Abstract
The primary hyperoxalurias are rare disorders of glyoxylate metabolism in which specific hepatic enzyme deficiencies result in overproduction of oxalate. Due to resulting severe hyperoxaluria, recurrent urolithiasis or progressive nephrocalcinosis are the principal manifestations. End stage renal failure occurs frequently and is followed by systemic oxalate deposition with its devastating effects. Due to lack of familiarity with the primary hyperoxalurias and their heterogeneous clinical expression, the diagnosis is often delayed until there is advanced disease. In recent years, improvements in medical management have been associated with better patient outcomes. Though there are several therapeutic options that can help to prevent early kidney failure, to date the only curative treatment is combined liver-kidney transplantation in those patients with type I primary hyperoxaluria. Promising areas of investigation are being identified. Knowledge of the spectrum of disease expression, early diagnosis, and initiation of treatment before renal failure ensues are essential to realize a benefit for patients.
Introduction
The primary hyperoxalurias (PH) type I and type II are relatively rare autosomal recessive inborn errors of glyoxylate metabolism, which result in markedly increased endogenous oxalate synthesis by the liver, and to a minor degree in PH II by other body cells. Type I primary hyperoxaluria (MIM 604285), is caused by deficient or absent activity of liver-specific peroxisomal alanine:glyoxylate aminotransferase (AGT, [1]). In some patients with PH type I enzyme is present but mistargeted to mitochondria where it is metabolically inactive. Primary hyperoxaluria type II is a somewhat milder but not benign variant (PHII, MIM 260000, 604296) that occurs as a result of deficient glyoxylate reductase/hydroxypyruvate reductase (GRHPR) enzyme activity [2]. A small number of patients have been described with a phenotype like that of PH I and PH II but with normal AGT and GRHPR enzyme activities. The specific etiology of the hyperoxaluria in such patients remains to be elucidated and they are referred to as non-I, non-II PH patients [3].
Among disorders causing hyperoxaluria, the primary hyperoxalurias are the most severe, ultimately leading to end-stage renal failure (ESRF) and if untreated, death in most of the patients [4]. Primary (endogenous) hyperoxaluria must be differentiated from the more common secondary forms. In secondary hyperoxaluria there is either dietary or other exposure to large amounts of oxalate or oxalate precursors or there is an underlying disorder that causes increased absorption of (dietary) oxalic acid from the intestinal tract. The latter is usually characterized by fat malabsorption. Among secondary causes of hyperoxaluria, those attributable to gastrointestinal disease (e.g. inflammatory bowel diseases (IBD), cystic fibrosis, status post bariatric surgery, short bowel syndrome (SBS) can lead to severe hyperoxaluria due to enhanced absorption of oxalate from the GI tract and may result in reduced renal function. Most other forms of absorptive secondary hyperoxaluria are of milder degree (0.55 to < 0.8 mmol/1.73m2/d) and usually carry a better prognosis. In contrast to IBD or SBS patients with PH show oxalate absorption within normal limits (<15%) [5].
Oxalate cannot be metabolized in mammals and is primarily eliminated via the kidneys as an end product of metabolism. Oxalate is freely filtered at the glomerulus and also secreted by the tubules. In all types of PH, very high urinary oxalate excretion, typically >1 mmol/1.73m2/24 hours (normal < 0.5), is observed. The urine becomes supersaturated for calcium oxalate resulting in formation of calcium oxalate complexes and crystals, which deposit in the renal parenchyma (nephrocalcinosis) and form stones in the urinary tract (urolithiasis), the clinical hallmarks of the primary hyperoxalurias (Figure 1). Progressive renal parenchymal inflammation and interstitial fibrosis from progressive nephrocalcinosis and recurrent urolithiasis along with secondary complications (urinary tract infection, obstruction) cause renal impairment, which progresses to end-stage renal failure (ESRF) over time [6–8]. Once renal function declines to a glomerular filtration rate below 30–40 ml/min per 1.73 m2 body surface area, renal excretion of oxalate is sufficiently compromised that plasma oxalate concentration rises (normal limits 1 – 6 µmol/l, [9]) and can rapidly exceed the supersaturation threshold for calcium oxalate as levels > 30 µmol/L are reached. Systemic deposition of calcium oxalate salts (oxalosis) then occurs in extra-renal tissues, including retina, myocardium, vessel walls, skin, bone, and the central nervous system among others (Figure 2). Long-term consequences include cardiomyopathy, cardiac conduction disturbances, vasculopathy, heart block, treatment resistant anemia, oxalate osteopathy resulting in debilitating bone and joint pain, retinopathy and if untreated, early death [4, 10]. Thus, the hepatic defect which primarily manifests within the urogenital tract, when advanced, becomes a devastating multisystemic disorder. Due to the risk of systemic oxalosis, renal replacement with dialysis or transplantation is required earlier in patients with PH then those with renal insufficiency from other causes. Yet, the systemic nature of the clinical manifestations may obscure the diagnosis for years. Awareness of PH as a possible cause and measurement of plasma as well as urinary oxalate are essential initial steps [7].
