Abstract
Following early embryonic germ cell migration, oocytes are surrounded by somatic cells and remain arrested at diplotene stage until luteinizing hormone (LH) surge. Strict regulation of both meiotic arrest and meiotic resumption during dormant stage are critical for future fertility. Inter-cellular signaling system between the somatic compartment and oocyte regulates these meiotic events and determines the follicle quality. As well as the collected number of eggs, their qualities are also important for in vitro fertilization (IVF) outcome. In spontaneous and IVF cycles, germinal vesicle (GV)–stage oocytes, premature GV breakdown, and persistence of first meiotic arrest limit the reproductive performance. Likewise, both women with premature ovarian aging and young cancer women are undergoing chemoradiotherapy under the risk of follicle loss because of unregulated meiotic events. Understanding of oocyte meiotic events is therefore critical for the prevention of functional ovarian reserve. High levels of cyclic guanosine monophophate (cGMP), cyclic adenosine monophophate (cAMP) and low phosphodiesterase (PDE) 3A enzyme activity inside the oocyte are responsible for maintaining of meiotic arrest before the LH surge. cGMP is produced in the somatic compartment, and natriuretic peptide precursor C (Nppc) and natriuretic peptide receptor 2 (Npr2) regulate its production. cGMP diffuses into the oocyte and reduces the PDE3A activity, which inhibits the conversion of cAMP to the 5′AMP, and cAMP levels are enhanced. In addition, oocyte itself has the ability to produce cAMP. Taken together, accumulation of cAMP inside the oocyte induces protein kinase activity, which leads to the inhibition of maturation-promoting factor and meiotic arrest also continues. By stimulating the expression of epidermal growth factor, LH inhibits the Nppc/Npr2 system, blocks cGMP synthesis, and initiates meiotic resumption. Oocytes lacking the functional of this pathway may lead to persistence of the GV oocyte, which reduces the number of good quality eggs. Selective regulation of somatic cell signals and oocyte meiotic events enhance progress in fertility preservation methods, which may give us the opportunity to prevent follicle loss in prematurely aging women and young women with cancer are undergoing chemoradiotherapy.
Keywords: oocyte meiosis, fertility preservation, cGMP, cAMP, PDEs, Nppc/Npr2
Introduction
Oocyte–somatic cell complex is not only an extraordinary cell in the female body but also it has the ability to maintain meiotic arrest and initiates meiotic resumption. From an evolutionary perspective, prophase I arrest is preserved in many animal species including human. Following primordial germ cell (PGC) migration from yolk sack to the genital ridge, oocytes go into first meiotic division and remain arrested at the dictyate/diplotene stage of prophase I until luteinizing hormone (LH) surge. Duration of dormant stage in human and animals is different. In human beings, completion of the primary arrest period takes place within the years. In contrast, the length of this period in others animals has been reported as months, days, and minutes.1–3 During the diplotene-stage arrest, oocyte accumulates a stockpile of organic and inorganic molecules, which are necessary for successful fertilization and implantation. Moreover, diplotene-stage oocytes are surrounded by pregranulosa cells, which are necessary for the initiation of meiotic division and prevent apoptotic follicle loss. Following LH surge, growing oocytes complete first meiotic division and undergo second meiotic arrest in metaphase stage until fertilization. The stage of second meiotic arrest varies between the species.4 Interestingly, the secondary meiotic arrest does not take place in some animals.4 After the fertilization, oocytes are released from second arrest and embryonic development begins.
Female infertility is not exclusively because of reduced oocyte number but is also the result of defective oocyte–somatic cells interaction, which reduces the fertilization and implantation capacity and is the reason for poor pregnancy outcome. Generation of new oocytes from embryonic stem cells has not been reported yet in humans. Hence, we are not able to prevent most of the infertility problems secondary to poor follicular development. Progress in the knowledge of the somatic cells–oocyte interaction has improved the in vitro fertilization embryo transfer (IVF-ET) outcome. By selectively regulating somatic cell signaling, it is possible to solve some problems related to female infertility. Previous studies and reviews have discussed in detail the molecular and hormonal control of meiotic arrest and meiotic resumption.5–14 In this review, we will try to find logical replies for three questions: (i) Which signaling molecules are responsible for maintaining first meiotic arrest? (ii) How does LH surge initiate meiotic resumption? (iii) Does the establishment of selective regulation methods to prevent follicle loss increase progress in fertility preservation technologies in prematurely aging women and young cancer patients who are undergoing chemoradiotherapy? Before start answering the questions, we will make a brief description about the oocyte–somatic cell interactions.
Insight into the Oocyte–somatic Cell Interactions
The strong relations between the granulosa cells and oocytes begin in the early embryogenesis and continue until fertilization. Following arrival in the genital ridge, PGCs are surrounded by the pregranulosa cells. These somatic cells prevent apoptosis of PGCs, and first meiotic division begins. Moreover, somatic pregranulosa cells protect PGCs from ovarian somatic signals, which disturb the proper establishment of the PGCs. Mural granulosa cells are the main sources of inhibitory signals responsible for first meiotic arrest,15,16 but orchestration by the oocyte is also required.17,18 If mural granulosa cells are removed from the cumulus granulosa cells, oocytes undergo meiotic resumption,19,20 suggesting the inhibitory action of mural granulosa cells. The size of antral space determines the size and volume of growing follicle in many species. While physically large animals have larger follicles, physically small animals have smaller follicles.21 Following antrum expansion, the follicle gradually reaches its full size and volume and somatic cells divide into mural and cumulus cells.22,23 After the establishment of the mural and somatic cells compartments, the oocyte acquires the ability to release from dormant stage and undergoes meiotic resumption.17,22,23 When preovulatory LH surge occurs, germinal vesicle (GV) of the dormant oocyte is broken, which leads to the second stage of first meiotic division.24 Germinal vesicle breakdown (GVBD) can be detected microscopically,24 and it is used for evaluating the follicle stage and quality in embryology laboratories. Following ovulation, while mural granulosa cells remain within the follicle wall, cumulus granulosa cells continue to surround oocyte. Until fertilization, cumulus granulosa cells provide energy for oocyte and maintain second meiotic arrest within the ampullary part of the tubes.
Presence of the cell-to-cell connections between the granulosa cells and oocyte (mural to mural, cumulus to cumulus, mural to cumulus, cumulus to oocyte) is crucial for transporting the somatic signals and maintaining first meiotic arrest. These cytoplasmic connections are referred to as a gap junctions. Communication between two different cell types (cumulus to oocyte) is realized by heterologous gap junctions. On the other hand, homologous gap junctions are present between two similar cell types such as cumulus cells to mural cells. They are the most important intercellular communication routes between the oocyte and somatic cells. Each channel comprises connexin proteins and lets the diffusion of many organic and inorganic regulatory substances. Accordingly, mice lacking connexin protein are infertile.25
In the next section, we will review the function and possible roles of several key factors in maintaining oocyte meiotic arrest, such as cyclic guanosine monophosphate (cGMP), cyclic adenosine monophosphate (cAMP), oocyte maturation inhibitor (OMI), phosphodiesterase enzymes (PDEs), maturation-promoting factor (MPF), protein phosphatases (PPs), and natriuretic peptide precursor C (Nppc), and natriuretic peptide receptor 2 (Npr2) system, which are expressed or regulated by somatic cells and/or oocyte (Table 1).
Table 1.
