Abstract
Background
Gamma-enolase, known also as neuron-specific enolase (NSE), is an enzyme of the glycolytic pathway, which is expressed predominantly in neurons and cells of the neuroendocrine system. As a tumour marker it is used in diagnosis and prognosis of cancer; however, the mechanisms enrolling it in malignant progression remain elusive. As a cytoplasmic enzyme gamma-enolase is involved in increased aerobic glycolysis, the main source of energy in cancer cells, supporting cell proliferation. However, different cellular localisation at pathophysiological conditions, proposes other cellular engagements.
Conclusions
The C-terminal part of the molecule, which is not related to glycolytic pathway, was shown to promote survival of neuronal cells by regulating neuronal growth factor receptor dependent signalling pathways, resulting also in extensive actin cytoskeleton remodelling. This additional function could be important also in cancer cells either to protect cells from stressful conditions and therapeutic agents or to promote tumour cell migration and invasion. Gamma-enolase might therefore have a multifunctional role in cancer progression: it supports increased tumour cell metabolic demands, protects tumour cells from stressful conditions and promotes their invasion and migration.
Keywords: gamma-enolase, cancer, glycolysis, cell survival, tumour marker
Introduction
Enolases (EC 4.2.1.11) are intracellular enzymes that catalyse the dehydration of 2-phospho-D-glycerate to phosphoenolpyruvate in the catabolic direction of the glycolytic pathway, a process converting glucose into pyruvate, which enables the formation of high-energy compounds of ATP and NADH. In the anabolic direction during gluconeo-genesis, they catalyse the reverse reaction of hydration of phosphoenolpyruvate to 2-phospho-D-glycerate. The glycolytic pathway and its enzymes are one of the most conserved and important metabolic networks in living organisms and therefore, enolases are among the most ubiquitously and abundantly expressed proteins.1–4 Despite being expressed in most cells, the gene that encodes for enolase is not a housekeeping gene since its expression varies during several developmental, metabolic or pathophysiological conditions.5 In addition to their innate glycolytic function, many enzymes of the glycolytic pathway, including enolase, were shown to possess various specific regulatory functions and to play a pleiotropic role in physiological and pathological processes, including cancer.1,2,6,7 In this paper we review the properties, distribution and function of gamma-enolase and its role in enhanced glycolysis and proliferation of tumour cells. Additionally, we expose new mechanisms through which gamma-enolase may promote cancer progression: aiding adaptation of tumour cells to stressful conditions by activating survival promoting signalling pathways and promoting migration of tumour cells. Finally, we discuss the role of gamma-enolase as a marker of exposure to carcinogenic pollutants and review the diagnostic and prognostic utility of gamma-enolase in cancer patients.
Properties and distribution of enolase
Enolases are functionally active as dimers, composed of non-covalently linked subunits alpha- (α), beta- (β) and gamma- (γ), facing each other in an antiparallel fashion, which may form five homodimeric or heterodimeric isoenzymes, expressed in a development and tissue-specific manner. The iso-enzyme αα (alpha-enolase) is localized in all foetal and in the majority of adult mammal tissues. During tissue development, it is replaced by other isoforms: in skeletal and heart muscles by αβ and ββ (beta-enolase), and in neuronal cells and cells of the diffuse neuroendocrine system by isoenzymes αγ and γγ (gamma-enolase). In mammals, each of the three isoenzymes is encoded by an independent loci.8,9 All enolase isoforms have a molecular range between 82 and 100 kDa and share high sequence identity and kinetic properties.1,6,10–12 However, each isoform possesses characteristic short variable regions, which are situated predominantly on the surface of the molecule and might be the sites of contact with different cytoskeleton elements or other cell components.13
Besides the peptide molecule, enolase requires a divalent metal ion for its stabilisation and catalytic activity. Six divalent metals have been demonstrated to activate enolase: Mg2+, Zn2+, Cd2+, Co2+, Mn2+ and Ni2+. The most abundant is Mg2+, which provides the highest activation strength.1,14,15 The metal ion is not firmly bound into the protein part of the molecule; therefore enolase is not a typical metalloenzyme, but defined as a “metal-ion-activated enzyme complex”.16 Enolase has two binding sites for Mg2+, both contributing to catalysis: binding to the first site, Mg2+ induces conformational changes of the active site enabling the binding of the substrates, whereas the binding of a second Mg2+ is an essential part of the catalytic apparatus.1,17–19
Enolase localizes predominantly in the cytosol however, variations in cellular localisation were observed for all three enolase isoforms. Alpha-enolase was observed in the nucleus, on the cell surface and in extracellular space. It may interact with different cytoplasmic, nuclear and membrane molecules and exhibits several other functions besides catalysis.1,20 The nuclear form of alpha-enolase was recognized as Myc promoter-binding protein-1 (MBP-1), an alternative splicing form involved in regulation of transcription by repressing the function of Myc and acting as a tumour suppressor.6,21–23 Alpha-enolase localizes also on cell surface of neuronal, endothelial and hematopoietic cells as well on pancreatic, breast and lung cancer cells. Its surface expression was shown to depend on the pathophysiological conditions of the cells and its C-terminal lysine residue acts as a plasminogen-binding receptor modulating peri-cellular fibrinolytic activity and promoting migration and metastasis of cancer cells. The cell surface alpha-enolase is catalytically active, maintaining its active dimeric form. Alpha-enolase was shown also to be secreted from cells by exosomes, cell derived vesicles, proposed to play an important role in intercellular communication.23–25 However, the mechanisms of surface translocation, membrane attachment, cell surface expression or secretion remain unknown.6,26–32 The properties and function of alpha-enolase in malignant disease have been extensively studied and reviewed.1,2,6,20,23
