Abstract
The South Durban Health Study is a population-based study that examined the relationship between exposure to ambient air pollutants and respiratory disease among school children with high prevalence of asthma who resided in two purposely-selected communities in north and south Durban, KwaZulu-Natal, South Africa. From these participants, a subgroup of 135 families was selected for investigation of household characteristics potentially related to respiratory health. In these households, a walkthrough investigation was conducted, and settled dust and air samples were collected for allergen and fungal measurements using standardised techniques. Asp f1 allergen was detected in all homes, and Bla g1 allergen was detected in half of the homes. House dust allergens, Der f1 and Der p1 exceeded concentrations associated with risk of sensitization and exacerbation of asthma in 3 and 13%, respectively, of the sampled homes, while Bla g1 exceeded guidance values in 13% of the homes. Although airborne fungal concentrations in sleep areas and indoors were lower than outdoor concentrations, they exceeded 1000 CFU/m3 in 29% of the homes. Multivariate analyses identified several home characteristics that were predictors of airborne fungal concentrations, including moisture, ventilation, floor type and bedding type. Airborne fungal concentrations were similar indoors and outdoors, which likely reduced the significance of housing and indoor factors as determinants of indoor concentrations.
Conclusion
Allergen concentrations were highly variable in homes, and a portion of the variability can be attributed to easily-recognised conditions.
Keywords: allergens, home characteristics, indoor air pollution, moisture
Introduction
Studies in both developing and developed countries have found rapid increases in the prevalence of paediatric asthma (Ehrlich, 2002; Abu-Ekteish et al., 2009). Exposure to indoor air pollutants, including allergens, has been recognised as an important factor in asthma and other respiratory diseases. In developed countries, exposure and sensitization to indoor allergens is considered a possible cause of asthma and known trigger of asthma attacks, especially in children (Pearce et al., 2000). Asthma exacerbation in children is associated with exposure to high concentrations of cockroach allergen (Bla g1, Bla g2, Per f1), house dust mite allergen (Der p1 and Der f1), animal allergen (Mus m1, Rat n1, Can f1, Fel d1), and fungal allergen (Asp f1, Alt a1, Cla h1, S. chartarum) (Rosenstreich et al., 1997; Garrett et al., 1998; Gold et al., 1999; Lau et al., 2000; Carlsten et al., 2010; Olmendo et al., 2011). Understanding the household risk factors associated with proliferation of allergens is an initial and important step in controlling exposure and reducing morbidity (IOM, 2000; Pearce et al., 2000; Arshad, 2010).
House dust mites (HDM) are common in warm and humid climates, and their allergens are found in reservoirs, such as bedding, floor coverings, and fabric covered furnishings (Peroni et al., 1994; van Strien et al., 1994; Custovic et al., 1998a). High surface concentrations of animal and insect allergens, e.g., mouse and cockroach allergens, are most likely in spaces where food is abundant, e.g., kitchens, dining rooms and living rooms (Rosenstreich et al., 1997; Olmendo et al., 2011). Numerous fungal species are abundant outdoors, but indoor infestations require spaces and surfaces with moisture or high water activity (Arruda et al., 1992; Dales et al., 1997; Garrett et al., 1998).
In developing countries, studies of residential indoor environments have focused on biomass fuels and combustion pollutants. Biological pollutants, such as allergens, have received scant attention (Manjra et al., 1995; Ezzati and Kammen, 2001; Tielsch et al., 2009), particularly in Africa. In this study, concentrations of allergens and airborne fungi are characterized in homes of primary school children in Durban, KwaZulu-Natal, South Africa. Also examined is the association of household characteristics and concentrations of these pollutants with the aim of contributing to the understanding of the relationship between allergens and childhood asthma.
Durban is an industrial port city located on the east coast of South Africa with a population of approximately three million people. Due to the prevailing subtropical climate, both the temperature and humidity are generally high, creating an environment that encourages the growth and proliferation of biological agents and pollutants. Very high prevalence rates of asthma (32%) have been reported among children attending schools in south Durban, an area containing numerous air pollutant-emitting industries, as compared to a prevalence rate of 17% for children living outside this area (Kistnasamy et al., 2008).
Methods
Sample selection and recruitment of participants
The homes selected for indoor environment assessment represent a subset of households participating in the South Durban Health Study (SDHS), an epidemiological study that examined the relationship between ambient air pollution and respiratory disease among school children (grades 3–6; Naidoo et al., 2007). The SDHS recruited children attending seven schools in two communities: four schools in the highly industrialised south Durban area, and three schools in northern Durban, an area without major industry. At each of these schools, a screening questionnaire was completed by caregivers and used to identify children with known or probable persistent asthma. Of the 423 children who participated in the SDHS, 81 (19%) were classified as persistent asthmatics, based on the screening questionnaire.
From the participating schools, households of children with known and probable asthma were given priority for recruitment into an indoor environment assessment sub-study. A total of 20 households from each school list were targeted. Child caregivers or other responsible adults living with the child were recruited by telephone or house visits. Individuals giving consent were then scheduled for indoor assessments. Their homes were visited during the day by trained technicians who could speak the local languages (isiZulu or English). Home visits for walkthrough assessment, settled dust collection and airborne fungal sampling were done from May 2004 to September 2005.
Each household recruited for the indoor environmental assessment provided written consent. All procedures in the study were approved by the respective ethics committees of the University of KwaZulu-Natal (South Africa) and the University of Michigan (USA) participating in the SDHS.
Home walkthrough assessment
The walkthrough assessment utilized a standardised instrument that was previously field-tested and used in Detroit, USA to investigate household characteristics as indicators of allergen concentrations (Parker et al., 2004). This instrument was modified to include questions about informally constructed homes common in Durban. It also included direct observations, questions directed to the child caregiver or the adult respondent regarding household characteristics in each room of the house, and activities of the child and other occupants. Information collected on housing characteristics included the type of house (single family house or flat/apartment, single or double storey, etc.), building age, moisture problems (sources or indicators), fungal growth, ventilation, and heating practices. Information on the types of floors, furnishings, bedding used by the child, and presence of stuffed toys was also collected. The rooms investigated in each house were the child’s sleep area, the child’s play area, the kitchen and the bathroom.
