Significance
Bacterial resistance to clinically relevant antibiotics has renewed public interest in identifying therapeutics with new scaffolds for the treatment of such infections. Analogs of orthosomycins could provide one such scaffold. One route to modifying these scaffolds is through rational engineering of the biosynthetic enzymes, requiring characterization of the biosynthetic pathway. A key feature of orthosomycin antibiotics is the orthoester linkage between carbohydrate groups, and our data suggest that a family of oxygenases is likely responsible for orthoester formation.
Keywords: antibiotic biosynthesis, oxidative cyclization, crystal structure, nonheme iron α-ketoglutarate–dependent oxygenases
Abstract
Orthosomycins are oligosaccharide antibiotics that include avilamycin, everninomicin, and hygromycin B and are hallmarked by a rigidifying interglycosidic spirocyclic ortho-δ-lactone (orthoester) linkage between at least one pair of carbohydrates. A subset of orthosomycins additionally contain a carbohydrate capped by a methylenedioxy bridge. The orthoester linkage is necessary for antibiotic activity but rarely observed in natural products. Orthoester linkage and methylenedioxy bridge biosynthesis require similar oxidative cyclizations adjacent to a sugar ring. We have identified a conserved group of nonheme iron, α-ketoglutarate–dependent oxygenases likely responsible for this chemistry. High-resolution crystal structures of the EvdO1 and EvdO2 oxygenases of everninomicin biosynthesis, the AviO1 oxygenase of avilamycin biosynthesis, and HygX of hygromycin B biosynthesis show how these enzymes accommodate large substrates, a challenge that requires a variation in metal coordination in HygX. Excitingly, the ternary complex of HygX with cosubstrate α-ketoglutarate and putative product hygromycin B identified an orientation of one glycosidic linkage of hygromycin B consistent with metal-catalyzed hydrogen atom abstraction from substrate. These structural results are complemented by gene disruption of the oxygenases evdO1 and evdMO1 from the everninomicin biosynthetic cluster, which demonstrate that functional oxygenase activity is critical for antibiotic production. Our data therefore support a role for these enzymes in the production of key features of the orthosomycin antibiotics.
Most currently available antibiotics are based upon one of a few scaffolds, with variants targeting the same biological process and having a common mechanism (1). However, bacterial resistance to structurally related compounds can occur rapidly and shorten the lifetime of a new antibiotic (2). Orthosomycins exhibit broad spectrum antibiotic properties against bacterial pathogens and hold therapeutic promise as they have a scaffold differing from other antibiotics and act on alternative targets. The orthosomycin everninomicin binds a unique site on the ribosome (3, 4) and is effective against resistant bacteria including methicillin resistant Staphylococcus aureus and vancomycin resistant enterococci (5). The everninomicin A congener (Ziracin) reached phase III clinical trials, but pharmacological complications prevented approval. However, everninomicin analogs or other natural congeners may still prove useful.
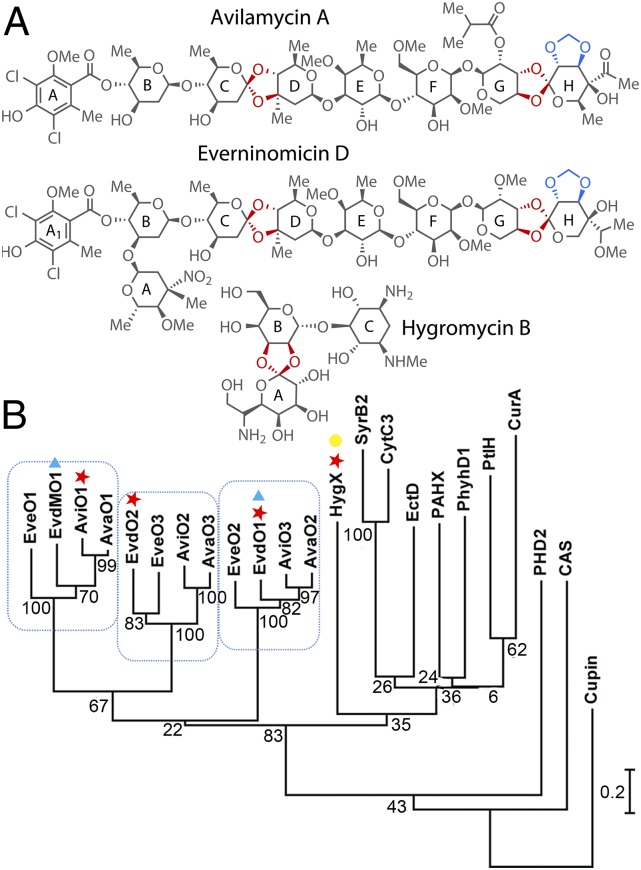
Produced by actinomycetes, orthosomycins are decorated oligosaccharides containing interglycosidic spirocyclic ortho-δ-lactone (orthoester) linkages, an uncommon functional group that enforces the interglycosidic angle necessary for antibiotic activity. The first orthosomycin described was hygromycin B (also called destomycin C), which was isolated from Streptomyces hygroscopicus in 1958 (6) and has one orthoester linkage (Fig. 1A, red). More complex orthosomycins include everninomicin and avilamycin, which each contain two orthoester linkages (Fig. 1A, red) and a methylenedioxy bridge (Fig. 1A, blue). Methylenedioxy bridge formation has previously been attributed to cytochrome P450 enzymes (7, 8). However, orthoester linkage biosynthesis has not previously been described. Orthoester linkage formation is associated with especially demanding steric constraints and substrates that are already highly oxygenated, and its biosynthesis results in a unique trioxaspiro functional group.
Fig. 1.
Orthosomycins and associated oxygenases. (A) Orthosomycins with orthoester linkages and methylenedioxy bridges in red. (B) Oxygenase phylogenetic analysis. Enzymes characterized structurally (red ★), through gene disruption (blue ▲), and for binding affinity (yellow ●) are indicated.
Everninomicin and avilamycin biosynthetic gene clusters encode upwards of 50 enzymes with many lacking assigned function. It has been suggested that within the avilamycin biosynthetic cluster in Streptomyces viridochromogenes, a set of putative α-ketoglutarate, nonheme iron-dependent [AKG/Fe(II)-dependent] oxygenases may be capable of catalyzing both orthoester linkage and methylenedioxy bridge formation (9, 10). AKG/Fe(II)-dependent oxygenases are powerful oxidants that catalyze a range of reactions, including oxidative cyclizations, hydroxylations, peroxidations, epoxidations, desaturations, and halogenations (11–14). This reactivity is suited to orthoester linkage and methylenedioxy bridge biosynthesis. However, practical challenges, including lack of knowledge of substrate identity and the difficulty in genetic manipulation of the producing organisms, have precluded validation of this prediction. We overcame these challenges by using the availability of closely related homologs (>70% identity) that synthesize different congeners of everninomicin and avilamycin. By combining our genomic and structural data (15), we propose that AKG/Fe(II)-dependent oxygenases can catalyze orthoester linkage formation.
Results
Comparative Genomic Analysis of AKG/Fe(II)-Dependent Oxygenases.
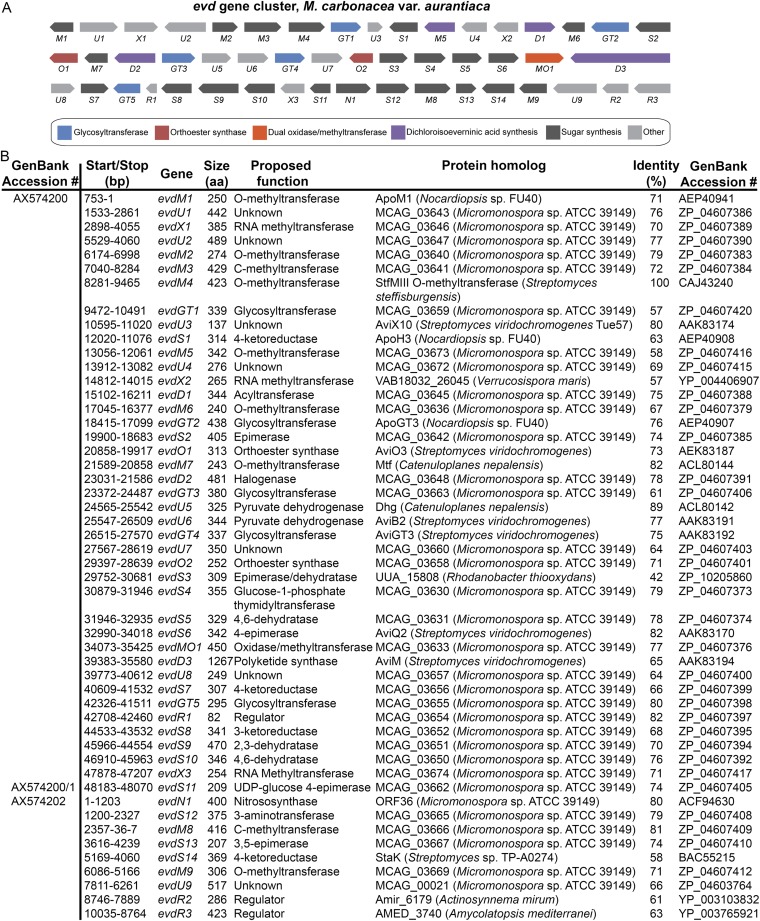
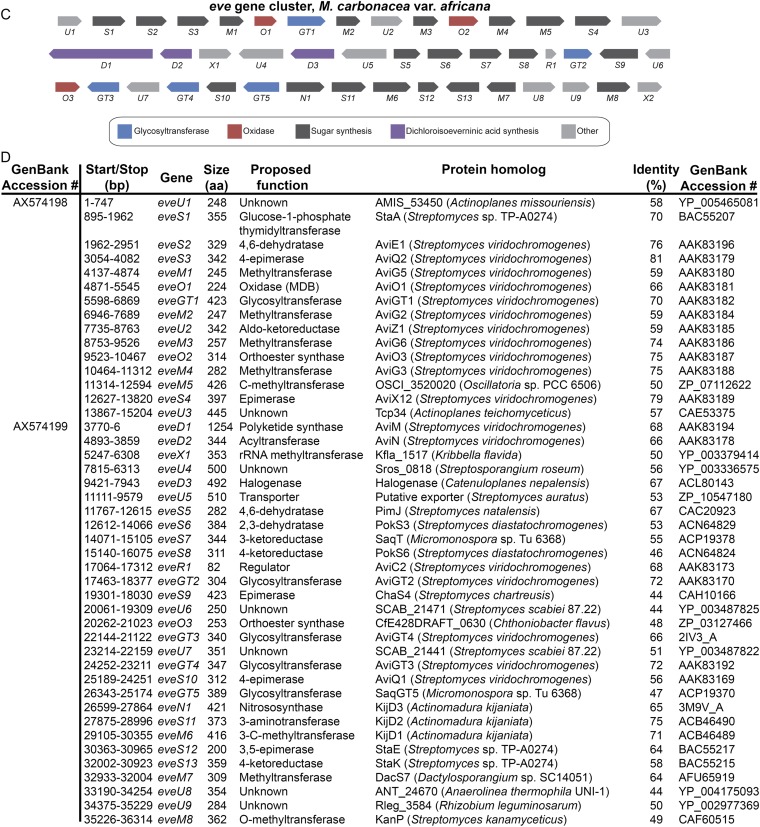
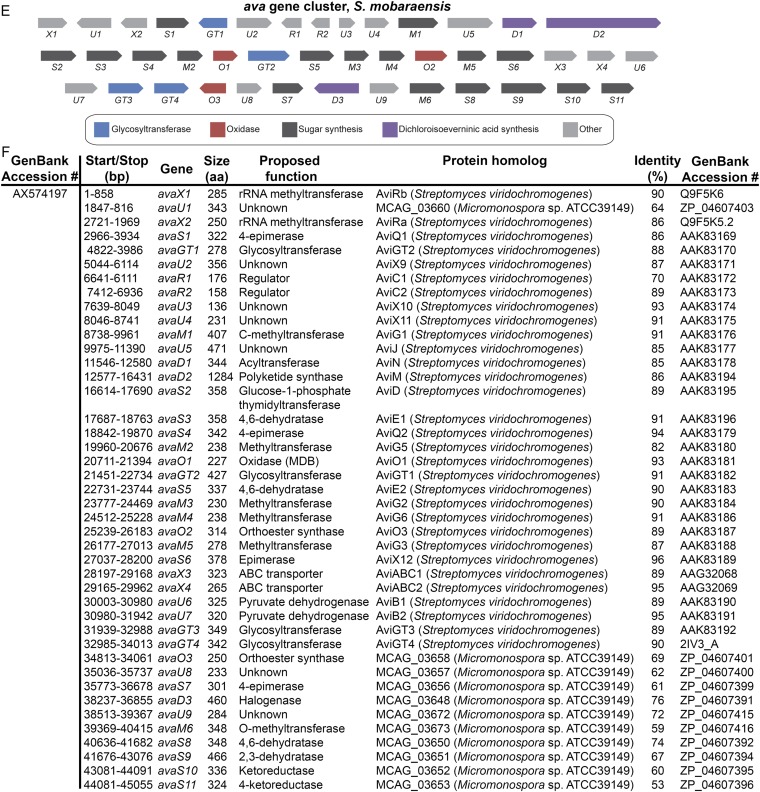
Our studies use five orthosomycin biosynthetic gene clusters available in GenBank: ava (avilamycin biosynthesis from Streptomyces mobaraensis), avi (avilamycin biosynthesis from S. viridochromogenes Tü57) (10), evd (everninomicin biosynthesis from Micromonospora carbonacea var. aurantiaca), eve (everninomicin biosynthesis from M. carbonacea var. africana), and hyg (hygromycin B biosynthesis from S. hygroscopicus). Of these, only the avi and hyg gene clusters include functional annotation. We therefore used comparative genomics to propose functions for the ava, evd, and eve clusters (Fig. S1). Each cluster contains ORFs encoding enzymes expected in oligosaccharide production, including putative glycosyltransferases, aminotransferases, sugar synthases, and sugar dehydrogenases. Of particular interest are 13 ORFs encoding putative AKG/Fe(II)-dependent oxygenases: three in each of the ava, avi, evd, and eve gene clusters and one in the hyg gene cluster. The number of putative oxygenases correlates with how many anticipated oxidative cyclizations are required for orthoester linkage and methylenedioxy bridge formation in each antibiotic. Furthermore, these are the only enzymes within each gene cluster that appear to have sufficient catalytic capacity for such oxidations.