Different subcellular localisation and interactions with other proteins were observed also for beta-enolase during maturation, normal function and regeneration of muscles. Specific interactions with macromolecules may address beta-enolase to the subcellular site where ATP, produced through glycolysis, is most needed for muscular contraction or regeneration.33–35 Increased expression of beta-enolase was detected in rhabdomyosarcoma tissue, which is, to our knowledge, the only evidence that this isoform might be involved in cancer.36,37
Gamma-enolase
Gamma-enolase, is a 433 amino acid long acidic dimeric protein, which includes two enolase isoenzymes, γγ and αγ, and is also referred as neuron-specific enolase (NSE). The subunit molecular mass is approximately 39 kDa, whereas Mr of the native form is 78 kDa which might vary on the subunit combination. Gamma-enolase localizes predominantly in neuronal cells and in neuroendocrine cells, particularly in those of the amine precursor uptake and decarboxylation (APUD) lineage, for example in the intestine, lung, thyroid and pituary gland and pancreas.8,38 It is found in lower amounts also in non-neuronal and non-neuroendocrine tissues or cells, such as erythrocytes, platelets, breast tissue, prostate and uterus.39–41 The γγ isoform is found predominantly in mature neurons and is also used as marker of neuronal maturation and differentiation, while the αγ isoenzyme localizes in higher amounts in non-neuronal cells.8,9
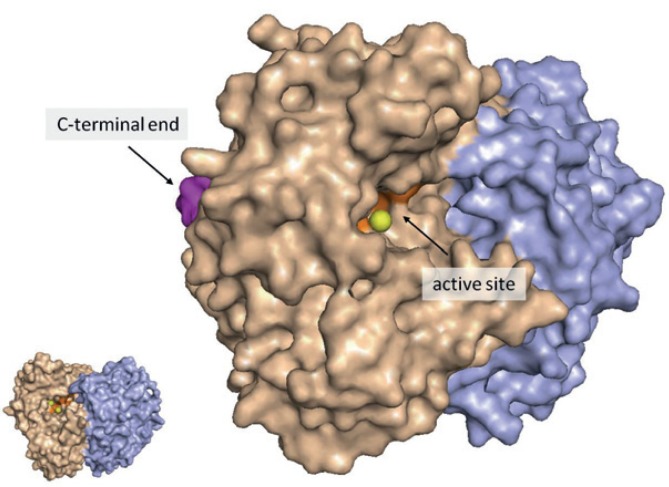
The C-terminal end of gamma-enolase contains a PDZ-binding motif (431S-433L: SVL) (Figure 1), which might enable an interaction with several proteins that contain a PDZ-domain and are involved in intracellular redistribution of molecules and signalling pathway events. Different gamma-enolase cellular localisation, which depends on the pathophysiological conditions of the cells, propose other cellular engagement besides glycolysis. In neuronal, glial and astrocytic cells, gamma-enolase was shown to associate with the plasma membrane, or even appear on the surface of cells42–45, which might occur through its hydrophobic domain in the N-terminal region (32A-43Y: AAVPSGASTGIY). Also, on the cell surface alpha-enolase may bind to plasminogen by C-terminal lysine.46 In contrast to alpha-enolase, gamma-enolase has no C-terminal lysine and does not bind plasminogen; therefore it might exert other functions on cell surface.43,46–48 Gamma-enolase was detected also in the nucleus of malignantly transformed urothelial and epithelial breast cells and in glioblastoma cells; however its role remains unknown.40,49–51 Significantly higher increase of gamma-enolase antigen levels than its catalytical activity was observed during exponential growth of small-cell lung cancer cells, proposing that cellular gamma-enolase exists also as an enzymatically inactive compound, that might possess other functions.52
FIGURE 1.
Position of gamma-enolase catalytical active site and the PDZ-binding motif containing C-terminal end. Subunits of the γγ-dimer are represented by separate colours (wheat and violet). The orange part represents the catalytical active site, yellow balls represent Mg2+ ions and the magenta part represents the C-terminal end of the molecule (the last 6 amino acids). For better representation, active site and C-terminal end are shown only in one subunit. The image was created using PyMOL (DeLano LLC Scientific). Gamma-enolase crystal structure (1TE6) was obtained from Protein Data Bank (PDB). The image was prepared by authors and has not been published elsewhere.
The function of gamma-enolase in increased glycolysis in cancer
It is generally known that glycolysis is drastically enhanced in tumour cells and is a hallmark of cancer progression.53,54 In tumours that outgrow its feeding circulation, cells are exposed to an environment with poor oxygen and nutrients supply50, which leads to a prevalence of aerobic glycolysis over mitochondrial oxidative phosphorylation.55–57 This metabolic switch referred also to as the Warburg effect, enables tumour cells to produce energy to survive and eventually proliferate regardless the presence of oxygen. Glycolysis alone, however, is energetically less efficient than oxidative phosphorylation. Therefore, reactions of the glycolytic pathway have to be drastically accelerated to satisfy the higher metabolic needs of proliferating tumour cells, which is evident from a net increase in glucose consumption and higher expression of glycolytic enzymes.55,58–60
Gamma-enolase is overly-expressed in tumours39 and its major contribution to tumour progression is, no doubt, the participation to accelerated glycolysis of cancer cells. For instance, malignant transformation of astrocytic61, breast40 and urothelial cells49 led to occurrence of gamma-enolase in originally gamma-enolase-negative cells and to colony formation and proliferation, which strongly suggests that transformed cells might obtain the ability to express gamma-enolase in order to adapt to increased metabolic needs of a neoplastic state.61,62 Further, malignantly transformed urothelial cells, which were able to proliferate and form tumours when inoculated into immune compromised mice, were shown to express higher levels of gamma-enolase, compared to less active and differentiated cells. Authors proposed that cells, which express gamma-enolase at higher rates, might have an advantage in tumour initiation and subsequent growth.49 Gamma-enolase was significantly up-regulated also in glioblastoma cells exposed to hypoxia and serum starvation, and additionally, its knock-down significantly diminished cell growth50, supporting the findings that the dependence of tumour cell growth on glycolysis is even more emphasized in stressful conditions.55,60,63 Finally, in non-small cell lung cancer cells, an alternative splicing form of c-H-ras, p19ras, was shown to specifically bind gamma-enolase and inhibit its enzymatic activity, resulting in diminished cell proliferation.58 The glycolytic function of gamma-enolase and its impact on promoting tumour cell growth represents a promising target for cancer therapy.64
The pro-survival function of gamma-enolase in cancer