Observations supporting the role of somatic signaling pathways responsible for prophase I arrest and resumption of meiosis in oocytes.
| STUDY OBSERVATIONS | INTERPRETATION OF DATA | PUBLISHED STUDIES |
|---|---|---|
| Prophase I arrested oocyte contains a large nucleus covered by a nuclear envelope called as the germinal vesicle (GV). | First detectable sign of meiotic resumption is GV breakdown (GVBD). First meiotic division is completed by extruding a first polar body. | 20 |
| High levels of cAMP inside the oocyte are essential for first meiotic arrest. Somatic cell-derived cGMP contributes to the maintenance of elevated intra-oocyte cAMP levels. | Sufficient amount of cGMP synthesis by somatic cells are essential for maintaining prophase I arrest in fully-grown oocytes. | 26,29 |
| Regulation of meiotic events in oocytes are strictly controlled by changes in the activity of Cdk1–cyclin B complex also known as maturation or meiosis promoting factor (MPF). | An increase in Cdk1–cyclin B activity stimulates to progress into metaphase of meiosis I. | 56,59 |
| Prophase I arrested oocytes do not resume meiosis until LH surge. | LH surge initiates the meiotic resumption by blocking somatic cell derived inhibitory signals. | 112 |
| Fully-grown oocytes resume meiosis spontaneously when exit from the follicle and cultured in vitro. | Mural and cumulus granulosa cells prevent the meiotic resumption. | 20 |
| Functional LH receptors on mural cells make them main target for LH action. LH suppresses somatic cells-derived inhibitory signals. | Due to absence of functional LH receptors on the cumulus cells and oocyte LH does not have a direct action on them. LH action on cumulus cells and oocyte are indirect rather than direct. | 99,113,114 |
| Following the preovulatory LH surge catalytic activity of oocyte-specific PDE3A increases. | PDE3A hydrolyses cAMP into the 5′AMP inside the oocyte and initiates meiotic resumption. | 92 |
| LH surge mediates the phosphorylation of Cx43 and Cx37 leading to the disruption of the gap junctional communication. LH surge increases the expression of MAPK and phosphorylates gap junctions. Carbenoxolone, a gap junction blocker, reduces cGMP concentrations inside the oocyte. | Granulosa cells derived cGMP are transmitted into the oocyte via dephosphorylated gap junctions. | 15,42,106,107,110 |
| Mural granulosa cells express Nppc and cumulus granulosa cells express Nppc receptor 2 (Npr2). cGMP production in somatic cells is regulated by Nppc/Npr2 system. | Nppc/Npr2 system is crucial for maintaining oocyte meiotic arrest. | 29,30,82 |
| Nppc/Npr2 system responsible for maintaining the high cGMP and cAMP concentration inside the oocyte during prophase I arrest. | Oocytes having Nppc/Npr2 mutation undergo early meiotic resumption. | 29,91 |
| Both OMI and Nppc originated from outer somatic cells and exert their effects on cumulus cells. The molecular weight of the Nppc and OMI ar~2000 dalton. | Nppc and OMI may be the same molecule responsible for prophase I arrest. | 29,89,97 |
| Ovulatory dose of hCG in patient undergoing IVF leads to decline in follicular fluid CNP concentration. | hCG suppresses follicular fluid CNP secretion. | 11 |
(i) Which signaling molecules are responsible for maintaining first meiotic arrest?
Somatic Cell cGMP Production and its Transport into the Oocyte
Although oocyte has the ability to synthesize endogenous cAMP from its sources, maintenance of successful meiotic arrest is required in addition to receiving inhibitory signals from the somatic compartment. One of the main inhibitory products of somatic cells is cGMP, which maintains cAMP levels within the oocyte that is responsible for prophase I arrest.26 When somatic cell-free oocytes or cumulus cell-enclosed oocytes were cultured with 8-Br-cGMP, a cGMP analog, inhibition of GVBD occurred, supporting the inhibitory role of cGMP on meiotic events.27
cGMP is generated by molecular interaction between the cumulus and mural granulosa cells.28,29 Oocyte-derived paracrine factors (ODPFs) regulate the production of cGMP by somatic cells. There is a close relationship between the somatic cell-derived cGMP and intra-oocyte cAMP levels. Inhibitory cGMP signals from the somatic compartment reach the oocyte via gap junctions.16,30 A relatively hypoxic environment within the cumulus cell-enclosed oocytes induces the cGMP synthesis by increasing the production of hypoxanthine and inosine substrates.31
Before the LH surge, accumulation of the cGMP within oocyte inhibits the enzymatic activity of PDE3A26,30 and conversion of the cAMP into the 5′AMP is blocked. As a result, high cAMP concentration within the oocyte inhibits nuclear membrane dissolution.26,30,32 Following LH surge, phosphorylation of the heterologous gap junctions inhibits the diffusion of cGMP into the oocyte.26,30 Moreover, LH peak decreases the cGMP synthesis in the granulosa cells, suggesting dual effect of the LH peak on cGMP regulation.26,30 Both suppression of the somatic production of cGMP and inhibition of its transport into the oocyte lead to an increase in oocyte-specific PDE3A activity.30 Release of the PDE3A enzyme from the inhibitory effect of cGMP leads to an increase in its enzymatic activity and a decrease in cAMP levels within the oocyte and initiates meiotic resumption.
Maintenance of Elevated cAMP Levels Inside the Oocyte
Several central and peripheral molecules exert their effects on reproductive tissue by triggering intracellular cAMP. It is exactly not known how this single molecule can regulate the activities of several enzymes and expression of some proteins within the oocyte. Intra-oocyte cAMP level is mainly responsible for maintaining prophase I meiotic arrest. Similarly, analogs of cAMP, such as dibutyryl-cAMP and 8-bromo-cAMP, increase the intra-oocyte cAMP levels and block follicle maturation.6,33,34 Main production site of cAMP is the oocyte. As shown in Figure 1A and B, cAMP is generated by the oocyte adenylyl cyclase (AC). The G-protein-coupled receptors 3 and 12 are present in the oocyte plasma membrane and regulate the activity of AC enzyme.17,18,35–37 GPR3 induces Gs expression and activates the AC enzyme inside the oocyte.17 When somatic cell-free mouse oocytes were cultured with forskolin, the activity of AC enzyme increases. This leads to an elevation of cAMP synthesis, and the onset of nuclear envelope dissolution is delayed.38 However, maintenance of meiotic arrest is required to sustain high levels of cAMP and cGMP inside the oocyte. Somatic compartment cells provide cGMP for oocyte.39,40 By inhibiting PDE3A, cGMP increases intra-oocyte cAMP levels. In agreement, cAMP levels inside the oocyte decrease when oocytes exit from the follicle,20 confirming somatic contribution.
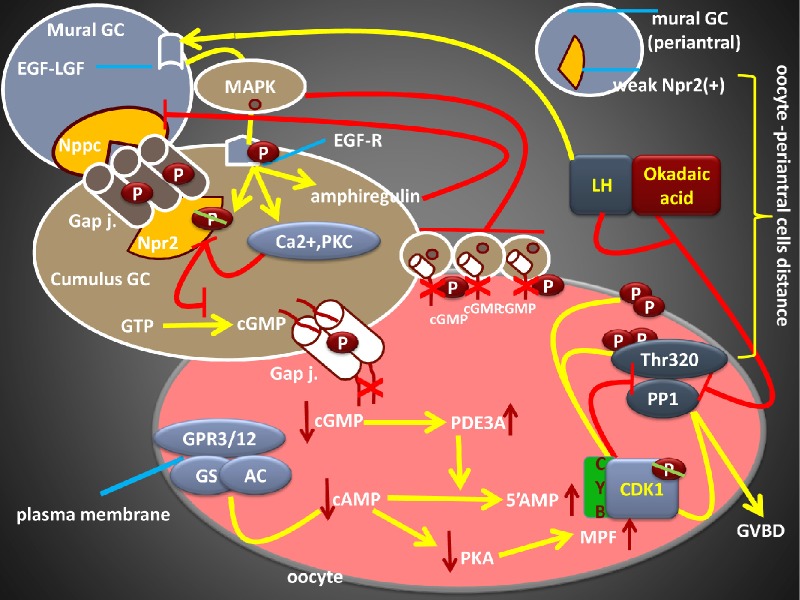
Figure 1.
