Abstract
Purpose
Polymorphism in miR-146a (rs2910164) has been reported to be associated with gastric cancer risk in the Chinese population. We aimed at evaluating the relationship between rs2910164 and the clinical characteristics and outcomes in stage IB–III gastric cancer patients treated with adjuvant chemotherapy after surgery.
Materials and methods
Ninety-eight patients with stage IB–III gastric cancer treated with surgical resection followed by adjuvant chemotherapy of oxaliplatin and fluoropyrimidines were included in the analysis. Genomic DNA was extracted from peripheral blood sample of all patients. Polymerase chain reaction-based restriction fragment length polymorphism assay was used to determine the genotypes.
Results
The 2-year disease-free survival rate was 63%, and the 3-year overall survival (OS) rate was 73.4%. In dominant model, we found that rs2910164 GC + CC (G: guanine, C: cytosine) genotype carriers were less likely to develop lymph node metastasis (P=0.059). The 3-year OS was significantly different for patients with or without lymph node metastasis (89.3% vs 63.7%, P=0.015) and for patients with stage I–III disease (100.0%, 88.6%, and 56.9%; P=0.018). The 3-year OS for GC + CC carriers was significantly higher than for GG carriers (75.1% vs 66.7%, P=0.041). After the multivariant Cox regression analysis, histological grade (P=0.033, relative risk: 5.116, 95% confidence interval: 1.145–22.865) and lymph node status (P=0.031, relative risk: 6.648, 95% confidence interval: 1.191–37.118) were found to be independent prognostic factors for these patients.
Conclusion
rs2910164 could be associated with the lymph node metastasis and prognosis of Chinese gastric cancer patients treated with oxaliplatin and fluoropyrimidines after surgical resection.
Keywords: gastric cancer, miR-146a, polymorphism, clinical characteristics, prognosis
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death worldwide.1 Approximately two-thirds of gastric cancer patients were diagnosed in developing countries, especially in the People’s Republic of China, Japan, and Korea.2 After World War II, the incidence rate of gastric cancer in developed countries has been declining,3 but gastric cancer is still one of the most common malignancies in the People’s Republic of China. Many gastric cancer patients in the People’s Republic of China are diagnosed at relatively later stages and should receive adjuvant chemotherapy with or without radiation after surgical resection. Oxaliplatin combined with fluoropyrimidines (including 5-fluorouracil [5-FU], capecitabine, S-1, etc) are most commonly used chemotherapy regimens.4,5 But some patients will develop recurrence or metastasis sooner or later; the prognosis of these patients is poor with median overall survival (OS) less than 12 months.6 It is very important for us to identify biomarkers that can predict the risk of recurrence or metastasis and the response to chemotherapy.
microRNAs (miRNAs) are evolutionarily conserved, single-stranded, small noncoding RNAs.7 It has been estimated that miRNAs regulate expression of approximately 10%–30% human genes by binding to the 3′ untranslated region of their target messenger RNAs (mRNAs).8 More than half of the known miRNAs are located in cancer-associated genomic regions.9 They play an important role in various biologic or pathologic processes, such as cell proliferation, differentiation, apoptosis, and tumor initiation and progression.10 Polymorphism or mutation in miRNA sequence or in their target gene sequence may influence the maturation or miRNA–mRNA interaction and thereby the expression level of their target genes.11 Some studies have shown that a G> C (G: guanine, C: cytosine) polymorphism in miR-146a precursor (rs2910164) results in increased level of mature miR-146a.12,13 rs2910164 has been reported to be associated with gastric cancer risk in the Chinese population.14,15 But it is still needs to be verified if rs2910164 could predict the prognosis of gastric cancer patients treated with oxaliplatin and fluoropyrimidines.
In our study, we enrolled 98 stage IB–III gastric cancer patients who received oxaliplatin and fluoropyrimidines as adjuvant chemotherapy, and explored the association between rs2910164 and the clinical characteristics and outcomes of these patients.
