Abstract
Background
Pain from the sacroiliac joint (SIJ) is an under-recognized cause of low back pain. The degree to which SIJ pain decreases quality of life has not been directly compared to other more familiar conditions of the lumbar spine.
Methods
Multivariate regression analysis of individual patient data from two prospective multicenter clinical trials of SIJ fusion and three prospective multicenter clinical trials of surgical treatments for degenerative lumbar spine conditions.
Results
Controlling for baseline demographic parameters as well as a validated disability score, quality of life scores (EuroQOL 5-D and SF-36) were, in most cases, lower in the SIJ cohorts compared to the three other spine surgery cohorts.
Conclusion
Patients with SIJ dysfunction considering surgery have decrements in quality of life as or more severe compared to patients with degenerative spondylolisthesis, spinal stenosis, and intervertebral disc herniation.
Keywords: spine surgery, disability, low back pain, sacroiliac joint pain, lumbar stenosis, intervertebral disc herniation, degenerative spondylolisthesis, sacroiliac joint fusion
Video abstract
Background
Chronic back pain is an exceedingly common and important worldwide health problem. Back pain rates are higher than cancer and chronic obstructive pulmonary disease as a cause of poor health, and lower back pain is the sixth most common cause of loss of global disability-adjusted life years.1
Degenerative conditions of the lumbar spine, including intervertebral disc herniation (IDH), spinal stenosis (SPS), and degenerative spondylolisthesis (DS), are accepted as common causes of lower back pain that often require definitive surgical treatment. The rate of lumbar fusion has risen 2.4-fold in the decade between 1998 and 2008, and the cost-per case has more than tripled during this period.2 Despite this increase in use, success rates from lumbar fusion, especially in patients with isolated degenerative disc disease, continue to be unacceptably low.3
One explanation for low success rates is the inability to accurately diagnose the source of lower back pain. Pain emanating from the sacroiliac joint (SIJ) is an under-recognized cause of chronic lower back pain. SIJ dysfunction can cause back and pelvic pain with radiation into the groin, legs, or hips,4 and can be mistaken for other causes of pain. Nonetheless, SIJ pain may be very common. In patients presenting for evaluation of low back pain, the SIJ was determined to be the source of lower back pain in 14%–22% of patients presenting for back pain evaluation.5,6 The SIJ is even more commonly (up to 40%7,8) suspected as a source of lower back pain in patients who have undergone prior lumbar fusion.
Currently available treatment options for SIJ dysfunction include physical therapy,9 SIJ steroid injections,10,11 Radio-frequency ablation of the neural structures posterior to the SIJ,12,13 and open14 or minimally invasive15–19 SIJ fusion. A recently published surgery vs non-surgery randomized trial of SIJ fusion using triangular titanium implants substantiates the use of this technology.20
Many surgeons do not consider SIJ dysfunction in their diagnostic workup of low back pain. This could be because of inadequate recognition of the importance of SIJ dysfunction as a contributor to poor health quality, disability, and pain. Although multiple studies have been published regarding SIJ pain, direct comparisons with other sources of back pain have not been published. In earlier work, we demonstrated that preoperative quality of life scores in patients with SIJ pain are low, indicating a substantial burden of disease.21 In this report, we used primary data sets to compare disability and quality of life scores in patients participating in two sets of clinical trials, one enrolling patients with SIJ pain and the other enrolling patients with three common spinal conditions (IDH, SPS, and DS) often treated surgically. The goal was to directly compare decreased quality of life across disease categories.
Methods
Data sources
Data for this study were taken from multicenter prospective clinical trials performed in the USA in two settings: two trials of minimally invasive SIJ fusion for SIJ dysfunction and three trials of commonly accepted surgical treatments for IDH, SPS, and DS. Four of the trials directly compared pain, disability, and quality of life scores in patients randomized to either surgical treatment or non-surgical care. The fifth trial was a single-arm SIJ study only but was included because enrollment criteria were identical to the randomized trial. All trials used similar assessments, as detailed in the later text. Only baseline (preoperative) scores were compared. Trial eligibility criteria are described in Table S1.
SIJ trials
Baseline scores were taken from two ongoing prospective multicenter clinical trials of SIJ fusion. Sacroiliac Joint Fusion With iFuse Implant System® ([SIFI], NCT01640353, N=172 subjects) is a prospective multicenter single-arm study of minimally invasive SIJ fusion using titanium triangular implants (iFuse Implant System®; SI-BONE, San Jose, CA, USA) with enrollment at 19 US centers. Investigation of Sacroiliac Fusion Treatment ([INSITE], NCT01681004) is a prospective multicenter randomized controlled trial of the same surgical treatment vs best-available non-surgical management, which included pain medications, physical therapy, SIJ steroid injections, and radiofrequency ablation of the lateral branches of the sacral nerve roots. INSITE (N=148 subjects) enrolled subjects at 19 US sites. Eligibility criteria were identical between INSITE and SIFI. The primary endpoints for these studies were success/failure endpoints based on SIJ pain responses. Twelve month results from INSITE20 and SIFI22 have been published.