Abbreviated pathways to illustrate inhibitory signals maintaining meiotic arrest before the LH surge. (A) Mild hypoxia within the somatic cells induces hypoxanthine (HX) synthesis, which increases the synthesis of inosine, which is the responsible substrate for GTP and cGMP synthesis. Nppc/Npr2 system controls cGMP production in the somatic cells. Npr2 is activated by the Nppc and produces cGMP from GTP. cGMP diffuses into the oocyte via gap junctions (two thick red lines). In the absence of LH surge, gap junctions undergo dephosphorylation and become activate. cGMP inhibits the PDE3A, which inhibits the hydrolysis of cAMP into 5′AMP. 8-Br-cGMP, a cGMP analog, also blocks the PDE3A activity. cGMP synthesis in somatic cells may overcome the hydrolytic activity of PDE5 enzyme (dashed red line). Note the compartmentalization of the PDEs. GPRs induce Gs expression and activate AC, which stimulates cAMP synthesis. Accumulation of cAMP inside the oocyte mediates meiotic arrest by increasing PKA. Activation or inhibition of PKA by cAMP regulates the functions of the Cdc25 and the Wee1B/Myt1. While the Cdc25 dephosphorylates Cdk1, Wee1B/Myt1 kinase phosphorylates it. E2, FSH, and ODPFs (GDF-9, BMP-15) contribute to maintain first meiotic arrest by inducing Npr2 mRNA in cumulus cells. ODPFs also control the activity of IMPDH. The action of IMPDH, which converts IMP into GMP, is blocked by mizoribine and mycophenolic acid. High PP1 activities inside the oocyte prevent nuclear envelope dissolution. Pharmacological inhibition of MPF with the roscovitine blocks GVBD. High follicular fluid (FF) CNP levels were noted before ovulation. (B) Abbreviated pathways to illustrate the role of LH surge on meiotic resumption. LH acts on the mural cells because of the absence of functional LHRs in the cumulus cells and oocyte. LH surge leads to disruption of arresting mechanisms, which would result in the activation of PDE3A. Inhibition of the Nppc/Npr2 by LH surge leads to a reduction in cGMP synthesis. LH surge mediates the phosphorylation (P) of connexin 43 and 37, leading to the closure of the gap junctions (thick red lines). Phosphorylation of gap-junction is a MAPK-dependent process. LH decreases granulosa cell nitric oxide expression and activates cGMP synthesis. LH surge also initiates the dephosphorylation of Cdk1 and the MPF becomes active. MPF phosphorylates both PP1 and nuclear envelope, which causes GVBD. OA increases the phosphorylated PP1 (inactive PP1) levels and leads to GVBD. Taken together, both the synthesis and transfer of cGMP into the oocyte are blocked. Decline in the cGMP concentration inside the oocyte allows PDE3A to hydrolyze cAMP into 5′AMP, leading to meiotic resumption. Yellow and blue arrows indicate excitatory signals, and red lines indicate inhibitory signals. See text for details. (Adapted from Refs. 5–14.)
Different molecular mechanisms may be responsible for maintaining of the first meiotic arrest in small and growing oocytes. Studies have demonstrated that increased levels of cAMP is obligatory for prophase I meiotic arrest in growing oocytes.18,35 On the other hand, when the size of oocytes is under 60 μm, they fail to resume meiosis despite exiting from the follicles.41 Thus, persistence of high intra-oocyte cAMP levels may not be obligatory for maintaining prophase I arrest in smaller oocytes. Taken together, maintaining elevated cAMP levels inside the oocytes is essential for prophase I arrest15,42 in growing oocytes. Nevertheless, small oocytes that do not have the ability to complete meiotic division high levels of cAMP inside the oocyte may not be necessary for maintaining prophase I arrest. The oocytes somehow produce sufficient amount of cAMP to maintain prophase I arrest.17,18 However, cGMP transport from granulosa cells contributes to the maintenance of increased cAMP levels inside the oocyte.15,42
PDE3A, PDE5, PDE9A, and PDE4 Enzymes Hydrolyze Somatic Cell-derived cGMP
Differentiation and maturation of follicles following LH surge is a cAMP-mediated process. However, the sustained exposure of cAMP inhibits oocyte maturation.43 This paradox might be explained by the compartmentalization hypothesis, which is very helpful for understanding the contribution of the different PDEs upon follicular maturation. According to this hypothesis, regulation of the cAMP concentration within the somatic compartment and oocyte is different because of the differential settlement of the PDE subtypes.44 In agreement with our results, Thomas et al have showed the compartmentalization of PDE4 and PDE3 within the bovine follicle.45
The enzymes that metabolize somatic cell-derived cGMP in a growing oocyte are not known in detail. PDE enzymes have an important role in cell-to-cell signaling between somatic compartment and the oocyte. PDEs have the ability to degrade both cAMP and cGMP.46 So far, more than 10 classes of PDE enzymes, such as PDE3A, PDE5, PDE9A, and PDE4, have been reported. PDE3A is the oocyte-specific enzyme that hydrolyzes cAMP into 5′AMP and leads to meiotic resumption.47 Inhibition of PDE3A activity in humans prevents both spontaneous and gonadotropin-induced follicular growth.48 Likewise, PDE3A mutant mice are infertile because of GV oocyte.49 In a recent study, Hanna et al have shown that PDE9A is the only cGMP-specific phosphodi-esterase present in the GV oocyte.50 Moreover, they have detected 17 of the 19 PDE genes assayed in the somatic cells. They have concluded that cGMP residues inside the oocyte are hydrolyzed by the PDE9A after the LH surge. Similarly, previous studies have demonstrated the presence of PDE4 activity in the somatic cells but not within the oocyte.44 While PDE4 enzyme hydrolyzes cAMP, it is not sensitive to cGMP. The oocyte is not responsive to the PDE4 inhibitors, suggesting that PDE4 enzyme is expressed only in the somatic cells.45
Although follicular somatic cells contain PDE5 enzyme antigen,51 PDE5 enzyme activity is not detected within the oocytes.26 Unlikely the oocyte PDE3A enzyme, PDE5 enzyme is not specific to somatic cells. Similarly, PDE5 enzyme activity is present in the different mammalian cell types and tissues in which hydrolysis of cGMP occurs.52 Likewise, PDE5 enzyme in the somatic compartment metabolizes granulosa cell-derived cGMP. Hydrolysis of somatic cGMP by the PDE5 enzyme causes meiotic resumption by decreasing cGMP concentration within the oocyte cytoplasm.26,51 Sildenafil and zaprinast are specific and potent PDE5 inhibitors.53,54 Both these inhibit the cGMP degradation in the cumulus cells. Culture medium containing sildenafil inhibits LH-dependent meiotic resumption in the oocytes.26 By using zaprinast, a recent in vitro study has investigated the possible roles of this drug on c-type natriuretic peptide (CNP)-induced inhibition of nuclear envelope dissolution in COCs. However, they did not find any significant effect on GVBD.11 In light of the above findings, authors have suggested that CNP-induced cGMP synthesis in somatic cells may overcome the hydrolytic activity of PDE5 enzyme and may continue to transport cGMP into oocytes to inhibit nuclear envelope breakdown. Nevertheless, it should be remembered that zaprinast not only inhibits PDE5 enzyme but also inhibits PDE6, 9, 10, and 11 enzymes.55 Therefore, the development of more specific PDE5 inhibitors will increase our knowledge of their functional role in GVBD.