Settled dust sampling and allergen quantification
Following the walkthrough assessment, two settled dust samples from the child’s sleep area were collected. One sample was collected from the bedding (pillows and mattress) and, if present, a second sample was collected from a carpet or rug near the bed. A vacuum cleaner (WapVs300s, Bellenberg, Germany) equipped with a specialised nozzle and a 40 mm diameter filter paper (Macherey-Nagel, Germany) on the inlet was used. Dust samples were collected from bedding materials by vacuuming for 2 min. Carpet/rug samples were collected by vacuuming a 1.0 m2 area for 2 min. After sampling, the dust-loaded filter was folded, wrapped with aluminium foil, and placed in a sealed polyethylene bag for transport. Additional settled dust samples were collected from fabric furniture and carpet or rugs in the child's play area using similar procedures. All settled dust samples were transported to the laboratory on the day of collection and stored at 4°C until analysis.
In the laboratory, settled dust samples were sieved through a No. 45 mesh screen (355 μm sieve) to remove large particles and fibres. If two settled dust samples were collected from a single room (sleep area or play area), they were pooled and analysed as a single sample. For extraction, 100 mg of fine settled dust sub-samples were weighed in test tubes and 2.0 ml of 0.05% Tween20 in phosphate-buffered saline was added. The samples were then suspended using a vortex mixer, mixed for 2 hr using a laboratory mixer, and then centrifuged at 2500 rpm for 20 minutes at 4 °C. Supernatants for allergen analysis were removed and stored at −20 °C (Earle et al., 2007). Allergens were measured using ELISA monoclonal antibodies assays (Indoor Biotechnologies, Wiltshire, UK) for house dust mite (Der p1 and Der f1) and fungi (Asp f1) on the children’s sleep area samples, and for cockroach allergen (Bla g1) on the children’s play area samples. Airborne fungi and mite allergen concentrations in settled dust were expressed as μg/g dust, and Bla g1 concentrations were expressed as U/g dust (Chapman et al., 1987). The estimated minimum detectable level (MDL) for Der p1 and Der f1 was 0.30 μg/g dust and 0.60 U/g for Bla g1, and the reproducibility was 18% for Der p1, 3% for Der f1 and 5% for Bla g1, based on previous inter-laboratory studies (Pollart et al., 1991; Ovsyannikova et al., 1994; Sawyer et al., 1998; van Strien et al., 2003; Arbes et al., 2005). Because of the few number of censored data points for HDM allergens (10% and 6% of Der p1 and Der f1 respectively), ½ MDL value (0.15 μg/g dust) was used to replace censored data for statistical analysis (Hewett and Ganser, 2007). The characteristics of the Bla g1 concentrations meant using a more robust (more precise and less bias) β-substitution method to substitute Bla g1 concentrations below MDL was necessary (Ganser and Hewett, 2010).
Airborne fungal sampling, identification and quantification
A sterilised two-stage Andersen sampler (Tisch Environmental, Ohio, USA) was used to collect viable airborne fungi from the child’s sleep area, the room most used by the child (play area), the kitchen, and the bathroom of each home. During sampling, any external windows and doors of the sampled rooms were closed. The sampler was placed at the centre of the room at least 0.5 m above the floor and away from obstacles that could affect airflow. Samples were collected for 5 min at 15 L/min using a calibrated vacuum pump (SKC Ltd, USA) onto malt extract agar (MEA) and dichloran (18%) glycerol agar (DG18) plates (Oxoid Ltd, Hampshire, USA). One to four airborne indoor fungal samples, and one airborne outdoor fungal sample were on each media collected during each home visit. After sampling, the media plates were sealed, marked, transported to the laboratory, and incubated in a temperature-regulated microbial cabinet between 25 and 28°C for 3 to 5 days. After incubation, fungal colonies were counted and visually identified as Cladosporium, Aspergillus, Fusarium, Penicillium and Rhizopus species. Unknown species were grouped and classified as “unknown”. Each of the five identified airborne fungal species was further isolated for confirmation by culturing onto MEA and DG18 agar plates for further observations of cellular, hyphal and spore morphology. Morphologies were defined by visual observation and microscopy, and organisms were identified using Samsonet al. (2000) as a reference. Concentrations of viable airborne fungi were expressed as colony forming units per cubic meter of air (CFU/m3). Airborne fungal concentrations below the MDL (13 CFU/m3) were set to ½ MDL for statistical analysis. Confirmatory analyses of the fungal genera were performed by the Medical Microbiology Laboratory of Inkosi Albert Luthuli Central Hospital in Durban, South Africa.
Airborne concentrations of each fungal species and the total fungal concentration measured in different rooms of the same house were averaged to create new variables: indoor Cladosporium, indoor Aspergillus, indoor Penicillium, indoor Fusarium, indoor Rhizopus, indoor unknown and indoor total. Several culture samples from the sleep area (n=4) and outdoors (n=9) showed excessively high concentrations of several species that made identification and quantification of other species impossible. For these samples, the concentrations were reported as the highest concentration found for each species and for the total. Airborne fungal species and total fungal concentrations on MEA and DG18 were similar, therefore only results of MEA are presented in this article.
Data analyses
Descriptive analyses were performed for home characteristics and other factors potentially associated with allergen and airborne fungal concentrations. Non-parametric Spearman rank correlations were used to investigate the association between concentrations of airborne fungi and allergens in settled dust. Multivariate analyses used stepwise regression and dust allergen concentrations and airborne fungal concentrations as dependent variables to test association with home characteristics variables such as season, neighbourhood and region, used as independent variables. Stata/IC version11.0 for Windows (StataCorp LP, Texas) was used for statistical analysis. Before a multivariate regression analysis was computed for different outcome variables, bivariate analyses between outcome variables and home characteristics, were done. Variables that were significant at the 95% confidence level in the bivariate analyses, together with other variables that were considered important potential determinants of indoor pollution concentrations as shown in other studies, were included in the final multivariate models.
Results
A total of 135 homes were visited for the indoor environment study: 53% (n=71) of them were situated in south Durban region, and the rest in north Durban. Using data from the screening questionnaire administered to caregivers for SDHS, 103 (77%) of the participating households had children classified as asthmatics or probable asthmatics.