Materials and methods
Patients and clinical samples
This was a retrospective biomarker study. It was approved by the Ethics Committee of Jiangxi Cancer Hospital. Inclusion criteria were:
patients aged 18–75, with histologically confirmed gastric adenocarcinoma;
according to American Joint Committee on Cancer Staging classification (7th ed, 2010), patients were at stage IB–III with tumors resectable when diagnosed;
patients at stage IB had one or several risk factors, such as poor differentiation, vascular invasion, neural invasion etc;
ECOG (Eastern Cooperative Oncology Group) performance status 0–2;
life expectancy of at least 3 months;
patients received surgical resection and adjuvant chemotherapy of oxaliplatin and fluoropyrimidines;
adequate bone marrow reserve as evidenced by absolute granulocyte count ≥1,500/μL and platelets ≥100,000/μL; adequate hepatic function as evidenced by serum bilirubin ≤ institutional upper limit of normal (IULN), serum transaminases (serum glutamic oxaloacetic transaminase or serum glutamic pyruvic transaminase) ≤2.5× IULN; measured or calculated creatinine clearance of >60 mL/min (utilizing G–K equation); cardiac ejection fraction within the institutional range as measured by echocardiogram or multigated acquisition scan;
patients gave written informed consent and had to be accessible for treatment and follow-up.
Exclusion criteria were:
patients at stage IA who received no adjuvant chemotherapy;
patients at stage IV with tumors unresectable;
patients received neoadjuvant chemotherapy ± radiotherapy;
patients received postoperative radiotherapy.
Between January 2011 and December 2012, 98 eligible patients were included in our study.
Chemotherapy regimens included 1) 84 patients who received mFOLFOX6: oxaliplatin 85 mg/m2 intravenous (IV) over 2 hours on day 1+ Leucovorin 400 mg/m2 IV over 2 hours on day 1+5-FU 400 mg/m2 IV bolus on day 1, 5-FU 2,400 mg/m2 continuous intravenous infusion 46 hours, every 2×24 weeks; 2) eight patients received XELOX: oxaliplatin 130 mg/m2 IV over 2 hours on day 1+ capecitabine 1,000 mg/m2 twice daily on day 1–14, every 3×24 weeks; 3) six patients received SOX: oxaliplatin 130 mg/m2 IV over 2 hours on day 1+ S-1 40-60 mg twice daily on day 1–14, every 3×24 weeks.
All patients were followed up according to National Comprehensive Cancer Network guidelines (every 3 months or whenever they experienced suspicious symptoms and signs indicating recurrence or metastasis). Follow-up included a history and physical examination, complete blood count, chemistry profile, and abdominal computed tomography with contrast. DFS (disease-free survival) was defined as time from the date of diagnosis to the date of recurrence or metastasis. OS was defined as time from the date of diagnosis to the date of death from any reason or last follow-up. The median follow-up time was 24 months.
DNA extraction and genotyping
The blood samples were collected before adjuvant chemotherapy. Genomic DNA was extracted from the peripheral blood sample of all patients. A 5 mL blood sample was collected from each patient. Genomic DNA was isolated by standard proteinase K digestion and phenol–chloroform extraction from the blood samples. DNA purity and concentration were determined by spectrophotometric absorbance measurements at 260 and 280 nm using an ultraviolet spectrophotometer. TE buffer was used as control.
Genotypes of rs2910164 were determined by polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP). Primers were 5′-CATGGGT TGTGTCAGTGTCAGAGCT-3′ (sense) and 5′-CCTT CAGAGCCTGAGACTCTGCC-3′ (antisense). PCR was done in a total volume of 10 μL, containing 5 ng genomic DNA, 2.5 pmol each primer, 4 μL double distilled H2O, and 5 μL PCR Mix. PCR consisted of 35 thermal cycles at 95°C for 30 seconds, 62°C for 40 seconds, and 72°C for 45 seconds, using a PTC 200 Thermal Cycler (Bio-Rad, Hercules, CA, USA). For RFLP analysis, PCR products were digested with SacI (5 U) at 37°C overnight, separated on a 3.0% agarose gel, and subsequently stained with ethidium bromide.16 A negative control (no DNA template) was added in each assay to monitor PCR contamination. A 10% masked random sample of subjects was tested twice by different persons, and the results were concordant for all the masked duplicate sets.
Statistical analysis
SPSS version 18.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The descriptive analysis of rs2910164 genotypes and clinicopathological features was expressed both in absolute values and percentages. The association between the genotypes and clinicopathological features was analyzed based on chi-square or Fisher’s exact probability tests. The 2-year DFS rates and 3-year OS rates were estimated by the Kaplan–Meier product limit method for each of the different genotypes and expressed in percentages. Comparisons were made with the log-rank test. Hazard ratios of recurrence/metastasis and death with 95% confidence intervals (CI) were estimated by using the Cox model. All statistical tests are two-sided tests, and P<0.05 was considered significant.