Crosstalk between the MPF Complex and PPs
Sleep and vigilance of the oocyte is regulated by phosphorylation and dephosphorylation events during the reproductive period. Changes in the levels and activity of cyclin-dependent kinase-1 (Cdk1)–cyclin B protein complex (MPF) inside the oocyte are established as the first step of meiotic division.56 While some denominates it as a MPF, others called it as a meiosis-promoting factor or M-phase-promoting factor.56 In meiotically competent oocytes, MPF is localized inside the nucleus.57 Oocytes undergo metaphase II when the concentration and activity of MPF increase inside the oocyte nucleus.58–60 Inhibition of MPF with the roscovitine prevents GVBD in the mouse oocytes.61 The effects of intra-oocyte cAMP levels on phosphorylation/dephosphorylation reactions and the regulation of MPF are not well understood. Phosphorylation reactions and MPF activation during meiotic division are regulated by cAMP, protein kinase A (PKA), and PPs.9,62–64 One of the main substance maintaining the first meiotic arrest is high PKA activity inside the oocyte.62,63 cAMP mediates first meiotic arrest by increasing PKA levels. In contrast, decline in the PKA activity inside the oocyte leads to GVBD.49 Before the LH surge, presence of high cAMP concentration inside the oocyte increases PKA activity and leads to the phosphorylation of Cdk1 and the inactive MPF complex.63 After the LH surge, decline in the intra-oocyte cAMP concentration leads to dephosphorylation of Cdk1 and MPF becomes active. Nevertheless, the effect of PKA on the Cdk1 phosphorylation status is indirect rather than direct. Namely, activation or inhibition of PKA by cAMP inside the oocyte regulates the functions of the Cdc25 phosphatase and the Wee1B/Myt1 kinase.62 While the Cdc25 dephosphorylates Cdk1, Wee1B/Myt1 kinase phosphorylates it. In good agreement, meiotic resumption does not take place in the Cdc25b knockout mice, supporting an indirect role of PKA.65
Another substance that has an important role in maintaining meiotic arrest is PPs. PP1 is localized both in the cytoplasm and the nucleus of oocytes, and PP2A is found within the cytoplasm.9,66,67 However, PP1 shows diverse subcellular localization in okadaic acid (OA)-mediated GVBD with higher nuclear PP1 expression in meiotically competent oocytes.9 OA is an inhibitor of PP1, PP2A, and Ser/Thr PPs and is generated from the marine black sponges and starfish.68–71 Exposure of oocyte to OA leads to premature GVBD.72 When incompetent mouse oocytes are given OA, their capacity for resuming meiosis increases, suggesting the high activity of PP1 in incompetent oocytes.73 Nevertheless, possible effects of OA administration on human oocytes are unknown.
Little is known about the specific targets and molecular regulators of the PP1. MPF and oocyte nuclear envelope are possible targets of PP1. Moreover, differential localization of PP1 inside the cytoplasm and nucleus might regulate meiotic maturation.9 There is a strong relationship between the Cdk1 and PPs. Cdk1 discomposes the structural stability of the oocyte nuclear membrane by transferring phosphates on lamins and causes the first morphological sign of nuclear membrane dissolution.5,74,75 Similarly, by removing phosphate from phosphoproteins, PPs regulate both meiotic and mitotic division.76,77 Moreover, phosphorylation of PP1 during meiotic maturation inhibits the PP1 activity and causes nuclear envelope dissolution.67,76,77
PP1 and PP2A have been detected in oocytes of some animals.72,76 Similar to cAMP, PPs have an antagonistic effect against MPF.64 If the activity of PP1 increases, the activity of MPF decreases. The opposite is also true. Both LH surge and OA administration inhibit (phosphorylated) the PP1 activity, and MPF is released from PP1-dependent inhibition. Moreover, inhibition of PP1 increases the phosphorylation of nuclear envelope and leads to GVBD.5 It is well known that Cdk1 inhibits enzymatic function of PP1 by phosphorylating it at Thr320.78,79 In good agreement, in the oocytes of Cdk1-null mice, Cdk1 phosphorylates the PP1, resulting in suppression of PP1 activity thus facilitating the activation of the main factors involved in GVBD.5 Consistent with this, the concentration of phosphorylated nuclear PP1 (inactive PP1) is elevated in OA-mediated GVBD.5,9 Taken together, the strong relationship between the MPF complex and PP1 seems to be regulating meiotic arrest and resumption of meisosis.9
Nppc/Npr2 System and its Regulators
The majority of the studies investigating the possible mechanisms regulating the first meiotic arrest and follicle maturation in mammals are based on the works conducted on model organisms such as rat and mouse. However, meiotic regulation sites and signals vary between two animals. Meiotic regulatory signals stem from outer somatic cells in rat, whereas in mice, regulatory signals stem from inner somatic cells.80 Although rat genome shows close similarity to the human genome,81 we should be careful while adapting the meiotic regulation findings obtained from this model organisms to humans.
A large number of peptides, including natriuretic peptides (NPs), are involved in maintaining reproductive processes. Evolutionary mechanisms controlling the oocyte meiotic arrest are preserved in the oocytes of many species.82–84 The NP regulate fluid homeostasis in vertebrates.85 They show varied effects in different tissues of the same and different species. Atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and c-type natriuretic peptide (CNP) have structural similarity. ANP and BNP use NPR-A, while the connection site of CNP is the NPR-B.86 This pattern is different in fish, where CNP uses NPR-B and NPR-A receptors.83 ANP is encoded by Nppa. Amino acid sequences of ANP and CNP in many species are similar. The possible roles of the ANP and BNP on meiotic division and oocyte maturation are controversial. Tornell et al have reported that ANP inhibits spontaneous maturation in rat oocytes.87 In contrast, Kawamura et al have showed that the expression of Nppa in the oocytes of mice is not affected by hCG administration.11 Further investigations are necessary to elucidate the possible role of ANP on meiotic maturation. In contrast, a recent in vitro study has shown that both BNP and CNP stimulate the generation of cGMP in porcine oocytes to maintain prophase I arrest.88
Nppc is a peptide that contains 22 amino acids with a molecular mass of ~2,000 dalton, and its sequence shows similarity between the species.89 Mural members of the somatic compartment cells express Nppc. On the other hand, Nppc receptor (Npr2) is a guanylyl cyclase and is expressed by cumulus cells28 and periantral mural granulosa cells. When the distance from the oocyte membrane increases, Npr2 expression on the periantral mural cells decreases. Nppc induces Npr2 expression and encodes CNP. Somatic cells of secondary and antral follicles have the ability to express Nppa mRNA. However, expression of Nppc was noted only in the granulosa cells of antral follicles.90 Interactions between the Nppc and Npr2 systems play an important role in controlling the prophase I arrest. Similarly, activation of Nppc/Npr2 system inhibits the nuclear membrane dissolution in cumulus cell-surrounded oocytes. Nevertheless, Nppc does not lead to GVBD in cumulus cell-free oocytes. Initiation of meiotic resumption in Npr2 or Nppc-null mice supports the inhibitory role of Nppc/Npr2 system.29 Relevantly, premature meiotic resumption occurs in the early stage of antral follicles of Npr2 and Nppc mutant mice.91
By increasing the Npr2 activity in cumulus cells, Nppc enhances cGMP synthesis.30,92 In a recent study, Zhang et al have demonstrated that outer somatic cells induce the synthesis of cGMP by secreting Nppc. Once released, Nppc binds and stimulates the Npr2 in the inner somatic cells and initiates cGMP synthesis.29 GTP is the last substrate for cGMP synthesis in somatic cells. Conversion of the GTP into cGMP in somatic cells is catalyzed by Npr2.29 Because somatic cells do not have sufficient vascular supply, they have to live in an relatively hypoxic milieu.93 Hypoxic environment within the somatic cells induces the production of hypoxanthine and inosine. Both are required for GTP synthesis.7,31,94
Somatic cell-derived cGMP is transferred into the oocyte cytoplasm through heterologous gap-junctional communication.29 Accumulation of cGMP inside the oocyte inhibits PDE3A activity and suppresses the conversion of cAMP into 5′AMP. Administration of the oocyte-specific PDE3A inhibitors increases the cAMP concentration inside the oocytes of many species,95,96 supporting the indirect and critical role of somatic cGMP on this enzyme. Taken together, all these chain reactions lead to accumulation of cGMP and cAMP inside the oocyte that maintains prophase I arrest until the LH surge.26,30,92 Nppc/Npr2 signaling pathway maintains the elevated cGMP concentrations both in the somatic compartment and inside the oocyte during the first meiotic arrest.