Characteristics of children’s homes
Most homes (70%) were single-family homes of formal brick construction. Relatively few (7%) were informally constructed with walls made of different materials from wood, corrugated steel, cardboard boxes, etc. Typically, homes had four or five rooms, but the range in the sample was from one to 10 rooms.
The frequency of selected home characteristics in children’s sleep and play areas, based on the walkthrough checklist, are shown in Table 1. The bedroom was the room used by the majority (87%) of children as a sleep area; the remainder of the children used a living room (6%) or other room (7%) in the house. Living rooms were frequently (80%) used as an indoor play area. Few of the sleep areas were heated during the cold season (10%) or cooled during the hot season (36%). In most sleep and play areas, smooth and/or impervious floors (cement, floor tiles or linoleum) were dominant (56% and 87%, respectively). Sleep areas had more evidence of moisture and visible fungal growth than play areas, which occurred mostly on interior roofs and/or ceilings rather than on walls and floors. Most children slept on beds with mattresses (93%); a few of them used mattresses on the floor (4%). Most children used either foam (44%) or polyester-filled (40%) pillows on their beds.
Table I.
House characteristics in 135 sleep and 112 play areas of visited homes of school children.
| Conditions found in the households | Sleep area [N=135] n (%) | Play area [N=112] n (%) | |
|---|---|---|---|
| heating | 13 (9.6) | ||
| use of fan / air conditioner | 50 (44.6) | 60 (53.6) | |
| chairs present | 46 (34.1) | 105 (93.7) | |
| cluttered spaces | 15 (11.1) | 4 (3.5) | |
| evidence of cockroaches | 49 (36.3) | 27 (27.3) | |
| evidence of rodents | 26 (19.3) | 16 (1432) | |
| opening windows | 131 (97.0) | 107 (95.5) | |
| fungal or mildew smell | 49 (36.3) | 11 (9.8) | |
| ceiling/ interior roof | presence of ceiling | 86 (63.7) | 81 (71.7) |
| visible water stains/ moisture | 48 (35.6) | 34 (30.1) | |
| visible fungal growth | 36 (26.7) | 18 (15.9) | |
| wall | visible water stains/ moisture | 30 (22.2) | 14 (12.4) |
| visible fungal growth | 16 (11.9) | 4 (3.5) | |
| floor | smooth non-porous | 83 (61.2) | 93 (93.9) |
| Cement | 18 (13.3) | 4 (4.0) | |
| wall-to-wall carpet | 34 (25.2) | 14 (12.5) | |
| dirt floor | 1 (0.7) | ||
| hard floor with rug/s | 32 (23.7) | 34 (30.4) | |
| type of bedding | bed with mattress | 129 (95.6) | |
| mattress on the floor | 5 (3.7) | ||
| sofa/ couch | 1 (0.7) | ||
| blankets on the floor | 1 (0.7) | ||
| type of blankets | cotton blankets | 40 (29.6) | |
| wool blankets | 65 (48.1) | ||
| Comforter | 67 (49.6) | ||
| acrylic blankets | 2 (1.5) | ||
| type of pillows | feather filled | 4 (3.0) | |
| polyester filled | 58 (43.0) | ||
| foam filled | 68 (50.4) | ||
| other type | 4 (3.0) |
Occurrence and concentrations of allergens and airborne fungi in children’s homes
Settled dust samples could not be collected from 9 (7%) of the homes visited because of the absence of electricity. In one-room homes, only a single settled dust sample from the sleep area could be collected. In these homes, 126 and 99 valid (composite) settled dust samples for allergen analysis were collected and analysed from sleep areas and play areas respectively, and 129 indoor and 129 outdoor air samples were collected and analysed.
Table 2 shows allergen concentrations in settled dust. Detection frequencies varied from 51% for Bla g1 to 100% for Asp f1. The average Asp f1 concentrations in south Durban were higher than concentrations in north Durban. Only one household had both HDM allergen concentrations in children’s settled dust bed samples below MDL. Of the HDM allergen concentrations, 3% (4 of 126) of homes had Der f1 concentrations above 10 μg/g dust, a concentration sometimes used as a threshold for the development and exacerbation of asthma (Platts-Mills et al.,1997), compared to 13% (13 of 126) Der p1 concentrations exceeding this value. The maximum Der f1 concentration (39.7 μg/g dust) exceeded the next highest value (22.9 μg/g dust) by almost two-fold. Although for cockroach allergen 49% of the homes were below MDL, 13% (13 of 99) exceeded 2 μg/g dust, a guideline value for asthma exacerbation (Platts-Mills et al., 1997). The highest Bla g1 concentration (24.4 U/g dust) detected in a home’s play area settled dust was over three times higher the second highest concentration (7.8 U/g) observed in another home.
Table II.
Fungal (Asp f1) and house dust mite (Der p1, Der f1) allergen concentrations in settled dust collected from children’s sleep areas and cockroach (Bla g1) allergen concentrations in settled dust collected from children’s play areas.
| Allergen | Sample size | Detection frequency (%) | Mean (SD) | Median | Minimum | Percentile
|
Maximum | |
|---|---|---|---|---|---|---|---|---|
| 90th | 95th | |||||||
| Asp f1 (μg/g) | 126 | 99 | 0.58 (0.24) | 0.47 | 0.32 | 0.99 | 1.09 | 1.37 |
| Der p1 (μg/g) | 126 | 90 | 6.89(13.51) | 1.98 | 0.15 | 18.26 | 46.57 | 71.42 |
| Der f1 (μg/g) | 126 | 94 | 1.96 (4.40) | 1.00 | 0.15 | 2.63 | 5.39 | 39.71 |
| Bla g1 (U/g) | 99 | 51 | 1.23 (2.65) | 0.66 | 0.25 | 2.87 | 3.31 | 24.40 |
Mean airborne concentrations of different fungal species in children’s sleep areas, indoors and outdoors are shown in Figure 1. Indoor and outdoor fungal compositions were similar, and Cladosporium was by far the most abundant of the identified species. Cladosporium concentrations averaged around 60% of the total airborne fungal concentration, and were typically ten or more times higher than other identified fungal species. Penicillium concentrations were slightly higher than other species identified, namely Aspergillus, Fusarium and Rhizopus. Airborne fungal aerosol compositions in the children's sleep areas and indoor spaces were similar to those outdoors, however, outdoor concentrations were higher (Figure 2).