Gamma-enolase was shown to act as a neurotropic factor in neuronal cells.7,65,66 This function is manifested through an additional active site, which is not a part of the catalytical apparatus involved in glycolysis, but localized at the C-terminal end of the molecule. For instance, a 30 amino acid long peptide, mimicking the C-terminal part of gamma-enolase, was shown to promote survival, differentiation and regeneration of neurons by activating signal transduction pathways which are normally triggered by the activation of Trk receptor: phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. Additionally, the C-terminal peptide of gamma-enolase was demonstrated to impair apoptosis and to interact with p75 neurotrophin receptor (p75NTR) and suppress the activation of its downstream effectors in apoptotic signalling. Despite having similar amino acid sequence in the C-terminal part, other enolase isoforms do not show a neurotropic function.7,43,46,67–69 Gamma-enolase neurotrophic effect is regulated by cathepsin X, a cysteine carboxymonopeptidase, which is frequently expressed in neuronal and glial cells.70,71 Cathepsin X was shown to sequentially cleave the final two amino acids (433L and 432V) at the C-terminal end of gamma-enolase and to disrupt the PDZ motif, through which gamma-enolase binds to the scaffold protein gamma-1-syntrophin. The latter mediates the translocation of gamma-enolase and its association with plasma membrane, which is a prerequisite for neurotrophic activity.43,46 Therefore, only C-terminally uncleaved gamma-enolase has a pro-survival activity. The protective function of gamma-enolase was observed also in brains of a mouse model of Alzheimer disease (Tg2576): C-terminally truncated gamma-enolase localized in immediate plaque vicinity and strongly colocalized with cathepsin X, while uncleaved gamma-enolase exhibiting neuroprotective activity, localized in microglia cells in close proximity of senile plaques. Additionally, using a mouse microglial cell model, gamma-enolase was shown to protect neuronal cells from amyloid-β peptide toxicity and cathepsin X reversed its function.66
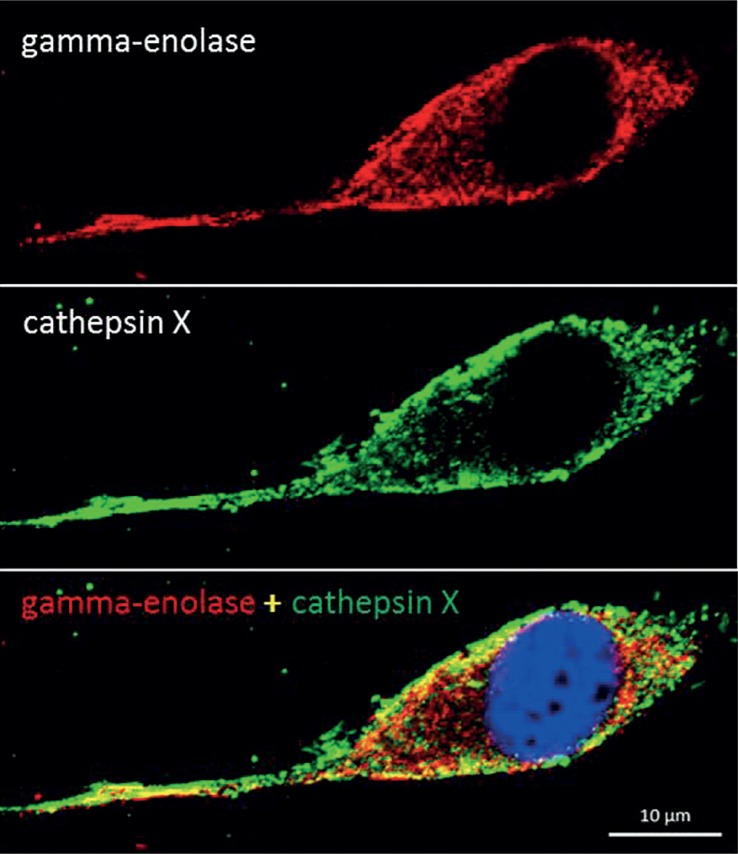
Gamma-enolase has been proposed to act as a pro-survival factor also in cancer cells. It was shown to support glioblastoma cell adaptation to cellular stress, such as serum starvation, hypoxia, chemotherapy and radiotherapy; however, no specific mechanism has yet been proposed.50 Both, starvation and hypoxia have been linked to progression of cancer and resistance to treatment by inducing biological changes in tumour cells, one of them being increased glycolysis.55,60,72 However, C-terminally uncleaved gamma-enolase might additionally support tumour cell adaptation to stressful conditions by activating survival promoting signalling pathways as it does in neuronal cells, and cathepsin X, which is present also in tumour cells71, might regulate its function (Figure 2). For instance, in glioblastoma cell lines, exposed to serum starvation or hypoxia, gamma-enolase expression was significantly increased50; moreover, significant increases in protein and phosphoprotein levels were observed also in PI3K/Akt and MAPK/ERK and anti-apoptotic signalling pathways73,74, which are triggered by gamma-enolase in neuronal cells. Separate analysis of expression and role of C-terminally uncleaved and truncated gamma-enolase in cancer cells and tumour tissue might provide new information on its involvement in tumour progression.
FIGURE 2.
Co-localization of gamma-enolase and cathepsin X in human glioblastoma cells U87-MG grown in serum-free medium for 72 h. U87-MG cells were grown in Eagle´s Minimum Essential Medium (EMEM, Sigma), supplemented with 10% (v/v) foetal bovine serum (HyClone), 1% L-glutamine (Sigma) and 1% penicillin/streptomycin (Sigma) at 37°C and humidified atmosphere with 5% CO2. For protein visualization, cells were seeded on glass coverslips at a concentration of 1 × 104 cells/ml in 24 well plates. After 24 h, complete growth medium was replaced with serum-free medium and cells were left to grow for additional 72 h. After treatment cells were fixed with 10% formalin for 30 min at room temperature and then permeabilized by 0.5% Tween®20 in phosphate buffered saline (PBS), pH 7.4 for 10 min. Non-specific binding was blocked with 3% bovine serum albumin (BSA) in PBS, pH 7.4 for 1.5 h at room temperature. Cells were then incubated with primary antibody against N-terminal end of gamma-enolase (10 μg/ml, goat polyclonal, Santa Cruz Biotechnology) and active cathepsin X (10 μg/ml, mouse monoclonal, 2F12) in 3% BSA in PBS pH 7.4 for 2 h at room temperature. After three washes with PBS, pH 7.4, cells were incubated with Alexa Fluor 555 donkey anti-goat (Molecular Probes™) and Alexa Fluor 488 donkey anti-mouse (Molecular Probes™) secondary antibody in 3% BSA in PBS, pH 7.4. After washing with PBS, ProLong® Gold Antifade Mountant with 4’,6-diamidino-2-phenylindole, dilactate (DAPI, Molecular Probes™) was used to mount coverslips on glass slides. Fluo rescence microscopy was performed by Carl Zeiss LSM 710 confocal microscope (Carl Zeiss Oberkochen) with ZEN 2012 image software. Gamma-enolase (red) and cathepsin X (green) staining showed co-localisation in the perimembrane region. The blue staining with DAPI represents the nucleus. The image was prepared by authors and has not been published elsewhere.
The role of gamma-enolase in migration of tumour cells
Recently, a study on glioma cells showed that gamma-enolase knockdown significantly reduced migration of cells; however, no specific mechanism has been proposed.75 An important prerequisite for cell migration is a dynamic remodelling of actin cytoskeleton. Remodelling is stimulated by several molecules that link migratory signals to the actin filaments and are upregulated in invasive and metastatic cancer cells.76 In neuroblastoma cells, gamma-enolase was shown to co-localize with actin filaments, an interaction that depends on the presence of gamma-1-syntrophin.43 Additionally, gamma-enolase C-terminal peptide was shown to regulate RhoA kinase, a regulator of actin cytoskeleton organization. Consequently, gamma-enolase induced actin polymerisation and its redistribution to growth cones of neurites.68 Similarly, alpha-enolase was shown to bind to actin and tubulin77 and to mediate invasiveness of tumour cells78 and sensitivity to microtubule targeted drugs.79 These results provide evidence, that gamma-enolase might be involved in migration of tumour cells through interactions with actin filaments and regulation of RhoA kinase function.