Fig. S1.
Pictorial annotation and deduced functional assignment for ORFs of the evd, eve, and ava gene clusters. Using antiSMASH (antismash.secondarymetabolites.org), ORFs were identified from GenBank nucleotide sequences. Each ORF was analyzed using Translated BLAST (Blastx). Based on the function of homologous proteins, gene names were assigned and functions of genes were proposed. (A) Pictorial annotation of the evd gene cluster colored by proposed function in orthosomycin biosynthesis. (B) Deduced evd gene functions and nearest homologs for each ORF. (C) Pictorial annotation of the eve gene cluster colored by proposed function in orthosomycin biosynthesis. (D) Deduced eve gene functions and nearest homologs for each ORF. (E) Pictorial annotation of the ava gene cluster colored by proposed function in orthosomycin biosynthesis. (F) Deduced ava gene functions and nearest homologs for each ORF.
Phylogeny of AKG/Fe(II) Oxygenases of Orthosomycin Biosynthesis.
Phylogenetic analysis of the 13 putative AKG/Fe(II)-dependent oxygenases of avilamycin, everninomicin, and hygromycin B biosynthesis demonstrates that these enzymes form a distinct subfamily that contains the 13 oxygenases studied here as well as several as-yet-uncharacterized close homologs (a subset is included in Fig. S1). This orthosomycin-associated subfamily is most closely related to the phytanoyl-CoA 2-hydroxylase (PhyH) subfamily of AKG/Fe(II) oxygenases (16–19). Our analysis further separates the orthosomycin-associated oxygenases into subgroups (Fig. 1B). The first three subgroups contain one oxygenase from each avilamycin and everninomicin gene cluster, whereas the fourth subgroup contains only HygX. Sequence identity between enzymes of different subgroups is 12–40%, which is consistent with action on distinct substrates. Enzymes belonging to the same subgroup exhibit sequence identities of 70–94%, suggesting that they catalyze the same reaction on either closely related or identical substrates (20). Sequence identities in this range further suggest that an enzyme can represent a subgroup.
Structures of S. viridochromogenes AviO1, M. carbonacea EvdO1 and EvdO2, and S. hygroscopicus HygX Oxygenases.
We determined crystal structures for a representative of each phylogenetic subgroup (Fig. 2A, Fig. S2 A–C, and Tables S1 and S2). Each enzyme adopts the double stranded β-helix motif with the active site housing a metallocenter between β-sheets containing antiparallel β-strands. Despite conservation of fold, the oligomerization state varies, with AviO1 and EvdO2 monomers, EvdO1 a dimer, and HygX a tetramer. Structural similarity searches support our sequence analysis suggesting that the orthosomycin-associated oxygenases are related to the PhyH subfamily of AKG/Fe(II)-dependent oxygenases (16–19). Of note, the halogenases SyrB2 and CytC3 cluster near the orthosomycin-associated oxygenases (13, 21).
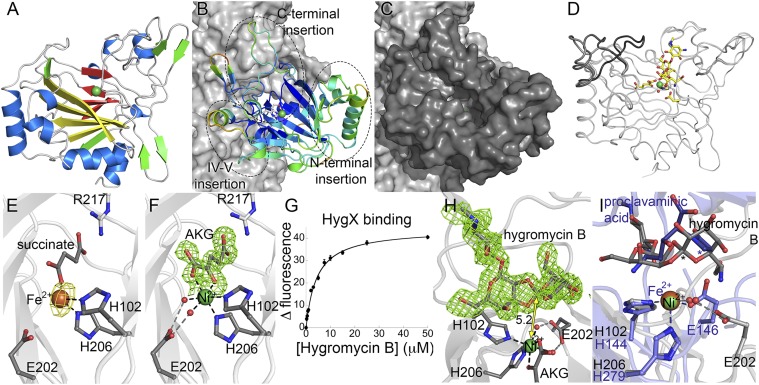
Fig. 2.
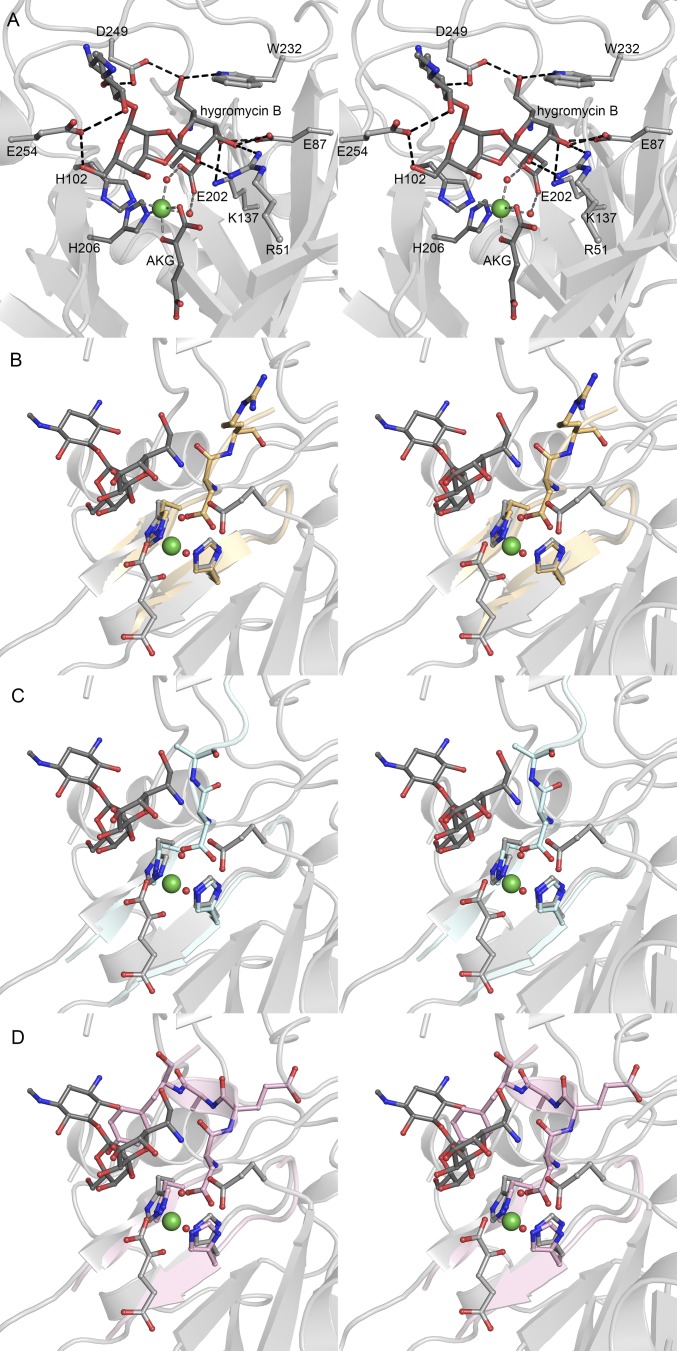
HygX structure. (A) HygX with major strands in yellow and minor strands in red. Ni2+and Fe2+ are green and orange spheres. (B) HygX A chain colored by crystallographic temperature factors where cool colors indicate low B-factors. Loop insertions are highlighted. (C) Surface representation in the same orientation as B. (D) Superposition of four chains of the HygX-AKG complex showing loop movement, with hygromycin B shown for reference. (E) Iron coordination with anomalous difference density in yellow and contoured at 5σ. (F) Active site metal coordination of HygX. |FO| − |FC| difference density is contoured around AKG at 2.5 σ, calculated before inclusion of AKG in the model. (G) Tryptophan fluorescence at 350 nm of 0.5 μM HygX, 0.05 mM NiCl2, 0.05 mM AKG, and varying concentrations of hygromycin B (Kd, 3.4 ± 0.5 μM). (H) 3σ |FO| − |FC| difference density calculated before hygromycin B placement. One possible site of hydrogen atom abstraction is 5.2 Å from the metal center. (I) HygX–AKG–hygromycin B product complex (in gray) aligned with the clavaminate synthase–AKG–proclavaminate substrate complex (blue; PDB ID code 1DRT). Asterisks mark sites of hydrogen atom abstraction.
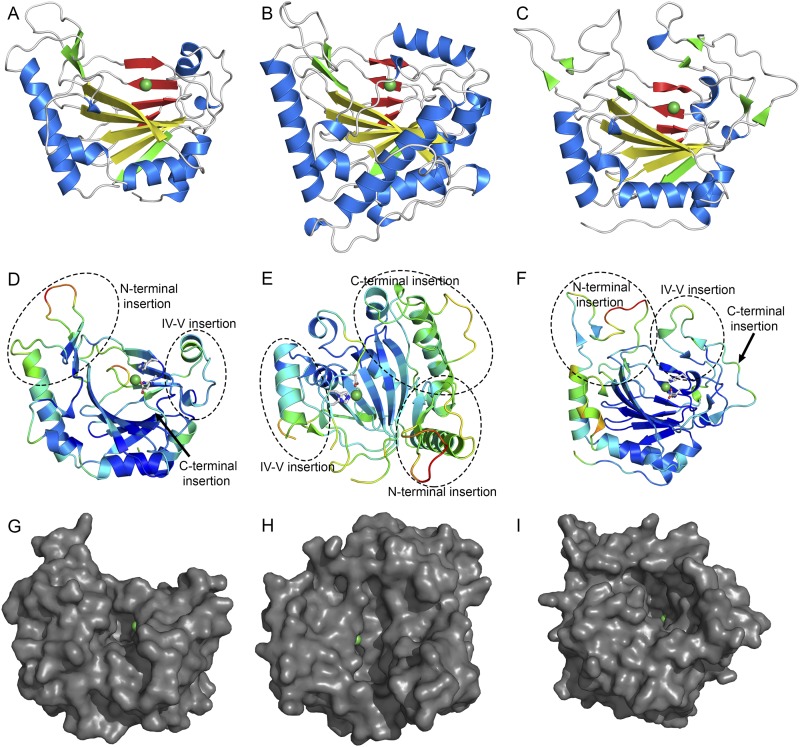
Fig. S2.
Orthosomycin-associated oxygenase structures. Cartoon representations of AviO1 (A), EvdO1 (B), and EvdO2 (C) in the same orientations. The major sheet is colored yellow, the minor sheet is red, and nickel ions are shown as green spheres. (D–F) Cartoon representation of AviO1 (D), EvdO1 (E), and EvdO2 (F) colored by crystallographic temperature factors. Warm colors indicate high B-factors and more thermal motion. Three insertion sites common to the PhyH subfamily of AKG/Fe(II)-dependent oxidases are labeled. The orientation of each maximizes the view of the three insertions and the binding site. (G–I) Surface representation of AviO1 (G), EvdO1 (H), and EvdO2 (I) showing the prime substrate binding site in the same orientation as used in D–F.
