Significance
Stable carbon isotopes give diet information for both modern and fossil mammals and can be used to classify diets as C4 grazers, C3–C4 mixed, or C3 browsers. We show that diets of some major African herbivore lineages have significantly changed over the past 4 million years by comparing fossils from the Turkana Basin in Kenya with modern mammals from East and Central Africa. Some fossil assemblages have no modern analogues in East and Central Africa, suggesting different ecological functions for some mammals in the past as compared with their modern counterparts. The development of modern tropical grassland ecosystems are products of the coevolution of both grasses and herbivores.
Keywords: carbon isotopes, evolution, diet
Abstract
A large stable isotope dataset from East and Central Africa from ca. 30 regional collection sites that range from forest to grassland shows that most extant East and Central African large herbivore taxa have diets dominated by C4 grazing or C3 browsing. Comparison with the fossil record shows that faunal assemblages from ca. 4.1–2.35 Ma in the Turkana Basin had a greater diversity of C3–C4 mixed feeding taxa than is presently found in modern East and Central African environments. In contrast, the period from 2.35 to 1.0 Ma had more C4-grazing taxa, especially nonruminant C4-grazing taxa, than are found in modern environments in East and Central Africa. Many nonbovid C4 grazers became extinct in Africa, notably the suid Notochoerus, the hipparion equid Eurygnathohippus, the giraffid Sivatherium, and the elephantid Elephas. Other important nonruminant C4-grazing taxa switched to browsing, including suids in the lineage Kolpochoerus-Hylochoerus and the elephant Loxodonta. Many modern herbivore taxa in Africa have diets that differ significantly from their fossil relatives. Elephants and tragelaphin bovids are two groups often used for paleoecological insight, yet their fossil diets were very different from their modern closest relatives; therefore, their taxonomic presence in a fossil assemblage does not indicate they had a similar ecological function in the past as they do at present. Overall, we find ecological assemblages of C3-browsing, C3–C4-mixed feeding, and C4-grazing taxa in the Turkana Basin fossil record that are different from any modern ecosystem in East or Central Africa.
The expansion of C4 biomass beginning in the late Miocene marks a major vegetation change in the history of Earth. Today C4 plants comprise ca. 50% of net primary productivity (NPP) in the tropics (1) yet contributed less than 1% of NPP only 10 million years ago. C4 plants are primarily grasses and sedges, although C4 photosynthesis is known to be used in ∼20 plant families (2, 3). C4 photosynthesis is an adaptation to low (ca. <500 ppm by volume) concentrations of CO2 in Earth’s atmosphere along with high growing-season temperatures (4). Although genetic evidence indicates an Oligocene origin of C4 photosynthesis in the grasses (5, 6), macrofossil evidence for C4 photosynthesis in grasses is extremely sparse (7, 8).
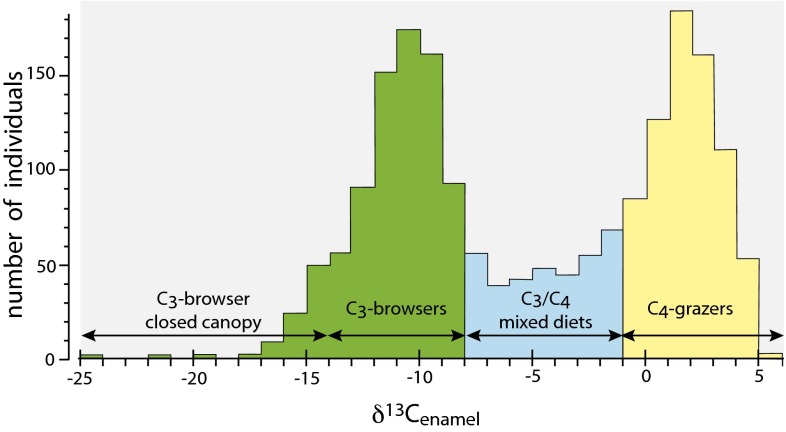
The expansion of C4 biomass has been documented through stable isotopes in paleosols (9–12), grass phytoliths (13), herbivore tooth enamel (14–16), and biomarkers in deep-sea sediments (17, 18). At 10 Ma in Africa, Asia, and North America, the δ13C values for equid tooth enamel indicate a diet dominated by C3 vegetation; by ca. 7 Ma, equids in Africa have a diet dominated (>75%) by C4 vegetation (14, 15). In East Africa today there is a distinct difference in diets of major herbivores, with most mammals either being predominantly browsing (>ca. 75% C3) or grazing (>ca. 75% C4), and there are relatively few mixed feeders (Fig. 1).
Fig. 1.
δ13C1750 values for tooth enamel (or equivalent) for >1,900 mammals from East and Central Africa (principal localities in SI Appendix, Table S1; data from Dataset S1).
A recent study of the early transition of C3 to C4 dietary change in the Turkana Basin from 10 Ma to ca. 4 Ma (15) showed that equids were the earliest mammals to fully exploit the C4 dietary resource, attaining a predominantly C4-grazing diet by 7 Ma. Other mammal groups (hippopotamids, elephantids, and bovids) changed to a C4 diet later than did the equids. In this paper we document dietary changes in the major Artiodactyla-Perissodactyla-Proboscidea (APP) taxa in the Turkana Basin between ca. 4 Ma and 1 Ma and compare those to dietary preferences of extant APP taxa in East and Central Africa. The Turkana Basin has an excellent stratigraphy (19–22) with excellent preservation of fossils from 4 to 1 Ma; this study focuses on fossils recovered from the Koobi Fora, Kanapoi, and Nachukui Formations of northern Kenya.
We compare dietary changes within the major APP taxa through the past 4 Ma in the formations listed above using >900 individual fossils that represent the major taxa collected within the principal stratigraphic intervals of these formations. Fossil mammalian diets are compared with those of >1,900 extant mammal individuals sampled from >30 different regions and habitats in eastern and central Africa. We compare the ecosystem structure (C3 browsers, C3/C4 mixed diets, and C4 grazers) through the Pliocene and Pleistocene and document changes in ungulate diets over time.