Figure 1.
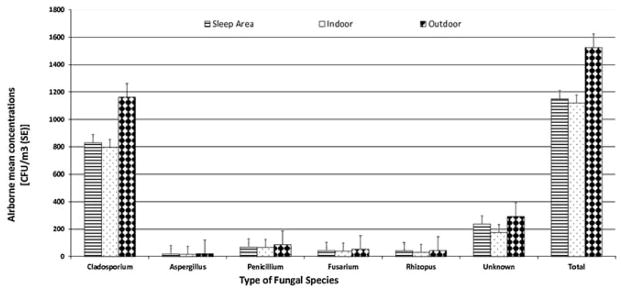
Mean airborne concentrations (standard error) of different fungal species (identified and unknown) sampled from sleep areas, indoors, and outdoors of the children’s homes.
Characterization of allergens and airborne fungi in low and middle – income homes of primary school children in Durban, South Africa
Figure 2.
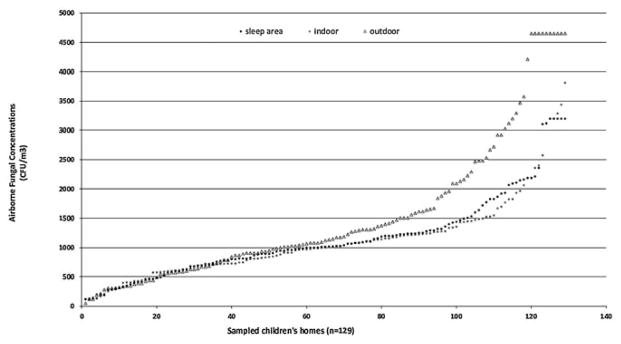
Distributions of total airborne viable fungal concentrations sampled in sleep, indoor and outdoor areas of visited children’s homes.
Characterization of allergens and airborne fungi in low and middle – income homes of primary school children in Durban, South Africa
Airborne fungal concentrations in Durban homes exceeded concentrations that have been associated with health effects in several studies (WHO, 2009). The median concentration was 667 CFU/m3 for Cladosporium, a dominant species in the samples collected from these homes. Thirty percent of the homes had Cladosporium concentrations above 1,000 CFU/m3, a concentration which has been associated with adverse respiratory health outcomes in other studies (WHO, 2009). The highest Cladosporium concentrations were 3,500 CFU/m3 indoors and 4,040 CFU/m3 outdoors, but these represent underestimates due to sampler overload. The number of homes with total airborne fungal concentrations that exceeded 1,000 CFU/m3 was 66 (51% of the 129) homes. Although Penicillium and Aspergillus were frequently detected in the sleep areas, the concentrations averaged 520 and 147 CFU/m3 respectively.
Home characteristics, allergen concentrations in dust, and airborne fungal concentrations
There were no significant correlations observed between Asp f1 settled dust concentrations and airborne fungal concentrations in sleep areas, indoors and outdoors. However, analysis stratified by some home characteristics showed significant correlations. Homes with rugs, or usage of fans in the sleep areas, had significant positive correlations between these two variables, namely, Asp f1 and airborne total fungal concentrations [(r= 0.53, p=0.011, n=22) and (r= 0.36, p=0.032, n=35) respectively]. In informal constructed homes, Asp f1 concentrations in settled dust were negatively correlated with airborne total fungal concentrations (r= −0.80, p=0.005, n=10).
Tables 3 and 4 show results of the multivariate models for different allergen and airborne fungal concentrations adjusted for season and region. Each of the final models had different significant predictors for each allergen, and the coefficients of determination (R2) were less than 0.3. Surprisingly, the presence of open windows and cement floors in sleep areas were significant and positive predictors of higher Der p1 and Der f1 allergen dust concentrations, respectively. Predictors of Bla g1 dust concentrations included smooth floors and visible roof and ceiling mould. No home characteristics were identified as significant predictors of airborne Aspergillus concentrations. Moisture and visible fungal growth on interior roof surfaces were negative predictors of higher airborne Penicillium and total fungal concentrations, but positive predictors of Cladosporium concentrations in air.
Table III.
Determinants of different allergen concentrations in children’s sleep areas (Asp f1 and HDM) and play areas (Bla g1) predicted using multivariate stepwise regression models.
| Dependent variable (Model adjusted R2) | Home characteristics (Predictor variables) | Coefficient | 95% CI | p-value |
|---|---|---|---|---|
| Aspf1 (0.2808) | flat / duplex home | 0.16 | 0.07–0.26 | 0.001* |
| opening windows | −0.48 | −0.72–−0.24 | <0.001* | |
| comforter | 0.07 | <0.01–0.15 | 0.065 | |
| Derf1 (0.0529) | cement floor | 2.51 | 0.17–4.86 | 0.036* |
| wall-to-wall carpet | 1.89 | 0.01–3.77 | 0.048* | |
| cotton blanket | 1.94 | −0.36–4.24 | 0.097 | |
| Derp1 (0.1363) | opening windows | 16.80 | 0.43–33.17 | 0.044* |
| comforter | −36.95 | −56.82 –−17.08 | <0.001* | |
| Wool blankets | −39.18 | −58.82 – −1954 | <0.001* | |
| cotton blanket | −30.83 | −51.33 – −10.34 | 0.004* | |
| blend of blankets | −30.88 | −51.20 – −10.57 | 0.003* | |
| Blag1 (0.0511) | smooth floors | 0.67 | −0.56 – 1.40 | 0.070 |
| visible roof or ceiling mould | −0.71 | −1.37 – −0.04 | 0.038* |
Variables included in the models: type of house, house age, opening windows, moisture and dampness signs, fungal growth, bedding, heating and presence of a ceiling.
Table showing variables with a p-value of <0.1 on the multivariate analysis
All models adjusted for season and region (south or north Durban)
p<0.05
Table IV.