Table S1.
Data collection, Ni-SAD phasing, and refinement statistics
| EvdO1 | EvdO1 Ni-SAD | EvdO2 | EvdO2-AKG | AviO1 | AviO1 Ni-SAD | |
| Data collection | ||||||
| PDB ID code | 4XBZ | 4XAB | 4XAC | 4XAA | ||
| Space group | P21 | P21 | P212121 | P212121 | P21212 | P21212 |
| Cell dimensions | ||||||
| a, b, c (Å) | 91.6, 109.4, 146.1 | 92.3, 109.0, 146.2 | 42.6, 65.8, 84.5 | 42.5, 65.4, 84.6 | 72.1, 115.1, 33.9 | 72.4, 115.4, 33.9 |
| α, β, γ (°) | 90, 108.3, 90 | 90, 108.3, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Wavelength, Å | 0.979 | 1.485 | 1.078 | 1.127 | 1.485 | 1.485 |
| APS Beamline | 21-ID-F | 21-ID-D | 21-ID-D | 21-ID-D | 21-ID-D | 21-ID-D |
| Resolution, Å | 2.3 | 2.9 | 1.75 | 1.87 | 2.3 | 2.5 |
| Rsym, % | 12.1 (41.8) | 8.6 (44.3) | 6.8 (29.6) | 7.4 (32.4) | 7.0 (44.0) | 11.1 (92.6) |
| I/σI | 15.2 (2.3) | 25.9 (6.2) | 18.1 (4.2) | 22.3 (5.8) | 26.7 (2.6) | 34.4 (2.5) |
| Completeness, % | 99.5 (99.1) | 98.1 (97.0) | 99.9 (99.6) | 97.2 (98.7) | 86.7 (61.1) | 98.6 (97.3) |
| Redundancy | 3.8 (3.1) | 7.7 (7.8) | 3.6 (3.6) | 5.7 (5.7) | 4.0 (2.8) | 8.5 (5.9) |
| Refinement | ||||||
| Resolution, Å | 2.3 | NA | 1.75 | 1.87 | 2.3 | NA |
| No. of reflections | 120,893 | NA | 24,667 | 19,679 | 11,436 | |
| Rwork/Rfree | 18.3/23.7 | 17.5/20.2 | 16.8/20.8 | 18.8/22.1 | ||
| No. of atoms | ||||||
| Protein | 19105 | 2006 | 2014 | 1700 | ||
| Ligand/ion | 44 | 11 | 16 | 1 | ||
| Water | 1333 | 182 | 144 | 62 | ||
| B-factors | ||||||
| Protein | 35.1 | 28.3 | 34.3 | 46.9 | ||
| Ligand/ion | 44.3 | 28.2 | 27.8 | 58.2 | ||
| Water | 38.5 | 36.2 | 41.3 | 46.5 | ||
| Ramachandran | ||||||
| Favored, % | 96.0 | 98.0 | 98.0 | 97.3 | ||
| Allowed, % | 3.8 | 2.0 | 2.0 | 2.7 | ||
| Outliers, % | 0.2 | 0.0 | 0.0 | 0.0 | ||
| rms deviations | ||||||
| Bond lengths, Å | 0.008 | 0.006 | 0.007 | 0.008 | ||
| Bond angles, ° | 1.07 | 1.05 | 1.11 | 1.10 |
All datasets were collected from a single crystal. Values in parentheses are for data in the highest-resolution shell. The EvdO1 Ni-SAD dataset was used only for phasing. A subset of frames from the AviO1 Ni-SAD dataset was used for refinement. NA, not applicable.
Table S2.
HygX data collection and refinement statistics
| HygX-apo | HygX-Fe | HygX-AKG | HygX-AKG-hygB | HygX-AKG Ni-SAD | |
| Data collection | |||||
| PDB ID code | 4XC9 | 4ZPI | 4XCA | 4XCB | |
| Space group | P21212 | P212121 | P212121 | P212121 | P212121 |
| Cell dimensions | |||||
| a, b, c (Å) | 112.1, 274.4, 52.8 | 54.4, 109.0, 221.6 | 53.4, 112.0, 177.8 | 50.3, 117.5, 185.7 | 53.5, 112.1, 178.2 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Wavelength, Å | 0.979 | 1.739 | 0.979 | 0.979 | 1.485 |
| APS Beamline | 21-ID-F | 21-ID-D | 21-ID-F | 21-ID-F | 21-ID-D |
| Resolution, Å | 2.4 | 2.5 | 2.30 | 1.60 | 2.95 |
| Rsym, % | 8.0 (44.3) | 9.4 (58.4) | 7.1 (23.4) | 7.5 (44.6) | 10.5 (33.0) |
| I/σI | 14.7 (2.4) | 18.8 (2.2) | 21.1 (6.8) | 20.0 (3.3) | 27.3 (9.0) |
| Completeness, % | 92.9 (91.1) | 87.8 (89.8) | 96.1 (97.8) | 93.8 (92.8) | 99.5 (99.9) |
| Redundancy | 3.7 (3.6) | 3.7 (3.3) | 4.4 (4.4) | 4.9 (4.4) | 7.3 (7.5) |
| Refinement | |||||
| Resolution, Å | 2.4 | 2.5 | 2.3 | 1.6 | NA |
| No. of reflections | 61,588 | 149,043 | 46,643 | 126,897 | |
| Rwork/Rfree | 18.4/24.6 | 17.1/22.4 | 17.6/23.6 | 22.2/25.3 | |
| No. of atoms | |||||
| Protein | 11732 | 7851 | 7985 | 8160 | |
| Ligand/ion | 48 | 28 | 55 | 188 | |
| Water | 498 | 140 | 554 | 578 | |
| B-factors | |||||
| Protein | 40.7 | 39.5 | 30.6 | 22.2 | |
| Ligand/ion | 49.9 | 56.6 | 37.5 | 20.2 | |
| Water | 38.7 | 34.8 | 33.1 | 25.5 | |
| Ramachandran | |||||
| Favored, % | 96.7 | 96.7 | 97.6 | 97.8 | |
| Allowed, % | 3.2 | 3.3 | 2.3 | 2.2 | |
| Outliers, % | 0.1 | 0.0 | 0.1 | 0.0 | |
| rms deviations | |||||
| Bond lengths, Å | 0.008 | 0.008 | 0.008 | 0.006 | |
| Bond angles, ° | 1.12 | 1.11 | 1.09 | 1.07 |
All datasets were collected from a single crystal. Values in parentheses are for data in the highest-resolution shell. The HygX-AKG Ni-SAD dataset was used only for phasing. hygB, hygromycin B; NA, not applicable.
Origins of Substrate Specificity.
Loop insertions between β-strands of the double stranded β-helix of AKG/Fe(II)-dependent oxygenases are proposed to control substrate specificity (22) and AviO1, EvdO1, EvdO2, and HygX all contain loop inserts to form large binding clefts (Fig. 2C, Fig. S2 D–F, and Movies S1–S4). Three notable insertions are located at the N- and C-termini and between β-strand IV and β-strand V of the β-helix (Fig. 2B and Fig. S2 D–I), common sites for specificity-inducing insertions. Smaller inserts in the core fold complement these major insertions.
All insertions have high crystallographic temperature factors, a statistic commonly interpreted as a metric of flexibility. Here, it suggests that substrate binding loops may change conformation upon substrate association. Analysis of the multiple copies of EvdO1 and the HygX in the crystallographic asymmetric units further supports flexibility of these inserts. Four dimers (eight protomers) are located in the EvdO1 asymmetric unit, and these have an average rms deviation of 0.21 Å for the two β-sheets of the double stranded β-helix but an rms deviation of 0.36 Å for the three long loop insertions. Two short loop insertions connecting the outermost β-strands of the double stranded β-helix (approximately residues 129–138 and 226–230) are disordered in four copies. Another striking conformational difference is observed by comparing loops of the four protomers of the HygX-AKG costructure (Fig. 2D) to suggest a movement of nearly 20 Å to promote active site closure. Upon hygromycin B binding, the loop adopts the fully closed conformation in all four chains. Conversely, succinate binding results in all four protomers adopting the most open ordered conformation.
Active Site.
Our structural studies exploited catalytically inactive Ni2+-substituted enzymes to minimize oxidative damage to the enzyme. This strategy has been used with great success in investigations of Fe(II)/AKG-dependent oxygenases, for example, in the histone demethylases (23–25). Other work has demonstrated that metal substitution does not alter the coordination within the error of crystallographic resolution (26). Metal coordination in canonical AKG/Fe(II)-dependent oxygenases involves two histidines and one acidic residue to form a conserved H-X-D/E…H motif known as the facial triad. Whereas AviO1, EvdO1, and EvdO2 retain the facial triad and the Ni2+ retains the octahedral coordination geometry typical of iron coordination in the orthosomycin associated AKG/Fe(II)-dependent oxygenases (Fig. S3 A–C), HygX exhibits an unexpected variation (Fig. 2 E and F). We ensured that this variation was not an artifact of metal substitution by determining the crystal structure of both HygX incorporated with Fe2+ and apo-HygX. We observed no significant change in the positions of the coordinating ligands associated with either the change in metal identity or the removal of the metal completely (Fig. 2E and Fig. S4). In this variation, the acidic residue of the H-X-D/E…H motif is substituted with a glycine, and a glutamic acid located four residues before the distal histidine completes the metal coordination sphere with either a long hydrogen bond or via water-mediated interactions to form a novel H-X-G…E-X3-H motif. The acidic ligand is absent in the halogenases (13, 21), with substrate halides replacing the protein-associated ligand. However, relocation of the acidic ligand is unprecedented.
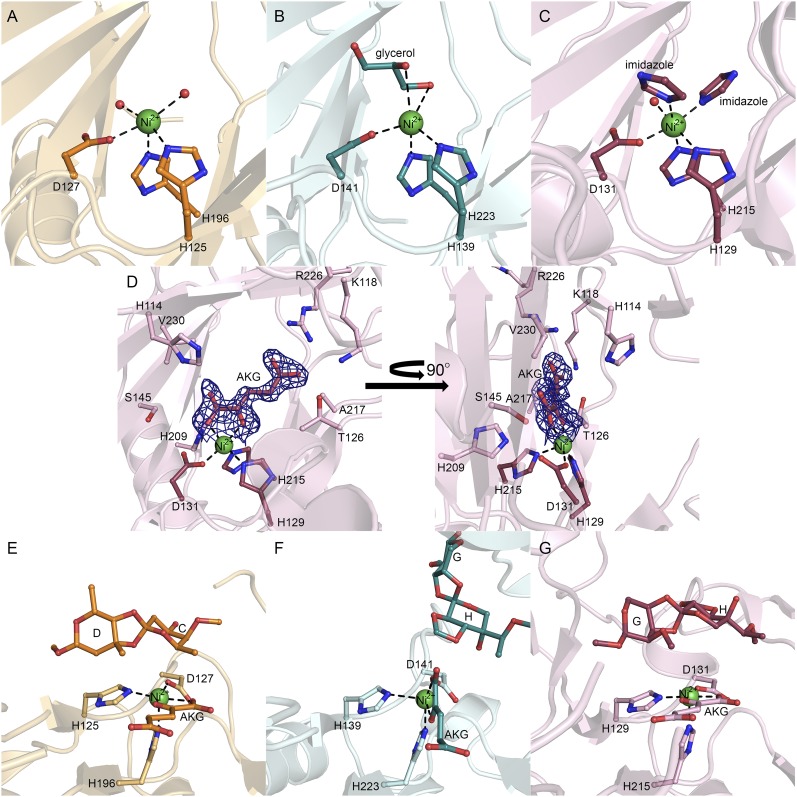
Fig. S3.