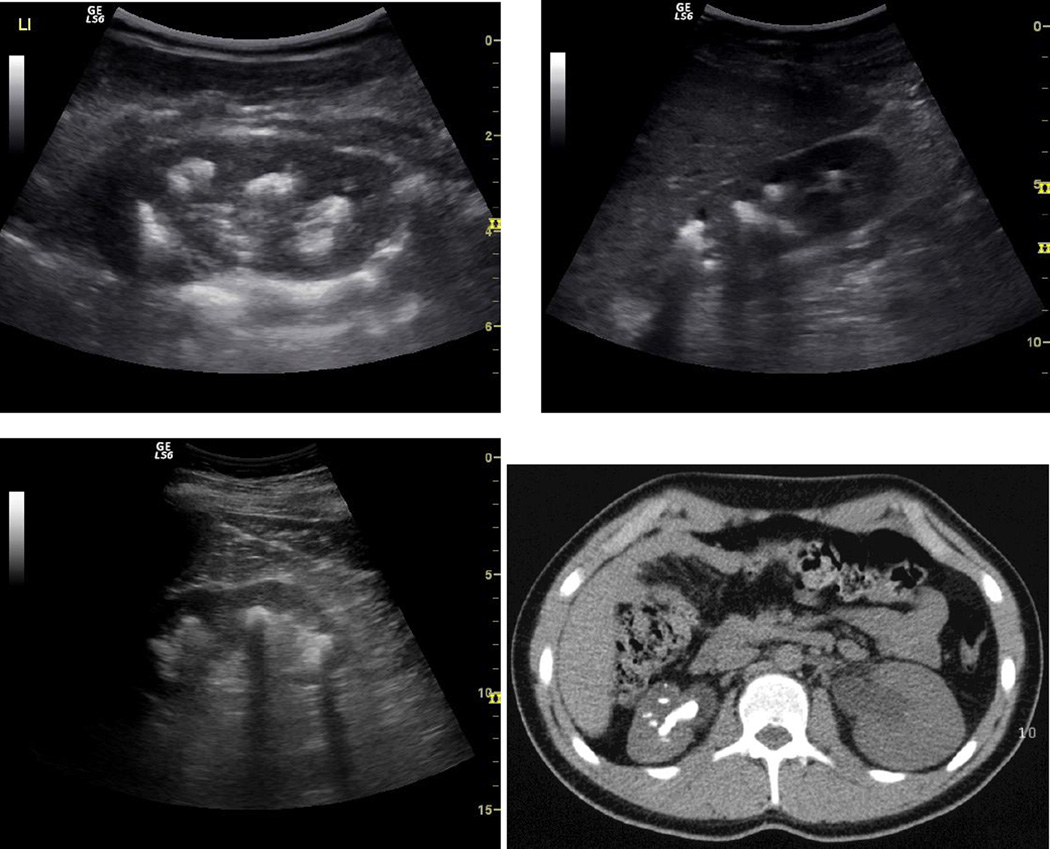
Figure 1.
a) Typical sonographical and x-ray findings of severe urolithiasis in patients with primary hyperoxaluria type 1 aged 5, 9 and 39 years of age and still normal or only slightly impaired renal function. b) Low enhanced CT scan showing a Staghorn calculus and 5 further smaller stones in the left kidney of a 16 year old patient with primary hyperoxaluria, uncompliant with regard to medication and fluid intake.
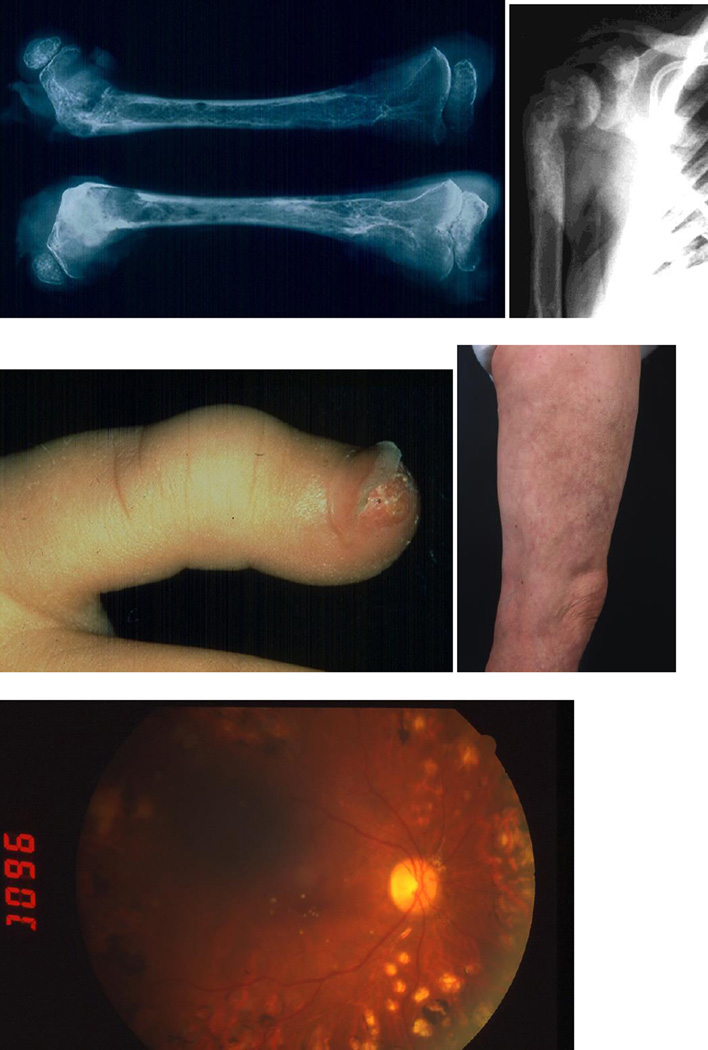
Figure 2.
Severe systemic oxalosis – calcium oxalate depositions in the bone (osteolytic lesions, epiphyseolysis), skin (crystal deposition at finger tip, livedo reticularis like picture) and retina in different patients with primary hyperoxaluria type 1.
Due to the nature of the metabolic defects in PH, marked hyperoxaluria is present from birth, yet there is marked heterogeneity of disease expression. Severe “infantile oxalosis” with early ESRF in PH type I occurs in some patients, while others lack clinically apparent sequela well into adulthood [4]. Although the primary hyperoxalurias are considered rare with a prevalence of 0.8 to 2.9 per 106 populations and an estimated incidence rate of 0.1 to 0.2 per 106 populations, they appear to be under diagnosed. This is suggested by the high proportion of patients (up to 35%) in whom diagnosis is made only after advanced renal failure has developed or, even worse, after early kidney graft failure from recurrent oxalosis following isolated kidney transplantation [6–8].