Factors influencing concentrations of various airborne fungal species and total fungal concentrations from children’s sleep areas– from multivariable regression models
| Dependent variable (Model adjusted R2) | Home characteristics (Predictor variables) | Coefficient | 95% CI | p-value |
|---|---|---|---|---|
| Cladosporium (0.1008) | opening windows | 701 | −129 – 1530 | 0.097 |
| informal constructed home | 499 | 82 – 916 | 0.019* | |
| visible ceiling or interior roof moisture | 321 | 77 – 565 | 0.010* | |
| Penicilium (0.2320) | visible ceiling or interior roof moisture | −54 | −92 – −16 | 0.006* |
| house made of bricks | 156 | 20 – 293 | 0.025* | |
| informal constructed home | 246 | 148 – 344 | <0.001* | |
| presence of a ceiling | −55 | −96 – −15 | 0.008* | |
| house made of wood | −142 | −297 – 12 | 0.071 | |
| Fusarium (0.1286) | opening windows | 54 | 6 – 101 | 0.028* |
| visible wall fungal growth | −20 | −42 – 1 | 0.065 | |
| home less than 25 years old | 20 | 6 – 34 | 0.004* | |
| Rhizopus (0.1034) | house made of bricks | 70 | 1 – 140 | 0.045* |
| informal constructed home | 62 | −2 – 126 | 0.057 | |
| Unknown fungal species (0.0945) | home older than 25 years | −116 | −239 – 7 | 0.064 |
| opening windows | 550 | 114 – 985 | 0.014* | |
| Total airborne fungi (0.1179) | house made of bricks | 792 | 45 – 1539 | 0.038* |
| visible wall fungal growth | −358 | −741 – 25 | 0.067 | |
| informal constructed home | 1193 | 510 – 1875 | 0.001* | |
| visible ceiling or roof moisture | 337 | 86 – 589 | 0.009* |
Variables included in the models: type of house, house age, opening windows, moisture and dampness signs, fungal growth, heating and presence of a ceiling
Table showing variables with a p-value of <0.1 on the multivariate analysis All models adjusted for season and region (south or north Durban)
p<0.05
Discussion
Durban homes showed allergen concentrations and compositions in settled dust that resemble those in some studies in both developing and developed countries (e.g., Chew et al., 1998; Addo-Yobo et al., 2001; Perfetti et al., 2004), but concentrations were lower than those seen in inner cities in the USA (e.g., Eggleston et al., 1999; Breysse et al., 2005). A moderate fraction of Durban homes had allergen and airborne fungal concentrations that exceeded suggested risk thresholds for sensitisation and/or asthma exacerbation and other respiratory illnesses (Platts-Mills et al., 1997; WHO, 2009). This is an important finding because most of the sampled homes had children classified as probable or known to be asthmatic. Total airborne fungal concentrations in sleep areas and total airborne indoor fungal concentrations were significantly associated with fungal allergen concentrations in settled dust, but only when some home characteristics were present (or absent), suggesting that dust as a reservoir of allergenic materials maybe influenced by the airborne fungi only under certain conditions. From the observed correlations it could be true that airborne fungal concentrations are influenced by reservoir fungal dust contamination. Overall, the lack of correlation among dust and air measurements across different homes in Durban suggests that multiple factors unaccounted for in this study may be affecting these measurements.
Fungal allergen (Asp f1) and airborne fungi in sleep areas
Asp f1 was detected in settled dust samples in all homes. The abundance of this fungus supports the hypothesis that fungi can rapidly proliferate when conditions are suitable, as found in Durban's subtropical climate. Fungi have been reported to grow from between 6 to 196 hr after spore deposition in appropriate conditions, and allergens can be produced within 12 hr after this growth (Arruda et al., 1992). This observation is supported by our findings of an association between interior roof or ceiling moisture damage and total airborne fungal and Cladosporium concentrations found in the children's sleep areas.
Dust concentrations of fungal allergen were significantly affected by housing type. Elevated concentrations of Asp f1 and total airborne fungi were seen in attached homes (flat/apartment/duplex) and in informal constructed homes (as compared to detached and formal family homes). One factor that may influence this association is the close proximity between attached dwellings, which can promote air exchange between dwellings that transports airborne fungi. Conditions such as high water activity and indoor climate in informal constructed homes can also promote indoor fungal growth and lead to higher concentrations (Danaviah et al., 2000).
The similarity of indoor and outdoor airborne fungal concentrations at the time of sampling suggests only limited indoor sources of fungi. However, the associations observed between home characteristics and airborne fungal concentrations suggest that several characteristics are important determinants of indoor fungal concentrations (Table 4). Opening windows in sleep areas was a significant determinant of lower concentrations of Asp f1 and total airborne fungi in sleep areas. Although natural ventilation possible with opened windows allows entry of outdoor airborne fungi, ventilation can help to reduce by dilution indoor air contaminants, and it can reduce the build-up of humidity, which can be especially important in smaller and more crowded houses and bedrooms. Opening windows may provide a practical approach to provide ventilation and control airborne fungi in the study communities, especially considering that none of the Durban homes used mechanical ventilation or particle filtration.
Bedding type was a significant determinant of Asp f1 concentrations in children’s beds. Unlike other microbes, fungal proliferation on surfaces needs sufficient water activity and nutrients. These fungal growth requirements are unlikely to be found in bedding, hence this finding appears coincidental. Possibly, bedding concentrations of Asp f1 may be a surrogate for household maintenance practices, which are likely to be worse in poorer households.
Other determinants of individual airborne fungal species concentrations identified in children's sleep areas were not consistent with the determinants of total airborne fungal concentrations. Concentrations of different airborne fungi were inversely associated with visible moisture, visible fungal growth and age of the homes, which is also inconsistent with the conditions fungi need to proliferate. Again, these results appear coincidental. Because indoor and outdoor concentrations of airborne fungi were very similar, these associations may reflect seasonality or some other unmeasured or uncontrolled factor. It is clear that airborne fungal concentrations determined using short-term indoor measurements require careful and cautious interpretation given the influence of outdoor concentrations and the great variation that can occur very rapidly (Chew et al., 2003; Cho et al., 2006).
Earlier studies examining indoor environments in Durban showed that Cladosporium is the dominant airborne fungal genus in the region (Danaviah et al., 2000; Berman, 2007). In the present study, association of Cladosporium allergen concentrations was not associated with airborne Cladosporium concentrations was not investigated. Airborne Cladosporium concentrations were not associated with visible fungal growth or moisture on surfaces. Asp f1 allergen concentrations in dust are relevant due to their potentially profound health effects.