Results
The distinction between C3 dicots and C4 grasses makes stable isotopes a very useful tracer of diet in tropical ecosystems. Although C4 dicots are known from Africa, they are uncommon in most biomes (23). Likewise, plants using the Crassulacean acid metabolism pathway (mostly succulents) are also uncommon in most African ecosystems and also often have chemical defenses that deter mammalian herbivory. In the discussion below, modern samples for plants and tooth enamel have their respective δ13C values corrected for the anthropogenic CO2 and are corrected to preindustrial values (see SI Appendix, Detailed Methods) and are reported as δ13C1750. Using data reported in ref. 24, we find the δ13C1750 values for C3 plants from forest floor in closed canopy (Ituri Forest), mesic (Aberdares, Nairobi region), and xeric (Turkana, Samburu, Laikipia) biomes to be ca. −32.6, −26.6, and −25.6‰, respectively. Mesic (panicoids) and xeric (chloridoids and Aristida) grasses have δ13C1750 values of −10.0 and −11.2‰, respectively. The isotopic distinction between mesic and xeric vegetation within C3 and C4 ecosystems has previously been noted for both C3 plants (25) and C4 plants (26, 27).
δ13C Assignments for C3 Browsing, Mixed C3/C4, and C4 Grazing.
Diets of African mammals are frequently discussed in terms of C3-dominated browsing, mixed feeding, and C4-dominated grazing. In this discussion, a browsing diet is dominated by C3 biomass (primarily dicots), whereas a grazing diet comprises primarily C4 biomass (mainly grasses). SI Appendix, Table S1 gives geographic, climatic, and ecological information for 30 geographic localities with measured δ13C on keratin, collagen, or enamel from APP mammals. In each collecting region we analyzed the different APP species to determine the characteristic δ13C value for individuals in that particular region; thus, each taxon has a region-specific diet. Dataset S1 presents δ13C1750 data for >1,900 individuals from East and Central Africa, distributed across >50 species of large mammals; SI Appendix, Table S2 summarizes data for individual species. For comparison between tissues, all values are reported as enamel values using isotope enrichments in SI Appendix, Detailed Methods. Analysis of these data using the Akaike Information Criterion indicates that there are multiple modes for these individual δ13C1750 values: A three-component mixture analysis identifies C3-browsing and C4-grazing components with modal δ13C1750 values of −10.9 ± 1.6 and 1.7 ± 1.6‰ for C3-browsing and C4-grazing taxa, respectively, with mixed feeders having intermediate values. Isotope enrichment values ε*enamel-diet are between 13.3 and 14.6‰ for ungulate mammals (24, 28), with the higher values being associated with bovid ruminants; we use 14.1‰ for all taxa in this paper (SI Appendix, Detailed Methods). Using these enrichment values and the xeric- and mesic-mixing lines for C3 dicots and C4 grasses, we have adopted a value of −8‰ as the boundary between C3 browsers (<−8‰) and mixed C3/C4 diets (>−8‰ to <−1‰), and a value of −1‰ for the boundary between C4 grazers (>−1‰) and mixed C3/C4 diets. Thus, defined isotopically, “mixed C3/C4 diets” have C3/C4 diet ratios between ca. 75/25 and ca. 25/75, given the uncertainties in the mixing lines (SI Appendix, Fig. S2).
Most samples used for stable isotope analysis of fossils are identified only to tribe for bovids and genus for other taxa; therefore, in the discussion below we evaluate taxonomic groups at the tribal level for bovids and at the generic level for other taxa using this diet classification (Table 1). We consider normalized proportions of C4 grazers (G), mixed C3–C4 diet generalists (M), and C3 browsers (B), referred to as G:M:B, using the isotope ranges described above and in SI Appendix. For comparison within a taxon in each time interval, we compare the fraction of individuals that are C4 grazers, mixed C3–C4 diet generalists, or C3 browsers. In contrast, for comparison of taxa with respect to all other taxa within a single ecosystem or a time slice, we use the average δ13C value to define the predominant mode of feeding: C4 grazer, mixed C3–C4 diet generalist, or C3 browser.
Table 1.
Modern East African large mammal taxon groups (tribe for Bovidae, genus for other taxa) used in this study
| Taxon | n | %G | %M | %B |
| Artiodactyla | ||||
| Bovidae | ||||
| Aepycerotini | 66 | 15 | 77 | 8 |
| Alcelaphini | 141 | 100 | 0 | 0 |
| Antilopini | 122 | 11 | 30 | 60 |
| Bovini | 167 | 84 | 13 | 4 |
| Caprini | 1 | 0 | 0 | 100 |
| Cephalophini | 63 | 0 | 2 | 98 |
| Hippotragini | 38 | 89 | 11 | 0 |
| Neotragini | 84 | 2 | 11 | 87 |
| Reduncini | 90 | 93 | 7 | 0 |
| Tragelaphini | 126 | 0 | 15 | 85 |
| Giraffidae | ||||
| Giraffa | 61 | 0 | 7 | 93 |
| Okapia | 2 | 0 | 0 | 100 |
| Hippopotamidae | ||||
| Choeropsis | 1 | 0 | 0 | 100 |
| Hippopotamus | 186 | 36 | 61 | 4 |
| Suidae | ||||
| Hylochoerus | 26 | 0 | 0 | 100 |
| Phacochoerus | 101 | 80 | 18 | 2 |
| Potamochoerus | 46 | 2 | 22 | 76 |
| Tragulidae | ||||
| Hyemoschus | 1 | 0 | 0 | 100 |
| Perissodactyla | ||||
| Equidae | ||||
| Equus | 157 | 91 | 8 | 1 |
| Rhinocerotidae | ||||
| Ceratotherium | 13 | 100 | 0 | 0 |
| Diceros | 145 | 0 | 6 | 94 |
| Proboscidea | ||||
| Elephantidae | ||||
| Loxodonta | 280 | 0 | 19 | 81 |
Classified by the percentage of individuals that are C4 grazers (G), mixed C3–C4 feeders (M), or C3 browsers (B) based on the isotope values (δ13C1750 values >−1‰, >−1‰ and <−8‰, and <−8‰, respectively). See SI Appendix for complete data.