Early diagnosis, molecular sub typing and prompt initiation of treatment is of vital importance for PH patients. This is best illustrated by recent clinical studies showing improved outcome with median age at ESRF being now > 30 years, compared to earlier case series reporting a 50% risk for ESRF by age of 15 years, and about 80% ESRF rate by age 30 [6,11,12]. Yet, due to lack of familiarity with the disease, and the misconception of physicians and patients that stones are mostly benign, delays of many years from onset of symptoms to diagnosis are common [7, 12]. Onset of calcium oxalate stone formation in childhood or adolescence, frequent recurrences of stone disease, multiple calcium oxalate stones, or nephrocalcinosis in patients with renal failure of any age are important clues to the diagnosis and always warrant metabolic screening and if appropriate further specific diagnostic testing [13].
The first sign or symptom is usually blood in the urine, pain, passage of a stone, or urinary tract infection. Patients with renal failure due to “infantile oxalosis” present with failure to thrive, anemia and acidosis. The majority of patients are symptomatic early in life and mostly before 10 years of age [4, 6]. In some cases, however, the disease may go unrecognized either due to the absence of symptoms or to incorrect diagnosis, until patients reach 30 to 50 years of age [4,12]. Some patients present during adulthood with kidney failure as the first symptom, often after earlier symptoms have been misinterpreted.
Diagnostic Evaluation
Diagnostic evaluation should be conducted in a stepwise manner beginning with ultrasound examination or other imaging of the kidneys and urinary tract (Figure 1). In patients with PH, nephrocalcinosis is a frequent finding in infancy and early childhood, whereas later in life urolithiasis without nephrocalcinosis is more often encountered [12, 13]. Oxalate should be measured, either in 24 h urines, or in “spot” urine samples in infants and smaller children. The latter values are expressed as (molar) creatinine ratios, for which age related normal values have been established (14–16, Table 1). Significantly elevated values should be repeated for confirmation. In patients with severe hyperoxaluria (> 0.8 mmol/1.73m2/24 h), glycolate (PH I) and L-glyceric acid (PH type II) should also be determined. High glycolate excretion is observed in many but not all patients with PH type I. However, normal urine glycolate does not exclude the diagnosis. Elevated urinary L-glyceric acid excretion is found in nearly all patients with PH type II though occasional patients with normal L-glyceric acid values have been reported [17]. In PH type I the urinary oxalate excretion tends to be higher (2.14 ± 1.29 mmol/1.73 m2/d) than in PH type II (1.46 ± 0.49 mmol/1.73 m2/d), though the degree of overlap precludes its use for diagnostic differentiation [18].
Table 1.
Normal age related values for urinary oxalate, glycolate and L-glycerate excretion in 24 h urine expressed in mmol (mg) per 1.73m2 body surface area per day and spot urine samples (expressed as molar creatinine ratio).
| Parameter Age | Normal values | ||
|---|---|---|---|
| Oxalate | < 0.50 mmol (< 45 mg)/1.73 m2/d | ||
| Glycolate | < 0.50 mmol (< 45 mg)/1.73 m2/d | ||
| L-Glyceric acid | < 5 µmol/l | ||
| Molar Creatinine ratios (mmol/mol) | Oxalate | Glycolate | L-Glycerate |
| 0– 6 months | < 325–360 | < 363–425 | 14–205 |
| 7– 24 months | < 132–174 | < 245–293 | 14–205 |
| 2– 5 years | < 98–101 | < 191–229 | 14–205 |
| 5– 14 years | < 70–82 | < 166–186 | 23–138 |
| > 16 years | < 40 | < 99–125 | < 138 |
In patients with good renal function plasma oxalate measurement is of little diagnostic benefit. However, plasma oxalate, glycolate and glycerate concentrations can be helpful parameters in patients with ESRF. Plasma oxalate levels in PH-ESRF patients are almost always higher (> 60–100 µmol/l) than those of non-PH-ESRF patients (20–60 µmol/l, [9]). An elevated plasma glycolate or glycerate level may be helpful for distinguishing PH type I from type II disease [19, 20]. The diagnosis can be confirmed by determination of AGT and GRHPR enzyme activity in tissue from a percutaneous liver biopsy. Diagnosis by liver biopsy is still considered the gold standard. However, it is invasive and bears some risks (e.g. bleeding).
With advancing knowledge, molecular genetics has now reached a level of sensitivity and specificity that makes it feasible for definitive diagnostic testing [21]. Screening of the three most common mutations of AGXT detects both mutant alleles in 34 %, while direct sequencing can identify both alleles in 95% of type I patients [22]. In an index case with a known mutation, carrier status can be quickly established. Disadvantageous for mutation analysis is the fact that mutations are distributed throughout the 11 exons of the AGXT gene and more than 100 mutations have already been described. Genotyping for type II PH has been less complex, with only 15 mutations of GRHPR identified to date. A private mutation may appear only in one family. Therefore, sequencing of all exons sometimes is necessary to identify both mutated alleles and can be a time consuming process. Prenatal diagnosis can be determined in patients with one or without any mutation carrier status using linkage analysis of extra or intragenic markers [23]. For this purpose samples from several family members, as well as of an index case with specific diagnosis are necessary.
Primary Hyperoxaluria Type I
PH type I accounts for the majority (70–80%) of all cases. Although disease severity and resulting phenotype varies widely even within affected families, about 90 % of all (western) PH I cases become symptomatic in childhood to adolescence with the cardinal features being recurrent urolithiasis and/or nephrocalcinosis [6–8, 24]. In some cases, however, the disease may go unrecognized for years either due to the absence, subtlety, or misinterpretation of symptoms. It is important to note that deficiency of hepatic AGT never causes liver disease.. Most patients develop chronic renal failure between 25 and 45 years of life, though renal failure may occur as early as infancy and some patients retain renal function into the sixth or seventh decade.
Median age at diagnosis in the Netherlands is 7.3 years. Data from the International Registry for Primary Hyperoxaluria reports median age for diagnosis to be 6.0 years in patients with retained renal function compared to 23 years in those with ESRF at time of diagnosis [6, 12]. Early diagnosis leading to appropriate clinical management appears associated with improved overall outcome.