Activation of Nppc/Npr2 system in somatic compartment cells is modulated by various stimuli. In addition to local factors generated by the somatic compartment, paracrine factors generated by oocytes induce the expression of Npr2 in cumulus cells.29 Some substances secreted by the oocytes, including growth differentiation factor-9 and bone morphogenetic protein-15, induce Npr2 activation and lead to an increase in cGMP synthesis.7,29 Nppc/Npr2-mediated cGMP synthesis is also controlled by the inosine monophosphate dehydrogenase (IMPDH), which is the rate-limiting enzyme in cGMP synthesis.7 The action of IMPDH, which converts inosine monophosphate (IMP) into the xanthosine monophosphate, is blocked by mizoribine, which is a purine synthesis inhibitor (4-carbamoyl-1-β-D-ribofuranosyl imidazololium-5-olate). IMPDH is also blocked by mycophenolic acid. Inhibition of IMPDH reduces the levels of intra-oocyte guanine. ODPFs regulate the activity of IMPDH enzyme and estradiol synthesis in somatic cells.7 A recent study has reported that estradiol participates in the maintenance of meiotic arrest by increasing the expression of Nppc/Npr2 system.82 Thus, the signals responsible for prophase I arrest are not exclusively from the somatic cells to oocytes but also from oocyte to the somatic cells supporting the bidirectional communication7 (Figs. 1 and 2).
Figure 2.
Abbreviated pathways to illustrate the LH effect on the Nppc/Npr2 system. EGF-LGF potentiates the effectiveness of LH signal, induces the expression of EGF-R, and inhibits the Npr2 activity. Likewise, after the LH surge, expression of mitogen-activated protein kinase (MAPK) increases and activates the EGF-R. Phosphorylation of EGF-R activates it. Decline in the expression levels of Npr2 after LH signal takes place by three possible mechanisms. First, activation of EGF-R increases the calcium levels inside the cumulus cells and reduces the Npr2 activity. Second, induction of EGF-R activity decreases Npr2 expression in the cumulus cells by means of dephosphorylation reactions. Third, by activating EGF-R, LH increases the secretion of amphiregulin, which leads to downregulation of the Nppc expression. Moreover, EGF-R activation inhibits the Nppc mRNA expression in the somatic cells. Taken together, EGF- and MAPK-mediated LH action in the Nppc/Npr2 system blocks the conversion of GTP to cGMP, and the oocyte undergoes meiotic resumption. Concentration of nuclear phosphorylated PP1 (inactive PP1) is elevated in OA-mediated GVBD. LH surge induces the activation of MAPK, which phosphorylates the gap-junction proteins and leads to their closure. Cdk1 inhibits PP1 by phosphorylating it at Thr320. Cdk1 also phosphorylates the nuclear envelope and initiates GVBD. Periantral mural cells signify weak Npr2 activity. When the distance from oocyte membrane increases, Npr2 activity in periantral mural cells decreases. (Adapted from Refs. 5–14.)
Are Nppc and OMI the Same Peptides?
Although Nppc demonstrates many properties similar to those reported for OMI, whether Nppc satisfies the criteria as an OMI remains to be determined. On the other hand, some similarities between the OMI and Nppc makes one think that OMI and Nppc are same the molecules. For example, (a) the molecular weight of the two substances are ~2,000 dalton;89,97 (b) both OMI and Nppc originated from the outer somatic cells and exert their effects on cumulus cells;29 (c) both of these inhibit the premature meiotic maturation;29 (d) follicular fluid contains both Nppc and OMI;11,98 and (e) cumulus cells do not show OMI expression, whereas weak Nppc activity is seen in the cumulus cells.29,98
Data supporting the similarity of OMI and Nppc come from two studies.11,28 It is well known that the effect of OMI on cumulus cells was blocked by the LH surge.19,97 Robinson et al have showed that Npr2 expression in mouse cumulus cells is dropped by LH.28 Likewise, it has been reported that administration of LH/hCG suppresses the Nppc synthesis in mice follicles.11 Moreover, Nppc administration inhibits both spontaneous and hCG-induced nuclear envelope dissolution in mice.11 Taken together, Nppc and OMI may be the molecules responsible for prophase I meiotic arrest, but further studies are necessary to elucidate this close relation between the OMI and Nppc.
(ii) How does LH surge initiate meiotic resumption?
The possible effects of LH surge on meiotic resumption are uncertain. The expression of LH receptor (LHR) within the COCs is site and cell specific. While some members of the COCs such as mural granulosa cells and theca cells express LHR, cumulus granulosa cells and oocyte do not contain functionally active LHR.99,100 Hence, the presence of functional LHRs on mural granulosa cells make them the main target for LH action. In contrast, LH action on cumulus cells and oocytes is indirect rather than direct. LH-related mural cell signals reach the cumulus cells and oocytes via local factors and gap junctions.101 Genome-wide analyses showed that LH peak stimulates the expression of endothelin-1, leptin,102,103 epidermal growth factor-like ligands (EGF-LGF),104 and insulin like-3 transcript105 and initiates meiotic resumption. Before the puberty, because of immature LH pulse generator, LH-catalyzing reactions do not take place within the somatic cells and the follicle remains arrested in prophase. Until puberty, LH-dependent reactions within the somatic cells are either inhibited or functionally inactive. After the puberty, LH surge enables the oocyte to progress into the completion of first meiotic division.
Both prophase I arrest and meiotic resumption are maintained by the cAMP levels inside the oocyte. Significant decline in cAMP concentrations inside the oocyte is required for the resumption of meiosis.97 By inhibiting its synthesis and transport, LH surge decreases cGMP concentrations inside the oocyte and initiates the meiotic resumption. It has been reported that closure of gap junctions by LH surge inhibits cGMP transport from the somatic compartment into the oocyte.106 In order to close gap junctions, LH phosphorylates the connexin 43 protein.107 The gap junction–blocking effect of the LH can be mimicked by carbenoxolone, a gap junction blocker.106 Administration of this agent leads to premature nuclear envelope dissolution. Paradoxically, LH induces cAMP synthesis in the somatic compartment.97 However, closure of gap-junctional communication by the LH surge inhibits the transfer of granulosa cell-derived cAMP into the oocyte. Taken together, decline in the cGMP concentrations inside the oocyte alleviates the suppressive effect of cGMP on PDE3A,26,30,92,108 which hydrolyzes cAMP inside the oocyte.
It is well known that LH inhibits the production of cGMP synthesis in somatic cells. As described in detail above, cGMP production in somatic cells is regulated by the Nppc/Npr2 system.30,92 On the other hand, little is known about the possible effects of LH surge on Nppc/Npr2 system. In goat, while FHS administration induces Nppc expression in somatic cells, LH reduces its expression.90 In contrast, neither FSH nor LH administration leads to significant changes on Nppa expression.90 In mouse, Npr2 expression in cumulus cells is blocked by LH.28 Likewise, elevated levels of follicular fluid Nppc were detected in humans undergoing IVF-ET before ovulation.11 Administration of ovulatory dose of hCG in patients undergoing IVF causes a decline in follicular fluid Nppc concentration, confirming its suppression by hCG.11 Taken together, Nppc/Npr2 is a system that is responsive to LH, FSH, and hCG.11,28,90
A possible mediator of the LH effect on the Nppc/Npr2 system is the epidermal growth factor (EGF).109 While EGF and EGF-like growth factors (EGF-LGF) are located on the mural granulosa cells, EGF receptors (EGF-R) are located on the cumulus granulosa cells. EGF-LGF potentiates the effectiveness of LH signal, induces the expression of EGF-R,91,108 and inhibits the Npr2 activity. Following LH surge, expression of cumulus cell-derived mitogen-activated protein kinase (MAPK) increases and activates the EGF-R.110 Little is known about how EGF-LGF initiates the meiotic resumption. Decline in the expression levels of Npr2 after the LH signal takes place by three possible mechanisms. First, activation of EGF-R increases calcium levels inside the cumulus granulosa cells and reduces Npr2 activity.109 Second, induction of EGF-R activity decreases Npr2 expression in the cumulus cells by means of dephosphorylation reactions.111 Third, by activating EGF-R, LH increases the secretion of amphiregulin, which leads to downregulation in Nppc expression.91 Moreover, EGF-R activation inhibits the Nppc mRNA expression in the somatic cells91 and causes a decline in Nppc concentration.28 To sum up, EGF-mediated LH action inhibits the expression of Nppc/Npr2 in the somatic cells and conversion of the GTP into the cGMP is blocked.11 As a result, both cAMP and cGMP concentrations decrease inside the oocyte, which leads to meiotic resumption29,108 (Fig. 2).