Dataset S2 presents δ13C data for tooth enamel from >900 individual specimens from the Turkana Basin ranging in age from ca. 4–1 Ma. The same isotopic ranges are used to distinguish between C3 browsing, mixed C3–C4 diets, and C4 grazing for both fossil and modern mammals. We assume the δ13C value of the atmosphere is constant for the Pleistocene and Pliocene and has the same value as the preindustrial atmosphere (see discussion in SI Appendix, Detailed Methods).
Diets of Mammalian Lineages in the Pliocene and Pleistocene.
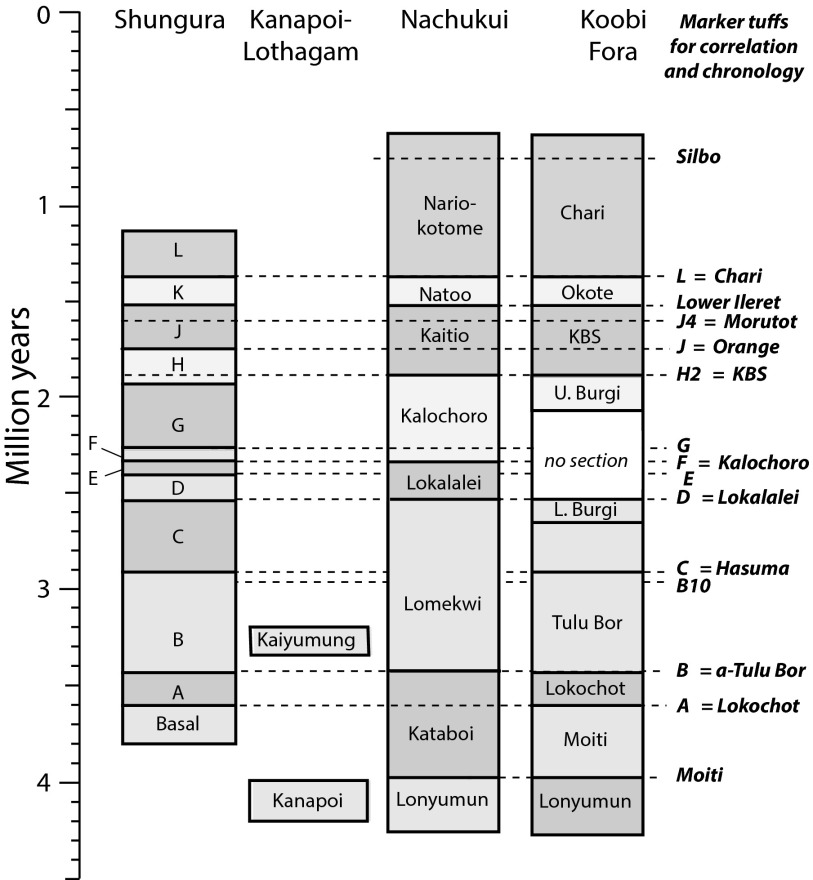
The mammalian lineages considered here derive from different members of the Kanapoi, Koobi Fora, and Nachukui Formations and are of comparable age to the Shungura Formation in the lower Omo Valley as shown in Fig. 2; K/Ar and 40Ar/39Ar dates from the sequence are derived from all four formations and many volcanic ashes are correlative between the formations. Time intervals used in this study are based on correlative marker horizons between the Koobi Fora and Nachukui Formations, and are as follows from oldest to youngest: >4 Ma, 4.0–3.6 Ma, 3.6–3.4 Ma, 3.4–3.0, 3.0–2.5 Ma, 2.5–2.35 Ma, 2.35–1.9 Ma, 1.9–1.5 Ma, 1.5–1.3 Ma, and 1.3–1.0 Ma.
Fig. 2.
Stratigraphic relationships in the Turkana Basin for major collecting geographic regions: Shungura, Nachukui, Koobi Fora, and Kanapoi Formations. Dashed lines show some important volcanic ash layers (tuffs) used for correlation between formations; tuff names are in bold. Stratigraphy and correlations based on earlier results (see SI Appendix, Detailed Methods).
The APP taxa for modern specimens is discussed using the normalized proportions of C4 grazing, C3–C4 mixed, and C3 browsing (G:M:B) for each taxon (SI Appendix). These results largely confirm previous isotope surveys (29, 30) for modern African bovids, hippos (31, 32), suids (33), and elephants (34) but expand the database severalfold. However, comparison of individual lineages of APP taxa show significant changes over time; a number of taxa had diets in the fossil record that are quite different from those of their modern representatives (e.g., Aepycerotini, Antilopini, Tragelaphini, and Loxodonta; SI Appendix, Figs. S4 and S5).
Discussion
Ecosystem and Dietary Change Through the Past 4 Ma.
Dietary change through time for individual lineages.
Many African taxa have diets that remained essentially the same (less than 2‰ change) for much of the past 4 million years (SI Appendix, Table S4 and Fig. S4). These include the taxa and lineages that are presently C4 grazers, Alcelaphini, Bovini, Reduncini, Ceratotherium (=Rhino-G), Metriochoerus-Phacochoerus and Equus, the C3–C4 mixed feeder Hippopotamus s. l., and the C3 browsers Neotragini, Giraffa, Diceros (=Rhino B), and Deinotherium. Of these, it is notable that modern Alcelaphini have δ13C1750 values that are consistently more positive relative to fossil Alcelaphini. Such differences could be due to several factors: a slight diagenetic exchange of 13C resulting in the fossils δ13C1750 values being slightly more negative relative to modern samples, a change in the atmospheric δ13C value causing a shift in the δ13C of plants and the derived dietary δ13C of enamel, an increase in the isotope enrichment (tooth enamel relative to diet) of alcelaphins that occurred in the past million years, or a slight difference in diet whereby many modern alcelaphins are true hypergrazers and the fossil alcelaphins were not. Diagenesis is unlikely to more strongly affect alcelaphins than other taxa, so diagenesis does not explain such differences. Studies of North Atlantic benthic marine carbonates show relatively constant δ13C values through the past ca. 6 Ma (see discussions in refs. 35 and 36), indicating that the δ13C of the atmosphere was similar through the past 4 Ma. At present, we cannot distinguish between the last two possibilities—a change in the isotope enrichment specific to alcelaphins, or a more C4-selective diet for alcelaphins than all other taxa—although we favor the latter.