House dust mite allergens (Der p1 and Der f1) in sleep areas
The occurrence of HDM allergens in the SDHS homes was high (90% and 94% for Der p1 and Der f1, respectively) in comparison to homes in the USA (80.2%; Leaderer et al., 2002) and elsewhere (Custovic et al., 1998b; Moscato et al., 2000). This is not surprising given that year-round climatic conditions in Durban are ideal for the proliferation of these allergen-producing organisms, e.g., the annual average temperature and humidity is 23°C and 75%, respectively. Potter et al. (2010) also documented that coastal areas of South Africa have higher HDM concentrations compared to (typically drier and cooler) inland areas. Although Der p1 concentrations were not correlated to Der f1 concentrations in the children’s sleep areas, both allergens seem to thrive in Durban homes. Der p1 had higher concentrations, which supports observation that only a single type of HDM allergen is dominant in a house (Moscato et al., 2000; Perfetti et al., 2004). Proliferation of one type of species in an environment is a likely result of the competition for food and water.
Few factors were associated with HDM allergen concentrations. The observed association of blanket type with HDM concentrations is similar to findings elsewhere. All blanket types (both natural and synthetic materials) were associated with lower Der p1 concentrations, contrary to expectations. Blankets made of cotton and other natural materials have been associated with lower concentrations of Der p1, suggesting that such material is not conducive for HDM proliferation (Hallam et al., 1999; Custovic et al., 2000). However, natural materials can provide protein as food for HDM (Sawyer et al., 1998). In the present study, although the association between Der f1 allergen concentrations and cotton blankets was not statistically significant, the positive association observed supports this hypothesis of natural fibres as a food source for HDMs. However, the higher HDM concentrations associated with cement floors (smooth floors) and presence of opened windows found in this study are inconsistent with Carrer et al. (2001), who shows lower concentrations with these factors. Further, that visible moisture and dampness were not significant predictors of HDM concentrations might imply that these factors did not significant affect humidity inside Durban homes, possibly because ambient conditions are already very humid.
Cockroach allergen (Bla g1) in play areas
Although Manjra et al. (1995) indicated that Per f1 allergen was the most prevalent cockroach allergen in Durban homes, 51% of the samples in the present study showed the presence of Bla g1 allergen. A much lower occurrence of cockroach allergen has been in homes in the USA in several studies, e.g., 30% for Bla g1 (Chew et al., 1998; Leaderer et al., 2002) and 24% for Bla g in family room floors and/or furnishings (Gold et al., 1999). Although the Bla g1 detection frequency was high, concentrations were much lower than those reported elsewhere (Eggleston et al., 1999). Because settled dust samples for Bla g1 analysis were collected from children’s “play areas” and most of these were living rooms, it is likely that higher detection frequency could have been observed from samples collected from the kitchens.
Although almost 50% of the samples had Bla g1 below detection limit, we modeled concentrations against home characteristics. Cockroach allergen proliferation has been associated with socioeconomic status and housekeeping practices, e.g., food storage and trash maintenance, and with older homes and informally constructed homes (Leaderer et al., 2002). In our study, observed associations, such as smooth floors in children’s play areas associated with higher concentrations of Bla g1 allergen, and visible interior roof and ceiling mould growth associated with lower Bla g1 concentrations are contrary to expectations. The low R2 (0.0511) for the Bla g1 model in table 3 suggests that our independent variables do not fully explain the variability in the allergen concentration.
Study limitations
The sample size of the sub-study limits our ability to observe relationships and account for interactions among variables. Moreover, the home assessments were conducted in different seasons, and seasonal influences on allergen concentrations, dampness, visible moisture, and ventilation practices may influence the findings.
All of the dust and air sampling data were cross-sectional in nature. Longitudinal data would have provided a more comprehensive assessment of allergen and airborne fungal concentrations. Additionally, repeated measurements in each household would help to ensure that observations were representative of everyday practise and conditions. The fungal aerosol sampling used a single growth media and incubation condition, and it represents a very short-term (5 min) sample that may not be representative. The general approach of collecting and incubating airborne fungi may underestimate some species due to the different media and incubation temperatures needed for optimal determination of these species (Samson et al., 2000). This applies to incubation temperatures needed for optimal growth of Aspergillus and Penicillium species which are between 35°C and 37°C (Samson, 2004). Also, only five fungal genera were identified in air samples, and some species may be misclassified as "unknown" although they may belong to one of the target genera. We may have missed the association between airborne fungal concentrations and allergen concentrations in settle dust allergen because we investigated Aspergillus allergen instead of the Cladosporium allergen which is the most prevalent.
Analysis, such as modeling Bla g1 concentrations, using data with approximately 50% of the dust samples having concentrations not detectable is problematic. In our dataset with characteristics that include single MDL, small sample size (n= 99), high left censored points (49%) and large standard deviation (2.65, β-substitution was a method of choice compared to maximum likelihood estimation (MLE), log-probit regression (LPR) and common substitution methods (MDL/2 and MDL/√2). Our dataset was not lognormal which is another important characteristic for using β-substitution as a substitution method (Hewett and Ganser, 2007; Ganser and Hewett, 2010). Because of the few censored data points seen for the airborne fungal concentrations and HDM and fungal dust allergens concentrations, our substitution method of choice is justified for these variables.
Conclusions
This study characterised concentrations of allergens in homes in a developing country, and included many homes in which a vulnerable population lived: children with asthma. Settled dust allergen and airborne fungal concentrations frequently exceeded concentrations and guidelines linked to respiratory illness, including asthma exacerbation and asthma development. While few of the "classical" determinants of high pollutant concentrations were identified, and results are somewhat limited due to the imprecision of environmental sampling of airborne fungi and ELISA measurements of Asp f1 allergen in settled dust, these two biological contaminants require further investigation in indoor environments in developing countries. Because allergen concentrations varied with certain home characteristics, such as house age and presence of opening windows, some low cost interventions to reduce allergen concentrations may be feasible. The study results suggest that developing countries should view allergen exposure as an important public health issue.
Acknowledgments
We wish to thank the caregivers and children who participated in the study. This research and the SDHS were supported by the Fogarty International Center of the U.S. National Institutes of Health (Grant #2 D43 TW00812-06), and eThekwini Municipality.