Results
Patient characteristics
The median age was 54 years (range: 19–73 years). More patients (n=69, 70.4%) were younger than 60 years. Males and females accounted for 70.4% (n=69) and 29.6% (n=29), respectively. Primary lesion was located in cardia for only eight patients (8.2%). Twenty-seven (27.6%) patients showed well to moderately differentiated cells, and 71 (72.4%) patients showed poor differentiation or signet ring cells. Most patients were in T3–4 stage (n=79, 80.6%) or had regional lymph node metastasis (n=69, 70.4%). Eleven (11.2%), 26 (26.5%), and 61 (62.2%) patients were in stage IB, II, and III, respectively. The 2-year DFS rate was 63%, and the 3-year OS rate was 73.4% for all patients enrolled.
Genotypes and clinicopathological features
The frequency of each rs2910164 genotype was 14.3% (14 specimens) for GG variant, 46.9% (46 specimens) for GC variant, and 38.8% (38 specimens) for CC variant. The results indicated that the risk of lymph node metastasis for GG carriers was higher than that for GC + CC carriers (P=0.059). rs2910164 genotypes were not associated with other clinicopathological features (Table 1).
Table 1.
rs2910164 genotypes and clinicopathological features
| Variables | Codominant model
|
Dominant model
|
Recessive model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GG, N=14 | GC, N=46 | CC, N=38 | P-value | GG, N=14 | GC + CC, N=84 | P-value | GG + GC, N=60 | CC, N=38 | P-value | |
| Age (years) | ||||||||||
| <60 | 8 | 34 | 27 | 8 | 61 | 42 | 27 | |||
| ≥60 | 6 | 12 | 11 | 0.482 | 6 | 23 | 0.240 | 18 | 11 | 0.911 |
| Sex | ||||||||||
| Male | 10 | 30 | 29 | 10 | 59 | 40 | 29 | |||
| Female | 4 | 16 | 9 | 0.538 | 4 | 25 | 1.000 | 20 | 9 | 0.308 |
| Location | ||||||||||
| Noncardia | 11 | 43 | 36 | 11 | 79 | 54 | 36 | |||
| Cardia | 3 | 3 | 2 | 0.144 | 3 | 5 | 0.085 | 6 | 2 | 0.478 |
| Differentiation | ||||||||||
| Well to moderate | 4 | 15 | 8 | 4 | 23 | 19 | 8 | |||
| Poor/signet-ring | 10 | 31 | 30 | 0.496 | 10 | 61 | 1.000 | 41 | 30 | 0.354 |
| Depth of invasion | ||||||||||
| T1-2 | 3 | 12 | 4 | 3 | 16 | 15 | 4 | |||
| T3-4 | 11 | 34 | 34 | 0.195 | 11 | 68 | 1.000 | 45 | 34 | 0.115 |
| LN metastasis | ||||||||||
| N0 | 1 | 17 | 11 | 1 | 28 | 18 | 11 | |||
| N+ | 13 | 29 | 27 | 0.101 | 13 | 56 | 0.059 | 42 | 27 | 0.911 |
| Anatomic stage | ||||||||||
| I-II | 3 | 21 | 13 | 3 | 34 | 24 | 13 | |||
| III | 11 | 25 | 25 | 0.222 | 11 | 50 | 0.238 | 36 | 25 | 0.565 |
Abbreviations: N, the absolute number of patients; G, guanine; C, cytosine; LN, lymph node.
Genotypes and clinical outcomes
We further investigated whether there was significant difference in DFS and OS among patients with different clinicopathological features and genotypes. The Kaplan–Meier analysis with log-rank test for DFS indicated that T stage and Anatomic stage were significantly associated with 2-year DFS rate. The 2-year DFS rate for patients with T1–2 was significantly higher than those in T3–4 (88.2% vs 56.5%, P=0.023). The 2-year DFS rate was significantly different for patients in stage IB, II, and III (100%, 72.7%, and 51.0%; P=0.019). N stage revealed a borderline significant association with DFS (P=0.095). No significant association was detected between rs2910164 genotypes and DFS.