Gamma-enolase as a marker of exposure to environmental carcinogenic pollutants arsenic and cadmium
Arsenic and cadmium exposure is linked to breast and bladder cancer occurrence. Exposure of breast epithelial and urothelial cells to As3+ or Cd2+ was shown to induce malignant transformation of cells and an increase of mRNA and protein levels of gamma-enolase in the cytoplasm and nucleus of cells, while expression of alpha-enolase did not change. Authors proposed that gamma-enolase might be translated as a possible biomarker for chronic environmental exposure to As3+ or Cd2+. Its expression in non-malignant cells was influenced also by methylation and histone modifications, induced by a histone deacetylase inhibitor (MS-275) and a methylation inhibitor (5-AZC), which proposed that gamma-enolase gene expression is controlled by methylation and histone modifications. The later provides evidence that environmental carcinogenic pollutants, such as cadmium and arsenic, might cause changes in epigenetic regulation of genes, which specifically affect the expression and function of gamma-enolase in breast epithelial cells and urothelial cells.40,49
Gamma-enolase in tumour tissues
Gamma-enolase is typically overexpressed in tumours of neurogenic and neuroendocrine origin and has been used as a marker for detection of neuroendocrine differentiation of tumour cells. It is considered the most important tumour marker for poorly differentiated neuroendocrine tumours, since a tumour is classified as a neuroendocrine tumour only when it expresses at least two neuroendocrine markers of which one is gamma-enolase.80,81 Immunohistochemistry of gamma-enolase is regularly used for differential diagnosis of small-cell lung cancer (SCLC) from other lung cancer histological subtypes (Table 1).82,83 Gamma-enolase increased expression was observed also in other tumours, including breast cancer, with increased staining in lymph node metastases compared to primary breast tumours84 or in glioblastomas, with higher levels in advanced stage tumours, which were related to shorter patient survival.50 Nevertheless, immunostaining of gamma-enolase in tumour tissue has limited diagnostic or prognostic utility, since many clinical studies provided contradictory results.80,85–87
TABLE 1.
Use of gamma-enolase as a tumour marker
| Neuroendocrine cancer | Proposed use | Use in clinical practice | Recommendations | Reference | |
|---|---|---|---|---|---|
| Tumour tissues | SCLC | Differential diagnosis from other lung cancer subtypes | Yes | EGTM, NACB | [82, 83] |
| Other neuroendocrine tumours (neuroblastoma, endocrine pancreatic tumours, seminoma, medullary thyroid carcinoma, phaeochromocytoma, ect.) | Diagnosis or detection of neuroendocrine differentiation of tumour | Yes | [80, 81, 95, 96] | ||
| Serum | SCLC | Differential diagnosis from other lung cancer subtypes when biopsy is not possible | Yes | EGTM, NACB | [82, 83] |
| Prognosis | Unknown | [82, 83, 97] | |||
| Post-operative surveillance | Yes | EGTM, NACB | [82, 83] | ||
| Monitoring efficacy of therapy | Yes | EGTM, NACB | [82, 83] | ||
| Detection of recurrent disease after primary surgery | Yes | NACB | [82, 83] | ||
| NSCLC | Monitoring therapy in advanced disease | No | [83] | ||
| Prognosis | Unknown | [83] | |||
| Testicular cancer (seminoma) | Diagnosis | Experimental | EGTM | [98] | |
| Carcinoids | Diagnosis | Unknown | [96, 99] | ||
| Monitoring efficacy of therapy | Yes | EGTM | [8, 39] | ||
| Detection of early relapse | Yes | [8, 39, 96] | |||
| Medullary thyroid carcinomas | Monitoring efficacy of therapy | Yes | EGTM | [8, 39] | |
| Detection of early relapse | Yes | [8, 39] | |||
| Phaeochromocytoma | Monitoring efficacy of therapy | Yes | EGTM | [8, 39] | |
| Detection of early relapse | Yes | [8, 39] | |||
| Endocrine pancreatic tumours | Diagnosis | Yes | [95, 96] | ||
| Monitoring efficacy of therapy | Yes | EGTM | [8, 39] | ||
| Detection of early relapse | Unknown | [8, 39, 99] | |||
| Paraganglioma | Diagnosis | Unknown | [99] | ||
| Neuroblastoma | Differential diagnosis | Unknown | [8] | ||
| Prognosis | Yes | ACS | [100] | ||
| Monitoring efficacy of therapy | Yes | EGTM | [8, 100] | ||
| Detection of recurrent disease | Yes | [97] |
ACS = American Cancer Society; EGTM = European Group for Tumour Markers; NACB = National Academy of Clinical Biochemistry; NSCLC = non-small-cell lung cancer; SCLC = small-cell lung cancer
Gamma-enolase in extracellular fluids of cancer patients
In general, gamma-enolase serum levels are better indicators than its tissue expression (Table 1).80 Levels of gamma-enolase are elevated in sera from patients with various cancers, however, its appearance in extracellular fluids without any apparent cellular damage is not clear.1,88 After stroke, brain injury or cardiac arrest, gamma-enolase is released into the cerebrospinal fluid and eventually into the bloodstream due to damage or death of neuronal cells or impairment of the blood-brain barrier integrity. For instance, levels of gamma-enolase in cerebrospinal fluid and serum have been used as a biomarker of cerebral injury and for the assessment of neurological disorders.38,89,90 Gamma-enolase is the only neuroendocrine tumour marker, which is used as a serum marker for follow up and monitoring of therapy effectiveness. Increased gamma-enolase levels in extracellular fluids are related to cancer progression and are typical for cancer in advances stages with distant metastases.8,39,80,84–87 The levels of gamma-enolase in non-treated cancer increase proportionally to the tumour mass, stage and number of metastases and are related to worse prognosis, however, the levels are not related to the location of metastases.39
Gamma-enolase is used in clinical practice in patients with SCLC and neuroblastoma. Its levels are significantly elevated compared to healthy subjects; however, specificity and sensitivity are too low to be used in screening.39,91 According to the recommendations of expert groups for the use of markers in lung cancer, gamma-enolase is recommended as an auxiliary marker in SCLC for differential diagnosis when biopsy is not possible and when other neuroendocrine tumours are excluded. Further, it is recommended for SCLC postoperative surveillance, for monitoring of therapy in advanced disease and for detection of recurrent disease.83,91 During chemotherapy, a transient rise of gamma-enolase serum levels occurs due to cytolysis of tumour cells, which disappears in case of successful treatment. However, persistently elevated levels show unsuccessful therapy. Gamma-enolase is not a recommended tumour marker in neuroblastoma; however, it is frequently used for differential diagnosis of neuroblastoma from nefroblastoma and for disease monitoring.8,91
Gamma-enolase is used as an auxiliary serum marker for follow-up and monitoring of therapy effectiveness in patients with carcinoids, melanoma, seminoma, feocromocitoma, medullary thyroid carcinoma, and endocrine pancreatic tumours. In patients with brain tumours, the levels of gamma-enolase in sera are not elevated, however, increased levels were reported in cerebrospinal fluid.39,91
Increased serum levels of gamma-enolase were reported also in patients with cancers of nonneuroendocrine origin, such as T-cell leukaemia92, B-cell lymphoma93 and malignant melanoma.94 In general, higher serum levels of gamma-enolase are related to worse prognosis and are the highest in patients with advanced metastatic stage.39
Gamma-enolase is usually measured in serum samples and less frequently in cerebrospinal fluid, pleural exudate or ascites. Its half-life in serum is estimated to be approximately 30 h.101 The αγ isoform is expressed in large amounts also in erythrocytes and in platelets, therefore it is important to separate blood cells from plasma or serum within 60 minutes from sample collection to prevent haemolysis of blood samples, which could lead to falsely elevated levels of gamma-enolase.80,102,103 Falsely elevated serum levels of gamma-enolase might be also due to various noncancerous pathological causes104, such as benign pulmonary diseases105, renal failure106, brain injuries, seizures, stroke38,107, severe hypoglycaemia108, benign liver diseases109 or systemic sclerosis.110
Concluding remarks
Glycolytic enzymes were shown to exert various specific regulatory functions and to play a pleiotropic role in physiological and pathological processes. Therefore, their participation to accelerated glycolysis could not be the only contribution to tumour progression.2 Alpha-enolase, the most exhaustively studied enolase isoform, was found to be one of the most frequently altered proteins in human pathologies and suggested as a universal cellular sensor that responds to multiple stimuli and reacts through multiple mechanisms.6,111 Gamma-enolase, sharing high-sequence identity with alpha-enolase, is also emerging as a multi-functional molecule. Different cellular localisation and interactions with other molecules strongly suggest its multiple cellular engagements.