Lumbar spine trials
Data for lumbar spinal conditions were taken from the Spine Patient Outcomes Research Trial (SPORT). SPORT is a set of National Institutes of Arthritis and Musculoskeletal and Skin Diseases-funded (U01-AR45444) prospective multi-center randomized controlled trials of surgical vs non-surgical treatment for three conditions: IDH (NCT00000410), SPS (NCT00000409), and DS (NCT00000411). SPORT was performed in collaboration with the Trustees of Dartmouth College. In each case, subjects were randomized at baseline to receive either immediate surgical treatment or non-surgical care. The primary endpoint of these studies was improvements in SF-36 physical function scores. SPORT data were provided by SPORT authors (Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth, NH, USA) for this analysis.
Assessments
Both SIJ trials and SPORT performed similar assessments at baseline and follow-up. SIJ trials included Oswestry Disability Index (ODI), while SPORT included the “MODEMS” version of ODI, which is very similar in structure and content. ODI is a validated, ten-question survey that measures disability due to back pain, with higher scores representing increased disability.22 Both trials included EuroQOL-5D (EQ-5D),23 a six-question general health survey. The first five questions (three responses each) result in 35=243 unique responses that can be mapped to time-trade off (TTO) health state utility.24 In both studies, US norms of TTO were used for this mapping. The TTO value extends from −0.3 (indicating very poor health) to 1.0 (indicating perfect health).
Both studies included SF-36, a 36-question general health survey that has been used in thousands of studies.25 As confirmed by SPORT authors, SPORT utilized version 1 of SF-36 and the SIJ trials utilized version 2. Both versions assess quality of life in eight subdomains and produce two summary scores (physical component summary [PCS] and mental component summary [MCS]). SF-36 subdomain assessments are identical across versions for four of the eight subdomains; for the other four domains, the survey questions and response categories were modified between versions.26 However, both survey versions report norm-based scores, with values based on popu lation means of 50 with standard deviations of 10. With the help of Optum (Lincoln, RI, USA), who owns and administers SF-36, individual values from the SIJ trials utilized version 2 (SIJ) trials were converted to norm-based scores using the same 1998 norms as used by SPORT. These adjustments allowed direct comparison of both summary scales (PCS and MCS) and individual norm-based subdomains.
Statistical methods
After combining data sets, statistical analysis consisted of tabular and graphical summaries. In addition, general linear models were used to compare EQ-5D TTO and SF-36 scores across studies controlling for age, sex, body mass index, and ODI (or MODEMS) scores. Both linear and squared terms were included in all models. Interaction terms did not add to the model fit and were therefore not used further. For each linear model, the IDH group was chosen as the reference level, since these patients had the highest scores and were youngest. The primary goal of the analysis was to determine the relative differences in quality of life scores among the four diagnoses while controlling for potential baseline covariates. All statistical analysis was done in R.27 Graphical analysis with smoothing was performed using the ggplot2 library.28
Results
Demographic characteristics of trial participants are shown in Table 1. Due to large sample sizes, all baseline demographic characteristics show statistically significant differences across studies. Across the studies, IDH participants were younger, DS and SPS patients were older, and IDH and SPS participants were less likely to be female.
Table 1.
Demographic characteristics
| SIJ
|
SPORT
|
||||
|---|---|---|---|---|---|
| INSITE N=148 |
SIFI N=171 |
DS N=607 |
IDH N=1,244 |
SPS N=654 |
|
| Female, Number (%) | 103 (69.6%) | 119 (69.6%) | 418 (68.9%) | 522 (42.0%) | 254 (38.8%) |
| Age (years), mean (SD) | 51.3 (11.2) | 50.8 (11.3) | 66.1 (10.3) | 41.7 (11.4) | 64.6 (11.7) |
| Body mass index, mean (SD) | 30.4 (6.5) | 29.3 (6.6) | 29.1 (6.2) | 28.0 (5.5) | 29.4 (5.6) |
Abbreviations: DS, degenerative spondylolisthesis; IDH, intervertebral disc herniation; INSITE, Investigation of Sacroiliac Fusion Treatment; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SIJ, sacroiliac joint; SPORT, Spine Patient Outcomes Research Trial; SPS, spinal stenosis.