Hippotragin bovids and suids of the Nyanzachoerus-Notochoerus lineage changed from a mixed feeding to a grazing diet during the interval represented by this stratigraphic sequence.
Aepycerotini, Antilopini, and Tragelaphini represent three bovid tribes whose diets have recently shifted to more negative δ13C values, implying that the fossil representatives of these taxa had a higher C4 component in their diet than their modern relatives (SI Appendix, Table S4 and Figs. S4 and S5). Fossil Aepycerotini in the Turkana Basin are enriched in 13C by several per mil compared with modern Aepyceros; only specimens from the Mara and adjoining Serengeti have δ13C values comparable to those of most of the fossil Aepycerotini. Fossil Antilopini in all except the lowest stratigraphic intervals have average δ13C values between ca. −1 and −3‰, indicating a strong C4 preference, which constrasts with modern antilopins that mostly prefer C3 browsing. Only the modern antilopin Eudorcas thomsonii has values similar to the Turkana Basin fossil Antilopini (SI Appendix, Table S2). Thus, the Antilopini have shifted toward browsing since the early to middle Pleistocene. Tragelaphini also have shifted from ca. −5‰ in the fossil record to ca. −10 to −12‰ in extant tragelaphins. Our survey of 126 modern tragelaphin individuals includes only 7 (i.e., ca. 6%) with δ13C values >−5‰, whereas 16 of 43 of fossil tragelaphins (i.e., ca. 37%) have δ13C values >−5‰. Tragelaphins from the Shungura Formation (Members C–G; from ca. 3.0–2.0 Ma) also had high δ13C values (37) similar to those measured on specimens from the Nachukui and Koobi Fora Formations in the equivalent time interval.
Loxodonta and Kolpochoerus-Hylochoerus are lineages that were primarily C4 grazers from 4 to 1 Ma, but are now C3 browsers (SI Appendix, Table S4 and Fig. S4). Both lineages have gone from average δ13C values ca. −1‰ between 4 and 1 Ma to the modern average δ13C1750 value of ca. −10 and −14‰, respectively. Such abrupt diet changes imply significant changes in the roles of these genera in the overall ecosystem, and perhaps a change in the ecosystems themselves.
Four C4-grazer lineages become extinct in this interval: Sivatherium, Notochoerus, Eurygnathohippus, and Elephas (SI Appendix, Table S4 and Fig. S4). Sivatherium was a browser at ca. 4 Ma and switched to grazing between 2 and 1 Ma, becoming extinct after adapting to a C4-grazing diet. Notochoerus was a C4-grazing suid; it became extinct in the basin by 1.6 Ma. Eurygnathohippus was a grazing three-toed equid related to hipparions that became extinct in the early Pleistocene. Elephas, a C4-grazing elephant, was present in the basin from 4 to 1 Ma ago, but it became extinct in Africa in the middle to late Pleistocene.
Cephalophins, neotragins, Giraffa, and the browsing rhino lineage represented by Diceros have been dedicated browsers throughout their known history. Deinotherium was similarly adapted throughout the 4–1 Ma time interval but became extinct in Africa in the middle Pleistocene; it has the most negative δ13C values of any taxon for all time intervals in the Turkana Basin for which we have analyses (Dataset S2).
Elephants and tragelaphin bovids are two groups often used for paleoecological interpretations, yet their respective fossil diets were very different from those of their modern closest relatives (SI Appendix, Table S4 and Figs. S4 and S5); therefore, the taxonomic presence of a lineage does not indicate that the earlier fossil representative of the lineage had an ecological function in the past similar to that of the modern representative. For example, Loxodonta is often considered to be a keystone species that strongly affects woody cover; although Loxodonta is now predominantly a C3 browser (SI Appendix, Table S2), in the late Pliocene and early Pleistocene Loxodonta was primarily a C4 grazer (SI Appendix, Table S4 and Figs. S4 and S5). Likewise, tragelaphins are commonly assumed to be indicators of forest or woodland (38, 39) because modern tragelaphins are browsers (e.g., see Table 1 and SI Appendix, Table S2); the strongly mixed C3–C4 diet of fossil tragelaphins suggests that they should not be considered as indicators of forest or woodland habitat for stratigraphic intervals in the Nachukui and Koobi Fora formations. Thus, the role of tragelephins in any fossil assemblage should be considered using the δ13C of specimens specific to that assemblage.
Ecosystem change through time.
This study demonstrates important changes in mammal diets and ecosystem structure through the past 4 million years. Three bovid tribes, the warthog lineage, Equus, and grazing rhinos have an essentially unchanged grazing regime through the Omo Group sequence; in contrast, the grazing giraffids, the grazing notochoere suids, grazing three-toed horses, and African representatives of grazing Elephas became extinct. The grazing gomphothere, Anancus, became extinct early in this record. Two bovid tribes, giraffes, and browsing rhinos remain dedicated browsers; browsing deinotheres became extinct. Three bovid tribes incorporate more C3 browsing in the diets of extant versus early Pleistocene representatives, whereas the formerly C4-grazing Kolpochoerus lineage culminates in the C3-browsing Hylochoerus and the formerly grazing Loxodonta switched to a C3 browsing-dominated diet. Hippos remain opportunistic feeders throughout.