N stage (P=0.015) and Anatomic stage (P=0.018) were also significantly associated with 3-year OS rate (Figures 1 and 2). Vascular invasion (P=0.092) and neural invasion (P=0.071) showed borderline significant association with OS. The 3-year OS rate of GC + CC genotype carriers was significantly higher than that of GG carriers (75.1% vs 66.7%, P=0.041) (Figure 3). Results are shown in Tables 2 and 3.
Figure 1.
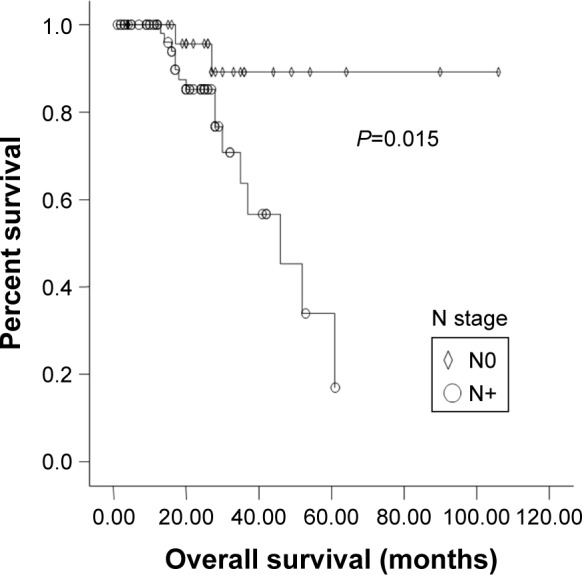
Kaplan-Meier graph of overall survival (OS) for patients with or without lymph node metastasis.
Abbreviations: N, regional lymph nodes; N0, without lymph node metastasis; N+, with lymph node metastasis.
Figure 2.
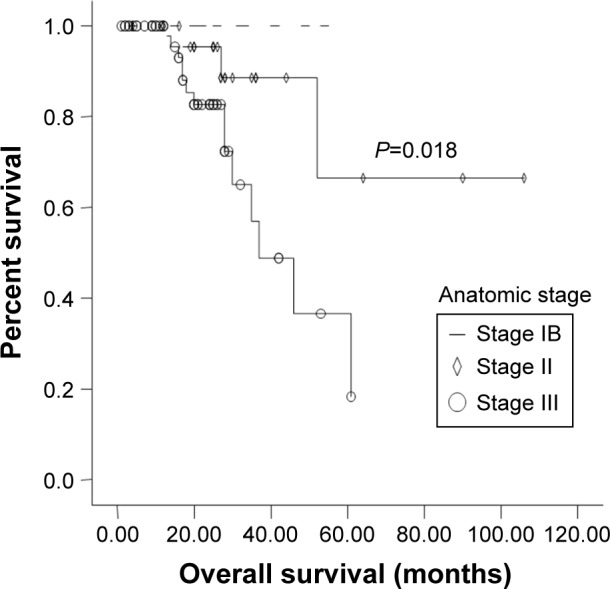
Kaplan-Meier graph of overall survival (OS) for patients at stage IB, II and III.
Note: The dotted line was the survival curve of patients with stage IB.
Figure 3.
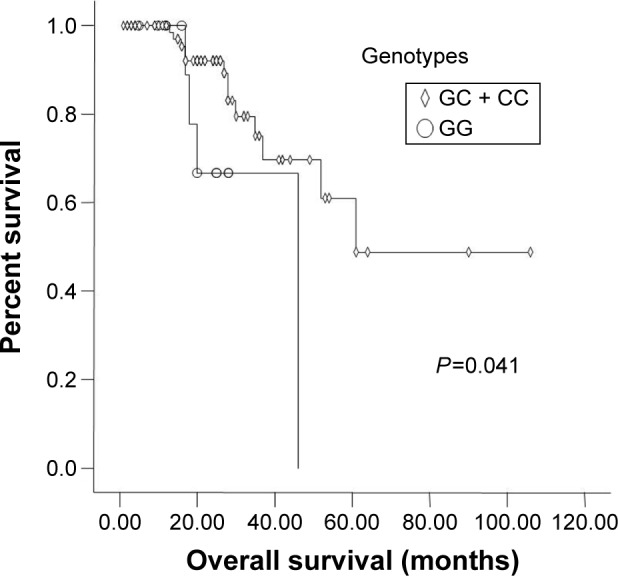
Kaplan-Meier graph of overall survival for different genotypes.