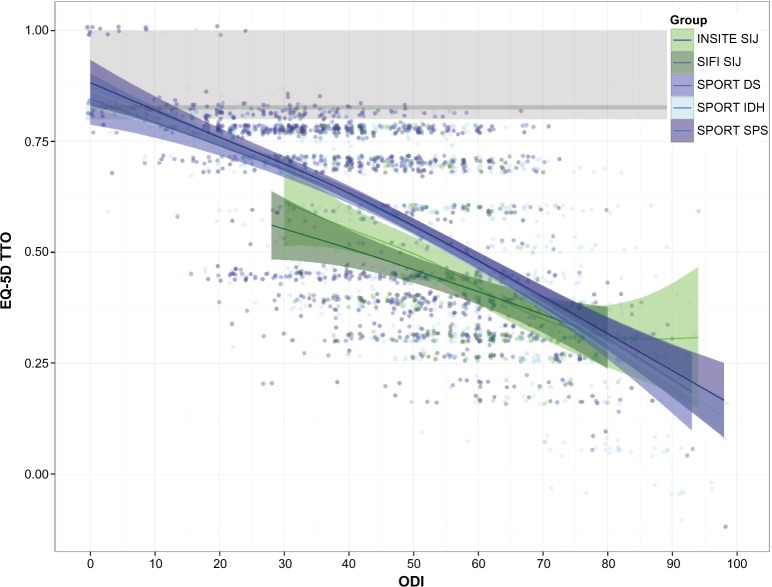
EQ-5D TTO had a modest but statistically significant (P<0.0001 each) linear relationship to age (increase of 0.002 points per year) and sex (0.05 points higher for men vs women). Not surprisingly, EQ-5D TTO strongly correlated with ODI (Pearson r=−0.687, P<0.0001, Figure 1), with increasing disability (higher ODI) showing decreased quality of life (lower EQ-5D TTO index). At any ODI level, EQ-5D TTO scores were lower for the SIJ trial subjects compared to SPORT subjects. To compare mean EQ-5D TTO values across trials, multivariate linear regression was performed controlling for age (including a term for age2), sex, body mass index, and ODI score (including ODI2). Compared to the IDH group, mean EQ-5D TTO scores were depressed by 0.023 and 0.01 points in the DS and SPS groups (P=0.0276 and 0.1461) respectively, and 0.057 and 0.084 points in the two SIJ groups (P<0.0001 each). In all populations, EQ-5D scores were substantially lower than population norms.29
Figure 1.
Baseline EQ-5D as a function of baseline ODI scores by trial.
Notes: SIJ trials are shown in green; SPORT trials are shown in blue. Individual patient scores are plotted as points and jittered slightly. Ribbons show family-wise 95% confidence limits (using geom_smooth from ggplot2). Gray line and bars show normal population median (82) and interquartile ranges (80–100).
Abbreviations: DS, degenerative spondylolisthesis; IDH, intervertebral disc herniation; INSITE, Investigation of Sacroiliac Fusion Treatment; ODI, Oswestry Disability Index; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SPORT, Spine Patient Outcomes Research Trial; SIJ, sacroiliac joint; SPS, spinal stenosis; TTO, time-trade off.
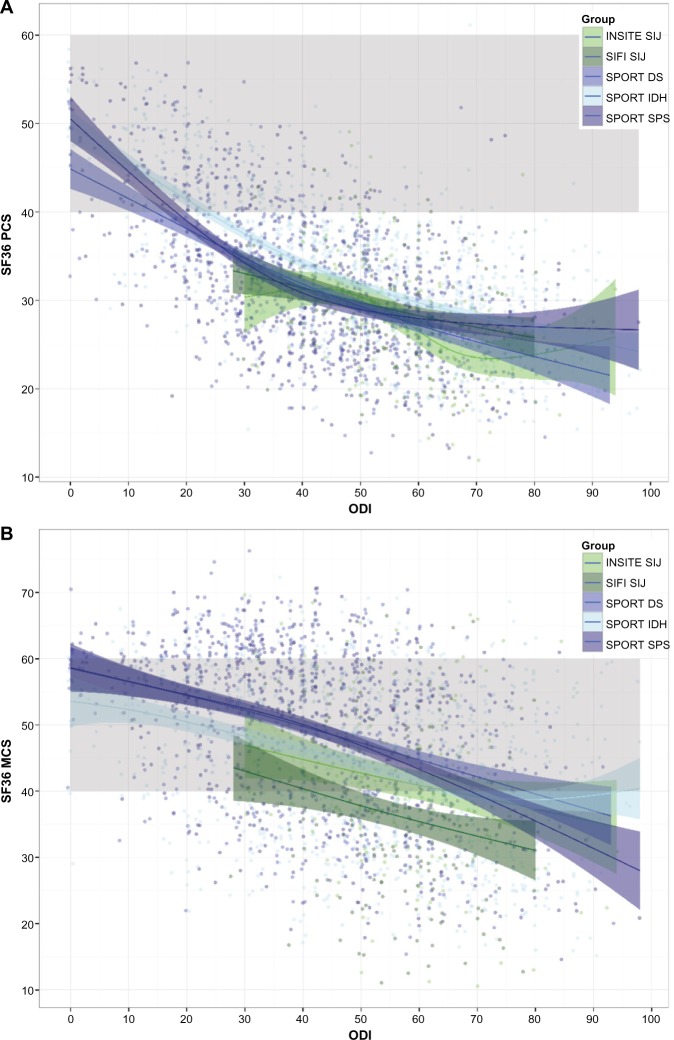
Similar to EQ-5D, SF-36 PCS and MCS showed the modest correlations with age (Pearson r of −0.63, P=0.0008 and r of −0.156, P<0.0001, respectively) and sex (2.5 points higher each for men vs women, P<0.0001) and strong associations with ODI scores (Pearson r −0.596 and −0.405, respectively, Figure 2). At any level of ODI, PCS scores were similar between the SIJ and SPORT populations, but MCS scores were lower in the SIJ trials. In similar multivariate regressions, compared to IDH, mean PCS scores were lower by 0.786 and 0.667 points in the DS and SPS groups (P=0.0523 and 0.0829, respectively), and mean PCS scores were 2.07 and 1.02 points lower in the two SIJ groups (P=0.0002 and 0.0512, respectively). Compared to IDH, mean MCS scores were higher by 2.0 and 1.17 points in the DS and SPS groups (P=0.0067 and 0.0932) and 1.17 and 6.43 points lower in the two SIJ groups (P=0.2476 and <0.0001).