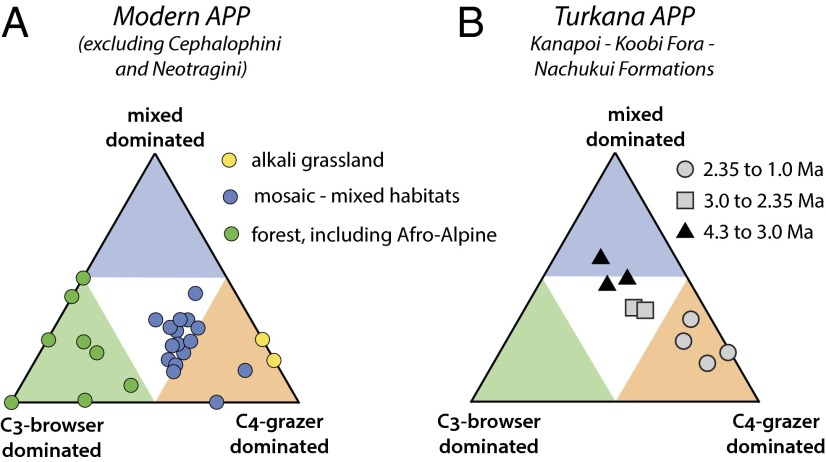
Modern ecosystems in Africa are characterized by having a large mammal fauna with distinctly different mixtures of G:M:B than faunas in the fossil record. Many of the modern ecosystems sampled are considered to be mosaics, including riparian forest with nearby wooded grassland or grasslands. Forest ecosystems (closed canopy forests, coastal and montane forests, and Afro-alpine in SI Appendix, Table S1) are dominated by C3 browsers and mixed C3–C4 feeders; pure grassland faunas have >80% C4 grazers, and most of the modern mosaic ecosystems have subequal numbers of C4 grazers and C3 browsers, with a minor number of C3–C4 mixed feeders (Figs. 3A and 4B).
Fig. 3.
Ternary diagram showing proportions of C3 browsers, C3–C4 mixed feeders, and C4 grazers from the orders Artiodactyla, Perrisodactyla, and Proboscidea (APP); each taxon in each locality or time interval is represented by the average δ13C for that taxon. Each point in the figure represents the respective proportions of APP taxa that are C3 browsers, C3–C4 mixed feeders, or C4 grazers at one modern locality, or one fossil assemblage from the Turkana Basin of a specific age range. The green, blue, and orange triangles represent regions where >50% of the taxa are C3 browsers, C3–C4 mixed feeders, or C4 grazers, respectively. (A) Modern ecosystems as described in SI Appendix, Table S1, using data from Dataset S1; Neotragini and Cephalophini are excluded for comparison with fossil assemblages (see SI Appendix, Fig. S3 for comparison with, and without, inclusion of Neotragini and Cephalophini). (B) Fossil assemblages for age ranges discussed in this paper from the Kanapoi, Nachukui, and Koobi Fora Formations; data from Dataset S2.
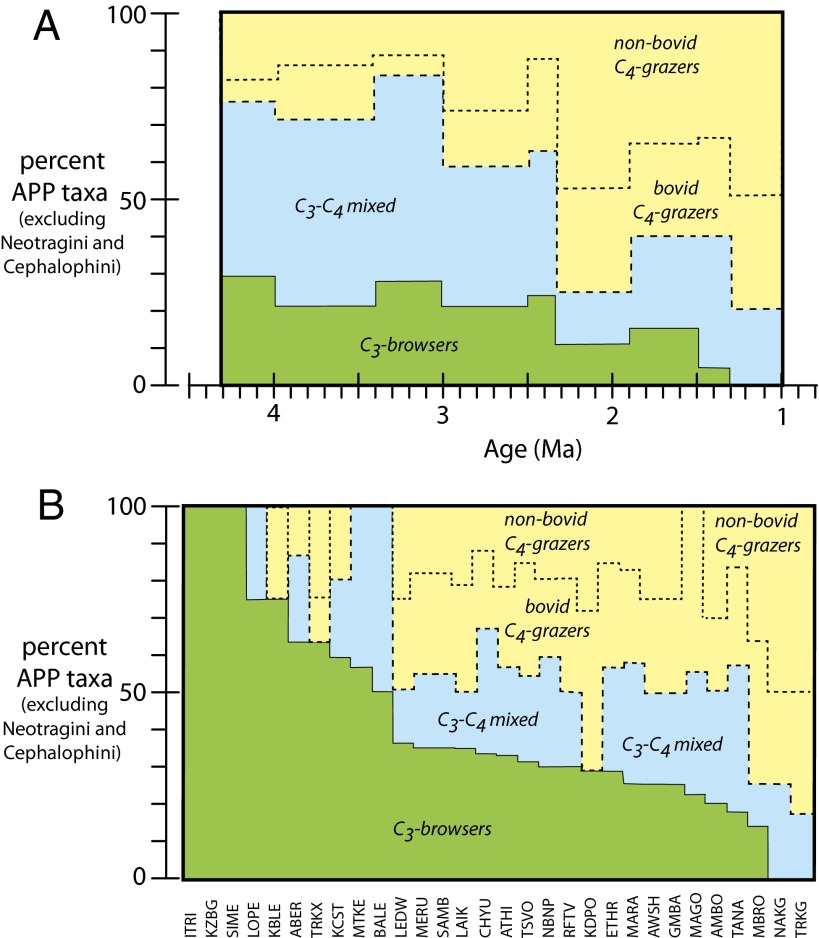
Fig. 4.
Trends over time for percentages of C3 browsers, C3–C4 mixed feeders, bovid C4 grazers, and nonbovid C4 grazers in the Kanapoi, Nachukui, and Koobi Fora Formations and modern ecosystems in East and Central Afria for APP taxa (excluding Neotragini and Cephalophini because of their rarity in the fossil assemblages). (A) Fossil assemblages from Kanapoi, Koobi Fora, and Nachukui Formations; time intervals as described in text (data from Dataset S2). (B) Modern ecosystems studied (see SI Appendix, Table S1; data from Dataset S1).
In this discussion we have assumed that C3 grasses are insignificant in the isotopic contribution to the C3 diet resources. If C3 grasses play a role in this story, strong selectivity would have to be in play because some lineages are essentially C4 grazers throughout the sequences (e.g., equids, Rhino-G, and alcelaphins). Although C3 grasses were possibly present, the selectivity for C4 grasses by some species and for C3 grasses by others must be invoked for such dietary differences.