(iii) Does the establishment of selective regulation methods to prevent follicle loss increase progress in fertility preservation technologies in prematurely aging women and young cancer patients undergoing chemoradiotherapy?
Differentiation and maturation of follicles are regulated by the signaling pathways that present between the oocyte and granulosa cells. Each member of this complex needs the support of the others, during both the dormant stage and folliculogenesis. This cell complex is very sensitive to the harmful effects of senescence, genetic and metabolic factors, and chemoradiotherapy. Defect in a member adversely affects the other. For instance, apoptosis of somatic cells causes the death of the oocyte and vice versa.
The pool of dormant-stage follicles inside the ovarian cortex determines individual-functional ovarian reserve. Sufficient amount of inhibitory molecules such as cGMP and cAMP is obligatory to maintain this dormancy. In addition to the oocyte’s own inhibitory molecules, somatic cell-derived inhibitory signals are also required to maintain meiotic arrest.6,15,42 In agreement with previous studies, cGMP is produced by somatic cells and diffuses into the oocyte28,29 and maintains sufficient amount of cAMP within the oocyte.26 Defect in this signaling pathways leads to obtaining a lower quality follicle in IVF cycles. The best example of the disturbed somatic cell signal is the persistence of meiotic arrest after the oocyte pick-up (OPU). Actually, management of the oocytes having meiotic arrest after the OPU is a very important problem in many IVF laboratories. Closure of intercellular communication of the GV oocyte by using gap junction blockers results in the resumption of meiosis and improves the oocyte stage. Moreover, placing a GV-stage oocyte in the phosphate-containing medium may elevate the GV oocyte to a higher stage.
The risk of follicle loss increases in young women with cancer and undergoing chemoradiotherapy. Both radiotherapy and chemotherapy carry a great risk for fertility outcome. Following chemoradiotherapy, PGCs may undergo irreversible damage leading to premature ovarian aging. Ovarian tissue cryopreservation, oocyte vitrification, and slow freezing at the GV stage are promising methods to improve the success rate of IVF-ET in these women. Nevertheless, cryoprotectants, cooling rate, storage time, and thawing methods may have a detrimental effect upon the follicle. There is no effective preventive measure to protect chemoradiotherapy-induced follicle loss in young women with cancer, who do not accept oocyte or ovarian tissue cryopreservation. Reducing the radiation exposure to the gonads by shielding or removing them from the field of radiation may preserve follicle loss. However, these preventive measures are invasive, and escape from follicular apoptosis is not possible. Targeted inhibition of both PDE3A and PDE9A before chemoradiotherapy may prevent the follicle maturation and lead to a decline in the apoptotic follicle death. Similar effects can be obtained by the administration of cGMP or cAMP analogs. By using selective signal modulators, if we can regulate the phosphorylation reactions inside the oocyte, we may prevent the follicle loss and extend the reproductive life span for women under the risk of premature ovarian aging.
Conclusion
Disturbance in oocyte meiotic events can lead to subfertility or premature aging by reducing the functional ovarian reserve. Selective regulation of somatic signals may be used in the treatment for infertile patients who suffer from the lack of good quality eggs. Management of the intercellular communication via gap junction blockers increases our control upon oocyte meiotic events. Accordingly, by using PDE inhibitors and/or cGMP or cAMP analogs, we can keep the oocytes at the GV stage and slow down their metabolism. As a consequence, we may inhibit chemoradiotherapy-induced follicle loss and preserve the future fertility of young female cancer survivors.
Footnotes
ACADEMIC EDITOR: Gabor Mocz, Editor in Chief
PEER REVIEW: Twelve peer reviewers contributed to the peer review report. Reviewers’ reports totaled 3,698 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived the study, wrote the first draft of manuscript, and drew the pictures: OC. Contributed to the writing of the manuscript: NC. Jointly developed the structure and arguments for the review and made critical revisions: SG, ETH, and SA. All authors contributed to the design and preparation of the manuscript, and reviewed and approved of the final manuscript.
REFERENCES
- 1.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24(2):86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T. Cell-cycle control during meiotic maturation. Curr Opin Cell Biol. 2003;15(6):654–663. doi: 10.1016/j.ceb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker M. Control of meiotic arrest. Rev Reprod. 1996;1(2):127–135. doi: 10.1530/ror.0.0010127. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama T, Tachibana K, Kishimoto T. Cytostatic Arrest: Post- Ovulation Arrest until Fertilization in Metazoan Oocytes. In: Verlhac M-H, Villeneuve A, editors. Oogenesis: The Universal Process. John Wiley & Sons, Ltd; Chichester, UK: 2010. pp. 357–387. [Google Scholar]
- 5.Adhikari D, Zheng W, Shen Y, et al. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet. 2012;21(11):2476–2484. doi: 10.1093/hmg/dds061. [DOI] [PubMed] [Google Scholar]
- 6.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130(6):791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 7.Wigglesworth K, Lee KB, O’Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci U S A. 2013;110(39):E3723–E3729. doi: 10.1073/pnas.1314829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol. 2014;382(1):480–487. doi: 10.1016/j.mce.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Swain JE, Bollen M, Liu XT, Ohl DA, Smith GD. Endogenous regulators of protein phosphatase-1 during mouse oocyte development and meiosis. Reproduction. 2004;128(5):493–502. doi: 10.1530/rep.1.00173. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Kong N, Xia G, Zhang M. Molecular control of oocyte meiotic arrest and resumption. Reprod Fertil Dev. 2013;25(3):463–471. doi: 10.1071/RD12310. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura K, Cheng Y, Kawamura N, et al. Preovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of preovulatory oocytes. Hum Reprod. 2011;26(11):3094–3101. doi: 10.1093/humrep/der282. [DOI] [PubMed] [Google Scholar]
- 12.Zhang MJ, Xia GL. Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell Mol Life Sci. 2012;69(8):1279–1288. doi: 10.1007/s00018-011-0867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod. 2010;16(9):654–664. doi: 10.1093/molehr/gaq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Stetina JR Orr-Weaver TL. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol. 2011;3(10):a005553. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe LA, Norris RP. Initiation of the meiotic prophase-to-metaphase transition in mammalian oocytes. In: Verlhac MH, Villenuve A, editors. Oogenesis: The Universal Process. John Wiley & Sons, Ltd; Chichester, UK: 2010. pp. 181–198. [Google Scholar]
- 16.Sela-Abramovich S, Galiani D, Nevo N, Dekel N. Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod. 2008;78(6):1111–1118. doi: 10.1095/biolreprod.107.065490. [DOI] [PubMed] [Google Scholar]
- 17.Mehlmann LM, Saeki Y, Tanaka S, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306(5703):1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 18.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287(2):249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Racowsky C, Baldwin KV. In vitro and in vivo studies reveal that hamster oocyte meiotic arrest is maintained only transiently by follicular fluid, but persistently by membrana/cumulus granulosa cell contact. Dev Biol. 1989;134(2):297–306. doi: 10.1016/0012-1606(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 20.Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med. 1935;62(5):665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers RJ, Irving-Rodgers HF, van Wezel IL, Krupa M, Lavranos TC. Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol. 2001;171(1–2):41–48. doi: 10.1016/s0303-7207(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 22.Erickson GF, Sorensen RA. In vitro maturation of mouse oocytes isolated from late, middle, and pre-antral Graafian follicles. J Exp Zool. 1974;190(1):123–127. doi: 10.1002/jez.1401900112. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of mouse oocyte. Dev Biol. 1976;50(2):531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- 24.Dekel N. Molecular control of meiosis. Trends Endocrinol Metab. 1995;6(5):165–169. doi: 10.1016/1043-2760(95)00079-w. [DOI] [PubMed] [Google Scholar]