PH I appears to affect about 1 in 120,000 live births in central Europe [8, 11]. However, the true incidence and prevalence are unknown. In both Europe and the U.S., PH accounts for less than 1% of children and adolescents with ESRF [4, 7]. The disease is seen with higher frequency in certain parts of the world, including the northern African countries and the Canary Islands [25]. Whether there are regional variations in disease expression remains to be determined, though early observations are of interest [26]. A recent Japanese survey of 59 patients documented a milder course (30 % with onset of symptoms after age 25 years) than was observed in an historical European cohort [27, 28]. A substantial proportion of patients (11– 30 %, Japan up to 75%) are diagnosed late with symptoms of advanced renal failure or early kidney graft failure.
PH I is evidenced by marked clinical heterogeneity of the disease. In addition to marked interfamilial clinical variability yet a more remarkable intrafamilial phenotype variation can be observed despite identical genotype [29]. In the majority of patients with PH I, systemic oxalosis is believed to occur progressively after the GFR falls below 30 to 40 ml/min per 1.73 m2. As systemic oxalosis produces a confusing multitude of symptoms partially resembling rheumatoid or autoimmune disorders/vasculitis, correct diagnosis is not uncommonly overlooked for years. Due to the autosomal-recessive nature of PH type I and the importance of early diagnosis, siblings of affected patients should always be screened. An algorithm to assist in diagnosis has been published recently [13].
Genetics of PH I
The single copy AGXT-gene is located on chromosome 2p37.3. Its genomic sequence consists of 11 exons spanning ~ 10kb, resulting in a 1,7 kb cDNA with an open reading frame of 1176 bp. The gene product AGT is a homodimeric protein exclusively expressed in human hepatocytes, each 43 kDa subunit containing 392 amino acids and holding one molecule of pyridoxal-phosphate as cofactor [30]. The large N-terminal domain contains most of the catalytic active site, the cofactor binding site, and the dimerization interface. The function of the smaller C-terminal domain is largely unknown apart from containing the atypical peroxisomal targeting sequence (PTS1, LysLysLeu) and a presumed ancillary sequence PTS1A required for proper peroxisomal transport [31]. The crystal structure of normal AGT has been solved and provides valuable knowledge of AGT folding and how specific mutations and polymorphisms effect protein folding, dimerization and catalytic activity [32].
Almost a hundred disease causing mutations in the AGXT gene have been reported so far. The spectrum includes mostly point mutations leading to missense, nonsense and a number of splicing mistakes. Minor deletions respectively insertions and a few major deletions spanning several exons or other complex mutations account for the remaining third. Mutations can be found throughout the 11 exons of the gene. Analysis limited to the 4 most common mutations (c.508G>A, c.33_34insC, c.731T>C, c.454T>A) or selected exons provides less sensitivity than liver biopsy. Nonetheless, with whole AGXT gene sequencing becoming increasingly available, diagnostic accuracy rivals biopsy [33, 34].
In Caucasians two main haplotypes are found, named AGT major (80 % allelic frequency) and AGT minor (20 % allelic frequency). In Japan, and China the minor allele frequency is only around 2 % [21]. The haplotypes differ in a number of polymorphisms occurring in the minor allele, among them a single base-pair change on position c.32 C>T (p.P11L). Strong linkage disequilibrium between these variants is observed, thus not accidentally occurring conjointly in the minor allele. Most of them have no relevant effect, but the P11L polymorphism influences the function of AGT in several ways: (1) reduction in AGT activity in-vitro up to 30 %, (2) impaired dimerization of AGT subunits in-vitro, and (3) introduction of a weak N-terminal mitochondrial mistargeting sequence that directs about 5 % of the enzyme to the mitochondria [35].
More than 20 polymorphisms of the AGXT gene are reported and the relevance of some variations is still unclear. For example c.836T>C is found compound heterozygously with causative mutations in patients displaying a PH I phenotype, but when expressed in vitro on the AGT major allele this variant shows normal activity. Genotype data must always be interpreted with regard to ethnic background of the population served by the reporting institutions.
Although minor haplotype homozygotes show reduction of catalytic activity, this alone is not sufficient to cause disease in the absence of specific mutations. In addition a number of missense mutations (including c.508G>A) are predicted to cause no phenotype on their own in absence of P11L. Others have different consequences whether present on the minor or major allele. The c.508 G>A mutation represents the most common mutation (20 – 40 % allelic frequency, AF) worldwide. In the presence of P11L there is a synergistic effect, resulting in slowing of the rate of AGT folding and dimerization, which allows mitochondrial import of unfolded AGT monomers. The insertion c33_34insC leads to a protein truncation and is the most common mutation found on the major allele (~12 % AF in Caucasians). Another frequently encountered missense mutation c.731T>C (I244T, 6–9 % AF) in patients from Spanish-African descent produces functionally inactive aggregates. Since it represents the causative mutation in about 90 % of PH I patients from the Canary Islands with almost all being homozygous it is believed to result from a founder effect in this population [25]. Unfortunately, despite the common genotype, affected individuals from the Canary Islands show no uniform phenotype.
The molecular consequences of some mutations have been studied in-vitro, in the E.coli system or in the mouse model where AGT is dually expressed in the mitochondria and peroxisomes. Caution is appropriate in extrapolating in-vitro observations to humans. Nonetheless, despite their limitations, these first studies provided the setting for the exploration of novel pharmacologic approaches. For example, the two most common mutations present on the minor allele result in conformational changes that can be modelled in cell culture, and their consequences modified by manipulations of culture conditions [36]. Unfortunately no studies on human hepatocytes are available, but these investigations serve as proof of principle that the understanding of the molecular pathology can lead to new therapeutic approaches.