Table 2.
Clinicopathological features and survival
| Variables | 2-year DFS (%) | P-value | 3-year OS (%) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 66.8 | 77.3 | ||
| ≥60 | 52.1 | 0.155 | 61.5 | 0.058 |
| Sex | ||||
| Male | 61.8 | 70.1 | ||
| Female | 65.5 | 0.395 | 67.9 | 0.282 |
| Location | ||||
| Noncardia | 50.0 | 75 | ||
| Cardia | 61.9 | 0.638 | 74.4 | 0.853 |
| Differentiation | ||||
| Well to moderate | 45.0 | 80.8 | ||
| Poorly/signet-ring | 45.9 | 0.313 | 70.8 | 0.220 |
| Depth of invasion | ||||
| T1–2 | 88.2 | 100.0 | ||
| T3–4 | 56.5 | 0.023 | 67.1 | 0.109 |
| LN metastasis | ||||
| N0 | 77.3 | 89.3 | ||
| N+ | 56.3 | 0.118 | 63.7 | 0.015 |
| Anatomic stage | ||||
| IB | 100.0 | 100.0 | ||
| II | 72.7 | 88.6 | ||
| III | 51.0 | 0.019 | 56.9 | 0.018 |
| Vascular invasion | ||||
| No | 61.7 | 78.7 | ||
| Yes | 56.0 | 0.531 | 44.4 | 0.092 |
| Neural invasion | ||||
| No | 64.3 | 78.1 | ||
| Yes | 53.0 | 0.201 | 29.2 | 0.071 |
Abbreviations: LN, lymph node; DFS, disease free survival rate; OS, overall survival rate.
Table 3.
miR-146a genotypes and survival
| Variables | 2-year DFS (%) | P-value | 3-year OS (%) | P-value |
|---|---|---|---|---|
| Codominant model | l | |||
| GG | 53.8 | 66.7 | ||
| GC | 67.1 | 75.5 | ||
| CC | 62.8 | 0.608 | 76.4 | 0.106 |
| Dominant model | ||||
| GG | 53.8 | 66.7 | ||
| GC + CC | 64.8 | 0.356 | 75.1 | 0.041 |
| Recessive model | ||||
| GG + GC | 63.8 | 72.4 | ||
| CC | 62.8 | 0.922 | 76.4 | 0.991 |
Abbreviations: G, guanine; C, cytosine; DFS, disease free survival rate; OS, overall survival rate.
After the multivariant Cox regression analysis, differentiation (P=0.033, relative risk: 5.116, 95% CI: 1.145–22.865) and lymph node metastasis (P=0.031, relative risk: 6.648, 95% CI: 1.191–37.118) were found to be independent prognostic factors for these patients.
Discussion
Some studies have investigated the relationship between miR-146a rs2910164 polymorphism and susceptibility of gastric cancer; most of them were performed in Chinese or Asian population. Recently, a meta-analysis of these studies confirmed that the G allele was associated with increased risk of gastric cancer, especially in the Asian population.14 In one meta-analysis, rs2910164 was found to be associated with increased gastric cancer risk, particularly in high quality studies with small sample size consisting of Caucasians.15
It has been reported that polymorphism in miR-146a and the expression level of miR-146a were associated with the prognosis of oropharyngeal cancer, bladder cancer, glioma, and prostate cancer.16–19 However, only a few studies have investigated the relationship of rs2910164 or miR-146a and the clinical features and outcomes of gastric cancer. In our study, we enrolled patients at stage IB—III who received adjuvant chemotherapy after surgical resection. The results showed that compared with GC + CC carriers, GG carriers were more likely to develop regional lymph node metastasis. The 3-year OS rate of GC + CC carriers was significantly higher than that for GG carriers.
Kogo et al20 investigated the clinical significance of miR-146a in gastric cancer cases. In clinical samples, they found that miR-146a level was significantly reduced in GG genotype compared with the CC genotype, and lower levels of miR-146a were associated with lymph node metastasis and venous invasion (P,0.05). Moreover, a lower level of miR-146a was an independent prognostic factor for OS (P=0.003). The median OS of patients in the miR-146a low- and highexpression groups was 1.1 and 3.1 years, respectively. Okubo et al21 investigated the association between rs11614913 in miR-196a2, rs2910164 in miR-146a, and rs3746444 in miR-499 and the clinicopathological characteristics and OS in gastric cancer patients. They found that among patients younger than 65 years of age, rs2910164 CG + GG genotype tended to be associated with worse OS than CC genotype (P=0.09). These results are consistent with those from our study, which indicated that patients with C allele usually had less aggressive tumors and better prognosis.