Active sites of orthosomycin-associated oxygenases AviO1, EvdO1, and EvdO2. Active site metal coordination of AviO1with Ni2+ (A), EvdO1 with Ni2+ (B), and EvdO2 with Ni2+ (C). Residues of the facial triad (conserved H-X-D/E…H motif) are shown in ball-and-stick representation. Nickel ions are shown as green spheres and water molecules as red spheres. All ligands (glycerol in EvdO1, imidazole in EvdO2) are shown in ball and stick. (D) AKG binding in EvdO2. One molecule of imidazole has been omitted for clarity. The left view is over the prime substrate binding site, and the right is rotated 90° about the y axis. (E–G) Docking poses for disaccharide product mimics containing orthoester linkages and/or methylenedioxy bridges. Ligands not originating from an experimental crystal structure are colored darker. (E) AviO1 with the C-D product mimic and AKG-docked. (F) EvdO1 with the G-H product mimic and AKG-docked. The G-H product mimic adopts an orientation where the methylenedioxy bridge faces the metal center. (G) EvdO2 with AKG bound and the G-H product mimic docked with the orthoester linkage facing the metal center.
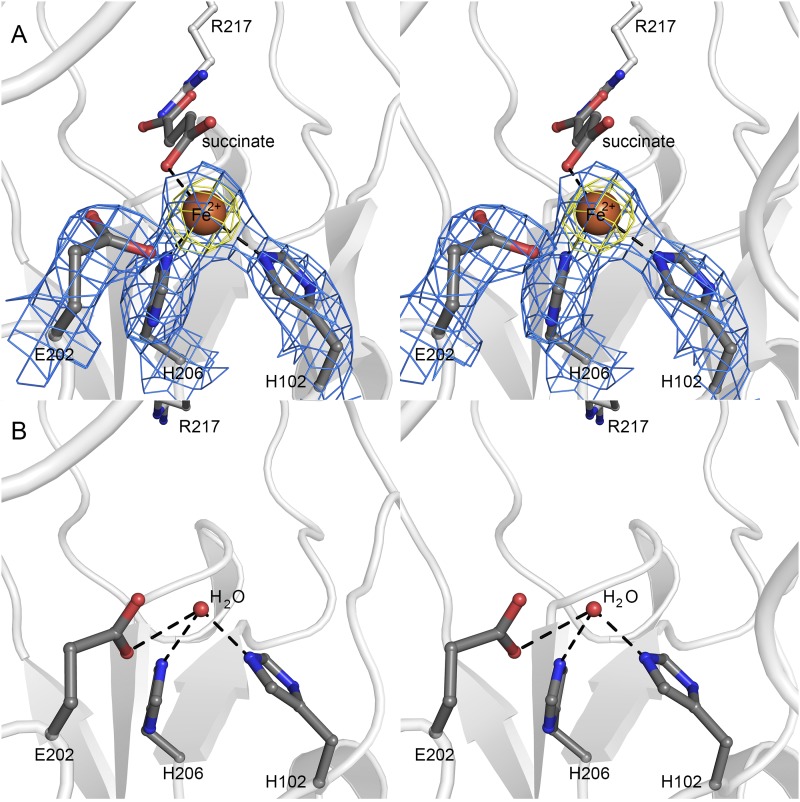
Fig. S4.
Metal substitution in HygX. (A) Stereoview of the iron binding site in HygX. Coordinating residues and ligands are shown in ball-and-stick representation. The iron ion is shown as a brown sphere; 2|FO| − |FC| density for the facial triad and iron are shown as blue mesh, contoured at 1.5σ, and the iron anomalous density is shown as yellow mesh and contoured at 5σ. (B) Active site of apo-HygX showing that removal of the metal ion is not associated with significant change in active site structure.
We determined costructures of these enzymes with AKG or succinate. Clear electron density appeared in the costructures of AKG with EvdO2 and HygX (Fig. 2F and Fig. S3D). AKG binding is accompanied by slight movements in many of the directly interacting side chains, and a large rotamer change of a conserved arginine (Arg226 of EvdO2 and Arg217 of HygX). As anticipated, the AKG binds directly to the metal center to replace two metal-coordinating solvent molecules in each complex with the 2-keto group trans to the acidic residue. Variability in the 1-oxo group coordination has been observed in other AKG/Fe(II)-dependent enzymes, which can be trans to either histidine residue (27). In EvdO2, the 1-oxo group is trans to the proximal histidine, whereas in HygX, the 1-oxo group is trans to the distal histidine. Following crystallization of HygX in 0.6 M succinate, weak electron density is observed for the 1- and 4-carboxylate groups in two of the four protomers. This may reflect the low affinity of succinate in the absence of prime substrate.
HygX Interactions with Hygromycin B.
The presence of two putative glycosyltransferases in the hyg gene cluster when hygromycin B contains one glycosidic linkage and one orthoester linkage suggests HygX may act upon a substrate with two glycosidic linkages. However, the precise substrate and its stereochemistry are unknown. Because the synthesis of a library of possible substrates is impractical, we assessed whether HygX catalyzed the terminal step of hygromycin B biosynthesis. As enzymes have affinity for their products, we measured HygX binding to hygromycin B using tryptophan fluorescence quenching (Kd, 3.4 ± 0.5 μM; Fig. 2G). A low-micromolar affinity is consistent with affinities observed between enzyme and product (28), suggesting HygX catalyzes the last step in hygromycin B biosynthesis. We also determined the costructure of HygX with AKG and hygromycin B to 1.6-Å resolution. Unambiguous electron density for hygromycin B showed a bridging oxygen approaching the metal center (Fig. 2H). The binding is highly specific, with the position stabilized by 10 direct and 5 water-mediated interactions (Fig. S5A) to promote an orientation in which the anomeric carbon of the destomic acid is 5.2 Å from the metal and 39° from the vertical position of an octahedrally coordinated metal.
Fig. S5.
Steric requirement for facial triad modification in HygX. (A) Stereoview of hygromycin B binding in HygX active site. Protein–ligand interactions are shown as black dashed lines and metal interactions with gray dashed lines. (B–D) Overlay of HygX with AviO1 (B), EvdO1 (C), and EvdO2 (D) show steric clashes with the acidic residue of the facial triad. In AviO1, EvdO1, and EvdO2, the protein backbone bends toward the active site to provide the acidic residue for coordination. Each illustration has had the major sheet of HygX excluded for clarity and is rotated 90° from A. HygX secondary structure is colored gray, AviO1 is pale orange, EvdO1 is light cyan, and EvdO2 is colored pink.
Structural comparisons of the HygX–hygromycin B costructure with the EvdO1, EvdO2, and AviO1 structures show that the hygromycin B ligand geometry would result in a steric clash if HygX used a canonical facial triad (Fig. S5). Facial triad modification in HygX both alleviates this steric clash and results in an increased volume of the prime substrate binding pocket. This facial triad is almost invariant across the family; the only known exception is replacement of the acidic residue with a halide substrate in halogenases (13, 21). The likelihood of AviO1, EvdO1, and EvdO2 performing chemical reactions similar to that catalyzed by HygX argues against the novel metal coordination tuning chemical reactivity toward oxidative ring closure. Instead, modification of the facial triad residues may have arisen solely for substrate accommodation.
Computational Analysis of Disaccharide Interactions with Orthosomycin-Associated Oxygenases.
Gene-disruption experiments of oxygenases in M. carbonacea var. aurantiaca have not as yet produced detectable accumulation of pathway intermediates that would be genuine substrates of these oxygenases. Furthermore, attempts to obtain substrate analogs via synthesis (everninomicin) or reduction (hygromycin B) were unsuccessful. However, the state of the art in computational methods has proven successful at discovering viable binding poses of known ligands (29, 30). Thus, we used two complementary in silico methods and our AviO1, EvdO1, and EvdO2 structures to explore whether productive binding modes of relevant ligands are plausible. We simplified docking calculations by using disaccharide mimics of products for two reasons. First, the carbohydrate chain lengths of the physiologically relevant substrates are unknown, but each product must contain at least two carbohydrates. Second, longer oligosaccharides have many degrees of conformational freedom that would dramatically increase the required search space for docking, reducing the confidence in the results. In contrast, the relative orientation of two sugars constrained by a rigidifying orthoester linkage removes uncertainty from the calculations.
We benchmarked our calculations by docking a talose-destomic acid disaccharide into the HygX structure determined without hygromycin B. The top-scoring binding poses reproduced the experimentally determined position with an rms deviation of 0.70 Å for all atoms and 0.34 Å for the five atoms of the trioxaspiro group. We then docked the C/D and G/H rings of avilamycin and everninomicin with AviO1, EvdO1, and EvdO2. In all cases, at least one pose oriented a disaccharide such that one oxygen atom of the orthoester linkage faces the metal center (Fig. S3 E–G). For EvdO1, in particular, the top pose oriented the G/H rings of everninomicin in a manner consistent with methylenedioxy bridge formation (Fig. S3F). In multiple instances, we observed clusters of highly related poses representing between 25–50% of the reported hits that were consistent with either orthoesterification or methylenedioxy bridge formation. These calculations suggest that the active site pockets can accommodate substrates in orientations consistent with orthoester linkage formation.
Everninomicin Congeners of M. carbonacea var. aurantiaca.
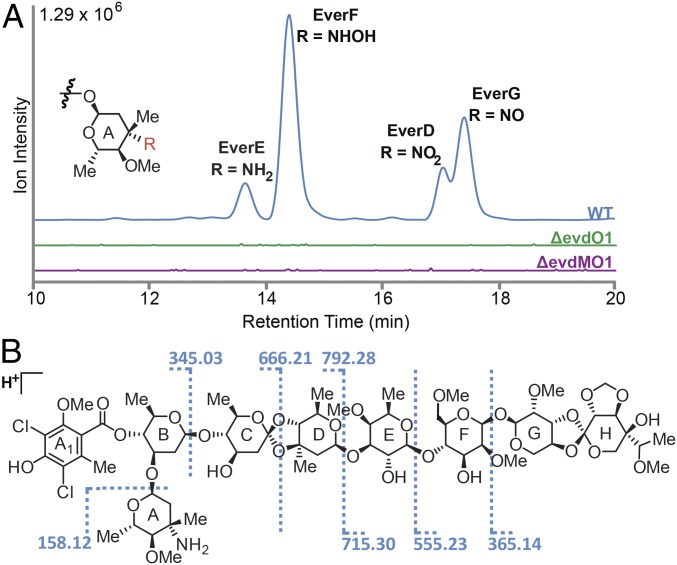
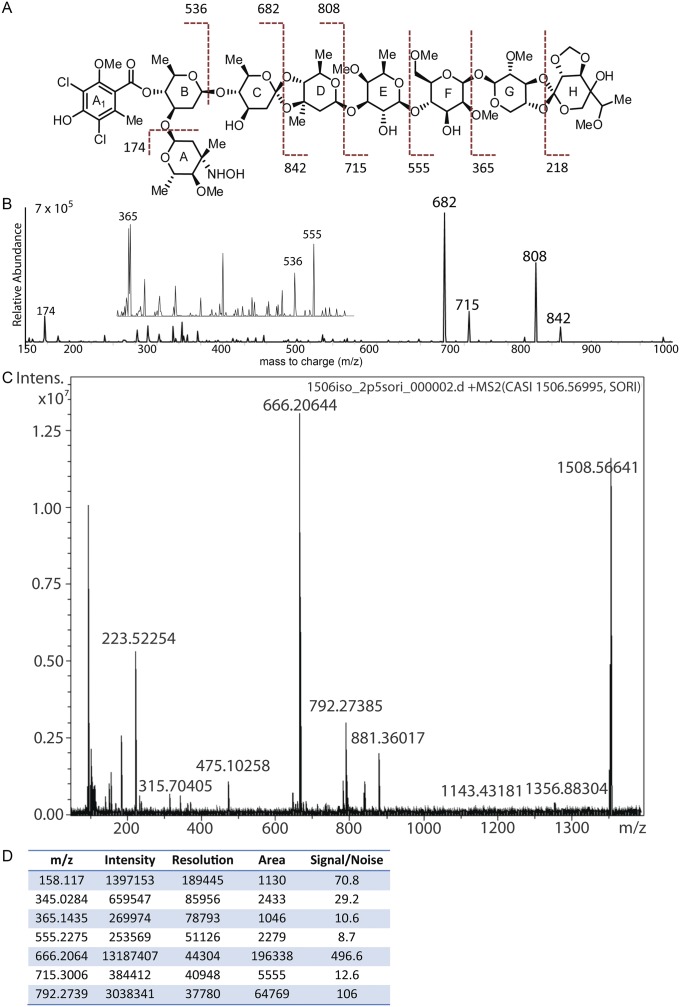
M. carbonacea var. africana produces fourteen previously described everninomicin congeners (31–34). Before genetic manipulation, we assessed which everninomicin congeners are produced by M. carbonacea var. aurantiaca because these have not been reported in the literature. We performed liquid chromatography–MS (LC/MS) analysis of crude extracts followed by tandem LC/MS. This analysis identified four congeners, everninomicins D–G, that vary in the oxidation state of the nitrogen group (-NH2, -NHOH, -NO, or NO2) decorating the A-ring (Fig. 3A, red, and Fig. S6). The structure of everninomicin E, the amino progenitor to the more oxidized species F and G, was confirmed by high resolution Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometric analysis. Fragmentation (MS2) provided unambiguous constitutional analysis for the monosaccharide substituents (Fig. 3B and Fig. S7), and the expected amine oxidation ladder products corresponding to everninomicins F–G were observed. Of note, everninomicins E, F, and G have not been previously reported.