Using this G:M:B ternary classification, the fossil record in the Turkana Basin shows distinctly different patterns for the early (4.3–3.0 Ma), middle (3.0–2.35 Ma), and later (2.35–1.0 Ma) time intervals compared with the modern ecosystems. Before ca. 2.35 Ma, the ecosystems had much higher percentages of C3–C4 mixed feeders than are found today in East and Central Africa, with all intervals having >40% C3–C4 mixed feeders. For comparison, only a few of the 30 modern ecosystems has such a high percentage of C3–C4 mixed feeders; those few are associated with forest or Afro-alpine montane ecosystems with few large mammalian herbivores (e.g., Bale and Mt Kenya). Fig. 3B shows the G:M:B ternary for the individual stratigraphic collection intervals in each of the Kanapoi, Koobi Fora, and Nachukui formations. After ca. 2.2 Ma there was an abrupt change to many more grazing taxa and overall a higher fraction of grazers than are found most of the modern ecosystems studied for comparison (Figs. 3B and 4).
The number of nonruminant grazers after 2.35 Ma is particularly striking, with between five and nine C4 grazers—in addition to grazing bovids—in these intervals. At the generic taxonomic level with which these comparisons are made, there are only three modern nonruminant C4 grazers in East and Central Africa: Phacochoerus, Equus, and Ceratotherium, although Hippopotamus is locally a grazer in some regions (e.g., Turkana, Nakuru). Many of the nonbovid C4-grazing fossil taxa are extinct (Sivatherium, Notochoerus, Eurygnathohippus, and Elephas) or have switched to browsing (the Kolpochoerus-Hylochoerus lineage and Loxodonta). The time interval from 2 to 1 Ma is noteworthy for the number of nonruminant grazers that are not part of the modern fauna.
Thus, there are several important ecological changes in the Turkana Basin over time: The earlier time interval (ca. 4.1–2.35 Ma) was dominated by C3–C4 mixed feeders, whereas the time interval from ca. 2.35–1.0 Ma was dominated by bovid and nonruminant C4 grazers (Fig. 4A). The timing of this shift in herbivore diet is consistent with previous studies that rely on taxonomic and morphological indicators (38, 39); however, the previously presumed diets are not always consistent with the isotope data. Modern analog collections from East and Central Africa do not represent ecosystems dominated by C3–C4 mixed feeders or nonruminant grazers (compare with Fig. 4B). After 1.0 Ma, there was a drastic transformation to the modern Africa dietary distribution, so that most nonruminant C4 grazers either became extinct or changed their diets to browsing. The timing of the Pleistocene diet changes since 1.0 Ma is uncertain and will come into focus as samples are analyzed from this and other basins (40, 41).
The paleosol record in the Turkana Basin (11, 12, 42) shows a decrease in woody cover with an increase in C4 biomass from 4 to 1 Ma, but changing from grassy woodland to wooded grasslands; no paleosols indicate open C4 grasslands. Comparisons between the dietary categories and paleosol ecological reconstructions for the Shungura Formations and the Koobi Fora–Nachukui Formations will be illuminating: From 4 to 1 Ma the Shungura Formation was more wooded than the Koobi Fora and Nachukui formations.
Summary Statement.
This study of the history of ecological change in the Kanapoi, Nachukui, and Koobi Fora Formations shows profound changes in ecosystem structure: For the period from 4.3 to 2.5 Ma, large mammal herbivorous taxa were dominated by C3–C4 mixed feeders. No modern dietary analog to this is found in East or Central Africa. From 2.5 to 1.0 Ma, grazing taxa, especially nonbovid grazers, became increasingly abundant; modern environments in East and Central Africa do not have such a high fraction of the nonbovid grazers. Many of the C4 grazing nonbovid herbivores became extinct between 2 and 0 Ma; in addition, some taxa that previously were C4 grazers or C3–C4 mixed feeders changed their diet to C3 browsing. More APP taxa were present in the basin for many of the stratigraphic intervals than exist in any modern equivalent environment (e.g., compare totals for SI Appendix, Tables S3 and S4); nowhere today in East or Central Africa is such taxonomic diversity found for the APP taxa as was found in the Turkana Basin from 4.3 to 1.0 Ma.
Interaction between the different large mammal herbivore taxa likely plays a role in diet change. In modern African ecosystems megaherbivores (>1,000 kg), particularly elephants and hippopotamus, maintain the structure and function of both wooded and grassy biomes (43, 44) and play a key role in determining the availability of food for mesoherbivores (4–450 kg; ref. 45). Therefore, changes in the diet of large herbivores throughout the 4–1 Ma time interval indicate significant alterations to mammalian dietary ecological structure and competitive interactions and may relate to shifts in vegetation structure. Ecological interactions with carnivores and primates, including hominins, may also be important for understanding the evolution of herbivore diets (46–48).
The interplay of grass expansion in the time period from 10 Ma to the present will be critical in understanding dietary changes that have occurred in the large mammal taxa in Africa. Although NPP of C4 grasses in the tropics has gone from ca. 1% at 10 Ma to ca. 50% today, there are no known C4-grass macrofossils (i.e., fossils exhibiting Kranz anatomy, fossil plants with δ13C values indicating C4 photosynthesis, or both) from Africa between 1 and 10 Ma. Which specific C4 grasses were predominant, or even present, in the Pleistocene or Pliocene of Africa (or elsewhere) is not known; such information will be key toward understanding the development of tropical grasslands and in understanding how fauna used the C4-grass dietary resources. Changes in digestibility, toxin level, palatability, nutrient distribution in space and in time, and relative abundances of the different C4 grasses likely all played an important role in the evolution of the mammalian diet in Africa. These factors may be important in understanding how different APP herbivores competed for dietary resources. It is well known that C4 photosynthesis is favored by low atmospheric CO2 concentrations (i.e., less than 500 ppm by volume; refs. 4 and 14); the interval from 4 Ma to the present was continually below this CO2 threshold (49–51). With each oscillation of CO2 in the atmosphere, tropical ecosystems are subjected to stresses that could have cumulative effects on ecosystem structure with respect to the comparative success of C3 and C4 lineages. The role of climate change, including changes in atmospheric CO2, will be better evaluated when details of extinctions and diet change are better known. These records are needed to evaluate the relationships between behavioral, morphological, and environmental change, which may not be synchronous (52).
This study of the dietary history of herbivores in the Turkana Basin shows that modern animals often have diets different from those of their closest fossil relatives. Likewise, for much of the past 4 million years, the large herbivorous fauna used dietary resources in different ways than do their modern analogs.