Gene encoding miR-146a is located on chromosome 5.22 Polymorphism (rs2910164) within the sequence of miR-146a precursor leads to a change from a G:U (uridine) pair to a C:U mismatch in its stem region. In tumor cell lines,12 researchers found that compared with the G allele, the amount of pre- and mature miR-146a from the C allele was reduced 1.9- and 1.8-fold, respectively. In clinical samples of gastric cancer, prostate cancer, thyroid cancer, liver cancer, breast cancer, and ovarian cancer, it also had been proved that rs2910164 was associated with tumor initiation and progression through influencing the expression level of miR-146a.12,13,23
miR-146a influences the biologic features by regulating the expression of the target genes such as BRMS1, TRAF6, ROCK1, CXCR4, BRCA1/2, etc. Kogo et al20 proved that expression of miR-146a significantly inhibited the cell’s capability for migration and invasion by suppressing EGFR (epidermal growth factor receptor) and IRAK1 (interleukin-1 receptor-associated kinase 1) level. EGFR and IRAK1 had been confirmed to be targets of miR-146a. EGFR promotes tumor cell growth by activating Raf/MEK/ERK and PI3K/PDK1/Akt signaling pathways.24 IRAK1 and subsequent NF-κB has been proved to be associated with poor prognosis and invasion in gastric cancer.25,26 Okubo et al21 demonstrated that rs2910164 CC and CG genotypes were associated with increased susceptibility to CIHM (CpG island hypermethylation) of DAP-kinase. It has been proved that CIHM is an important mechanism in gene silencing, and CIHM of tumor suppressor genes is also highly involved in gastric carcinogenesis.27 All the results from our study and from the literature indicated that by regulating the expression level of miR-146a, rs2910164 might influence the biologic behavior of tumor cells and thereby the prognosis of gastric cancer patients.
Previous studies enrolled both early-stage and metastatic gastric cancer patients, and the chemotherapy regimens were inconsistent. However, all patients in our study received adjuvant chemotherapy of oxaliplatin and fluoropyrimidines. Since GG carriers had a poorer prognosis than GC + CC carriers, we assumed that GG carriers may be less sensitive to platinum-based chemotherapy. BRCA1 and BRCA2 are also target genes of miR-146a. Gu et al28 found that in BRCA1/2 wild-type ovarian cancer patients receiving platinum-based chemotherapy, upregulation of miR-146a was associated with better prognosis. They demonstrated that upregulation of miR-146a reduced the expression level of BRCA1/2 and then impeded repair-related pathway and increased the sensitivity of platinum. We are now doing further research in gastric cancer patients and cell lines to clarify if rs2910164 is associated with platinum sensitivity in vivo and in vitro.
The allele frequency of rs2910164 G has been found to be different across ethnicities, ranging from 0.362 in an Asian population to 0.774 in a Caucasian population.29 And since most studies investigating rs2910164 and gastric cancer were conducted in Chinese and Asian population, data should be extrapolated to other ethnic groups cautiously. There are some other limitations of our study. First, this was a retrospective study which enrolled only stage IB–III patients who received adjuvant chemotherapy, so selection bias might exist. Second, the sample size was relatively small, prospective large-scale studies are needed to confirm the conclusions. Finally, since we did not explore the mechanisms, further studies on the biological mechanisms are warranted.