Though mutation analysis is valuable for diagnosis, thus far, there has been limited success in establishing genotype-phenotype correlations. Siblings who share the same genotype, but in whom clinical expression differs widely have been noted in a number of families worldwide [29]. Genotype-phenotype correlation has been described for some mutations of the AGXT gene [23, 34, 37]. Patients who are compound heterozygous for the c.508G>A mutation appear to respond to pharmacologic doses of pyridoxine with a partial reduction, or in those homozygous, with a sometimes complete normalization of urine oxalate excretion [38]. While most helpful in clinical management, partial pyridoxine response appears insufficient for the majority of c.508G>A heterozygotes to overcome the deleterious consequences of the disease in the long-run. With the constitution of the pan-European hyperoxaluria consortium (OxalEurope) joining forces with the Mayo Clinic database soon more than 500 PH cases will be available for genotype-phenotype studies. By collection of a large cohort of families and subsequent careful analysis we may be able to better identify genetic and environmental modifiers responsible for the variability in disease expression.
Primary Hyperoxaluria Type II
Type II is a monogenic, autosomal recessive disorder predominantly affecting hepatic glyoxylate metabolism, though GRHPR is also expressed in lesser amounts in most body tissues [23]. As a result of reduced enzyme activity increased amounts of glyoxylate and hydroxypyruvate are available for conversion by lactate dehydrogenase (LDH) to both oxalate and L-glyceric acid. Excessive amounts of both metabolites are excreted by the kidney. The hyperoxaluria leads to recurrent nephrolithiasis and less frequently, nephrocalcinosis. An asymptomatic clinical course for many years or unilateral presentation of stones initially with calculi appearing in the contra-lateral kidney only after nephrectomy or late in the course is not uncommon (7, 39). Until the late 1990’s only about 30 cases have been reported in the literature. Now with better awareness and molecular diagnostic tools available this entity is recognized more often.
Although the clinical course can be very similar to PH type I, patients with type II primary hyperoxaluria appear to have less severe stone formation, as well as less nephrocalcinosis and better preservation of renal function over time when compared to patients with type I disease [18]. Thus far no case of infantile renal failure with oxalosis resulting from GRHPR deficiency has been reported. Taking the small numbers and therefore the relative paucity of long-term data into account there is still a risk of renal impairment. Once severe renal impairment is reached the patient is also at high risk for systemic oxalosis.
The GRHPR gene is located on chromosome 9 (9p11) and consists of 9 exons, which encode a 328 amino acid (36 kDa) protein. To date 15 causative mutations in the GRHPR gene have been described, with the one base pair deletion G (c.103delG) in exon 2 being the most common in patients of Caucasian descent (allelic frequency of approximately 40 %). The spectrum of mutations identified includes deletions, insertions, missense, and nonsense mutations, all resulting in loss of protein expression or catalytic activity. A surprisingly high rate of homozygous mutations is found, with an unusually high proportion of minor deletions and insertions. The data for c.103delG indicates that this founder mutation is of north European descent.
Since PH II patients do not benefit from pyridoxine, since liver transplantation to date has not been confirmed to correct the hyperoxaluria, and since such patients may do reasonably well with kidney transplant alone, distinguishing between type I and type II PH is of paramount importance. Without careful testing, patients may be misclassified which may result in inappropriate therapy.
Non I, non II Primary Hyperoxaluria
The etiology of the hyperoxaluria in non-I, non-II PH remains to be elucidated. Indeed, since the diagnosis is one of exclusion (patients have normal AGT and GRHPR enzyme activity, normal enteric oxalate absorption, and no identifiable cause of secondary hyperoxaluria) it is unknown at present whether the patients described represent one or several different disorders. Little is known of the long term outcome of patients with this form of primary hyperoxaluria, since few patients have been well characterized to date [40]. Most patients have been diagnosed in infancy or early childhood, with marked hyperoxaluria identified during evaluation for urinary tract stones. To date, renal failure has not been identified, though the mean age of the patients described is quite young.
Treatment
Treatment should be initiated as soon as accurate and reproducible baseline measurements of urine oxalate excretion have been obtained. A large daily fluid intake (> 3 litres per 1.73 m2 per day) is essential. If this is impossible, placement of a gastrostomy tube should be considered to ensure adequate fluid administration. In case of fever, vomiting, diarrhea or other significant fluid losses patients with PH should receive i.v. fluids. As only a very small proportion of urinary oxalate is derived from the diet in patients with PH, dietary oxalate restriction is of limited benefit [5]. Nevertheless, nutrients extremely rich in oxalate and an excessive intake of ascorbic acid, a precursor of oxalate, should be avoided.
Pyridoxal phosphate is an essential cofactor of AGT and pharmacological doses of pyridoxine may significantly reduce hyperoxaluria in some patients with type I PH. Approximately 30 % of PH I patients have some degree of sensitivity to pyridoxine [4, 24, 35]. Patients with certain mutations in particular, respond to pyridoxine, and may even demonstrate normalization of urinary oxalate [33, 38]. In order to assess pyridoxine responsiveness (defined as > 30 % reduction in urinary oxalate excretion), pyridoxine is started at a dose of 5 mg/kg body weight per day. Recent data suggests that maximum benefit is likely to be achieved at less than 10 mg/kg/day [23], though higher doses may occasionally be considered on a trial basis. Occasionally, a much smaller daily dose (10–20 mg) is also effective [4]. A trial of at least 3–6 months of pyridoxine, with effectiveness determined by measurement of urinary oxalate excretion, is warranted in all PH I patients. For those who respond favorably, pyridoxine should be continued indefinitely or until liver transplantation. Evaluating pyridoxine response in patients who are in renal failure is difficult. Many such patients lack urine output, and in those who still have urine, other factors such as systemic oxalate deposits may influence urine oxalate excretion. Genotyping for mutations associated with pyridoxine response can be helpful in such patients.
