophic cascades in boreal and temperate ecosystems. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Terborgh J, Estes JA, editors. Island Press; Washington, DC: 2010. pp. 141–161. [Google Scholar]
- 81.Terborgh J, Nuñez VP, Balukjian B, Silman M. Vegetation dynamics of predator-free land-bridge islands. J Ecol. 2006;94:253–263. [Google Scholar]
- 82.Ripple WJ, Beschta RL. Linking a cougar decline, trophic cascade, and catastrophic regime shift in Zion National Park. Biol Conserv. 2006;133:397–408. [Google Scholar]
- 83.Rooney TP. High white-tailed deer densities benefit graminoids and contribute to biotic homogenization of forest ground-layer vegetation. Plant Ecol. 2009;202:103–111. [Google Scholar]
- 84.Brown JH, Heske EJ. Control of a desert-grassland transition by a keystone rodent guild. Science. 1990;250(4988):1705–1707. doi: 10.1126/science.250.4988.1705. [DOI] [PubMed] [Google Scholar]
- 85.Aunapuu M, et al. Spatial patterns and dynamic responses of arctic food webs corroborate the exploitation ecosystems hypothesis (EEH) Am Nat. 2008;171(2):249–262. doi: 10.1086/524951. [DOI] [PubMed] [Google Scholar]
- 86.Silliman BR, Bertness MD. A trophic cascade regulates salt marsh primary production. Proc Natl Acad Sci USA. 2002;99(16):10500–10505. doi: 10.1073/pnas.162366599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;404(6775):278–281. doi: 10.1038/35005072. [DOI] [PubMed] [Google Scholar]
- 88.Johnson DJ, Beaulieu WT, Bever JD, Clay K. Conspecific negative density dependence and forest diversity. Science. 2012;336(6083):904–907. doi: 10.1126/science.1220269. [DOI] [PubMed] [Google Scholar]
- 89.Carson WP, Anderson JT, Leigh EG, Jr, Schnitzer SA. In: Challenges associated with testing and falsifying the Janzen-Connell Hypothesis: a review and critique. Tropical Forest Community Ecology. Carson WP, Schnitzer SA, editors. Wiley-Blackwell; Oxford: 2008. pp. 210–241. [Google Scholar]
- 90.Alvarez-Loayza P, Terborgh J. Fates of seedling carpets in an Amazonian floodplain forest. J Ecol. 2011;99:1045–1054. [Google Scholar]
- 91.Terborgh J. Enemies maintain hyperdiverse tropical forests. Am Nat. 2012;179(3):303–314. doi: 10.1086/664183. [DOI] [PubMed] [Google Scholar]
- 92.Bagchi R, et al. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature. 2014;506(7486):85–88. doi: 10.1038/nature12911. [DOI] [PubMed] [Google Scholar]
- 93.Hammond DS, Brown VK. In: Disturbance, phenology and life-history characteristics: factors influencing distance/density-dependent attack on tropical seeds and seedlings. Dynamics of Tropical Communities. Newbery DM, Prins HHT, Brown ND, editors. Blackwell Scientific; Oxford: 1996. pp. 51–78. [Google Scholar]
- 94.Marhaver KL, Vermeij MJA, Rohwer F, Sandin SA. Janzen-Connell effects in a broadcast-spawning Caribbean coral: Distance-dependent survival of larvae and settlers. Ecology. 2013;94(1):146–160. doi: 10.1890/12-0985.1. [DOI] [PubMed] [Google Scholar]
- 95.Zaret TM, Paine RT. Species introduction in a tropical lake: A newly introduced piscivore can produce population changes in a wide range of trophic levels. Science. 1973;182(4111):449–455. doi: 10.1126/science.182.4111.449. [DOI] [PubMed] [Google Scholar]
- 96.Witte F, et al. The destruction of an endemic species flock: Quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fishes. 1992;34:1–28. [Google Scholar]
- 97.Smith AP, Quinn DG. Patterns and causes of extinction and decline in Australian conilurine rodents. Biol Conserv. 1996;77:243–267. [Google Scholar]
- 98.O’Donnell CFJ. Predators and the decline of New Zealand forest birds: An introduction to the hole-nesting and predator programme. NZ J Zool. 1996;23:213–219. [Google Scholar]
- 99.Steadman DW. Prehistoric extinctions of pacific island birds: Biodiversity meets zooarchaeology. Science. 1995;267(5201):1123–1131. doi: 10.1126/science.267.5201.1123. [DOI] [PubMed] [Google Scholar]
- 100.Savage JA. Extinction of an island forest avifauna by an introduced snake. Ecology. 1987;68:660–668. [Google Scholar]
- 101.Dorcas ME, et al. Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park. Proc Natl Acad Sci USA. 2012;109(7):2418–2422. doi: 10.1073/pnas.1115226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burney DA, Flannery TF. Fifty millennia of catastrophic extinctions after human contact. Trends Ecol Evol. 2005;20(7):395–401. doi: 10.1016/j.tree.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 103.Martin PS. The discovery of America: The first Americans may have swept the Western Hemisphere and decimated its fauna within 1000 years. Science. 1973;179(4077):969–974. doi: 10.1126/science.179.4077.969. [DOI] [PubMed] [Google Scholar]
- 104.Leigh EG, Jr, Hladik A, Hladik CM, Jolly A. The biogeography of large islands, or how does the size of the ecological theatre affect the evolutionary play? Rev Ecol. 2007;62:105–168. [Google Scholar]
- 105.Milchunas DG, Lauenroth WK. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr. 1993;63:327–366. [Google Scholar]