Fig. 3.
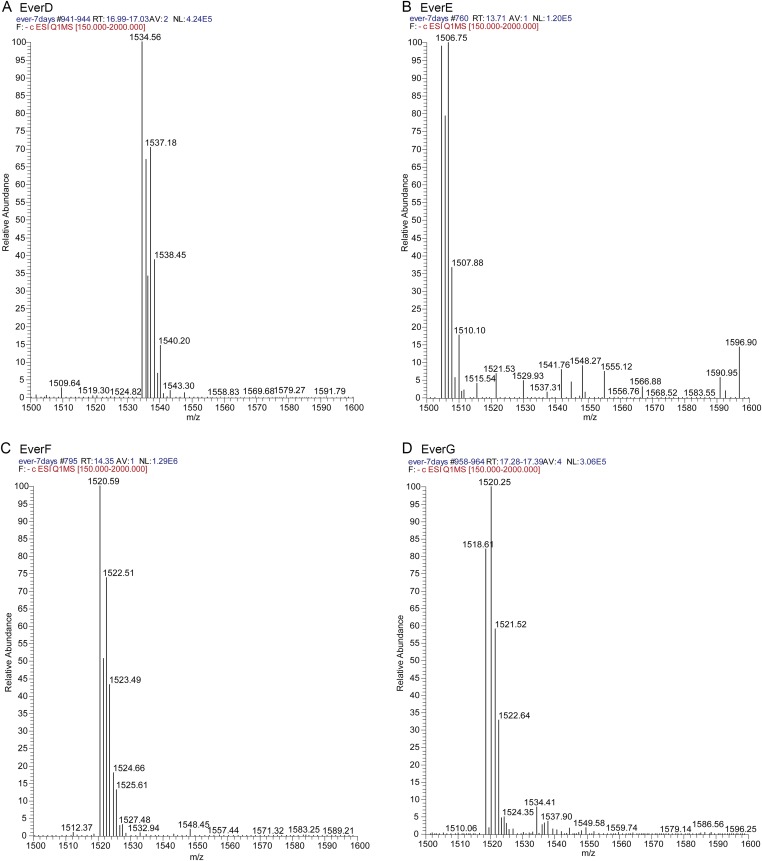
Oxygenases within the evd gene cluster are required for everninomicin production. (A) LC/MS of wild-type and deletion strains of M. carbonacea var. aurantiaca crude extracts. The chromatogram shows summed ion intensities in negative mode for everninomicins D–G: D, m/z = 1,534.5 [M−H]−; E, m/z = 1,504.5 [M−H]−; F, m/z = 1,520.5 [M−H]−; and G, m/z = 1,518.5 [M−H]−. No evidence of abundant accumulated metabolites related to everninomicin was observed. Spectra are in Fig. S6. (B) FT-ICR-MS2 of everninomicin E: m/z = 1,506.571 [M+H]+. Dashed lines indicate positions of cleavage and masses observed during fragmentation. Spectra are in Fig. S7.
Fig. S6.
Mass spectra of everninomicins D (A), E (B), F (C), and G (D). Data were collected in negative mode on a TSQ Quantum Access Max triple-stage quadrupole mass spectrometer equipped with a HESI electrospray ionization source. LC was performed in line prior mass spectrometric analysis. Refer to SI Materials and Methods for a detailed description of the methods used.
Fig. S7.
Fragmentation and mass spectrometric analysis of everninomicin congeners. (A) Mass spectrometric fragmentation pattern for everninomicin F. Dashed lines indicate positions of cleavage during fragmentation experiments. (B) Spectrum for fragmentation of everninomicin F (m/z = 1,522.5 [M+H]+). Collision energy was set to 40 V. (C) Spectrum for fragmentation of everninomicin E (m/z = 1,506.56691 [M+H]+) Fragmentation pattern in Fig 3B. (D) Table of masses and corresponding intensities for spectrum in Fig. S6C.
Targeted Gene Disruption in M. carbonacea var. aurantiaca.
We probed the functions of the evdO1 and evdMO1 genes in the production of these congeners by targeted gene disruption. Our approach used modified two-step PCR targeting based on the λ-Red recombination system followed by intergeneric conjugation with Escherichia coli to transfer plasmid DNA from donor E. coli (35). Genetic manipulation in actinomycetes is not well developed, and lack of sporulation by M. carbonacea renders conjugation difficult in this organism (36, 37). Thus, we adapted our conjugation method to transform mycelia. A second complication is M. carbonacea sensitivity to nalidixic acid, the counterselection marker commonly used to remove donor E. coli following conjugation. Thus, we titered the nalidixic acid to stunt the growth of E. coli, affording the slower-growing M. carbonacea time and space to grow. We then determined that each disruption resulted from a double crossover event using both PCR analysis and Southern hybridization (Fig. S8 and Table S3). LC/MS analysis of crude extracts from the ΔevdO1 and ΔevdMO1 strains revealed loss of measurable production of all everninomicin congeners (Fig. 3A), with no measurable accumulation of mass features readily identifiable as everninomicin biosynthetic intermediates. The loss of everninomicin production confirms the importance of oxygenase activity in everninomicin biosynthesis.
Fig. S8.
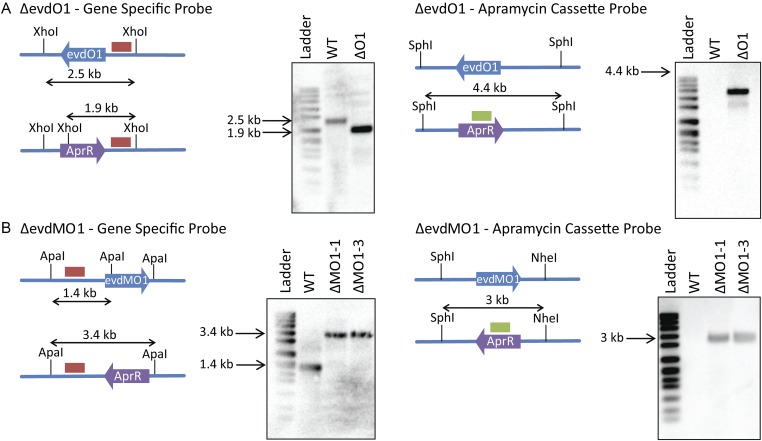
Southern hybridization of targeted deletion mutants verifying a double crossover event. (A) Southern blot analysis of ΔevdO1. Diagrams depict the relative shifts expected for replacement of evdO1 with the apramycin cassette. XhoI and SphI are restriction endonucleases used to cleave the genomic DNA into predictably sized fragments. Blots show predicted shifts were observed experimentally, thus confirming the double crossover. Ladder is DNA molecular-weight marker VII, DIG-labeled (product no. 11669940910; Roche Life Sciences). WT is wild-type M. carbonacea var aurantiaca, and ΔO1 is the ΔevdO1 knockout strain. (B) Southern blot analysis of ΔevdMO1. Diagrams depict the relative shifts expected for replacement of evdMO1 with the apramycin cassette. ApaI, KpnI, and NheI are restriction endonucleases used to cleave the genomic DNA into predictably sized fragments. Blots show predicted shifts were observed experimentally, thus confirming the double crossover. Ladder is DNA molecular-weight marker VII, DIG-labeled (product no. 11669940910; Roche Life Sciences). WT is wild-type M. carbonacea var aurantiaca, and ΔMO1-1 and ΔMO1-3 are ΔevdMO1 knockout strains.
Table S3.
Primers used for gene disruption and validation experiments in this study
| Name | Sequence |
| EvdO1-Red-F | 5′-CGGGCCCGCGACCGCTGATCAGAAGGGTGTGGACTGATGATTCCGGGGATCCGTCGACC-3′ |
| EvdO1-Red-R | 5′-CTGTCGCCCGGAACGCTCATCGGATGCCCCCCGAGCTCATGTAGGCTGGAGCTGCTTC-3′ |
| EvdMO1-Red-F | 5′-TTTCCCGCGCGCACCCGAACACTAGGCTTGGAATCCATGATTCCGGGGATCCGTCGACC-3′ |
| EvdMO1-Red-R | 5′-GTGGGGTCGCCGCAGGCGGCATCCGCGTCCGGCCGGTCATGTAGGCTGGAGCTGCTTC-3′ |
| AprUp | 5′-ATTCCGGGGATCCGTCGACC-3′ |
| AprDn | 5′-TGTAGGCTGGAGCTGCTTC-3′ |
| NeoUp | 5′-TGAATGAACTGCAGGACGAG-3′ |
| NeoDn | 5′-AATATCACGGGTAGCCAA-3′ |
| EvdO1-Southern-For | 5′-TCAGTCCACACCCTTCTGAT-3′ |
| EvdO1-Southern-Rev | 5′-GGCCTGTACCTGATGACGAG-3′ |
| EvdMO1-Southern-For | 5′-GTATGGCTCACTGCCTGGTC-3′ |
| EvdMO1-Southern-Rev | 5′-GGTGCACGATCGGATGAT-3′ |
| Apr-Southern-For | 5′-ACCGACTGGACCTTCCTTCT-3′ |
| Apr-Southern-Rev | 5′-TCGCTATAATGACCCCGAAG-3′ |
Discussion
Substrate Identity and Selectivity.
Genomic analysis, gene disruption, and structural characterization with ligands can suggest function for natural product biosynthetic enzymes (15, 38, 39), and our data strongly support a role for HygX in catalyzing orthoester linkage formation. Together, these data suggest a fully decorated three-ringed compound lacking the orthoester linkage as the substrate and narrows the possibilities for HygX substrates to two alternative anomers at C1 of the destomic acid moiety. The lack of readily available potential precursor molecules renders chemical synthesis of either of these anomers enormously challenging.
Reasonable sequence identities between HygX and each of the remaining 12 AKG/Fe(II)-dependent oxygenases in everninomicin and avilamycin biosynthesis suggest roles for the latter enzymes in carbohydrate-associated oxidative ring closures. Previous experimental assessment of the function of orthosomycin-associated oxygenases is limited to gene disruption of aviO1, aviO2, and aviO3 of avilamycin biosynthesis. Deletion of aviO2 (evdO2 homolog) from S. viridochromogenes Tü57 resulted in a new peak in the mass spectrum, interpreted as the loss of the acetyl group on C4 of the H-ring (see Fig. 1A for location) (40). Given that an AKG/Fe(II)-dependent oxygenase is unlikely to catalyze acetylation and that the homologous oxygenase is present in the evd and eve gene clusters despite the absence of an acetyl group on everninomicin congeners, an alternative interpretation of this result is that AviO2 catalyzes a ring closure before acetylation and that this ring closure is required for substrate recognition by an acetylating enzyme. Consistent with this interpretation, our computational studies suggest that the homolog EvdO2 can reasonably orient the G/H rings.
The assignment of function to the remaining enzymes is more tenuous. Deletion of both aviO1 (evdMO1 homolog) and aviO3 (evdO1 homolog) from S. viridochromogenes Tü57 resulted in loss of detectable avilamycin production (40), mirroring our results of loss of detectable everninomicin production upon the deletion of evdO1 and evdMO1. The lack of any detectable species precludes assignment of precise catalytic function.
Preliminary Reaction Scheme.
All characterized AKG/Fe(II)-dependent oxygenases have conserved aspects to their reaction cycle. For example, these powerful oxidants function via two sequential half reactions that require sequential binding of three substrates: AKG, prime substrate, and O2. The first half-reaction converts the cosubstrate AKG to succinate, whereas the second half-reaction converts prime substrate to product. A combination of kinetic, biochemical, and structural studies support a unified mechanism for cosubstrate catalysis that generates the highly reactive Fe(IV)= O oxidizing species (41–44).