Grant sponsor: EThekwini Municipality, KwaZulu Natal, Durban, South Africa
Grant no: 1a-103 - Health Study
Grant Sponsor: Sponsored in part by US NIH Fogarty International Center grant 2 D43 TW000812
Footnotes
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the manuscript.
References
- Arbes SJ, Sever M, Vaughn B, Mehta J, Lynch JT, Mitchell H, Hoppin JA, Spencer HL, Sandler DP, Zeldin DC. Feasibility of using subject-collected dust samples in epidemiologic and clinical studies of indoor allergens. Environ Health Persp. 2005;113(6):665–669. doi: 10.1289/ehp.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Ekteish F, Otoom S, Shehabi I. Prevalence of asthma in Jordan: Comparison between Bedouins and urban schoolchildren using the International Study of Asthma and Allergies in Childhood phase III protocol. Allergy and Asthma Proceedings. 2009;30(2):181–185. doi: 10.2500/aap.2009.30.3208. [DOI] [PubMed] [Google Scholar]
- Addo-Yobo EO, Custovic A, Taggart SC, Craven M, Bonnie B, Woodcock A. Risk factors for asthma in urban Ghana. J Allergy Clin Immunol. 2001;108(3):363–368. doi: 10.1067/mai.2001.117464. [DOI] [PubMed] [Google Scholar]
- Arshad SH. Does exposure to indoor allergens contribute to the development of asthma and allergy? Curr Allergy Asthma Rep. 2010;10:49–55. doi: 10.1007/s11882-009-0082-6. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Bernstein IL, Gallagher JS. The prevalence of house dust mites, dermatophagoides spp, and associated environmental conditions in homes in Ohio. J Allergy Clin Immunol. 1982;69:527–532. doi: 10.1016/0091-6749(82)90178-6. [DOI] [PubMed] [Google Scholar]
- Arruda LK, Platts-Mills TA, Longbottom JL. Aspergillus fumigatus: identification of 16, 18 and 45 kd antigens recognized by human IgG and IgE antibodies and murine monoclonal antibodies. J Allergy Clin Immunol. 1992;89:1166–1176. doi: 10.1016/0091-6749(92)90301-h. [DOI] [PubMed] [Google Scholar]
- Berman D. Pollen monitoring in South Africa. Current Allergy Clin Immunol. 2007;20:184–187. [Google Scholar]
- Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, Kanchanaraksa S, Swartz LJ, Callahan KA, Butz AM, Rand CS, Diette GB, Krishnan JA, Moseley AM, Curtin-Brosnan J, Durkin NB, Eggleston PA. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98(2):167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Carrer P, Maroni M, Alcini D, Cavallo D. Allergens in indoor air: environmental assessment and health effects. Sci Total Environ. 2001;270:33–42. doi: 10.1016/s0048-9697(00)00791-9. [DOI] [PubMed] [Google Scholar]
- Carlsten C, Dimich-Ward H, Becker AB, Ferguson A, Chan HW, DyBuncio A, Chan-Yeung M. Indoor allergen exposure, sensitization, and development of asthma in a high-risk birth cohort. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e740–746. doi: 10.1111/j.1399-3038.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Chapman MD, Heymann PW, Wilkins SR, Brown MJ, Platts-Mills TA. Monoclonal immunoassays for major dust mite (Dermatophagoides) allergens, Der p1 and Der f1, and house extracts. J Allergy Clin Immunol. 1987;80:184–194. doi: 10.1016/0091-6749(87)90128-x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Burge HA, Dockery DW, Muilenberg ML, Weiss ST, Gold DR. Limitations of a home characteristics questionnaire as a predictor of indoor allergen levels. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1536–1541. doi: 10.1164/ajrccm.157.5.9708011. [DOI] [PubMed] [Google Scholar]
- Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Cho JH, Hee Min K, Paik NW. Temporal variation of airborne fungi concentrations and related factors in subway stations in Seoul, Korea. Int J Hyg Environ Health. 2006;209(3):249–255. doi: 10.1016/j.ijheh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Custovic A, Fletcher A, Pickering CA, Francis HC, Green R, Smith A, Chapman M, Woodcock A. Domestic allergens in public places III: house dust mite, cat, dog and cockroach allergens in British hospitals. Clin Exp Allergy. 1998a;28:53–59. doi: 10.1046/j.1365-2222.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson A, Chapman MD, Woodcock A. Allergen avoidance in the treatment of asthma and atopic disorders. Thorax. 1998b;53:63–72. doi: 10.1136/thx.53.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custovic A, Hallam C, Woodcock H, Simpson B, Houghton N, Simpson A, Woodcock A. Synthetic pillows contain higher levels of cat and dog allergen than feather pillows. Pediatr Allergy Immunol. 2000;11:71–73. doi: 10.1034/j.1399-3038.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- Dales RE, Miller D, McMullen E. Indoor air quality and health: validity and determinants of reported home dampness and moulds. Int J Epidemiol. 1997;26(1):120–125. doi: 10.1093/ije/26.1.120. [DOI] [PubMed] [Google Scholar]
- Danaviah S, Gqaleni N, Chuturgoon AA, Dutton MF, Lalloo UG, Jeena PM. Indoor airborne moulds and their relevance to paediatric respiratory health – a study of an informal settlement in Durban, South Africa. In: Seppännen O, Säteri J, editors. Healthy Buildings 2000: Exposure, human responses and building investigations. Vol. 1. SIY Indoor Air Information Oy; Helsinki, Finland: 2000. pp. 293–298. [Google Scholar]
- Earle CD, Kind EM, Tsay A, Pittman K, Saric B, Vailes L, Godbout R, Oliver KG, Chapman MD. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119:428–433. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Eggleston PA, Wood RA, MD, Rand C, Nixon WJ, Chen PH, Lukk P. Removal of cockroach allergen from inner-city homes. J Allergy Clin Immunol. 1999;104:842–846. doi: 10.1016/s0091-6749(99)70296-4. [DOI] [PubMed] [Google Scholar]
- Ehrlich RI. The prevalence of asthma in South Africa. Current Allergy Clin Immunol. 2002;15(1):4–8. [Google Scholar]
- Ezzati M, Kammen D. Indoor air pollution from biomass combustion as a risk factor for acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358(9282):619–624. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- Ganser GH, Hewett P. An accurate substitution method for analyzing censored data. J Occup Environ Hyg. 2010;7(4):233–44. doi: 10.1080/15459621003609713. [DOI] [PubMed] [Google Scholar]