- 25.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385(6616):525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 26.Vaccari S, Weeks JL, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81(3):595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bu SB, Xie HR, Tao Y, Wang JH, Xia GL. Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endocrinol. 2004;223(1–2):85–93. doi: 10.1016/j.mce.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Robinson JW, Zhang M, Shuhaibar LC, et al. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev Biol. 2012;366(2):308–316. doi: 10.1016/j.ydbio.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris RP, Ratzan WJ, Freudzon M, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downs SM, Coleman DL, Wardbailey PF, Eppig JJ. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low-molecular weight fraction of porcine follicular-fluid. Proc Natl Acad Sci U S A. 1985;82(2):454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187(1–2):153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 33.Aktas H, Wheeler MB, Rosenkrans CF, Jr, First NL, Leibfried-Rutledge ML. Maintenance of bovine oocytes in prophase of meiosis I by high [cAMP]i. J Reprod Fertil. 1995;105(2):227–235. doi: 10.1530/jrf.0.1050227. [DOI] [PubMed] [Google Scholar]
- 34.Homa ST. Effects of cyclic AMP on the spontaneous meiotic maturation of cumulus-free bovine oocytes cultured in chemically defined medium. J Exp Zool. 1988;248(2):222–231. doi: 10.1002/jez.1402480214. [DOI] [PubMed] [Google Scholar]
- 35.Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005;288(2):397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase A in mammalian oocytes. Dev Biol. 2002;246(2):441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- 37.Albertini DF, Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974;63(1):234–250. doi: 10.1083/jcb.63.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97(2):264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 39.Magnusson C, Hillensjo T. Inhibition of maturation and metabolism in rat oocytes by cyclic amp. J Exp Zool. 1977;201(1):139–147. doi: 10.1002/jez.1402010117. [DOI] [PubMed] [Google Scholar]
- 40.Eppig JJ. The participation of cyclic adenosine monophosphate (cAMP) in the regulation of meiotic maturation of oocytes in the laboratory mouse. J Reprod Fertil Suppl. 1989;38:3–8. [PubMed] [Google Scholar]
- 41.Hirao Y, Miyano T, Kato S. Acquisition of maturational competence in in vitro grown mouse oocytes. J Exp Zool. 1993;267(5):543–547. doi: 10.1002/jez.1402670509. [DOI] [PubMed] [Google Scholar]
- 42.Edry I, Sela-Abramovich S, Dekel N. Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle. Mol Cell Endocrinol. 2006;252(1–2):102–106. doi: 10.1016/j.mce.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Tsafriri A, Zor U, Lamprech SA, Lindner HR. In-vitro induction of meiotic division in follicle-enclosed rat oocytes by LH, cyclic Amp and prostaglandin-E2. J Reprod Fertil. 1972;31(1):39–50. doi: 10.1530/jrf.0.0310039. [DOI] [PubMed] [Google Scholar]
- 44.Tsafriri A, Chun SY, Zhang R, Hsueh AJW, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178(2):393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 45.Thomas RE, Armstrong DT, Gilchrist RB. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol. 2002;244(2):215–225. doi: 10.1006/dbio.2002.0609. [DOI] [PubMed] [Google Scholar]
- 46.Dousa TP. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55(1):29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 47.Sasseville M, Albuz FK, Cote N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian follicle. Biol Reprod. 2009;81(2):415–425. doi: 10.1095/biolreprod.108.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Van der Elst J. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Hum Reprod. 2007;22(5):1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- 49.Masciarelli S, Horner K, Liu C, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114(2):196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna CB, Yao S, Wu XM, Jensen JT. Identification of phosphodiesterase 9A as a cyclic guanosine monophosphate-specific phosphodiesterase in germinal vesicle oocytes: a proposed role in the resumption of meiosis. Fertil Steril. 2012;98(2):487.e–495.e. doi: 10.1016/j.fertnstert.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Ning G, Chen X, et al. PDE5 modulates oocyte spontaneous maturation via cGMP-cAMP but not cGMP-PKG signaling. Front Biosci. 2007;13:7087–7095. doi: 10.2741/3212. [DOI] [PubMed] [Google Scholar]
- 52.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 53.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8(2):47–52. [PubMed] [Google Scholar]
- 54.Lugnier C, Schoeffter P, Le Bec A, Strouthou E, Stoclet JC. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- 55.Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108(5):671–680. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- 56.Labbé JC, Capony JP, Caput D, et al. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;8(10):3053. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitra J, Schultz RM. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci. 1996;109:2407–2415. doi: 10.1242/jcs.109.9.2407. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988;126(2):242–252. doi: 10.1016/0012-1606(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 59.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356(1–2):65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maller JL. On the importance of protein phosphorylation in cell cycle control. Mol Cell Biochem. 1993;12(7–128):267–281. doi: 10.1007/BF01076777. [DOI] [PubMed] [Google Scholar]
- 61.Mermillod P, Tomanek M, Marchal R, Meijer L. High developmental competence of cattle oocytes maintained at the germinal vesicle stage for 24 hours in culture by specific inhibition of MPF kinase activity. Mol Reprod Dev. 2000;55(1):89–95. doi: 10.1002/(SICI)1098-2795(200001)55:1<89::AID-MRD12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 62.Eppig JJVM, Marin-Bivens C, De La Fuente R. Regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY, editors. The Ovary. Amsterdam: Elsevier Academic Press; 2004. pp. 113–129. [Google Scholar]
- 63.Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci U S A. 2002;99(26):16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, Kishimoto T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun. 2012;3:1059. doi: 10.1038/ncomms2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lincoln AJ, Wickramasinghe D, Stein P, et al. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30(4):446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- 66.Smith GD, Sadhu A, Mathies S, Wolf DP. Characterization of protein phosphatases in mouse oocytes. Dev Biol. 1998;204(2):537–549. doi: 10.1006/dbio.1998.9043. [DOI] [PubMed] [Google Scholar]
- 67.Swain JE, Wang X, Saunders TL, Dunn R, Smith GD. Specific inhibition of mouse oocyte nuclear protein phosphatase-1 stimulates germinal vesicle breakdown. Mol Reprod Dev. 2003;65(1):96–103. doi: 10.1002/mrd.10258. [DOI] [PubMed] [Google Scholar]
- 68.Tachibana K, Scheuer PJ, Tsukitani Y, et al. Okadaic acid, a cytotoxic polyether from 2 marine sponges of the genus halichondria. J Am Chem Soc. 1981;103(9):2469–2471. [Google Scholar]