Gamma-enolase primary role in cancer is the participation to the accelerated glycolysis, which supports increased tumour cell metabolic demands and enables their proliferation. Its C-terminal end might protect tumour cells from stressful conditions and action of therapeutic agents by activating survival-promoting signalling pathways and regulating apoptosis. An additional role of gamma-enolase in cancer progression is its involvement in actin remodelling and consequently in promotion of migration and invasion of tumour cells. These findings suggest that the role of this well-known tumour marker, whose expression is altered during development and progression of a variety of cancers, is pleiotropic and still has to be defined. Future work should be focused on elucidation of gamma-enolase cellular redistribution, interactions with other molecules and involvement in cell signalling. Understanding these processes, together with the tools enabling effective inhibition of gamma-enolase glycolytic activity, might provide new opportunities for cancer treatment.
Acknowledgments
We thank Dr. Bojan Doljak for constructing Figure 1. This project was supported by Research Agency of the Republic of Slovenia (grants P4-0127 and J4-4123 to JK).
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–20. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–50. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Masoudi-Nejad A, Asgari Y. Metabolic cancer biology: Structural-based analysis of cancer as a metabolic disease, new sights and opportunities for disease treatment. Semin Cancer Biol. 2015;30:21–9. doi: 10.1016/j.semcancer.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 5.McAlister L, Holland MJ. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem. 1982;257:7181–8. [PubMed] [Google Scholar]
- 6.Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R. Alpha-enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995;21:191–8. doi: 10.1016/0168-0102(94)00849-b. [DOI] [PubMed] [Google Scholar]
- 8.Suresh MR. Cancer Markers. In: Wild D, editor. The immunoassay handbook. Third edition. Oxford, UK: Elsevier; 2005. pp. 664–94. [Google Scholar]
- 9.Marangos PJ, Parma AM, Goodwin FK. Functional properties of neuronal and glial isoenzymes of brain enolase. J Neurochem. 1978;31:727–32. doi: 10.1111/j.1471-4159.1978.tb07847.x. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher L, Rider CC, Taylor CB. Enolase isoenzymes: III. Chromatographic and immunological characteristics of rat brain enolase. Biochim Biophys Acta. 1976;452:245–52. doi: 10.1016/0005-2744(76)90077-2. [DOI] [PubMed] [Google Scholar]
- 11.Giallongo A, Feo S, Moore R, Croce CM, Showe LC. Molecular cloning and nucleotide sequence of a full-length cDNA for human alpha enolase. Proc Natl Acad Sci U S A. 1986;83:6741–5. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feo S, Oliva D, Barbieri G, Xu WM, Fried M, Giallongo A. The gene for the muscle-specific enolase is on the short arm of human chromosome 17. Genomics. 1990;6:192–4. doi: 10.1016/0888-7543(90)90467-9. [DOI] [PubMed] [Google Scholar]
- 13.Lebioda L, Stec B. Mapping of isozymic differences in enolase. Int J Biol Macromol. 1991;13:97–100. doi: 10.1016/0141-8130(91)90055-y. [DOI] [PubMed] [Google Scholar]
- 14.Faller LD, Johnson AM. Calorimetric studies of the role of magnesium ions in yeast enolase catalysis. Proc Natl Acad Sci U S A. 1974;71:1083–7. doi: 10.1073/pnas.71.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer JM. Specificity and mechanism of action of metal ions in yeast enolase. FEBS Letters. 1985;182:8–14. [Google Scholar]
- 16.Vallee BL. Zinc and metalloenzymes. Adv Protein Chem. 1955;10:317–84. doi: 10.1016/s0065-3233(08)60108-4. [DOI] [PubMed] [Google Scholar]
- 17.Faller LD, Baroudy BM, Johnson AM, Ewall RX. Magnesium ion requirements for yeast enolase activity. Biochemistry. 1977;16:3864–9. doi: 10.1021/bi00636a023. [DOI] [PubMed] [Google Scholar]
- 18.Brewer JM. Yeast enolase: mechanism of activation by metal ions. CRC Crit Rev Biochem. 1981;11:209–54. doi: 10.3109/10409238109108702. [DOI] [PubMed] [Google Scholar]
- 19.Brewer JM, Ellis PD. 31P-nmr studies of the effect of various metals on substrate binding to yeast enolase. J Inorg Biochem. 1983;18:71–82. doi: 10.1016/0162-0134(83)85041-7. [DOI] [PubMed] [Google Scholar]
- 20.Ko-Jiunn L, Neng-Yao S. The role of enolase in tissue invasion and metastasis of pathogens and tumor cells. J Cancer Mol. 2007;3:45–8. [Google Scholar]
- 21.Ghosh AK, Steele R, Ray RB. Functional domains of c-myc promoter binding protein 1 involved in transcriptional repression and cell growth regulation. Mol Cell Biol. 1999;19:2880–6. doi: 10.1128/mcb.19.4.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feo S, Arcuri D, Piddini E, Passantino R, Giallongo A. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1) FEBS Lett. 2000;473:47–52. doi: 10.1016/s0014-5793(00)01494-0. [DOI] [PubMed] [Google Scholar]
- 23.Capello M, Ferri-Borgogno S, Cappello P, Novelli F. Alpha-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–74. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 24.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–31. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 26.Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, Castagnoli C, et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer. 2009;125:639–48. doi: 10.1002/ijc.24355. [DOI] [PubMed] [Google Scholar]