- Garret MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998;28:459–467. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- Hallam C, Custovic A, Simpson B, Houghton N, Simpson A, Woodcock A. Mite allergens in feather and synthetic pillows. Allergy. 1999;55:407–408. doi: 10.1034/j.1398-9995.1999.00066.x. [DOI] [PubMed] [Google Scholar]
- Hewett P, Ganser GH. A Comparison of Several Methods for Analyzing Censored Data. Ann of Occup Hyg. 2007;51:611–632. doi: 10.1093/annhyg/mem045. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Clearing the air: Asthma and indoor air exposures. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Kistnasamy EJ, Robins TG, Naidoo R, Batterman S, Mentz GB, Jack C, Irusen E. The relationship between asthma and ambient air pollutants among primary school students in Durban, South Africa. Int J Environ Health. 2008;2(3/4):365–385. [Google Scholar]
- Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, Wahn U. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford TR, Gold DR, Kim Y, Jankun TM, Ren P, McSharry C, Platts-Mills T, Chapman MD, Bracken MB. Dust mite, cockroach, cat and dog allergen concentrations in homes of asthmatic children in the Northern United States: Impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjra A, Prescott R, Potter P. Cockroach allergy in Durban. Current Allergy Clin Immun. 1995;8:3–7. [Google Scholar]
- Moscato G, Perfetti L, Galdi E, Pozzi V, Minoia C. Levels of house-dust-mite allergen in homes of nonallergic people in Pavia, Italy. Allergy. 2000;55(9):873–878. doi: 10.1034/j.1398-9995.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- Naidoo R, Gqaleni N, Batterman S, Robins T, et al. [accessed 10 December 2011];Multipoint Plan: Project 4: Health Study and Health Risk Assessment (South Durban Health Study) Report. 2007 http://doeh.ukzn.ac.za/Libraries/Documents/SDHS_FINAL_Report_revision_February_2007.sflb.ashx.
- Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, Mellins RB, Hoepner L, Andrews H, Lopez-Pintado S, Quinn JW, Perera FP, Miller RL, Jacobson JS, Perzanowski MS. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128(2):284–92. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Vailes LD, Li Y, Heymann PW, Chapman MD. Monoclonal antibodies to group II Dermatophagoides spp. allergens: murine immune response, epitope analysis, and development of a two-site ELISA. J Allergy Clin Immunol. 1994;94(3 Pt 1):537–546. doi: 10.1016/0091-6749(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Pearce N, Douwes J, Beasley R. Is allergen exposure the major primary cause of asthma? Thorax. 2000;55:424–431. doi: 10.1136/thorax.55.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EA, Baldwin GT, Israel B, Salinas MA. Application of Health Promotion Theories and Models for Environmental Health. Health Educ Behav. 2004;31:491–509. doi: 10.1177/1090198104265601. [DOI] [PubMed] [Google Scholar]
- Perfetti L, Ferrari M, Galdi E, Pozzi V, Cottica D, Grignani E, Minoia C, Moscato G. House dust mites (Der p1, Der f 1), cat (Fel d 1) and cockroach (Bla g 1) allergens in indoor work-places (offices and archives) Sci Total Environ. 2004;328:15–21. doi: 10.1016/j.scitotenv.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Peroni DG, Boner AL, Vallone G, Antolini I, Warner JO. Effective allergen avoidance at high altitude reduces allergen-induced bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1994;149:1442–1446. doi: 10.1164/ajrccm.149.6.8004296. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(6 Pt 1):S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Pollart SM, Mullins DE, Vailes LD, Hayden ML, Platts-Mills TA, Sutherland WM, Chapman MD. Identification, quantitation, and purification of cockroach allergens using monoclonal antibodies. J Allergy Clin Immunol. 1991;87(2):511–521. doi: 10.1016/0091-6749(91)90010-l. [DOI] [PubMed] [Google Scholar]
- Potter PC. Common indoor and outdoor aero-allergens in South Africa: identification of the allergen that is responsible for symptoms is the key to the management of the allergic patient. CME. 2010;28(9):426–432. [Google Scholar]
- Rosenstreich D, Eggleston PA, Kattan M, Baker D, Slavin R, Gergen P, Mitchell H, McNiff-Mortiner K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among the inner-city children with asthma. New England J Med. 1997;337:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hoekstra ES, Lund F, Filtenborg O, Frisvad JC. In: Introduction to food and airborne fungi. 6. Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O, editors. Utrecht, Netherlands: Centraal Bureauvoor Schimmelcultures (CBS); 2000. [Google Scholar]
- Sawyer G, Kemp M, Shaw R, Patchett K, Siebers R, Lewis S, Beasley R, Crane J, Fitzharris P. Biologic pollution in infant bedding in New Zealand: High allergen exposure during a vulnerable period. J Allergy Clin Immunol. 1998;102:765–770. doi: 10.1016/s0091-6749(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Thulasiraj RD, Coles CL, Sheeladevi S, Yanik EL, Rahmathullah L. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int J Epidemiol. 2009;38:1351–1363. doi: 10.1093/ije/dyp286. [DOI] [PubMed] [Google Scholar]
- van Strien RT, Verhoeff AP, Brunekreef B, Van Wijnen JH. Mite antigen in house dust: relationship with different housing characteristics in The Netherlands. Clin Exp Allergy. 1994;24(9):843–853. doi: 10.1111/j.1365-2222.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- van Strien R, Koopman L, Kerkhof M, Oldenwening M, de Jongste J, Gerritsen J, Neijens HJ, Aalberse RC, Smit HA, Brunekreef B. Mattress encasings and mite allergen levels in the prevention and incidence of asthma and mite allergy study. Clin Exp Allergy. 2003;33(4):490–495. doi: 10.1046/j.1365-2222.2003.01626.x. [DOI] [PubMed] [Google Scholar]
- Heseltine E, Rosen J, editors. WHO. WHO guidelines for indoor air quality: dampness and mould. Copenhagen, Denmark: WHO Regional Office for Europe; 2009. [PubMed] [Google Scholar]