- 69.Bialojan C, Ruegg JC, Takai A. Effects of okadaic acid on isometric tension and myosin phosphorylation of chemically skinned guinea-pig taenia-coli. J Physiol. 1988;398:81–95. doi: 10.1113/jphysiol.1988.sp017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen P. Classification of protein-serine threonine phosphatases—identification and quantitation in cell-extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- 71.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 72.Picard A, Capony J, Brautigan D, Doree M. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J Cell Biol. 1989;109(6):3347–3354. doi: 10.1083/jcb.109.6.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.deVantery C, Stutz A, Vassalli JD, SchorderetSlatkine S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev Biol. 1997;187(1):43–54. doi: 10.1006/dbio.1997.8599. [DOI] [PubMed] [Google Scholar]
- 74.Holaska JM, Wilson KL, Mansharamani M. The nuclear envelope, lamins and nuclear assembly. Curr Opin Cell Biol. 2002;14(3):357–364. doi: 10.1016/s0955-0674(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 75.Heald R, Mckeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- 76.Rime H, Huchon D, Jessus C, Ozon R, Goris J, Merlevede W. Characterization of MPF activation by okadaic acid in Xenopus oocyte. Cell Differ Dev. 1990;29(1):47–58. doi: 10.1016/0922-3371(90)90023-p. [DOI] [PubMed] [Google Scholar]
- 77.Gavin A-C, Tsukitani Y, Schorderet-Slatkine S. Induction of M-phase entry of prophase-blocked mouse oocytes through microinjection of okadaic acid, a specific phosphatase inhibitor. Exp Cell Res. 1991;192(1):75–81. doi: 10.1016/0014-4827(91)90159-r. [DOI] [PubMed] [Google Scholar]
- 78.Van Eynde A, Wera S, Beullens M, et al. Molecular-cloning of NIPP-1, a nuclear inhibitor of protein phosphatase-1, reveals homology with polypeptides involved in RNA processing. J Biol Chem. 1995;270(47):28068–28074. doi: 10.1074/jbc.270.47.28068. [DOI] [PubMed] [Google Scholar]
- 79.Dohadwala M, da Cruz e Silva EF, Hall FL, et al. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1994;91(14):6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Downs SM. Mouse versus rat: profound differences in meiotic regulation at the level of the isolated oocyte. Mol Reprod Dev. 2011;78(10–11):778–794. doi: 10.1002/mrd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S, Shetty J, Hou L, et al. Human, mouse, and rat genome large-scale rearrangements: stability versus speciation. Genome Res. 2004;14(10A):1851–1860. doi: 10.1101/gr.2663304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology. 2011;152(11):4377–4385. doi: 10.1210/en.2011-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoue K, Naruse K, Yamagami S, Mitani H, Suzuki N, Takei Y. Four functionally distinct C-type natriuretic peptides found in fish reveal evolutionary history of the natriuretic peptide system. Proc Natl Acad Sci U S A. 2003;100(17):10079–10084. doi: 10.1073/pnas.1632368100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thorndyke MC, Goldsworthy GJ. Neurohormones in Invertebrates. Vol. 33. Cambridge University Press; Cambridge, UK: 1988. [Google Scholar]
- 85.Takei Y. Structural and functional evolution of the natriuretic peptide system in vertebrates. Int Rev Cytol. 2000;194:1–66. doi: 10.1016/s0074-7696(08)62394-3. [DOI] [PubMed] [Google Scholar]
- 86.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In: Schmidt HHH W, et al., editors. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin, Heildelberg, Germany: [DOI] [PMC free article] [PubMed] [Google Scholar]; Handb Exp Pharmacol. 2009;(191):341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tornell J, Carlsson B, Billig H. Atrial natriuretic peptide inhibits spontaneous rat oocyte maturation. Endocrinology. 1990;126(3):1504–1508. doi: 10.1210/endo-126-3-1504. [DOI] [PubMed] [Google Scholar]
- 88.Zhang W, Yang Y, Liu W, et al. Brain natriuretic peptide and C-type natriuretic peptide maintain porcine oocyte meiotic arrest. J Cell Physiol. 2015;230(1):71–81. doi: 10.1002/jcp.24682. [DOI] [PubMed] [Google Scholar]
- 89.Ogawa Y, Itoh H, Yoshitake Y, et al. Molecular cloning and chromosomal assignment of the mouse C-type natriuretic peptide (CNP) gene (Nppc): comparison with the human CNP gene (NPPC) Genomics. 1994;24(2):383–387. doi: 10.1006/geno.1994.1633. [DOI] [PubMed] [Google Scholar]
- 90.Peng JY, Xin HY, Han P, et al. Identification and gene expression analyses of natriuretic peptide system in the ovary of goat (Capra hircus) Gene. 2013;524(2):105–113. doi: 10.1016/j.gene.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 91.Tsuji T, Kiyosu C, Akiyama K, Kunieda T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol Reprod Dev. 2012;79(11):795–802. doi: 10.1002/mrd.22114. [DOI] [PubMed] [Google Scholar]
- 92.Richards JS. New signaling pathways for hormones and cyclic adenosine 3′, 5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15(2):209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 93.Basini G, Bianco F, Grasselli F, Tirelli M, Bussolati S, Tamanini C. The effects of reduced oxygen tension on swine granulosa cell. Regul Pept. 2004;120(1–3):69–75. doi: 10.1016/j.regpep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Kjellmer I, Andine P, Hagberg H, Thiringer K. Extracellular increase of hypoxanthine and xanthine in the cortex and basal ganglia of fetal lambs during hypoxia-ischemia. Brain Res. 1989;478(2):241–247. doi: 10.1016/0006-8993(89)91504-7. [DOI] [PubMed] [Google Scholar]
- 95.Nogueira D, Albano C, Adriaenssens T, et al. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69(3):1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 96.Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17(8):2079–2084. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 97.Tsafriri A, Pomerantz SH. Oocyte maturation inhibitor. Clin Endocrinol Metab. 1986;15(1):157–170. doi: 10.1016/s0300-595x(86)80047-0. [DOI] [PubMed] [Google Scholar]
- 98.Tsafriri A, Dekel N, Bar-Ami S. The role of oocyte maturation inhibitor in follicular regulation of oocyte maturation. J Reprod Fertil. 1982;64(2):541–551. doi: 10.1530/jrf.0.0640541. [DOI] [PubMed] [Google Scholar]
- 99.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56(4):976–984. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- 100.Peng XR, Hsueh AJ, Lapolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129(6):3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- 101.Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978;78(1):58–75. doi: 10.1083/jcb.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawamura K, Ye Y, Liang CG, et al. Paracrine regulation of the resumption of oocyte meiosis by endothelin-1. Dev Biol. 2009;327(1):62–70. doi: 10.1016/j.ydbio.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 103.Ye Y, Kawamura K, Sasaki M, et al. Leptin and ObRa/MEK signalling in mouse oocyte maturation and preimplantation embryo development. Reprod Biomed Online. 2009;19(2):181–190. doi: 10.1016/s1472-6483(10)60070-3. [DOI] [PubMed] [Google Scholar]
- 104.Park JY, Su YQ, Ariga M, Law E, Jin SLC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 105.Kawamura K, Kumagai J, Sudo S, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A. 2004;101(19):7323–7328. doi: 10.1073/pnas.0307061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147(5):2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- 107.Granot I, Dekel N. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994;269(48):30502–30509. [PubMed] [Google Scholar]
- 108.Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140(5):655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y, Kong N, Li N, et al. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology. 2013;154(9):3401–3409. doi: 10.1210/en.2013-1133. [DOI] [PubMed] [Google Scholar]
- 110.Fan HY, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abbey-Hosch SE, Smirnov D, Potter LR. Differential regulation of NPR-B/GC-B by protein kinase c and calcium. Biochem Pharmacol. 2005;70(5):686–694. doi: 10.1016/j.bcp.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 112.Channing CP, Tsafriri A. Mechanism of action of luteinizing hormone and follicle-stimulating hormone on the ovary in vitro. Metabolism. 1977;26(4):413–468. doi: 10.1016/0026-0495(77)90108-1. [DOI] [PubMed] [Google Scholar]
- 113.McGee EA Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 114.van Tol HT, van Eijk MJ, Mummery CL, van den Hurk R, Bevers MM. Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev. 1996;45(2):218–224. doi: 10.1002/(SICI)1098-2795(199610)45:2<218::AID-MRD15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]