Figure 2.
Baseline SF-36 PCS (A) and MCS (B) as a function of baseline ODI scores by trial.
Notes: SIJ trials are shown in green; SPORT trials are shown in blue. Individual patient scores are plotted as points and jittered slightly. Ribbons show family-wise 95% confidence limits (using geom_smooth from ggplot2). Gray band shows expected range for normal population (50±10) whereby data are presented as mean ± standard deviation.
Abbreviations: ODI, Oswestry Disability Index; INSITE, Investigation of Sacroiliac Fusion Treatment; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SPORT, Spine Patient Outcomes Research Trial; MCS, mental component summary; SIJ, sacroiliac joint; DS, degenerative spondylolisthesis; IDH, intervertebral disc herniation; SPS, spinal stenosis; PCS, physical component summary.
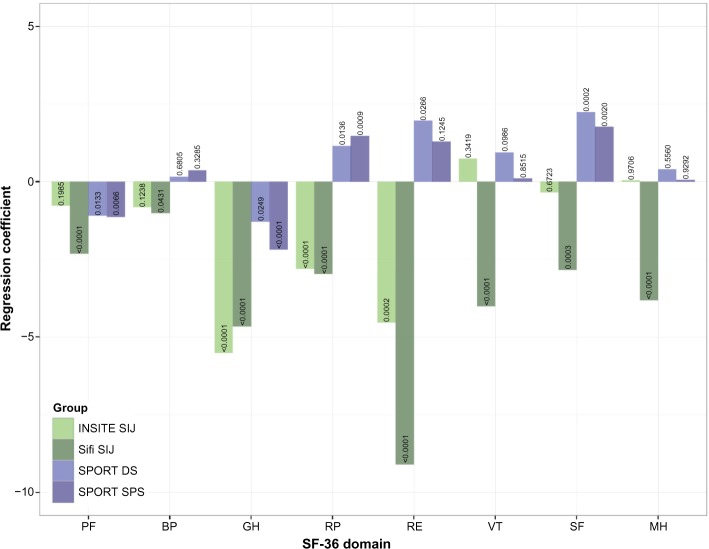
Norm-based SF-36 subdomain scores were low for all groups (Table 2). Figure 3 shows multivariate regression coefficients for the difference in means for each subdomain from the IDH reference group by study controlling for the same factors as in other regressions. Adjusting for differences in demographic characteristics and ODI, most subdomains showed lower scores in the SIJ cohorts compared to both IDH (reference group) and the other non-SIJ cohorts.
Table 2.
SF-36 norm-based subdomain scores, raw mean (SD)
| SIJ
|
SPORT
|
||||
|---|---|---|---|---|---|
| INSITE | SIFI | DS | IDH | SPS | |
| PF | 25.5 (8.0) | 24.6 (6.7) | 29.5 (9.4) | 31.1 (10.7) | 29.7 (9.8) |
| BP | 28.4 (4.8) | 28.5 (5.2) | 34.2 (8.2) | 31.5 (8.6) | 34.3 (8.5) |
| GH | 43.3 (10.0) | 44.5 (9.7) | 48.8 (9.1) | 50.4 (8.8) | 48.1 (9.1) |
| RP | 26.4 (8.2) | 26.3 (7.5) | 33.1 (8.6) | 31.7 (7.7) | 33.3 (9.0) |
| RE | 33.3 (14.4) | 29.1 (14.7) | 42.5 (13.7) | 40.5 (14.0) | 42.1 (14.2) |
| VT | 39.0 (9.8) | 34.6 (8.7) | 43.5 (10.5) | 41.3 (9.6) | 43.1 (10.4) |
| SF | 29.9 (10.1) | 28.1 (9.5) | 39.1 (12.2) | 33.2 (12.2) | 38.3 (12.1) |
| MH | 41.8 (11.6) | 38.3 (12.1) | 47.1 (10.7) | 43.1 (11.4) | 46.8 (11.4) |
Abbreviations: BP, bodily pain; DS, degenerative spondylolisthesis; GH, general health; IDH, intervertebral disc herniation; INSITE, Investigation of Sacroiliac Fusion Treatment; MH, mental health; PF, physical function; RE, role emotional; RP, role physical; SF, social functioning; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SIJ, sacroiliac joint; SPORT, Spine Patient Outcomes Research Trial; SPS, spinal stenosis; VT, vitality.
Figure 3.
Multivariate regression coefficients for SF-36 subdomains by domain and study (IDH as reference group).