Conclusion
In conclusion, for the first time in gastric cancer patients at stage IB–III who received adjuvant oxaliplatin and fluoropyrimidines, we demonstrated that GG carriers were more likely to develop regional lymph node metastasis, and the 3-year OS rate of GC + CC carriers was significantly higher than GG carriers. These results indicated that rs2910164 may influence the biologic features and prognosis of these patients. However, since the sample size was small and we did not explore the mechanism, further investigations are needed to verify these results.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed November 4, 2014]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–1425. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Ma N, Wang F, et al. Results of a randomized and controlled clinical trial evaluating the efficacy and safety of combination therapy with Endostar and S-1 combined with oxaliplatin in advanced gastric cancer. Onco Targets Ther. 2013;25(6):925–929. doi: 10.2147/OTT.S46487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Berezikov E, Guryev V, van de Belt J, et al. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120(1):21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353(17):1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 10.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 11.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10(6):389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jazdzewski K, Murray EL, Franssila K, et al. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2008;105(20):7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Feng NH, Li PC, et al. A functional polymorphism in Pre-miR-146a gene is associated with prostate cancer risk and mature miR-146a expression in vivo. Prostate. 2010;70(5):467–472. doi: 10.1002/pros.21080. [DOI] [PubMed] [Google Scholar]
- 14.Fu B, Song P, Lu M, et al. The association between miR-146a gene rs2910164 polymorphism and gastric cancer risk: a meta-analysis. Biomed Pharmacother. 2014;68(8):923–928. doi: 10.1016/j.biopha.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Xie WQ, Tan SY, Wang XF. MiR-146a rs2910164 polymorphism increases risk of gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20(41):15440–15447. doi: 10.3748/wjg.v20.i41.15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Zhou ZY, Chen JG, et al. Effect of miR-146a polymorphism on biochemical recurrence risk after radical prostatectomy in southern Chinese population. Genet Mol Res. 2014;13(4):10615–10621. doi: 10.4238/2014.December.18.3. [DOI] [PubMed] [Google Scholar]
- 17.Guan X, Sturgis EM, Song X, et al. Pre-microRNA variants predict HPV16-positive tumors and survival in patients with squamous cell carcinoma of the oropharynx. Cancer Lett. 2013;330(2):233–240. doi: 10.1016/j.canlet.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Chu H, Li P, et al. Genetic variants in miRNAs predict bladder cancer risk and recurrence. Cancer Res. 2012;72(23):6173–6182. doi: 10.1158/0008-5472.CAN-12-0688. [DOI] [PubMed] [Google Scholar]
- 19.Permuth-Wey J, Thompson RC, Burton NL, et al. A functional polymorphism in the pre-miR-146a gene is associated with risk and prognosis in adult glioma. J Neurooncol. 2011;105(3):639–646. doi: 10.1007/s11060-011-0634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kogo R, Mimori K, Tanaka F, et al. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17(13):4277–4284. doi: 10.1158/1078-0432.CCR-10-2866. [DOI] [PubMed] [Google Scholar]
- 21.Okubo M, Tahara T, Shibata T, et al. Association between common genetic variants in pre-microRNAs and the clinicopathological characteristics and survival of gastric cancer patients. Exp Ther Med. 2010;1(6):1035–1040. doi: 10.3892/etm.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labbaye C, Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J Hematol Oncol. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Ambrosone CB, DiCioccio RA, et al. A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis. 2008;29(10):1963–1966. doi: 10.1093/carcin/bgn172. [DOI] [PubMed] [Google Scholar]
- 24.McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee BL, Lee HS, Jung J, et al. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11(7):2518–2525. doi: 10.1158/1078-0432.CCR-04-1282. [DOI] [PubMed] [Google Scholar]
- 26.Yamanaka N, Morisaki T, Nakashima H, et al. Interleukin 1beta enhances invasive ability of gastric carcinoma through nuclear factor-kappaB activation. Clin Cancer Res. 2004;10(5):1853–1859. doi: 10.1158/1078-0432.ccr-03-0300. [DOI] [PubMed] [Google Scholar]
- 27.Tahara T, Shibata T, Nakamura M, et al. Association between cyclin D1 polymorphism with CpG island promoter methylation status of tumor suppressor genes in gastric cancer. Dig Dis Sci. 2010;55(12):3449–3457. doi: 10.1007/s10620-010-1206-5. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Zhang M, Peng F, et al. The BRCA1/2-directed miRNA signature predicts a good prognosis in ovarian cancer patients with wild-type BRCA1/2. Oncotarget. 2015;6(4):2397–2406. doi: 10.18632/oncotarget.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian T, Xu Y, Dai J, et al. Functional polymorphisms in two pre-microRNAs and cancer risk: a meta-analysis. Int J Mol Epidemiol Genet. 2010;1(4):358–366. [PMC free article] [PubMed] [Google Scholar]