Methods
Modern samples of APP taxa from East and Central Africa, and fossil samples from the well-dated Turkana Basin in northern Kenya, were analyzed for δ13C using standard methods (SI Appendix, Detailed Methods).
Ecological comparisons for modern taxa were made based on regional ecological grouping in restricted geographic areas, such as are presented in national parks or reserves (SI Appendix, Table S1). We used the classification of White (53) for discussion of African vegetation (SI Appendix, Classification of African Vegetation).
Fossil samples were grouped by stratigraphic age, using stratigraphic boundaries that are correlated between the Koobi Fora, Nachukui, Kanapoi, and Shungura Formations (Fig. 2).
Supplementary Material
Acknowledgments
Many people assisted in helping collect samples and in other ways. In particular, we thank Dr. Samuel Andanje, who passed away on May 4, 2015, as this paper was in review. We thank David Augustine, Kay Behrensmeyer, Francio Busenene, Dena Crain, David Daballan, Dan Davis, Mohammed Dhidha, Iain Douglas-Hamilton, Nick Georgiadis, Terese Hart, Peter Jenkins, Cedric Khayale, Samuel Kasiki, Mahala Kephart, Jackson King’oo, Hans Klingel, John Lanham, Chris Leadismo, Jonathan Leakey, Nigel Leakey, Richard Leakey, Francis Lomojo, Anthony Macharia, Emma Mbua Nina Mudida, Charles Musyoki, Patrick Omondi, Marcello Onen, David Patterson, Richard Potts, Jessica Rothman, Ben Seegmiller, Fraser Smith, Kes Smith, Katya Viehl, George Wittemyer, Solomon Yirga, Truman Young, and Nick van der Merwe. Many museums and organizations have been of great assistance, and we thank Addis Ababa University, the American Museum of Natural History, the Centre de Recherche en Science Naturelles, Lwiro of the Democratic Republic of Congo, the Ethiopian Wildlife Conservation Department, the Field Museum, Kenya Wildlife Service, Mpala Research Center, the National Museums of Kenya, Save The Elephants, the Turkana Basin Institute, the Uganda Wildlife Authority, and the University of Cape Town. Isotope analyses were done in the Stable Isotope Ratio Facility for Environmental Research laboratory at the University of Utah and at the University of Cape Town. Ivory was imported with Convention on International Trade in Endangered Species (CITES) permit 02US053837/9. This work was funded by the F. Brown Rosenblatt Fund of the University of Utah (UU); the Fulbright Foundation; the Geochemistry Laboratory (UU); the Geological Society of America; the Global Change and Sustainability Center (UU); the LSB Leakey Foundation; National Science Foundation Grants 9310546, 9604024, 0617010, 0621542, 0722598, 0819611, 1260535, and 2010079300; the Packard Foundation; National Geographic Grants 7767-04 and 9349-13; and the Wenner-Gren Foundation.
Footnotes
The authors declare no conflict of interest.
2Deceased May 4, 2015.
See Commentary on page 11428.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513075112/-/DCSupplemental.
References
- 1.Lloyd J, et al. Contributions of woody and herbaceous vegetation to tropical savanna ecosystem productivity: A quasi-global estimate. Tree Physiol. 2008;28(3):451–468. doi: 10.1093/treephys/28.3.451. [DOI] [PubMed] [Google Scholar]
- 2.Sage RF, Li M, Monson RK. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 Plant Biology. Academic; New York: 1999. pp. 551–584. [Google Scholar]
- 3.Sage RF, Christin PA, Edwards EJ. The C4 plant lineages of planet Earth. J Exp Bot. 2011;62(9):3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- 4.Ehleringer JR, Cerling TE, Helliker B. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- 5.Christin PA, et al. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr Biol. 2008;18(1):37–43. doi: 10.1016/j.cub.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 6.Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA. The age of the grasses and clusters of origins of C4 photosynthesis. Glob Change Biol. 2008;14(12):2963–2977. [Google Scholar]
- 7.Tidwell WD, Nambudiri EMV. Tomlisonia thomassonii, gen. et sp. nov., a permineralized grass from the upper Miocene Ricardo Formation, California. Rev Palaeobot Palynol. 1989;60(1):165–177. [Google Scholar]
- 8.Thomasson JR, Nelson ME, Zakrzewski RJ. A fossil grass (gramineae: chloridoideae) from the miocene with kranz anatomy. Science. 1986;233(4766):876–878. doi: 10.1126/science.233.4766.876. [DOI] [PubMed] [Google Scholar]
- 9.Quade J, Cerling TE, Bowman JR. Development of Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature. 1989;342:163–166. [Google Scholar]
- 10.Fox DL, Koch PL. Tertiary history of C4 biomass in the Great Plains, USA. Geology. 2003;31(9):809–812. [Google Scholar]
- 11.Cerling TE. Development of grasslands and savannas in East Africa during the Neogene. Palaeogeogr Palaeoclimatol Palaeoecol. 1992;5:241–247. [Google Scholar]
- 12.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;307:75–89. [Google Scholar]
- 13.Strömberg CAE, McInerney FA. The Neogene transition from C3 to C4 grasslands in North America: assemblage analysis of fossil phytoliths. Paleobiology. 2011;37:50–71. [Google Scholar]
- 14.Cerling TE, et al. Global change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 15.Uno KT, et al. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc Natl Acad Sci USA. 2011;108(16):6509–6514. doi: 10.1073/pnas.1018435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita ZA, Secord R, Boardman GS. A new stable isotope record of Neogene paleoenvironments and mammalian paleoecologies in the western Great Plains during the expansion of C4 grasslands. Palaeogeogr Palaeoclimatol Palaeoecol. 2014;399:160–172. [Google Scholar]
- 17.Feakins SJ, deMenocal PB, Eglinton TI. Biomarker records of late Neogene changes in northeast African vegetation. Geology. 2005;33(12):977–980. [Google Scholar]