Notes: All regressions controlled for age, age2, sex, BMI, ODI, and ODI2. Small numbers in bars indicate P-value for regression coefficient.
Abbreviations: BMI, body mass index; BP, bodily pain; DS, degenerative spondylolisthesis; IDH, intervertebral disc herniation; GH, general health; INSITE, Investigation of Sacroiliac Fusion Treatment; MH, mental health; ODI, Oswestry Disability Index; PF, physical function; RE, role emotional; RP, role physical; SPORT, Spine Patient Outcomes Research Trial; SPS, spinal stenosis; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SIJ, sacroiliac joint; SF, social functioning; VT, vitality.
Discussion
Chronic low back pain is a costly and complex illness that markedly impairs quality of life and is unquestionably associated with high annual health care expenditures. Pathology of the SIJ, resulting in SIJ dysfunction, is a common cause of low back pain. Unfortunately, due to a historical lack of effective surgical treatments for SIJ dysfunction, the condition has, until recently, been largely ignored by the surgical community. With the availability and increasing popularity30 of minimally invasive surgical techniques to treat the SIJ, interest in the impact of SIJ dysfunction has increased.
In a previous report based on the SIJ trials examined herein,21 we compared health utility values in the same SIJ cohorts to both a normal cohort as well as reported health state utilities available through a national clearinghouse of utilities used in published cost-effectiveness analyses. The decrement in health quality associated with SIJ dysfunction was marked and consistent with major diseases, such as liver cirrhosis and chronic obstructive pulmonary diseases, and slightly more burdensome than lumbar stenosis and DS. Moreover, observed values for the SIJ population were similar to those reported in other prominent spinal and other orthopedic (eg, hip and knee osteoarthritis) conditions for which surgery is commonly provided.
In the current study, we extend these findings by directly comparing individual patient health quality of life scores across two sets of prospective clinical trials involving patients with either SIJ dysfunction or three common spine conditions (DS, SPS, and IDH). This analysis showed that the decrement in health state utility for SIJ dysfunction was at least as severe as those seen in DS, SPS, and IDH. In most cases, the decrement was larger. This analysis confirms prior work and suggests that SIJ dysfunction is a cause of prominent decrements in quality of life at least as severe as those in other spinal conditions for which surgery is commonly provided. As SIJ dysfunction may be misdiagnosed as a degenerative spine condition, it is important that surgeons carefully examine and distinguish the cause of chronic lower back pain so as to provide treatments directed at the correct underlying disease.
Not surprisingly, quality of life measurements (EQ-5D and SF-36 scores) were strongly inversely correlated with ODI disability scores. Although ODI scores varied between trials, our analyses comparing baseline quality of life values in these trials controlled for individual ODI scores. In multivariate analyses that also accounted for baseline demographic factors, quality of life scores were as depressed in all cases and more depressed in most cases in the SIJ cohorts compared to the other cohorts.
Advantages of our study are as follows. The primary input data for the analysis consist of two carefully performed sets of large, multicenter prospective trials, including four randomized controlled trials and one single-arm study. Both study sets had large sample sizes, represent multicenter experience, and employed similar assessment tools. Both study sets focused on diseases of the lower back for which surgical treatments are commonly provided.
Limitations of our findings include the following. Studies were performed in different time periods (the SIJ studies enrolled subjects in the 2012–2015 time frame, whereas SPORT studies enrolled subjects in the early 2000s). The two study sets used slightly different versions of ODI and SF-36 surveys. However, the version differences were accounted for by using norm-based scores (for SF-36) and adjusting to the same normal population (1998 norms).
Conclusion
Based on individual data from multicenter clinical trials, the decrement in quality of life in patients with SIJ dysfunction is as or more marked compared to patients with DS, SPS, and IDH.
Table S1.