- 27.He P, Naka T, Serada S, Fujimoto M, Tanaka T, Hashimoto S, et al. Proteomics-based identification of alpha-enolase as a tumor antigen in non-small lung cancer. Cancer Sci. 2007;98:1234–40. doi: 10.1111/j.1349-7006.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seweryn E, Pietkiewicz J, Bednarz-Misa IS, Ceremuga I, Saczko J, Kulbacka J, et al. Localization of enolase in the subfractions of a breast cancer cell line. Z Naturforsch C. 2009;64:754–8. doi: 10.1515/znc-2009-9-1023. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima K, Hamanoue M, Takemoto N, Hattori T, Kato K, Kohsaka S. Plasminogen binds specifically to alpha-enolase on rat neuronal plasma membrane. J Neurochem. 1994;63:2048–57. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- 30.Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: identification of .alpha.-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–91. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 31.Dudani AK, Cummings C, Hashemi S, Ganz PR. Isolation of a novel 45 kDa plasminogen receptor from human endothelial cells. Thromb Res. 1993;69:185–96. doi: 10.1016/0049-3848(93)90044-o. [DOI] [PubMed] [Google Scholar]
- 32.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 33.Merkulova T, Lucas M, Jabet C, Lamandé N, Rouzeau JD, Gros F, et al. Biochemical characterization of the mouse muscle-specific enolase: developmental changes in electrophoretic variants and selective binding to other proteins. Biochem J. 1997;323:791–800. doi: 10.1042/bj3230791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller A, Demeurie J, Merkulova T, Geraud G, Cywiner-Golenzer C, Lucas M, et al. Fibre-type distribution and subcellular localisation of alpha and beta enolase in mouse striated muscle. Biol Cell. 2000;92:527–35. doi: 10.1016/s0248-4900(00)01103-5. [DOI] [PubMed] [Google Scholar]
- 35.Merkulova T, Dehaupas M, Nevers MC, Créminon C, Alameddine H, Keller A. Differential modulation of alpha, beta and gamma enolase isoforms in regenerating mouse skeletal muscle. Eur J Biochem. 2000;267:3735–43. doi: 10.1046/j.1432-1327.2000.01408.x. [DOI] [PubMed] [Google Scholar]
- 36.Royds JA, Variend S, Timperley WR, Taylor CB. An investigation of beta enolase as a histological marker of rhabdomyosarcoma. J Clin Pathol. 1984;37:905–10. doi: 10.1136/jcp.37.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royds JA, Variend S, Timperley WR, Taylor CB. Comparison of beta enolase and myoglobin as histological markers of rhabdomyosarcoma. J Clin Pathol. 1985;38:1258–60. doi: 10.1136/jcp.38.11.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34:2881–6. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 39.Lamerz R. NSE (neuron-specific enolase) γ-enolase. In: Thomas L, editor. Clinical laboratory diagnostics: use and assessment of clinical laboratory results. 1st. edition. Frankfurt/Main, Germany: TH-Books Verlagsgesellschaft; 1998. pp. 979–81. [Google Scholar]
- 40.Soh MA, Garrett SH, Somji S, Dunlevy JR, Zhou XD, Sens MA, et al. Arsenic, cadmium and neuron specific enolase (ENO2, γ-enolase) expression in breast cancer. Cancer Cell Int. 2011;11:41. doi: 10.1186/1475-2867-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haimoto H, Takahashi Y, Koshikawa T, Nagura H, Kato K. Immunohistochemical localization of gamma-enolase in normal human tissues other than nervous and neuroendocrine tissues. Lab Invest. 1985;52:257–63. [PubMed] [Google Scholar]
- 42.Vinores SA, Herman MM, Rubinstein LJ. Electron-immunocytochemical localization of neuron-specific enolase in cytoplasm and on membranes of primary and metastatic cerebral tumours and on glial filaments of glioma cells. Histopathology. 1986;10:891–908. doi: 10.1111/j.1365-2559.1986.tb02588.x. [DOI] [PubMed] [Google Scholar]
- 43.Hafner A, Obermajer N, Kos J. gamma-1-syntrophin mediates trafficking of gamma-enolase towards the plasma membrane and enhances its neurotrophic activity. Neurosignals. 2010;18:246–58. doi: 10.1159/000324292. [DOI] [PubMed] [Google Scholar]
- 44.Burack WR, Shaw AS. Signal transduction: hanging on a scaffold. Curr Opin Cell Biol. 2000;12:211–6. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 45.Ponting CP, Phillips C, Davies KE, Blake DJ. PDZ domains: targeting signalling molecules to sub-membranous sites. Bioessays. 1997;19:469–79. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- 46.Obermajer N, Doljak B, Jamnik P, Fonovic UP, Kos J. Cathepsin X cleaves the C-terminal dipeptide of alpha- and gamma-enolase and impairs survival and neuritogenesis of neuronal cells. Int J Biochem Cell Biol. 2009;41:1685–96. doi: 10.1016/j.biocel.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 47.McAleese SM, Dunbar B, Fothergill JE, Hinks LJ, Day IN. Complete amino acid sequence of the neurone-specific gamma isozyme of enolase (NSE) from human brain and comparison with the non-neuronal alpha form (NNE) Eur J Biochem. 1988;178:413–7. doi: 10.1111/j.1432-1033.1988.tb14465.x. [DOI] [PubMed] [Google Scholar]
- 48.Butterfield DA, Lange ML. Multifunctional roles of enolase in Alzheimer’s disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111:915–33. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soh M, Dunlevy JR, Garrett SH, Allen C, Sens DA, Zhou XD, et al. Increased neuron specific enolase expression by urothelial cells exposed to or malignantly transformed by exposure to Cd2+ or As3+ Toxicol Lett. 2012;212:66–74. doi: 10.1016/j.toxlet.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan T, Skaftnesmo KO, Leiss L, Sleire L, Wang J, Li X, et al. Neuronal markers are expressed in human gliomas and NSE knockdown sensitizes glioblastoma cells to radiotherapy and temozolomide. BMC Cancer. 2011;11:524. doi: 10.1186/1471-2407-11-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loja T, Chlapek P, Kuglik P, Pesakova M, Oltova A, Cejpek P, et al. Characterization of a GM7 glioblastoma cell line showing CD133 positivity and both cytoplasmic and nuclear localization of nestin. Oncol Rep. 2009;21:119–27. [PubMed] [Google Scholar]
- 52.Splinter TA, Verkoelen CF, Vlastuin M, Kok TC, Rijksen G, Haglid KG, et al. Distinction of two different classes of small-cell lung cancer cell lines by enzymatically inactive neuron-specific enolase. Br J Cancer. 1992;66:1065–9. doi: 10.1038/bjc.1992.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–44. [PubMed] [Google Scholar]