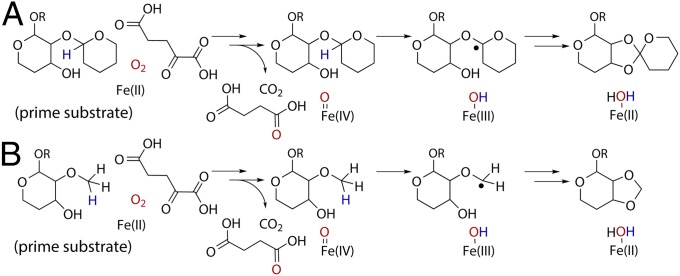
Numerous schemes for prime substrate catalysis are observed in the superfamily. The most relevant to orthoester linkage and methylenedioxy bridge formation is oxidative cyclization by clavaminate synthase, where hydrogen atom abstraction from substrate (45) is followed by ring closure. Comparison of hygromycin B in the HygX costructure to that of the proclavaminic acid substrate bound to clavaminate synthase (14) aligns an anomeric carbon of hygromycin B with the carbon atom of proclavaminate that undergoes hydrogen atom abstraction (Fig. 2I). This carbon atom is 5.4 Å from the metal in the clavaminate synthase–proclavaminate costructure and 5.2 Å in the HygX–hygromycin B costructure. The C1 carbon of the hygromycin B precursor is one logical site for hydrogen atom abstraction (Fig. 4A), although alternative positions, such as the hydroxyl group, cannot be excluded (46). Analogous abstraction possibilities exist to initiate methylenedioxy bridge formation (Fig. 4B).
Fig. 4.
Mechanistic possibilities for orthosomycin-associated oxygenases. Generation of the Fe(IV)=O species is conserved within the superfamily and not shown. (A) The first step of orthoester linkage formation may be radical generation by hydrogen atom abstraction, as suggested by structural parallels with clavaminate synthase catalyzed oxidative ring closure. The regiochemistry of the preexisting glycosidic bond is not known. (B) Methylenedioxy bridge formation may also use hydrogen atom abstraction by the Fe(IV)=O species. The position of the methoxy group at C2 or C3 is unknown.
In the context of this reaction scheme, several possible mechanisms could support orthoester linkage and methylenedioxy bridge formation following initial hydrogen atom abstraction. Options include oxidative radical coupling (with or without ligand transfer or oxygen rebound), nucleophilic capture of an oxocarbenium ion, or a ketene acetal intermediate (47). Because P450 enzymes are the only characterized enzymes that catalyze methylenedioxy bridge formation, we examined that mechanism closely. The paucity of P450 enzymes that can act as pure dehydrogenases resulted in a proposal of Fe-centered radical chemistry with an oxygen rebound mechanism followed by water elimination from the hydroxylated intermediate (48). Oxygen rebound leading to hydroxylation and elimination on a C2/C3 methoxy of the H-rings is supported by the AKG/Fe(II)-dependent superfamily scaffold. However, AKG/Fe(II)-dependent enzymes can also catalyze dehydrogenation of a C2/C3 methoxy group without ligand insertion and/or oxygen rebound, which could allow for methylenedioxy formation by quenching an oxonium intermediate. Other possible mechanisms for methylenedioxy formation are possible. For instance, in the event of a C2-deoxy H-ring precursor, C2 oxygenation and sequential oxidative desaturation circumvents the oxonium intermediate.
Potential for Engineering Altered Reactivity into AKG/Fe(II)-Dependent Enzymes.
Whereas Ziracin stalled in phase III clinical trials, everninomicin analogs still hold promise for clinical use. Everninomicin synthesis is challenging (49), likely making de novo production of analogs prohibitively expensive, but analogs could be produced by engineering the biosynthetic enzymes in the producing organism. The loss of detectable everninomicin production for the ΔevdO1 and ΔevdMO1 strains indicates that functional oxygenase activity is critical for everninomicin production. Thus, any bioengineering requires that substrate selectivity of the AKG/Fe(II)-dependent oxygenases be modified.
Within the AKG/Fe(II)-dependent superfamily, the halogenases are among the more closely related to the orthosomycin associated oxygenases. Excepting HygX, these are the only enzymes with a modified metal-coordinating facial triad. Interestingly, the ability of the AKG/Fe(II)-dependent halogenase SyrB2 to catalyze halogenation or hydroxylation is influenced by subtle changes to either the halide binding site or the substrate (50) and can be tuned to catalyze unnatural reactions, such as nitration of unactivated carbon centers (51). Interestingly, sequence-similarity searches using the 13 enzymes studied here also identify as-yet-uncharacterized very close homologs (a subset is listed in Fig. S1), which presents an opportunity to explore further reactions catalyzed by the oxygenase superfamily and investigate the capability of these oxygenases to accept expanded substrates.
Materials and Methods
Protocols for gene cloning, protein overexpression, purification, crystallization, data collection, structure determination and refinement, gene disruption, and mass spectral analysis are available in SI Materials and Methods.
SI Materials and Methods
Materials.
Hygromycin B was obtained from Calbiochem as a 50 mg/mL sterile-filtered solution dissolved in 25 mM Hepes (pH 7.2). All other materials were purchased from Sigma-Aldrich.
Bacterial Culture Conditions.
M. carbonacea var. aurantiaca and deletion mutants were grown on solid TSB medium [Oxoid; Tryptone Soy Broth; 2% (wt/vol) agarose]. E. coli strains were grown in LB medium. Intergeneric conjugations were performed on AS1 media [0.1% yeast extract, 0.5% soluble starch, 0.02% l-alanine, 0.02% l-arginine, 0.05% l-asparagine, 0.25% NaCl, 1% Na2SO4, 2% (wt/vol) agarose at pH 7.5, supplemented with 10 mM MgCl2]. LB broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl) was used for electrocompetent cell preparation. Apramycin (50 μg/mL), nalidixic acid (25 μg/mL), chloramphenicol (30 μg/mL), and kanamycin (50 μg/mL) were used when required for selection as described below.
Gene Replacement.
The genes evdO1 and evdMO1 were individually deleted in M. carbonacea var. aurantiaca using a modification of the PCR-targeted Streptomyces gene-replacement strategy described by Gust et al. (35). λ Red competent cells were prepared by inoculating 1% of a fresh overnight culture of E. coli BW25113/pIJ790 containing cosmid CA (containing the evd gene cluster) into 10 mL of LB medium containing 20 mM MgSO4, kanamycin, chloramphenicol, and 10 mM l-arabinose. The culture was grown at 30 °C with shaking to an OD600 of 0.6. The cells were recovered by centrifugation at 3,000 × g for 10 min at 4 °C. The pellet was washed three times with 10 mL ice-cold 10% glycerol. The washed pellet was then resuspended in 100 μL of ice-cold 10% glycerol and kept on ice.
The apramycin-resistance cassette containing the aac(3)IV-resistance marker, oriT, and flippase recombinase target (FRT) regions was amplified by PCR using primers EvdO1-Red-F and EvdO1-Red-R for the evdO1 knockout and EvdMO1-Red-F and EvdMO1-Red-R for the evdMO1 knockout (primer sequences are found in Table S3). PCR products were then directly transformed via electroporation into the arabinose-induced strain BW25113/pIJ790 containing cosmid CA in which gene replacement of evdO1 or evdMO1 was enabled via λ Red-mediated homologous recombination. Transformed E. coli were plated on LB containing apramycin and incubated overnight at 37 °C to promote loss of pIJ790. Colonies were inoculated into LB containing apramycin and kanamycin and grown overnight at 37 °C with shaking. The resultant cosmid was isolated, confirmed by sequencing, and then transformed by electroporation into nonmethylating E. coli strain ET12567 containing plasmid pUZ8002, which contains genes necessary for conjugal transfer of the cosmid. This strain was maintained at 37 °C in liquid LB medium containing kanamycin, apramycin, and chloramphenicol.
Intergeneric Conjugation of M. carbonacea var. aurantiaca for Gene Disruption.
M. carbonacea var. aurantiaca was grown from a streaked glycerol stock on TSB agar for 7 d. Mycelia were inoculated into 10 mL of TSB medium in a 50-mL Falcon tube and incubated with shaking at 30 °C for 5 d. Conjugal acceptor mycelia were prepared by adding a 1% inoculum of these cultures into 30 mL of liquid TSB with shaking at 30 °C for 48 h. The culture was then centrifuged at 3,000 × g for 10 min, and the pellet was resuspended in 5 mL of fresh TSB; 300-μL aliquots were transferred into sterile 1.5-mL Eppendorf tubes and homogenized using a sterile plastic cell homogenizer. Donor E. coli ET12567/pUZ8002 cells were prepared by inoculating 1% of a freshly prepared overnight LB culture into 10 mL LB medium in a 50-mL Falcon tube containing apramycin, chloramphenicol, and kanamycin and grown to an OD600 of 0.4 at 37 °C with shaking. The culture was then centrifuged at 3,000 × g for 10 min, and the pellet was washed three times with 10 mL fresh LB medium. After the final wash, the pellet was resuspended in 1 mL of LB medium. Donor (E. coli) and recipient (M. carbonacea) bacteria (300 μL each) were mixed then plated onto fresh AS1 agar plates. After 16 h of incubation at 30 °C, apramycin-resistant transformants were separated from the E. coli donor strain by overlaying the conjugation mixture with nalidixic acid (12.5 µg/mL) and apramycin (50 µg/mL). After 5–7 d of incubation at 30 °C, potential double-crossover mutants, identified by restreaking colonies picked by sterile cotton swab and testing for apramycin resistance and kanamycin sensitivity on solid TSB medium, were confirmed by PCR amplification of the kanamycin- and apramycin-resistance genes using the following primers, the sequences of which are reported in Table S3: AprUp and AprDn for amplifying the apramycin resistance gene; NeoUp and NeoDn for amplifying the kanamycin resistance gene. The mutants were further analyzed by Southern hybridization. Gene-specific probes were designed upstream of the genes of interest. The evdO1 probe (785 bp) was amplified using primers EvdO1-Southern-For and EvdO1-Southern-Rev. The evdMO1 probe (798 bp) was amplified using primers: EvdMO1-Southern-For and EvdMO1-Southern-Rev. An apramycin cassette probe was designed which hybridized to the apramycin resistance gene. The apramycin probe (884 bp) was amplified using primers: Apr-Southern-For and Apr-Southern-Rev. All probes were labeled with digoxigenin (DIG) using DIG High Prime DNA Labeling and Detection Starter Kit II (catalog no. 11585614910; Roche). Hybridization and detection were performed using the aforementioned DIG Starter Kit.
Production of Everninomicins D–G.
Seed cultures of mutant strains were generated by inoculating a loop of mycelia from TSB agar into 100 mL of 2997 Germination Medium [0.3% beef extract, 0.5% tryptose, 0.1% dextrose, 2.4% (wt/vol) soluble starch, 0.5% yeast extract, 0.1% calcium carbonate, 50 μg/mL apramycin] for 5 d at 35 °C in a 500-mL Erlenmeyer flask with shaking (5). For everninomicin production, 25 mL of the seed culture was added to 500 mL of apramycin-free Production Medium (0.5% yeast extract, 0.1% corn steep solids, 0.1% calcium carbonate, 3% glucose) in a 2-L baffled Fernbach flask and grown with shaking at 30 °C for 7 d. Diaion HP-20 resin (100 mL, previously preequilibrated with methanol and washed with water) was added to the fermentation cultures and incubated for 60 min with shaking; the combined resin and mycelia were collected by centrifugation at 3,000 × g and then extracted successively by resuspension and centrifugation at 3,000 × g with 250 mL of methanol and 250 mL of acetone and evaporated to dryness by rotary evaporation. The resulting crude extract was resuspended in 25 mL of solvent-grade methanol and was filtered through a fritted glass funnel containing silica gel (9 × 2 cm) via vacuum filtration and concentrated to dryness. Extracts were resuspended at a final concentration of 200 mg/mL in HPLC-grade methanol before analysis by LC/MS.
The extracts were analyzed in both negative- and positive-ion modes using a TSQ Quantum Access Max triple stage quadrupole mass spectrometer (Thermo Scientific) equipped with a HESI electrospray ionization source. Injections of 20 μL were separated on Accucore C18 (150 × 4.6 mm; Thermo Scientific) using a Finnigan Surveyor LC Pump Plus (Thermo Scientific). Mobile phases were as follows: 95% (vol/vol) water/5% (vol/vol) acetonitrile with 10 mM ammonium acetate (A) and 5% (vol/vol) water/95% (vol/vol) acetonitrile with 10 mM ammonium acetate (B). Gradient conditions were as follows: 0–1 min, 100% (A); 1–20 min, linear gradient to 100% (B); 20–26 min, 100% (B); 26–7 min, linear gradient to 100% (A); and 27–30 min, 100% (A). The flow rate was maintained at 1 mL/min with 15 μL sent to an Accela PDA detector (Thermo Scientific) and 5 μL subjected to mass spectral analysis. Nitrogen was used for both the auxiliary and sheath gas set to 10 and 54 psi, respectively. Positive-ion mode values were as follows: capillary temperature, 275 °C; spray voltage, 4.5 kV; capillary offset, 35V; tube lens voltage, 133V; and skimmer offset, 5V. Negative-ion mode values were as follows: capillary temperature, 275 °C; spray voltage, 3.0 kV; capillary offset, −35 V; tube lens voltage, −132 V; and skimmer offset 5 V. For fragmentation studies, a collision energy of 30 V was used.