Eligibility criteria for SIJ and SPORT studies
| SIJ studies |
| SIFI: NCT01640353 |
| INSITE: NCT01681004 |
| Inclusion criteria |
| 1. Age 21–70 years at time of screening |
| 2. Patient has lower back pain for >6 months inadequately responsive to conservative care |
| 3. Diagnosis of sacroiliac joint disruption or degenerative sacroiliitis based on ALL of the following: |
| a) Patient has pain at or close to the posterior superior iliac spine with possible radiation into buttocks, posterior thigh or groin and can point with a single finger to the location of pain (Fortin Finger Test) |
| b) Patient has at least three of five physical examination maneuvers specific for the SIJ |
| c) Patient has improvement in lower back pain numeric rating scale of at least 50% after injection of local anesthetic into affected SIJ(s) |
| d) One or more of the following: |
| i. SIJ disruption: Asymmetric SIJ widening on X-ray or CT scan or leakage of contrast on diagnostic arthrography |
| ii. Degenerative sacroiliitis: Radiographic evidence of SIJ degeneration, including sclerosis, osteophytes, subchondral cysts, or vacuum phenomenon on CT or plain film, or due to prior lumbosacral spine fusion |
| 4. Baseline Oswestry Disability Index score of at least 30% |
| 5. Baseline SIJ pain score of at least 50 on 0–100 mm visual analog scale |
| 6. Patient has signed study-specific informed consent form |
| 7. Patient has the necessary mental capacity to participate and is physically able to comply with study protocol requirements |
| Exclusion criteria |
| 1. Severe back pain due to other causes, such as lumbar disc degeneration, lumbar disc herniation, lumbar spondylolisthesis, lumbar spinal stenosis, lumbar facet degeneration, and lumbar vertebral body fracture |
| 2. Other known sacroiliac pathology such as |
| a) Sacral dysplasia |
| b) Inflammatory sacroiliitis (eg, ankylosing spondylitis or other HLA-associated spondyloarthropathy) |
| c) Tumor |
| d) Infection |
| e) Acute fracture |
| f) Crystal arthropathy |
| 3. History of recent (<1 year) major trauma to pelvis |
| 4. Previously diagnosed osteoporosis (defined as prior T-score <−2.5 or history of osteoporotic fracture) |
| 5. Osteomalacia or other metabolic bone disease 6. Chronic rheumatologic condition (eg, rheumatoid arthritis) |
| 7. Any condition or anatomy that makes treatment with the iFuse Implant System® infeasible |
| 8. Chondropathy |
| 9. Known allergy to titanium or titanium alloys |
| 10. Use of medications known to have detrimental effects on bone quality and soft-tissue healing |
| 11. Prominent neurologic condition that would interfere with physical therapy |
| 12. Current local or systemic infection that raises the risk of surgery |
| 13. Patient currently receiving or seeking worker’s compensation, disability remuneration, and/or involved in injury litigation |
| 14. Currently pregnant or planning pregnancy in the next 2 years |
| 15. Patient is a prisoner or a ward of the state |
| 16. Known or suspected drug or alcohol abuse |
| 17. Diagnosed psychiatric disease (eg, schizophrenia, major depression, personality disorders) that could interfere with study participation |
| 18. Patient is participating in an investigational study or has been involved in an investigational study within 3 months prior to evaluation for participation |
| SPORT, Intervertebral Disc Herniation: NCT00000410 |
| Inclusion criteria |
| 1. Duration of symptoms: 6 or more weeks |
| 2. Treatments tried: Non-steroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Persistent radicular pain provoked by moderate exercise, sitting, increased abdominal pressure, decreased mobility, list (scoliosis), straight leg raising |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health which makes spinal surgery too life-threatening to be an appropriate alternative, dramatic improvement with conservative care, or inability (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: A patient with a history of any invasive malignancy (except non-melanoma skin cancer) is ineligible unless he or she has been treated with a curative intent AND there has been no clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurological deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 6 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
| SPORT, Degenerative Spondylolisthesis: NCT00000409 |
| Inclusion criteria |
| 1. Duration of symptoms: 12 or more weeks |
| 2. Treatments tried: Nonsteroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Pain in low back, buttocks, or lower extremity that becomes worse with lumbar extension. Must be confirmed by evidence of central or central-lateral compression of the cauda equina by a degenerative lesion of the facet joint, disc, or ligamentum flavum on MRI, computed tomography scans, or myelograms |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health that makes spinal surgery too life-threatening to be an appropriate alternative, patient has improved dramatically with conservative care, or the patient is unable (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: Patients with a history of any invasive malignancy (except non-melanoma skin cancer) are ineligible unless they have been treated with curative intent AND have not had any clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurologic deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 12 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
| SPORT, Spinal Stenosis: NCT00000411 |
| Inclusion criteria |
| 1. Duration of symptoms: 12 or more weeks |
| 2. Treatments tried: Nonsteroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Pain in low back, buttocks, or lower extremity that becomes worse with lumbar extension. Must be confirmed by evidence of central or central-lateral compression of the cauda equina by a degenerative lesion of the facet joint, disc, or ligamentum flavum on MRI, computed tomography scans, or myelograms |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health that makes spinal surgery too life-threatening to be an appropriate alternative, patient has improved dramatically with conservative care, or the patient is unable (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: Patients with a history of any invasive malignancy (except non-melanoma skin cancer) are ineligible unless they have been treated with curative intent AND have not had any clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurologic deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 12 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
Abbreviations: CT, computed comography; HLA, human leukocyte antigen; INSITE, Investigation of Sacroiliac Fusion Treatment; MRI, magnetic resonance imaging; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SIJ, sacroiliac joint; SPORT, Spine Patient Outcomes Research Trial.
Acknowledgments
This paper was not prepared in collaboration with investigators of SPORT and does not necessarily reflect the opinions or conclusions of SPORT investigators.
Footnotes
Disclosure
DJC and WCR are SI-BONE employees. The authors report no other conflicts of interest in this work.