The aim of treatment with alkali citrate is the reduction of the urinary calcium oxalate saturation [41]. Citrate predominantly binds to calcium, forming a soluble complex and reducing the precipitation of calcium with other substances like oxalate. The daily dosage of alkali citrate is 0.1 – 0.15 g/kg body weight (0.3 – 0.5 mmol/kg) of a sodium or sodium/potassium citrate preparation. Citrate is metabolized in the liver to bicarbonate, the alkali load leads to reduction of the intra-tubular reabsorption of citrate and hence, more citrate is excreted via the urine [42]. Binding of calcium and citrate complexes is further increased at a higher urinary pH. Alkali citrate preparations lead to decreased stone production or lesser expression of nephrocalcinosis [41]. The effect of alkali therapy, and the patient’s compliance, is checked by measuring urinary pH and citrate excretion. The therapeutic effect of orthophosphate is comparable to that of alkali citrate medication. Long term follow up reports of orthophosphate treatment suggest efficacy for patients with PH [43].
The role of the GI tract in oxalate physiology is becoming better understood. A greater proportion of body oxalate appears to be eliminated by the GI tract when renal function is compromised. Colonic secretion and absorption of oxalate are regulated by physiologic stimuli including the renin-angiotensin system [33]. Recently, the anion exchanger SLC26A6 has been shown to be involved in gastrointestinal and renal oxalate excretion [44]. Certain micro-organisms in the colon, such as Oxalobacter formigenes contain enzymes that metabolize oxalate, reducing luminal concentrations and thus oxalate absorption. Oxalate secretory pathways for intestinal oxalate elimination have also been identified in the large intestine [45, 46]. A physiologic interaction of O. formigenes with rat colonic mucosa to modulate the handling of oxalate in colonized animals, with induction of enteric secretion/excretion, has also been described [47]. This has led to the hypothesis that this organism might be helpful in the treatment not only of patients with secondary, but also with primary hyperoxaluria [47]. A recent pilot study has shown a reduction of urinary oxalate excretion in the majority of PH patients treated with Oxalobacter [48]. Oral administration of Oxalobacter or oxalate degrading enzymes is currently undergoing study as a potential treatment.
Renal replacement therapy
With reduced capacity for renal clearance of excess oxalate in chronic renal failure, hyperoxalemia and plasma supersaturation for calcium oxalate ensue with risk of systemic oxalosis. Therefore, specific renal replacement strategies are required. When the GFR falls to less than 30 ml/min per 1.73m2, prompt renal replacement with transplantation or dialysis is needed in most PH patients. Dialysis treatment may need to be considered even earlier in some patients [49]. If dialysis is needed as a bridge to transplantation, intensive dialysis, e.g. hemodialysis on 5 to 6 days of the week for 3–4 hours per session plus nightly peritoneal dialysis, is often needed to match daily oxalate production rates and thus minimize the likelihood of systemic oxalosis.
In some patients with PH not even such intensive regimens are able to remove sufficient oxalate [49–51]. Therefore transplantation should be performed as early as possible [52]. Doing so will not only reduce injurious effects of oxalate on body tissues, but also reduce the oxalate burden that must be eliminated by the transplanted kidney [4].
Transplantation
Except for those patients with a complete response to pyridoxine (normalizaton of urine oxalate), early disease recurrence is a significant risk in isolated kidney transplantation in PH type I. Though reasonable outcomes of kidney only transplantation (including mostly living donors) were observed in North America [53], this approach has largely been replaced by combined liver/kidney transplantation. With the metabolic defect in the liver and the problem being overproduction of oxalate without other avenues for its disposition, it is clearly necessary to perform total hepatectomy, even though the liver is normal in every other aspect. Actuarial patient and liver allograft survivals at five years following combined liver and kidney transplantation are 80% and 72%, respectively [52]. Specific risk factors for renal graft failure due to recurrent oxalosis are young age (< 5 years) and a long period on dialysis (> 2 years) prior to transplantation. The latter is related to the large body oxalate burden that accumulates over time on dialysis [4, 52]. Administration of very generous fluids and of alkali citrate or neutral phosphate during the first months or even years after transplantation is essential because of the slow mobilisation of the accumulated body calcium oxalate stores and thus persistent hyperoxaluria [54]. The hyperoxaluria can cause injury to the renal allograft and must be carefully managed until it resolves after all oxalate stores have been mobilized and eliminated [55]. Whether or not dialysis is performed for the first days after transplantation depends on kidney graft function and degree of hyperoxalemia and hyperoxaluria to which the transplanted kidney is exposed.
The rationale of pre-emptive liver transplantation, instead of waiting until ESRF occurs, appears attractive at first look [56]. However, the risk of losing a graft or even a patient who might have lived many years longer without transplantation raises serious ethical questions. Thus far, over a dozen patients with PH I not in renal failure have been treated with pre-emptive liver transplantation achieving reasonable results [56]. However, the heterogeneity of the disease has to be kept in mind, when discussing a pre-emptive transplant option, as patients may remain well and with stable kidney function over many years under adequate treatment.
In patients with PH type II the potential benefit of liver transplantation is unclear. Hence, and as the disease most often is not as severe as the type I PH, isolated kidney transplantation is the treatment of choice in this group of patients.
Outlook
The long term outlook for patients with PH has improved substantially over the past two decades due to improved understanding of the metabolic pathways, molecular genetics, and pathophysiology of these inherited disorders. Recent work elucidating the role of the gastrointestinal tract in oxalate elimination and the prospect of molecular stabilization of mutant AGT leading to enhanced enzyme activity provide exciting prospects for the future treatments. Knowledge of the spectrum of disease expression, early diagnosis, and initiation of treatment before renal failure ensues are essential to realize the benefit for patients.