- 55.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golpour M, Akhavan Niaki H, Khorasani HR, Hajian A, Mehrasa R, Mostafazadeh A. Human fibroblast switches to anaerobic metabolic pathway in response to serum starvation: a mimic of warburg effect. Int J Mol Cell Med. 2014;3:74–80. [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C-A, Chao Y, Shiah S-G, Lin W-W. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim Biophys Acta. 2013;1833:1147–56. doi: 10.1016/j.bbamcr.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Jang SM, Kim JW, Kim CH, Kim D, Rhee S, Choi KH. p19(ras) Represses proliferation of non-small cell lung cancer possibly through interaction with Neuron-Specific Enolase (NSE) Cancer Lett. 2010;289:91–8. doi: 10.1016/j.canlet.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Amoêdo Ní D, Valencia JP, Rodrigues MF, Galina A, Rumjanek FD. How does the metabolism of tumour cells differ from that of normal cells. Biosci Rep. 2013;33:e00080. doi: 10.1042/BSR20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sedoris KC, Thomas SD, Miller DM. Hypoxia induces differential translation of enolase/MBP-1. BMC Cancer. 2010;10:157. doi: 10.1186/1471-2407-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vinores SA, Bonnin JM, Rubinstein LJ, Marangos PJ. Immunohistochemical demonstration of neuron-specific enolase in neoplasms of the CNS and other tissues. Arch Pathol Lab Med. 1984;108:536–40. [PubMed] [Google Scholar]
- 62.Vinores SA, Marangos PJ, Bonnin JM, Rubinstein LJ. Immunoradiometric and immunohistochemical demonstration of neuron-specific enolase in experimental rat gliomas. Cancer Res. 1984;44:2595–9. [PubMed] [Google Scholar]
- 63.Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- 64.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 65.Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J Neurochem. 1991;57:1178–84. doi: 10.1111/j.1471-4159.1991.tb08277.x. [DOI] [PubMed] [Google Scholar]
- 66.Hafner A, Glavan G, Obermajer N, Zivin M, Schliebs R, Kos J. Neuroprotective role of gamma-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell. 2013;12:604–14. doi: 10.1111/acel.12093. [DOI] [PubMed] [Google Scholar]
- 67.Hattori T, Ohsawa K, Mizuno Y, Kato K, Kohsaka S. Synthetic peptide corresponding to 30 amino acids of the C-terminal of neuron-specific enolase promotes survival of neocortical neurons in culture. Biochem Biophys Res Commun. 1994;202:25–30. doi: 10.1006/bbrc.1994.1888. [DOI] [PubMed] [Google Scholar]
- 68.Hafner A, Obermajer N, Kos J. gamma-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012;443:439–50. doi: 10.1042/BJ20111351. [DOI] [PubMed] [Google Scholar]
- 69.Pišlar AH, Kos J. C-terminal peptide of gamma-enolase impairs amyloid-beta-induced apoptosis through p75(NTR) signaling. Neuromolecular Med. 2013;15:623–35. doi: 10.1007/s12017-013-8247-9. [DOI] [PubMed] [Google Scholar]
- 70.Wendt W, Zhu X-R, Lübbert H, Stichel CC. Differential expression of cathepsin X in aging and pathological central nervous system of mice. Expl Neurol. 2007;204:525–40. doi: 10.1016/j.expneurol.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Kos J, Vižin T, Fonović UP, Pišlar A. Intracellular signaling by cathepsin X: Molecular mechanisms and diagnostic and therapeutic opportunities in cancer. Semin Cancer Biol. 2015;31:76–83. doi: 10.1016/j.semcancer.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets. 2009;9:381–90. doi: 10.2174/156800909788166637. [DOI] [PubMed] [Google Scholar]
- 73.Levin VA, Panchabhai SC, Shen L, Kornblau SM, Qiu Y, Baggerly KA. Different changes in protein and phosphoprotein levels result from serum starvation of high-grade glioma and adenocarcinoma cell lines. J Proteome Res. 2010;9:179–91. doi: 10.1021/pr900392b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levin VA, Panchabhai S, Shen L, Baggerly KA. Protein and phosphoprotein levels in glioma and adenocarcinoma cell lines grown in normoxia and hypoxia in monolayer and three-dimensional cultures. Proteome Sci. 2012;10:5. doi: 10.1186/1477-5956-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan T, Skaftnesmo KO, Leiss L, Sleire L, Wang J, Li X, et al. Neuronal markers are expressed in human gliomas and NSE knockdown sensitizes. BMC Cancer. 2011;11:524. doi: 10.1186/1471-2407-11-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walsh JL, Keith TJ, Knull HR. Glycolytic enzyme interactions with tubulin and microtubules. Biochim Biophys Acta. 1989;999:64–70. doi: 10.1016/0167-4838(89)90031-9. [DOI] [PubMed] [Google Scholar]
- 78.Trojanowicz B, Winkler A, Hammje K, Chen Z, Sekulla C, Glanz D, et al. Retinoic acid-mediated down-regulation of ENO1/MBP-1 gene products caused decreased invasiveness of the follicular thyroid carcinoma cell lines. J Mol Endocrinol. 2009;42:249–60. doi: 10.1677/JME-08-0118. [DOI] [PubMed] [Google Scholar]
- 79.Georges E, Bonneau AM, Prinos P. RNAi-mediated knockdown of alpha-enolase increases the sensitivity of tumor cells to antitubulin chemotherapeutics. Int J Biochem Mol Biol. 2011;2:303–8. [PMC free article] [PubMed] [Google Scholar]
- 80.Kasprzak A, Zabel M, Biczysko W. Selected markers (chromogranin A, neuron-specific enolase, synaptophysin, protein gene product 9.5) in diagnosis and prognosis of neuroendocrine pulmonary tumours. Pol J Pathol. 2007;58:23–33. [PubMed] [Google Scholar]
- 81.Tapia FJ, Polak JM, Barbosa AJ, Bloom SR, Marangos PJ, Dermody C, et al. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981;1:808–11. doi: 10.1016/s0140-6736(81)92682-9. [DOI] [PubMed] [Google Scholar]
- 82.Lopez J, Carl A, Burtis Edward R. Ashwood and David. In: Bruns E, editor. Tietz textbook of clinical chemistry and molecular diagnosis. 5th edition. St. Louis, USA: Elsevier; 2012. [Google Scholar]
- 83.Stieber P, Hatz R, Holdenrieder S, Molina R, Nap M, von Pawel J, et al. National Academy of Clinical Biochemistry Guidelines for the use of tumor markers in lung cancer. Section 3P. AACC press; 2006. [citated 2015 Jan 25]. Available at http://www.nacb.org. [Google Scholar]