Protein Expression and Purification.
All genes were synthesized (Mr. Gene for EvdO1 and EvdO2, Genscript for AviO1, GeneArt for HygX) and subcloned into either pET28a(+) (EvdO1, EvdO2, HygX) or pET23 (AviO1). The resulting vector transformed into E. coli BL21(DE3). Cultures were grown at 37 °C in LB (40 μg/mL kanamycin) with shaking to an OD600 of 0.4, when the temperature was lowered to 18 °C. Protein expression was induced 45 min later at an OD600 of 0.6–0.9 by the addition of 0.5 mM IPTG. The cultures continued to shake at low temperature for 16 h and then were harvested by centrifugation at 5,000 × g for 15 min and stored at −20 °C.
Cell pellets were thawed and resuspended in 15 mL of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole; pH 8.0) per liter of culture and supplemented with one Complete EDTA-free protease inhibitor mixture tablet (Roche Applied Science). The sample was lysed by sonication. After cell lysis, all purification steps were performed at 4 °C. Crude lysate was clarified by centrifugation at 40,000 × g for 1 h. The supernatant was passed over a Ni-NTA column (Qiagen) equilibrated with lysis buffer. The column was then washed with lysis buffer containing 20 mM imidazole. Protein was eluted using lysis buffer with 250 mM imidazole and immediately diluted 1:1 with lysis buffer. The sample was dialyzed into storage buffer (25 mM Tris, 75 mM NaCl at pH 7.4).
To incorporate Fe2+, HygX was first incubated with 0.5 mM EDTA for 1 h to remove any Ni2+ and then dialyzed extensively against PBS. The sample was then buffer-exchanged to 50 mM Mops (pH 7.0) using a PD-10 column (GE Healthcare Life Sciences). (NH4)2Fe(SO4)2 was added to a final concentration of 1 mM and allowed to incubate for 30 min. The sample was then run over the PD-10 column to remove excess iron. HygX-Fe was concentrated to 12 mg/mL, flash frozen, and stored at −80 °C.
The histidine tag was removed from EvdO2 and AviO1 before crystallization. Thrombin (20 U) was added and incubated overnight at 4 °C to remove the N-terminal hexahistidine tag. Cleaved proteins were passed over a Ni-NTA column to separate unprocessed sample, and the flow-through was collected. Samples were further purified through size-exclusion chromatography on a Superdex 200 10/300 GL column equilibrated in storage buffer. Fractions were analyzed using SDS/PAGE, pooled, and concentrated to 18 mg/mL (EvdO1), 6 mg/mL (EvdO2), 16 mg/mL (AviO1), or 16 mg/mL (HygX). Proteins were flash-frozen and stored at −80 °C in aliquots.
Tryptophan Fluorescence Quenching Assay.
The Kd of hygromycin B binding to HygX was determined by monitoring the quenching of intrinsic fluorescence from the single tryptophan residue of HygX upon hygromycin B binding. Using a Cary Eclipse Varian fluorescence spectrometer, sample fluorescence was measured at 20 °C with both emission and excitation slits set at 10 nm and detector voltage set to 800 V. The emission wavelength was set to 280 nm, and spectra collected were from 300 to 400 nm, with 350 nm used for calculating binding affinity. Each sample contained 990 μL of 0.5 μM HygX in 25 mM Tris (pH 7.4), 75 mM NaCl, 0.05 mM AKG, and 0.05 mM NiCl2, which was then mixed with 10 μL of hygromycin B (diluted in the above buffer) of varying concentrations. Spectra were measured in triplicate, and the experiment repeated three times. Because hygromycin B at higher concentrations has background fluorescence between 300 and 400 nm, the experiment was repeated using only buffer and subtracted from the measurement taken with HygX present. Change in fluorescence resulting from changing hygromycin B concentration was plotted against hygromycin B concentration and fit to a single binding-site model using Kaleidagraph Version 4.0.
Crystallization.
Crystals were grown using the hanging-drop vapor-diffusion method at room temperature in 3-μL drops containing an equal ratio of protein to reservoir solution. Crystals of EvdO1 (18 mg/mL in storage buffer plus 0.4 mM NiCl2) appeared after 3 d with a reservoir solution of 100 mM sodium citrate tribasic (pH 5.1) and 13% (wt/wt) PEG8000. EvdO2 (6 mg/mL in storage buffer) crystallized in 100 mM imidazole (pH 8.0), 38% (wt/wt) PEG8000, and 250 mM NaCl. The EvdO2-AKG cocrystals used fully formed EvdO2 crystals soaked with freshly prepared 200 mM AKG in 100 mM imidazole (pH 8.0). AviO1 (16 mg/mL in storage buffer) crystallized in 100 mM CAPS (pH 10.5), 1.2 M NaH2PO4, 0.8 M K2HPO4, and 200 mM Li2SO4. HygX (16 mg/mL in storage buffer) crystallized from 100 mM Bis-Tris (pH 6.8), 100 mM MgCl2, and 12% (wt/wt) PEG8000. HygX-AKG crystals were grown by incubating HygX (16 mg/mL in storage buffer) with 3 mM AKG for 30 min before setting up drops; crystallization conditions consisted of 50 mM CsCl, 100 mM Mes (pH 6.5), and 30% (wt/wt) Jeffamine M-600. HygX–AKG–hygromycin B crystals were grown from HygX (16 mg/mL in storage buffer plus 1 mM NiCl2, 3 mM AKG, and 5 mM hygromycin B) using a reservoir containing 100 mM Mes (pH 6.3) and 18% (wt/wt) PEG20000. HygX-Fe crystallized in 0.6 M succinic acid (pH ∼7). All crystals except those grown from Jeffamine M-600 were cryoprotected by creating an artificial mother liquor of the reservoir solution containing a cryoprotectant and soaking the crystals for one minute before cryocooling by plunging into liquid nitrogen. For EvdO1 and AviO1, the crystallization conditions were supplemented by 20% of a 50/50 (vol/vol) glycerol/ethylene glycol mix. For EvdO2 and HygX, the crystallization conditions were supplemented 17% (vol/vol) ethylene glycol.
Crystallographic Data Collection, Processing, Structure Determination, and Refinement.
Diffraction data were collected on the LS-CAT beamlines of the Advanced Photon Source (Argonne, IL) on Mar300 CCD detectors. All data were processed and scaled using the HKL2000 suite of programs (52) (Tables S1–S3). Structures of EvdO1, AviO1, and HygX were determined through single-wavelength anomalous diffraction (SAD)-phasing from anomalous signal from bound nickel ions using data collected in wedges at 1.484 Å. This wavelength was experimentally determined using X-ray fluorescence scans around the Fe and Ni K-edges using an XFlash 1001 SD detector (Bruker-AXS). The HygX-Fe2+ dataset was collected at 1.739 Å, a wavelength identified through X-ray fluorescence scans as maximizing the anomalous signal from Fe2+.
Nickel-binding sites were determined using the program HKL2MAP and SHELXC/D/E (53, 54) and input into the AutoSol routine of PHENIX for phasing and density modification (55). EvdO2 was determined using molecular replacement with PHASER (56, 57). To develop the search model for EvdO2, human phytanoyl-CoA dioxygenase phyhd1 (58) (PDB ID code 3OBZ) was structurally aligned with human phytanoyl-CoA 2-hydroxylase (19) (PDB ID code 2A1X), and all nonconserved secondary structure, ligands and water molecules were removed. Costructures of EvdO2 with AKG and HygX with hygromycin B were determined by isomorphous replacement from the unliganded structure. All structures were improved using AutoBuild of PHENIX (56, 59). Model building was performed in COOT (60) with composite omit maps calculated in CNS (61). Refinement was performed using phenix.refine (62). The costructure of HygX–AKG–hygromycin B contains significant disorder at the N termini in two of the four protomers. Omit maps and additional refinement with strict restraints were used to minimize model bias during refinement of this structure. Importantly, clear electron density of a quality expected for a 1.6-Å resolution structure are observed for two chains and these were the chains used for computational docking controls and all figures.
Computational Docking with Glide.
The polypeptides of EvdO1, EvdO2, AviO1, and HygX, each from structures without AKG or prime substrate bound, were prepared as receptors for in silico modeling and docking with Maestro (Schrodinger) by first removing all water molecules, next adding AKG in each of the two possible orientations observed in the superfamily, and adding protons to each structure (63). Structures were minimized using the molecular mechanics force field OPLS_2005. Docking grids were generated using Glide Receptor Grid Generation. The centroid of the HygX grid was identified using the position of hygromycin B; centroids of the EvdO1, EvdO2, and AviO1 grids were identified by overlaying the HygX–hygromycin B–AKG ternary structure and approximating the product binding site based upon the position of the orthoester linkage in hygromycin B. Docking ligands were either products or product mimics (hygromycin B, the C-D rings fused with an orthoester linkage, or the G-H rings fused with an orthoester linkage and containing the methylenedioxy bridge). These were built in the Maestro GUI workspace and prepared for docking by adding explicit hydrogen atoms in the Ligprep module.
Docking simulations were carried out using the Glide XP subroutine of Maestro. The calculations were benchmarked by identifying parameters that docked the talose-destomic acid disaccharide of hygromycin B into the structure of HygX into a position that replicated the position of these groups in cocrystal structure with the hygromycin B trisaccharide. Each disaccharide contained an orthoester linkage and was docked into EvdO1, EvdO2, and AviO1. During docking, ligand flexibility was permitted, while the protein receptor was held rigid. Relative free energy of binding was estimated with the extended precision scoring function.
Computational Docking with Surflex-Dock.
The receptors sites of EvdO1, EvdO2, AviO1, and HygX were prepared as described above. The protein preparation tool in Sybyl (Tripos) was then used to repair sidechains, cap the N and C termini, calculate charged groups, and add protons to each structure. The receptors were then minimized using the Tripos force field. Surflex-Dock protomols were computed using the position of hygromycin B; the EvdO1, EvdO2, and AviO1 protomols were seeded by overlaying the HygX–hygromycin B–AKG ternary structure and approximating the product binding site based upon the position of the orthoester linkage in hygromycin B. Ligands were either products or product mimics (hygromycin B, the C-D rings fused with an orthoester linkage, or the G-H rings fused with an orthoester linkage and containing the methylenedioxy bridge).
Docking calculations were carried out using the Surflex-Dock method (64) from within Sybyl, using the standard geom parameters, with the 20 top-scoring poses reported. The calculations were benchmarked by verifying that the talose-destomic acid disaccharide of hygromycin B was successfully placed into the structure of HygX in a position that replicates the location of these groups in the cocrystal structure with the hygromycin B trisaccharide. Each disaccharide contained an orthoester linkage and was docked into EvdO1, EvdO2, and AviO1. During docking, ligand flexibility was permitted, while the protein receptor was held rigid. Poses were ranked using the empirically derived Surflex-Dock scoring function (65).
Supplementary Material
Acknowledgments
We thank Dr. Jeffrey Spraggins of the Vanderbilt Mass Spectrometry Research Center for help with the Fourier transform ion cyclotron resonance mass spectrometric analysis of everninomicin E. This work was supported by National Institutes of Health (NIH) Grant T32 HL007751 (to K.M.M.), the Vanderbilt Summer Science Academy (J.L.M.), the Vanderbilt Institute of Chemical Biology (B.O.B.), Office of Naval Research Grant N00014-09-1-012 (to B.O.B.), and American Heart Association Grant 12GRNT11920011 (to T.M.I.). The Vanderbilt crystallization facility was supported by NIH Grant S10 RR026915. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4XBZ, 4XAB, 4XAC, 4XAA, 4XC9, 4XCA, 4XCB, and 4ZPI).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500964112/-/DCSupplemental.