References
- 1.Danpure CJ. Primary hyperoxaluria: from gene defects to designer drugs. Nephrol Dial Transplant. 2005;20:1525–1529. doi: 10.1093/ndt/gfh923. [DOI] [PubMed] [Google Scholar]
- 2.Cregeen DP, Williams EL, Hulton S, Rumsby G. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat. 2003;22:497–506. doi: 10.1002/humu.9200. [DOI] [PubMed] [Google Scholar]
- 3.Monico CG, Persson M, Ford GC, Rumsby G, Milliner DS. Potential mechanisms of marked hyperoxaluria not due to primary hyperoxaluria I or II. Kidney Int. 2002;62:392–400. doi: 10.1046/j.1523-1755.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- 4.Leumann E, Hoppe B. The primary hyperoxalurias. J Am Soc Nephrol. 2001;12:1986–1993. doi: 10.1681/ASN.V1291986. [DOI] [PubMed] [Google Scholar]
- 5.Sikora P, von Unruh GE, Beck B, Feldkötter M, Zajaczkowska M, Hesse A, Hoppe B. [13C2]oxalate absorption in children with idiopathic calcium oxalate urolithiasis or primary hyperoxaluria. Kidney Int. 2008;73:1181–1186. doi: 10.1038/ki.2008.63. [DOI] [PubMed] [Google Scholar]
- 6.Van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol Dial Transplant. 2003;18:273–279. doi: 10.1093/ndt/18.2.273. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe B, Langman C. A United States survey on diagnosis, treatment and outcome of patients with primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 8.Cochat, et al. Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol. 2006;21:1075–1081. doi: 10.1007/s00467-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe B, Kemper MJ, Bökenkamp A, Portale AA, Cohn RA, Langman CB. Plasma calcium-oxalate supersaturation in children with primary hyperoxaluria and end stage renal disease. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann G, Krieg T, Weber M, Sidhu H, Hoppe B. Unusual painful sclerotic like plaques on the legs of a patient with late diagnosis of primary hyperoxaluria type I. Br J Dermatol. 2004;151:1104–1107. doi: 10.1111/j.1365-2133.2004.06247.x. [DOI] [PubMed] [Google Scholar]
- 11.Kopp N, Leumann E. Changing pattern of primary hyperoxaluria in Switzerland. Nephrol Dial Transplant. 1995;10:2224–2227. doi: 10.1093/ndt/10.12.2224. [DOI] [PubMed] [Google Scholar]
- 12.Lieske JC, et al. International Registry for primary hyperoxaluria and Dent’s disease. Am J Nephrol. 2005;25:290–296. doi: 10.1159/000086360. [DOI] [PubMed] [Google Scholar]
- 13.Milliner DS. The primary hyperoxalurias: an algorithm for diagnosis. Am J Nephrol. 2005;25:154–160. doi: 10.1159/000085407. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe B, Leumann E. Hyperoxaluria. In: Blau N, Hoffmann G, Leonard J, Clarke J, editors. Physician's Guide to the Treatment and Follow-up of Metabolic Diseases. Springer Verlag Heidelberg; 2005. pp. 279–285. [Google Scholar]
- 15.Leumann EP, Dietl A, Matasovic A. Urinary oxalate and glycolate excretion in healthy infants and children. Pediatr Nephrol. 1990;4:493–497. doi: 10.1007/BF00869828. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe B, Leumann E, Milliner D. Urolithiasis in childhood. In: Geary D, Schäfer F, editors. Comprehensive Pediatric Nephrology. New York: Elsevier/WB Saunders; 2008. pp. 499–525. [Google Scholar]
- 17.Rumsby G, Sharma A, Cregeen DP, Solomon LR. Primary hyperoxaluria type 2 without L-glycericaciduria: is the disease under-diagnosed? Nephrol Dial Transplant. 2001;16:1697–1699. doi: 10.1093/ndt/16.8.1697. [DOI] [PubMed] [Google Scholar]
- 18.Milliner DS, Wilson DM, Smith LH. Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int. 2001;59:31–36. doi: 10.1046/j.1523-1755.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 19.Marangella M, Petrarulo M, Vitale C, Cosseddu D, Linari F. Plasma and urine glycolate assays for differentiating the hyperoxaluria syndromes. J Urol. 1192;148:986–989. doi: 10.1016/s0022-5347(17)36796-4. [DOI] [PubMed] [Google Scholar]
- 20.Marangella M, Petrarulo M, Mandolfo S, Bitale C, Cosseddy D, Linari F. Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron. 1992;60:64–70. doi: 10.1159/000186708. [DOI] [PubMed] [Google Scholar]
- 21.Danpure CJ. Molecular aetiology of primary hyperoxaluria type 1. Nephron Exp Nephrol. 2004;98:e39–e44. doi: 10.1159/000080254. [DOI] [PubMed] [Google Scholar]
- 22.Monico CG, Rossetti S, Schwanz HA, Olson JB, Lundquist PA, Dawson DB, Harris PC, Milliner DS. Comprehensive mutation screening in 55 probands with type 1 primary hyperoxaluria shows feasibility of a gene-based diagnosis. J Am Soc Nephrol. 2007;18:1905–1914. doi: 10.1681/ASN.2006111230. [DOI] [PubMed] [Google Scholar]
- 23.Rumsby G, Williams E, Coulter-Mackie M. Evaluation of mutation screening as a first line test for the diagnosis of the primary hyperoxalurias. Kidney Int. 2004;66:959–963. doi: 10.1111/j.1523-1755.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe B, Latta K, von Schnakenburg C, Kemper MJ on behalf of the Arbeitsgemeinschaft für pädiatrische Nephrologie. Primary Hyperoxaluria: the German experience. Am J Nephrol. 2005;25:276–281. doi: 10.1159/000086358. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo V, Alvarez A, Torres A, Torregrosa V, Hernández D, Salido E. Presentation and role of transplantation in adult patients with type 1 primary hyperoxaluria and the I244T AGXT mutation: Single-center experience. Kidney Int. 2006;70:1115–1119. doi: 10.1038/sj.ki.5001758. [DOI] [PubMed] [Google Scholar]
- 26.Coulter-Mackie MB, Rumsby G. Genetic heterogeneity in primary hyperoxaluria type 1: impact on diagnosis. Mol Genet Metab. 2004;83:38–46. doi: 10.1016/j.ymgme.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149:518–522. doi: 10.1007/BF01957682. [DOI] [PubMed] [Google Scholar]