- 18.Feakins SJ, et al. Northeast African vegetation change over 12 my. Geology. 2013;41(3):295–298. [Google Scholar]
- 19.Cerling TE, Brown FH. Tuffaceous marker horizons in the Koobi Fora region and the lower Omo Valley. Nature. 1982;299:216–221. [Google Scholar]
- 20.McDougall I, Brown FH. Precise 40Ar/39Ar geochronology for the upper Koobi Fora Formation, Turkana Basin, northern Kenya. J Geol Soc London. 2006;163:205–220. [Google Scholar]
- 21.McDougall I, Brown FH. Geochronology of the pre-KBS Tuff sequence, Omo Group, Turkana Basin. J Geol Soc London. 2008;165:549–562. [Google Scholar]
- 22.McDougall I, et al. New single crystal 40Ar/39Ar ages improve timescale for deposition of the Omo Group, Omo-Turkana Basin, East Africa. J Geol Soc London. 2012;169:213–226. [Google Scholar]
- 23.Sage RF, Wedin DA, Li M. The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, editors. C4 Plant Biology. Academic; New York: 1999. pp. 313–373. [Google Scholar]
- 24.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120:347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 25.Ehleringer JR, Cooper TA. Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia. 1988;76:562–566. doi: 10.1007/BF00397870. [DOI] [PubMed] [Google Scholar]
- 26.Hattersley PW. 13C values of C4 types in grasses. Aust J Plant Physiol. 1982;9:139–154. [Google Scholar]
- 27.Buchmann N, Brooks JR, Rapp KD, Ehleringer JR. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 1996;9:392–402. [Google Scholar]
- 28.Passey BH, et al. Carbon isotopic fractionation between diet, breath, and bioapatite in different mammals. J Archaeol Sci. 2005;32:1459–1470. [Google Scholar]
- 29.Cerling TE, Harris JM, Passey BH. Dietary preferences of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84:456–471. [Google Scholar]
- 30.Sponheimer M, et al. Diets of southern African Bovidae: Stable isotope evidence. J Mammal. 2003;84:471–479. [Google Scholar]
- 31.Boisserie JT, et al. Diets of modern and late Miocene hippopotamids: Evidence from carbon isotope composition and microwear of tooth enamel. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;221:153–174. [Google Scholar]
- 32.Cerling TE, et al. Stable isotope ecology of modern Hippopotamus amphibius in East Africa. J Zool (Lond) 2008;276:204–212. [Google Scholar]
- 33.Harris JM, Cerling TE. Dietary adaptations of extant and Neogene African suids. J Zool (Lond) 2002;256:45–54. [Google Scholar]
- 34.Cerling TE, Harris JM, Leakey MG. Browsing and grazing in modern and fossil proboscideans. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 35.Cerling TE, Harris JM, Leakey MG, Passey BH, Levin NE. Stable carbon and oxygen isotopes in East African mammals: Modern and fossil. In: Werdelin L, Sanders W, editors. Cenozoic Mammals of Africa. Univ of California Press; Oakland, CA: 2010. pp. 949–960. [Google Scholar]
- 36.Tipple BJ, Meyers SR, Pagani M. Carbon isotope ratio of Cenozoic CO2: A comparative evaluation of available geochemical proxies. Paleoceanography 25.3. 2010 doi: 10.1029/2009PA001851. [DOI] [Google Scholar]
- 37.Bibi F, Souron A, Bocherens H, Uno K, Boisserie JR. Ecological change in the lower Omo Valley around 2.8 Ma. Biol Lett. 2013;9(1):20120890. doi: 10.1098/rsbl.2012.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed KE. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol. 1997;32(2-3):289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- 39.Bobe R, Behrensmeyer AK. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;207(3–4):399–420. [Google Scholar]
- 40.Chritz KL, Marshall F, Zagal ME, Kirera F, Cerling TE. Environments and livestock disease risk of early herders in the later Holocene of the Lake Victoria Basin, Kenya. Proc Natl Acad Sci USA. 2015;112(12):3674–3679. doi: 10.1073/pnas.1423953112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrett ND, et al. Stable isotope paleoecology of Late Pleistocene Middle Stone Age humans from the Lake Vicoria basin, Kenya. J Hum Evol. 2014;82:1–14. doi: 10.1016/j.jhevol.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 43.Laws RM. Elephants as agents of habitat and landscape change in East Africa. Oikos. 1970;21:1–15. [Google Scholar]
- 44.Owen-Smith RN. Megaherbivores: The Influence of Very Large Body Size on Ecology. Cambridge Univ Press; Cambridge, UK: 1992. [Google Scholar]
- 45.Fritz H, Duncan P, Gordon IJ, Illius AW. Megaherbivores influence trophic guilds structure in African ungulate communities. Oecologia. 2002;131:620–625. doi: 10.1007/s00442-002-0919-3. [DOI] [PubMed] [Google Scholar]
- 46.Lewis ME. Carnivoran paleoguilds of Africa: Implications for hominid food procurement strategies. J Hum Evol. 1997;32(2-3):257–288. doi: 10.1006/jhev.1996.0103. [DOI] [PubMed] [Google Scholar]
- 47.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110(26):10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartoli G, Hönisch B, Zeebe RE. Atmospheric CO2 decline during the Pliocene intensification of Northern Hemisphere glaciations. Paleoceanography. 2011;26(4) doi: 10.1029/2010PA002055. [DOI] [Google Scholar]
- 50.Zhang YG, Pagani M, Liu Z, Bohaty SM, Deconto R. A 40-million-year history of atmospheric CO2. Philos Trans A Math Phys Eng Sci. 2013;371(2001):20130096. doi: 10.1098/rsta.2013.0096. [DOI] [PubMed] [Google Scholar]
- 51.Martínez-Botí MA, et al. Plio-Pleistocene climate sensitivity evaluated using high-resolution CO2 records. Nature. 2015;518(7537):49–54. doi: 10.1038/nature14145. [DOI] [PubMed] [Google Scholar]
- 52.Lister AM. The role of behaviour in adaptive morphological evolution of African proboscideans. Nature. 2013;500(7462):331–334. doi: 10.1038/nature12275. [DOI] [PubMed] [Google Scholar]
- 53.White F. 1983. The Vegetation of Africa: A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa. Natural Resources Research Report XX (UNESCO, Paris)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.