References
- 1.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325(5944):1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RN, Low DE, Pfaller MA. Epidemiologic trends in nosocomial and community-acquired infections due to antibiotic-resistant gram-positive bacteria: The role of streptogramins and other newer compounds. Diagn Microbiol Infect Dis. 1999;33(2):101–112. doi: 10.1016/s0732-8893(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 3.Adrian PV, et al. Evernimicin (SCH27899) inhibits a novel ribosome target site: Analysis of 23S ribosomal DNA mutants. Antimicrob Agents Chemother. 2000;44(11):3101–3106. doi: 10.1128/aac.44.11.3101-3106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belova L, Tenson T, Xiong L, McNicholas PM, Mankin AS. A novel site of antibiotic action in the ribosome: Interaction of evernimicin with the large ribosomal subunit. Proc Natl Acad Sci USA. 2001;98(7):3726–3731. doi: 10.1073/pnas.071527498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waitz JA, Horan AC. 1988. Micromonospora carbonacea var africana. US Patent 4,735,903.
- 6.Mann RL, Bromer WW. The isolation of a second antibiotic from Streptomyces hygroscopicus. J Am Chem Soc. 1958;80(11):2714–2716. [Google Scholar]
- 7.Díaz Chávez ML, Rolf M, Gesell A, Kutchan TM. Characterization of two methylenedioxy bridge-forming cytochrome P450-dependent enzymes of alkaloid formation in the Mexican prickly poppy Argemone mexicana. Arch Biochem Biophys. 2011;507(1):186–193. doi: 10.1016/j.abb.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Ikezawa N, et al. Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells. J Biol Chem. 2003;278(40):38557–38565. doi: 10.1074/jbc.M302470200. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann C, et al. Genes encoding enzymes responsible for biosynthesis of L-lyxose and attachment of eurekanate during avilamycin biosynthesis. Chem Biol. 2005;12(10):1137–1143. doi: 10.1016/j.chembiol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Weitnauer G, et al. Biosynthesis of the orthosomycin antibiotic avilamycin A: Reductions from the molecular analysis of the avi biosynthetic gene cluster of Streptomyces viridochromogenes Tü57 and production of new antibiotics. Chem Biol. 2001;8(6):569–581. doi: 10.1016/s1074-5521(01)00040-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, et al. Evidence that the fosfomycin-producing epoxidase, HppE, is a non-heme-iron peroxidase. Science. 2013;342(6161):991–995. doi: 10.1126/science.1240373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strieker M, Kopp F, Mahlert C, Essen LO, Marahiel MA. Mechanistic and structural basis of stereospecific Cbeta-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem Biol. 2007;2(3):187–196. doi: 10.1021/cb700012y. [DOI] [PubMed] [Google Scholar]
- 13.Blasiak LC, Vaillancourt FH, Walsh CT, Drennan CL. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature. 2006;440(7082):368–371. doi: 10.1038/nature04544. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, et al. Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nat Struct Biol. 2000;7(2):127–133. doi: 10.1038/72398. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, et al. Discovery of new enzymes and metabolic pathways by using structure and genome context. Nature. 2013;502(7473):698–702. doi: 10.1038/nature12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter K, et al. Synthesis of 5-hydroxyectoine from ectoine: Crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD. PLoS One. 2010;5(5):e10647. doi: 10.1371/journal.pone.0010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khare D, et al. Conformational switch triggered by α-ketoglutarate in a halogenase of curacin A biosynthesis. Proc Natl Acad Sci USA. 2010;107(32):14099–14104. doi: 10.1073/pnas.1006738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You Z, Omura S, Ikeda H, Cane DE, Jogl G. Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis. J Biol Chem. 2007;282(50):36552–36560. doi: 10.1074/jbc.M706358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonough MA, et al. Structure of human phytanoyl-CoA 2-hydroxylase identifies molecular mechanisms of Refsum disease. J Biol Chem. 2005;280(49):41101–41110. doi: 10.1074/jbc.M507528200. [DOI] [PubMed] [Google Scholar]
- 20.Tian W, Skolnick J. How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol. 2003;333(4):863–882. doi: 10.1016/j.jmb.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 21.Wong C, Fujimori DG, Walsh CT, Drennan CL. Structural analysis of an open active site conformation of nonheme iron halogenase CytC3. J Am Chem Soc. 2009;131(13):4872–4879. doi: 10.1021/ja8097355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aik W, McDonough MA, Thalhammer A, Chowdhury R, Schofield CJ. Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases. Curr Opin Struct Biol. 2012;22(6):691–700. doi: 10.1016/j.sbi.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury R, et al. Ribosomal oxygenases are structurally conserved from prokaryotes to humans. Nature. 2014;510(7505):422–426. doi: 10.1038/nature13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan S, Trievel RC. Structural and functional analysis of JMJD2D reveals molecular basis for site-specific demethylation among JMJD2 demethylases. Structure. 2013;21(1):98–108. doi: 10.1016/j.str.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Couture J-F, Collazo E, Ortiz-Tello PA, Brunzelle JS, Trievel RC. Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nat Struct Mol Biol. 2007;14(8):689–695. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
- 26.Horton JR, Upadhyay AK, Hashimoto H, Zhang X, Cheng X. Structural basis for human PHF2 Jumonji domain interaction with metal ions. J Mol Biol. 2011;406(1):1–8. doi: 10.1016/j.jmb.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, et al. Crystal structure of a clavaminate synthase-Fe(II)-2-oxoglutarate-substrate-NO complex: Evidence for metal centered rearrangements. FEBS Lett. 2002;517(1-3):7–12. doi: 10.1016/s0014-5793(02)02520-6. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Even A, et al. The moderately efficient enzyme: Evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry. 2011;50(21):4402–4410. doi: 10.1021/bi2002289. [DOI] [PubMed] [Google Scholar]
- 29.Isin B, Estiu G, Wiest O, Oltvai ZN. Identifying ligand binding conformations of the β2-adrenergic receptor by using its agonists as computational probes. PLoS One. 2012;7(12):e50186. doi: 10.1371/journal.pone.0050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh PK, et al. Plasticity of the quinone-binding site of the complex II homolog quinol:fumarate reductase. J Biol Chem. 2013;288(34):24293–24301. doi: 10.1074/jbc.M113.487082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganguly AK, Sarre OZ, Greeves D, Morton J. Letter: Structure of everninomicin D-1. J Am Chem Soc. 1975;97(7):1982–1985. doi: 10.1021/ja00840a078. [DOI] [PubMed] [Google Scholar]
- 32.Wright DE. The Orthosomycins, a new family of antibiotics. Tetrahedron. 1979;35:1207–1237. [Google Scholar]
- 33.Ganguly AK, et al. The structure of new oligosaccharide antibiotics, 13-384 components 1 and 5. Heterocycles. 1989;28(1):83–88. [Google Scholar]
- 34.Chu M, et al. Isolation and characterization of novel oligosaccharides related to Ziracin. J Nat Prod. 2002;65(11):1588–1593. doi: 10.1021/np020093t. [DOI] [PubMed] [Google Scholar]
- 35.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA. 2003;100(4):1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosted TJ, Wang TX, Alexander DC, Horan AC. Characterization of the biosynthetic gene cluster for the oligosaccharide antibiotic, Evernimicin, in Micromonospora carbonacea var. africana ATCC39149. J Ind Microbiol Biotechnol. 2001;27(6):386–392. doi: 10.1038/sj.jim.7000189. [DOI] [PubMed] [Google Scholar]
- 37.Flett F, Mersinias V, Smith CP. High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett. 1997;155(2):223–229. doi: 10.1111/j.1574-6968.1997.tb13882.x. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Nandurkar NS, Thorson JS. Characterization of the calicheamicin orsellinate C2-O-methyltransferase CalO6. ChemBioChem. 2014;15(10):1418–1421. doi: 10.1002/cbic.201402119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCoy JG, et al. Structural characterization of CalO2: A putative orsellinic acid P450 oxidase in the calicheamicin biosynthetic pathway. Proteins. 2009;74(1):50–60. doi: 10.1002/prot.22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treede I, et al. Genes involved in formation and attachment of a two-carbon chain as a component of eurekanate, a branched-chain sugar moiety of avilamycin A. Appl Environ Microbiol. 2005;71(1):400–406. doi: 10.1128/AEM.71.1.400-406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM., Jr Evidence for hydrogen abstraction from C1 of taurine by the high-spin Fe(IV) intermediate detected during oxygen activation by taurine:α-ketoglutarate dioxygenase (TauD) J Am Chem Soc. 2003;125(43):13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 42.Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. The first direct characterization of a high-valent iron intermediate in the reaction of an α-ketoglutarate-dependent dioxygenase: A high-spin FeIV complex in taurine/α-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry. 2003;42(24):7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 43.Proshlyakov DA, Henshaw TF, Monterosso GR, Ryle MJ, Hausinger RP. Direct detection of oxygen intermediates in the non-heme Fe enzyme taurine/α-ketoglutarate dioxygenase. J Am Chem Soc. 2004;126(4):1022–1023. doi: 10.1021/ja039113j. [DOI] [PubMed] [Google Scholar]
- 44.Riggs-Gelasco PJ, et al. EXAFS spectroscopic evidence for an Fe=O unit in the Fe(IV) intermediate observed during oxygen activation by taurine:α-ketoglutarate dioxygenase. J Am Chem Soc. 2004;126(26):8108–8109. doi: 10.1021/ja048255q. [DOI] [PubMed] [Google Scholar]
- 45.Iwata-Reuyl D, Basak A, Townsend CA. β-Secondary kinetic isotope effects in the clavaminate synthase-catalyzed oxidative cyclization of proclavaminic acid and in related azetidinone model reactions. J Am Chem Soc. 1999;121(49):11356–11368. [Google Scholar]
- 46.Borowski T, de Marothy S, Broclawik E, Schofield CJ, Siegbahn PEM. Mechanism for cyclization reaction by clavaminic acid synthase. Insights from modeling studies. Biochemistry. 2007;46(12):3682–3691. doi: 10.1021/bi602458m. [DOI] [PubMed] [Google Scholar]
- 47.Beau J-M, Jaurand G, Esnault J, Sinaÿ P. Synthesis of the disaccharide C•D fragment found in everninomicin-C and -D, avalamycin-A and -C and curamycin-A: Stereochemistry at the spiro-ortholactone center. Tetrahedron Lett. 1987;28(10):1105–1108. [Google Scholar]
- 48.Bjorklund JA, et al. Cryptic stereochemistry of berberine alkaloid biosynthesis. J Am Chem Soc. 1995;117(5):1533–1545. [Google Scholar]
- 49.Nicolaou KC, et al. Total synthesis of everninomicin 13,384-1--Part 1: Retrosynthetic analysis and synthesis of the A1B(A)C fragment. Chemistry. 2000;6(17):3095–3115. doi: 10.1002/1521-3765(20000901)6:17<3095::aid-chem3095>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Matthews ML, et al. Substrate positioning controls the partition between halogenation and hydroxylation in the aliphatic halogenase, SyrB2. Proc Natl Acad Sci USA. 2009;106(42):17723–17728. doi: 10.1073/pnas.0909649106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews ML, et al. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase. Nat Chem Biol. 2014;10(3):209–215. doi: 10.1038/nchembio.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 53.Sheldrick GM. Experimental phasing with SHELXC/D/E: Combining chain tracing with density modification. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pape T, Schneider TR. HKL2MAP: A graphical user interface for macromolecular phasing with SHELX programs. J Appl Cryst. 2004;37(5):843–844. [Google Scholar]
- 55.Terwilliger TC, et al. Decision-making in structure solution using Bayesian estimates of map quality: The PHENIX AutoSol wizard. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 6):582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, et al. Crystal structure of PHYHD1A, a 2OG oxygenase related to phytanoyl-CoA hydroxylase. Biochem Biophys Res Commun. 2011;408(4):553–558. doi: 10.1016/j.bbrc.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 59.Terwilliger TC, et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr. 2008;64(Pt 1):61–69. doi: 10.1107/S090744490705024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2(11):2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 62.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 4):352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friesner RA, et al. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 64.Jain AN. Surflex-Dock 2.1: Robust performance from ligand energetic modeling, ring flexibility, and knowledge-based search. J Comput Aided Mol Des. 2007;21(5):281–306. doi: 10.1007/s10822-007-9114-2. [DOI] [PubMed] [Google Scholar]
- 65.Jain AN. Scoring noncovalent protein-ligand interactions: A continuous differentiable function tuned to compute binding affinities. J Comput Aided Mol Des. 1996;10(5):427–440. doi: 10.1007/BF00124474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.