References
- 1.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA. Fusion surgery for lumbar degenerative disc disease: still more questions than answers. Spine J. 2015;15(2):272–274. doi: 10.1016/j.spinee.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Fortin JD, Aprill CN, Ponthieux B, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: Clinical evaluation. Spine. 1994;19(13):1483–1489. [PubMed] [Google Scholar]
- 5.Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;(217):266–280. [PubMed] [Google Scholar]
- 6.Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi: 10.1097/BRS.0b013e31818b8882. [DOI] [PubMed] [Google Scholar]
- 7.Liliang P-C, Lu K, Liang C-L, Tsai Y-D, Wang K-W, Chen H-J. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med Malden Mass. 2011;12(4):565–570. doi: 10.1111/j.1526-4637.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 8.DePalma MJ, Ketchum JM, Saullo TR. Etiology of Chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12(5):732–739. doi: 10.1111/j.1526-4637.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 9.Jackson R, Porter K. Current Concepts of Orthopaedic Physical Therapy. 3rd ed. Wisconsin, USA: American Physical Therapy Association, Orthopaedic Section; 2006. The pelvis and sacroiliac joint: physical therapy patient management utilizing current evidence. [Google Scholar]
- 10.Luukkainen R, Nissilä M, Asikainen E, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1999;17(1):88–90. [PubMed] [Google Scholar]
- 11.Luukkainen RK, Wennerstrand PV, Kautiainen HH, Sanila MT, Asikainen EL. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol. 2002;20(1):52–54. [PubMed] [Google Scholar]
- 12.Cohen SP, Hurley RW, Buckenmaier CC, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med Malden Mass. 2012;13(3):383–398. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 14.Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J Off J North Am Spine Soc. 2005;5(5):520–528. doi: 10.1016/j.spinee.2005.02.022. discussion 529. [DOI] [PubMed] [Google Scholar]
- 15.Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495–502. doi: 10.2174/1874325001206010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. doi: 10.1155/2013/536128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings J, Jr, Capobianco RA. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res. 2013;7(1):12. doi: 10.1186/1750-1164-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaetani P, Miotti D, Risso A, et al. Percutaneous arthrodesis of sacroiliac joint: a pilot study. J Neurosurg Sci. 2013;57(4):297–301. [PubMed] [Google Scholar]
- 19.Duhon B, Cher D, Wine K, Lockstadt H, Kovalsky D, Soo C-L. Safety and 6-month effectiveness of minimally invasive sacroiliac joint fusion: a prospective study. Med Devices Evid Res. 2013;6:219–229. doi: 10.2147/MDER.S55197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whang PG, Cher D, Polly D, et al. Sacroiliac joint fusion using triangular titanium implants vs non-surgical management: six-month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015;9(6):1–18. doi: 10.14444/2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cher D, Polly D, Berven S. Sacroiliac Joint pain: burden of disease. Med Dev Evid Res. 2014;7:73–81. doi: 10.2147/MDER.S59437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Group EuroQol – a new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 26.Ware JE. User Manual for SF-36v2 Health Survey. 3rd ed. Lincoln, RI: QualityMetric, Inc; 2011. [Google Scholar]
- 27.R Core Team 2013 . R: A Language and Environment for Statistical Computing. Vienna, Austria: [Accessed: September 1, 2013]. Available from: http://www.R-project.org/ [Google Scholar]
- 28.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Accessed October 1, 2014]. Available from: http://had.co.nz/ggplot2/book. [Google Scholar]
- 29.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorio MP, Polly DW, Jr, Ninkovic I, Ledonio CGT, Hallas K, Andersson G. Utilization of minimally invasive surgical approach for sacroiliac joint fusion in surgeon population of ISASS and SMISS membership. Open Orthop J. 2014;8:1–6. doi: 10.2174/1874325001408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Eligibility criteria for SIJ and SPORT studies
| SIJ studies |
| SIFI: NCT01640353 |
| INSITE: NCT01681004 |
| Inclusion criteria |
| 1. Age 21–70 years at time of screening |
| 2. Patient has lower back pain for >6 months inadequately responsive to conservative care |
| 3. Diagnosis of sacroiliac joint disruption or degenerative sacroiliitis based on ALL of the following: |
| a) Patient has pain at or close to the posterior superior iliac spine with possible radiation into buttocks, posterior thigh or groin and can point with a single finger to the location of pain (Fortin Finger Test) |
| b) Patient has at least three of five physical examination maneuvers specific for the SIJ |
| c) Patient has improvement in lower back pain numeric rating scale of at least 50% after injection of local anesthetic into affected SIJ(s) |
| d) One or more of the following: |
| i. SIJ disruption: Asymmetric SIJ widening on X-ray or CT scan or leakage of contrast on diagnostic arthrography |
| ii. Degenerative sacroiliitis: Radiographic evidence of SIJ degeneration, including sclerosis, osteophytes, subchondral cysts, or vacuum phenomenon on CT or plain film, or due to prior lumbosacral spine fusion |
| 4. Baseline Oswestry Disability Index score of at least 30% |
| 5. Baseline SIJ pain score of at least 50 on 0–100 mm visual analog scale |
| 6. Patient has signed study-specific informed consent form |
| 7. Patient has the necessary mental capacity to participate and is physically able to comply with study protocol requirements |
| Exclusion criteria |