- 106.Terborgh J. In: The trophic cascade on islands. The Theory of Island Biogeography Revisited. Losos JB, Ricklefs RE, editors. Princeton Univ Press; Princeton: 2009. pp. 116–142. [Google Scholar]
- 107.Krug AZ, Jablonski D, Valentine JW. Species-genus ratios reflect a global history of diversification and range expansion in marine bivalves. Proc Biol Sci. 2008;275(1639):1117–1123. doi: 10.1098/rspb.2007.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hairston NG. Species packing in Desmognathus salamanders: Experimental demonstration of predation and competition. Am Nat. 1986;127:266–291. [Google Scholar]
- 109.Gurevitch J, Morrison JA, Hedges LV. The interaction between competition and predation: A meta-analysis of field experiments. Am Nat. 2000;155(4):435–453. doi: 10.1086/303337. [DOI] [PubMed] [Google Scholar]
- 110.Werner EE, Gilliam JF, Hall DJ, Mittlebach GG. An experimental test of the effects of predation risk on habitat use in fish. Ecology. 1983;64:1540–1548. [Google Scholar]
- 111.Miller JRB, Ament JM, Schmitz OJ. Fear on the move: Predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J Anim Ecol. 2014;83(1):214–222. doi: 10.1111/1365-2656.12111. [DOI] [PubMed] [Google Scholar]
- 112.Sargeant AB, Allen SH. Observed interactions between coyotes and red foxes. J Mammal. 1989;70(3):631–633. [Google Scholar]
- 113.Ottersen G, et al. Ecological effects of the North Atlantic Oscillation. Oecologia. 2001;128:1–14. doi: 10.1007/s004420100655. [DOI] [PubMed] [Google Scholar]
- 114.Korpimäki E, Krebs CJ. Predation and population cycles of small mammals. BioSci. 1996;46:754–764. [Google Scholar]
- 115.Carson WP, Root RB. Herbivory and plant species coexistence: Community regulation by an outbreaking phytophageous insect. Ecol Monogr. 2000;70:73–99. [Google Scholar]
- 116.Oksanen L. Trophic exploitation and arctic phytomass patterns. Am Nat. 1983;122:45–52. [Google Scholar]
- 117.Milchunas DG, Salas OE, Lauenroth WK. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am Nat. 1988;132(1):87–106. [Google Scholar]
- 118.Berger J. In: Fear-mediated food webs. Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Terborgh J, Estes JA, editors. Island Press; Washington, DC: 2010. pp. 241–253. [Google Scholar]
- 119.Chase JM, et al. The interaction between predation and competition: A review and synthesis. Ecol Lett. 2002;5:302–315. [Google Scholar]
- 120.Roughgarden J, Feldman M. Species packing and predation pressure. Ecology. 1975;56:489–492. [Google Scholar]
- 121.Holt RD. Density-dependent mortality, non-linear competitive interactions and species coexistence. J Theor Biol. 1985;116:479–493. [Google Scholar]
- 122.Chesson P. Mechanisms of maintenance of species coexistence. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 123.Kuang JJ, Chesson P. Interacting coexistence mechanisms in annual plant communities: Frequency-dependent predation and the storage effect. Theor Popul Biol. 2010;77(1):56–70. doi: 10.1016/j.tpb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 124.Holt RH. Prey communities in patchy environments. Oikos. 1987;50:276–290. [Google Scholar]
- 125.Hairston NG, Smith FE, Slobodkin LB. Community structure, population control and competition. Am Nat. 1960;94:421–425. [Google Scholar]
- 126.Harper JL. In: The role of predation in vegetational diversity. Diversity and Stability in Ecological Systems. Woodwell GM, Smith HH, editors. Brookhaven National Laboratory; Upton, NY: 1969. pp. 48–61. [PubMed] [Google Scholar]
- 127.Connell JH. A predator-prey system in the marine region. I. Balanus glandula and several predatory species of Thais. Ecol Monogr. 1970;40:49–78. [Google Scholar]
- 128.Dayton PK. Competition, disturbance, and community organization: The provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr. 1971;41:351–389. [Google Scholar]
- 129.Lubchenco J. Diversity in a marine intertidal community: Importance of herbivore food preference and algal competitive abilities. Am Nat. 1978;112:23–39. [Google Scholar]
- 130.Terborgh J, Foster RB, Nuñez VP. Tropical tree communities: A test of the non-equilibrium hypothesis. Ecology. 1996;77:561–567. [Google Scholar]
- 131.Yu DW, Terborgh JW, Potts MD. Can high tree species richness be explained by Hubbell’s null model? Ecol Lett. 1998;1:193–199. [Google Scholar]
- 132.McGill BJ. A test of the unified neutral theory of biodiversity. Nature. 2003;422(6934):881–885. doi: 10.1038/nature01583. [DOI] [PubMed] [Google Scholar]
- 133.Dornelas M, Connolly SR, Hughes TP. Coral reef diversity refutes the neutral theory of biodiversity. Nature. 2006;440(7080):80–82. doi: 10.1038/nature04534. [DOI] [PubMed] [Google Scholar]
- 134.Clark JS. Beyond neutral science. Trends Ecol Evol. 2009;24(1):8–15. doi: 10.1016/j.tree.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Ricklefs RE, Renner SS. Global correlations in tropical tree species richness and abundance reject neutrality. Science. 2012;335(6067):464–467. doi: 10.1126/science.1215182. [DOI] [PubMed] [Google Scholar]