- 84.Hao X, Sun B, Hu L, Lahdesmaki H, Dunmire V, Feng Y, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100:1110–22. doi: 10.1002/cncr.20095. [DOI] [PubMed] [Google Scholar]
- 85.Miremadi A, Pinder SE, Lee AH, Bell JA, Paish EC, Wencyk P, et al. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. 2002;40:215–22. doi: 10.1046/j.1365-2559.2002.01336.x. [DOI] [PubMed] [Google Scholar]
- 86.Sawaki M, Yokoi K, Nagasaka T, Watanabe R, Kagawa C, Takada H, et al. Prognostic importance of neuroendocrine differentiation in Japanese breast cancer patients. Surg Today. 2010;40:831–5. doi: 10.1007/s00595-009-4179-2. [DOI] [PubMed] [Google Scholar]
- 87.Allen FJ, Van Velden DJ, Heyns CF. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol. 1995;75:751–4. doi: 10.1111/j.1464-410x.1995.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 88.Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–95. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- 89.Rundgren M, Cronberg T, Friberg H, Isaksson A. Serum neuron specific enolase - impact of storage and measuring method. BMC Res Notes. 2014;7:726. doi: 10.1186/1756-0500-7-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan SM. Biomarkers of cerebral injury in cardiac surgery. Anadolu Kardiyol Derg. 2014;14:638–45. doi: 10.5152/akd.2014.5321. [DOI] [PubMed] [Google Scholar]
- 91.Sturgeon C. Practice guidelines for tumor marker use in the clinic. Clin Chem. 2002;48:1151–9. [PubMed] [Google Scholar]
- 92.Fujiwara H, Arima N, Ohtsubo H, Matsumoto T, Kukita T, Kawada H, et al. Clinical significance of serum neuron-specific enolase in patients with adult T-cell leukemia. Am J Hematol. 2002;71:80–4. doi: 10.1002/ajh.10190. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Liu P, Chen X, Geng Q, Lu Y. Serum neuron-specific enolase is correlated with clinical outcome of patients with non-germinal center B cell-like subtype of diffuse large B-cell lymphoma treated with rituximab-based immunochemotherapy. Med Oncol. 2012;29:2153–8. doi: 10.1007/s12032-011-0049-z. [DOI] [PubMed] [Google Scholar]
- 94.Lorenz J, Dippold W. Neuron-specific enolase-a serum marker for malignant melanoma. J Natl Cancer Inst. 1989;81:1754–5. doi: 10.1093/jnci/81.22.1754. [DOI] [PubMed] [Google Scholar]
- 95.Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis, and treatment. Chin J Cancer. 2013;32:312–24. doi: 10.5732/cjc.012.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008;14:5377–84. doi: 10.3748/wjg.14.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeYoung C, Edelman M. Prognostic Factors for Small-Cell Lung Cancer. In: Syrigos K, Nutting C, Roussos C, editors. Tumors of the chest. Berlin, Heidelberg: Springer; 2006. pp. 189–97. [Google Scholar]
- 98.Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 99.Lamberts SWJ, Hofland LJ, Nobels FRE. Neuroendocrine tumor markers. Front Neuroendocrinol. 2001;22:309–39. doi: 10.1006/frne.2001.0218. [DOI] [PubMed] [Google Scholar]
- 100.Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, et al. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res. 2004;10:4–12. doi: 10.1158/1078-0432.ccr-1051-2. [DOI] [PubMed] [Google Scholar]
- 101.Johnsson P, Blomquist S, Lührs C, Malmkvist G, Alling C, Solem J-O, et al. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann Thorac Surg. 2000;69:750–4. doi: 10.1016/s0003-4975(99)01393-4. [DOI] [PubMed] [Google Scholar]
- 102.Ramont L, Thoannes H, Volondat A, Chastang F, Millet MC, Maquart FX. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin Chem Lab Med. 2005;43:1215–7. doi: 10.1515/CCLM.2005.210. [DOI] [PubMed] [Google Scholar]
- 103.Marangos PJ, Campbell IC, Schmechel DE, Murphy DL, Goodwin FK. Blood platelets contain a neuron-specific enolase subunit. J Neurochem. 1980;34:1254–8. doi: 10.1111/j.1471-4159.1980.tb09967.x. [DOI] [PubMed] [Google Scholar]
- 104.Trape J, Filella X, Alsina-Donadeu M, Juan-Pereira L, Bosch-Ferrer A, Rigo-Bonnin R. Increased plasma concentrations of tumour markers in the absence of neoplasia. Clin Chem Lab Med. 2011;49:1605–20. doi: 10.1515/CCLM.2011.694. [DOI] [PubMed] [Google Scholar]
- 105.Collazos J, Esteban C, Fernandez A, Genolla J. Measurement of the serum tumor marker neuron-specific enolase in patients with benign pulmonary diseases. Am J Respir Crit Care Med. 1994;150:143–5. doi: 10.1164/ajrccm.150.1.8025740. [DOI] [PubMed] [Google Scholar]
- 106.Filella X, Cases A, Molina R, Jo J, Bedini JL, Revert L, et al. Tumor markers in patients with chronic renal failure. Int J Biol Markers. 1990;5:85–8. [PubMed] [Google Scholar]
- 107.DeGiorgio CM, Gott PS, Rabinowicz AL, Heck CN, Smith TD, Correale JD. Neuron-specific enolase, a marker of acute neuronal injury, is increased in complex partial status epilepticus. Epilepsia. 1996;37:606–9. doi: 10.1111/j.1528-1157.1996.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 108.Strachan MW, Abraha HD, Sherwood RA, Lammie GA, Deary IJ, Ewing FM, et al. Evaluation of serum markers of neuronal damage following severe hypoglycaemia in adults with insulin-treated diabetes mellitus. Diabetes Metab Res Rev. 1999;15:5–12. doi: 10.1002/(sici)1520-7560(199901/02)15:1<5::aid-dmrr2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 109.Collazos J, Genolla J, Ruibal A. Neuron-specific enolase concentrations in serum in benign liver diseases. Clin Chem. 1991;37:579–81. [PubMed] [Google Scholar]
- 110.Massabki PS, Silva NP, Lourenco DM, Andrade LE. Neuron specific enolase concentration is increased in serum and decreased in platelets of patients with active systemic sclerosis. J Rheumatol. 2003;30:2606–12. [PubMed] [Google Scholar]
- 111.Petrak J, Ivanek R, Toman O, Cmejla R, Cmejlova J, Vyoral D, et al. Deja vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics. 2008;8:1744–9. doi: 10.1002/pmic.200700919. [DOI] [PubMed] [Google Scholar]