| 1. Severe back pain due to other causes, such as lumbar disc degeneration, lumbar disc herniation, lumbar spondylolisthesis, lumbar spinal stenosis, lumbar facet degeneration, and lumbar vertebral body fracture |
| 2. Other known sacroiliac pathology such as |
| a) Sacral dysplasia |
| b) Inflammatory sacroiliitis (eg, ankylosing spondylitis or other HLA-associated spondyloarthropathy) |
| c) Tumor |
| d) Infection |
| e) Acute fracture |
| f) Crystal arthropathy |
| 3. History of recent (<1 year) major trauma to pelvis |
| 4. Previously diagnosed osteoporosis (defined as prior T-score <−2.5 or history of osteoporotic fracture) |
| 5. Osteomalacia or other metabolic bone disease 6. Chronic rheumatologic condition (eg, rheumatoid arthritis) |
| 7. Any condition or anatomy that makes treatment with the iFuse Implant System® infeasible |
| 8. Chondropathy |
| 9. Known allergy to titanium or titanium alloys |
| 10. Use of medications known to have detrimental effects on bone quality and soft-tissue healing |
| 11. Prominent neurologic condition that would interfere with physical therapy |
| 12. Current local or systemic infection that raises the risk of surgery |
| 13. Patient currently receiving or seeking worker’s compensation, disability remuneration, and/or involved in injury litigation |
| 14. Currently pregnant or planning pregnancy in the next 2 years |
| 15. Patient is a prisoner or a ward of the state |
| 16. Known or suspected drug or alcohol abuse |
| 17. Diagnosed psychiatric disease (eg, schizophrenia, major depression, personality disorders) that could interfere with study participation |
| 18. Patient is participating in an investigational study or has been involved in an investigational study within 3 months prior to evaluation for participation |
| SPORT, Intervertebral Disc Herniation: NCT00000410 |
| Inclusion criteria |
| 1. Duration of symptoms: 6 or more weeks |
| 2. Treatments tried: Non-steroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Persistent radicular pain provoked by moderate exercise, sitting, increased abdominal pressure, decreased mobility, list (scoliosis), straight leg raising |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health which makes spinal surgery too life-threatening to be an appropriate alternative, dramatic improvement with conservative care, or inability (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: A patient with a history of any invasive malignancy (except non-melanoma skin cancer) is ineligible unless he or she has been treated with a curative intent AND there has been no clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurological deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 6 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
| SPORT, Degenerative Spondylolisthesis: NCT00000409 |
| Inclusion criteria |
| 1. Duration of symptoms: 12 or more weeks |
| 2. Treatments tried: Nonsteroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Pain in low back, buttocks, or lower extremity that becomes worse with lumbar extension. Must be confirmed by evidence of central or central-lateral compression of the cauda equina by a degenerative lesion of the facet joint, disc, or ligamentum flavum on MRI, computed tomography scans, or myelograms |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health that makes spinal surgery too life-threatening to be an appropriate alternative, patient has improved dramatically with conservative care, or the patient is unable (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: Patients with a history of any invasive malignancy (except non-melanoma skin cancer) are ineligible unless they have been treated with curative intent AND have not had any clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurologic deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 12 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
| SPORT, Spinal Stenosis: NCT00000411 |
| Inclusion criteria |
| 1. Duration of symptoms: 12 or more weeks |
| 2. Treatments tried: Nonsteroidal anti-inflammatory medical therapy and physical therapy |
| 3. Surgical screening: Pain in low back, buttocks, or lower extremity that becomes worse with lumbar extension. Must be confirmed by evidence of central or central-lateral compression of the cauda equina by a degenerative lesion of the facet joint, disc, or ligamentum flavum on MRI, computed tomography scans, or myelograms |
| 4. Tests: MRI to confirm diagnosis and level(s) |
| Exclusion criteria |
| 1. Previous lumbar spine surgery |
| 2. Not a surgical candidate for any of these reasons: Overall health that makes spinal surgery too life-threatening to be an appropriate alternative, patient has improved dramatically with conservative care, or the patient is unable (for any reason) to undergo surgery within 6 months |
| 3. Possible pregnancy |
| 4. Active malignancy: Patients with a history of any invasive malignancy (except non-melanoma skin cancer) are ineligible unless they have been treated with curative intent AND have not had any clinical signs or symptoms of the malignancy for at least 5 years |
| 5. Current fracture, infection, and/or deformity (greater than 15° of lumbar scoliosis, using Cobb measure technique) of the spine |
| 6. Age less than 18 years |
| 7. Cauda Equina syndrome or progressive neurologic deficit (usually requiring urgent surgery) |
| 8. Unavailability for follow-up (planning to move, no telephone, etc) or inability to complete data surveys |
| 9. Symptoms less than 12 weeks |
| 10. Patient currently enrolled in any experimental “spine-related” study |
Abbreviations: CT, computed comography; HLA, human leukocyte antigen; INSITE, Investigation of Sacroiliac Fusion Treatment; MRI, magnetic resonance imaging; SIFI, Sacroiliac Joint Fusion with iFuse Implant System®; SIJ, sacroiliac joint; SPORT, Spine Patient Outcomes Research Trial.