- 28.Takayama T, Nagata M, Ichiyama A, Ozono S. Primary hyperoxaluria type 1 in Japan. Am J Nephrol. 2005;25:297–302. doi: 10.1159/000086361. Erratum in: Am J Nephrol. 2005;25:416. [DOI] [PubMed] [Google Scholar]
- 29.Hoppe B, Danpure CJ, Rumsby G, et al. A vertical (pseudodominant) pattern of inheritance in the autosomal recessive disease primary hyperoxaluria type I. Lack of relationship between genotype, enzymic phenotype and disease severity. Am J Kidney Dis. 1997;29:36–44. doi: 10.1016/s0272-6386(97)90006-8. [DOI] [PubMed] [Google Scholar]
- 30.de la Chapelle A, Wright FA. Linkage disequilibrium mapping in isolated populations: the example of Finland revisited. Proc Natl Acad Sci. 1998;95:12416–12423. doi: 10.1073/pnas.95.21.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson JC, Dise RS, Ritchie MD, Hanks SK. Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity. J Biol Chem. 2002;277:29028–29035. doi: 10.1074/jbc.M111697200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Roe SM, Hou Y, Bartlam M, Rao Z, Pearl LH, Danpure CJ. Crystal structure of alanine:glyoxylate aminotransferase and the relationship between genotype and enzymatic phenotype in primary hyperoxaluria type 1. J Mol Biol. 2003;331:643–652. doi: 10.1016/s0022-2836(03)00791-5. [DOI] [PubMed] [Google Scholar]
- 33.van Woerden CS, Groothoff JW, Wijburg FA, Annink C, Wanders RJ, Waterham HR. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 2004;66:746–752. doi: 10.1111/j.1523-1755.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams E, Rumsby G. Selected exonic sequencing of the AGXT gene provides a genetic diagnosis in 50% of patients with primary hyperoxaluria type 1. Clin Chem. 2007;53:1216–1221. doi: 10.1373/clinchem.2006.084434. [DOI] [PubMed] [Google Scholar]
- 35.Danpure CJ, Lumb MJ, Birdsey GM, Zhang X. Alanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting in human hereditary kidney stone disease. Biochim Biophys Acta. 2003;1647:70–75. doi: 10.1016/s1570-9639(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 36.Lumb MJ, Danpure CJ. Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations. J Biol Chem. 2000;275:36415–36422. doi: 10.1074/jbc.M006693200. [DOI] [PubMed] [Google Scholar]
- 37.Pirulli D, Marangella M, Amoroso A. Primary hyperoxaluria: genotype-phenotype correlation. J Nephrol. 2003;16:297–309. [PubMed] [Google Scholar]
- 38.Monico CG, Rossetti S, Olson JB, Milliner DS. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 39.Kemper MJ, Conrad S, Müller-Wiefel DE. Primary hyperoxaluria type 2. Eur J Pediatr. 1997;156:509–512. doi: 10.1007/s004310050649. [DOI] [PubMed] [Google Scholar]
- 40.Monico CG, Persson M, Ford GC, Rumsby G, Milliner DS. Potential mechanisms of marked hyperoxaluria not due to primary hyperoxaluria I or II. Kidney Int. 2002;62:392–400. doi: 10.1046/j.1523-1755.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- 41.Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol. 1993;7:207–211. doi: 10.1007/BF00864405. [DOI] [PubMed] [Google Scholar]
- 42.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 43.Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH. Results of long term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331:1553–1558. doi: 10.1056/NEJM199412083312304. [DOI] [PubMed] [Google Scholar]
- 44.Hatch M, Freel RW, Vaziri ND. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J Am Soc Nephrol. 1999;10:S324–S328. [PubMed] [Google Scholar]
- 45.Jiang Z, Asplin JR, Evan AP, et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 46.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res. 2005;33:1–16. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 47.Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp. reduces urinary oxalate excretion promoting enteric oxalate excretion. Kidney Int. 2006;69:1–8. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 48.Hoppe B, Beck B, Gatter N, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type I (PH I) Kidney Int. 2006;70:1305–1311. doi: 10.1038/sj.ki.5001707. [DOI] [PubMed] [Google Scholar]
- 49.Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006;70:1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 50.Hoppe B, Graf D, Offner G, et al. Oxalic acid elimination in children with chronic renal failure: comparison between hemodialysis and peritoneal dialysis. Pediatr Nephrol. 1996;10:488–492. doi: 10.1007/s004670050145. [DOI] [PubMed] [Google Scholar]
- 51.Bunchman TE, Swartz RD. Oxalate removal in type I hyperoxaluria or acquired oxalosis using HD and equilibration PD. Perit Dial Int. 1994;14:81–84. [PubMed] [Google Scholar]
- 52.Jamieson NV European PHI Transplantation Study Group. A 20-year experience of combined liver/kidney transplantation for primary hyperoxaluria (PH1): the European PH1 transplant registry experience 1984–2004. Am J Nephrol. 2005;25:282–289. doi: 10.1159/000086359. [DOI] [PubMed] [Google Scholar]
- 53.Saborio P, Scheinman JI. Transplantation for primary hyperoxaluria in the United States. Kidney Int. 1999;56:1094–1100. doi: 10.1046/j.1523-1755.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 54.Hoppe B, Kemper MJ, Bokenkamp A, Portale AA, Cohn RA, Langman CB. Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 55.Monico CG, Milliner DS. Combined liver-kidney and kidney alone transplantation in primary hyperoxaluria. Liver Transplantation. 2001;7:954–963. doi: 10.1053/jlts.2001.28741. [DOI] [PubMed] [Google Scholar]
- 56.Nolkemper D, Kemper MJ, Burdelski M, et al. Long term results of pre-emptive liver transplantation in primary hyperoxaluria type 1. Pediatr Transplant. 2000;3:177–181. doi: 10.1034/j.1399-3046.2000.00107.x. [DOI] [PubMed] [Google Scholar]