Abstract
Introduction
A 24 year old man with primary hyperoxaluria type 1 (PH1) presented with a rapidly progressive axonal and demyelinating sensorimotor polyradiculoneuropathy shortly after the onset of end stage renal disease. His plasma oxalate level was markedly elevated at 107 µmol/L (normal: <1.8 µmol/L).
Methods
A sural nerve biopsy was performed. Teased fiber, paraffin and epoxy sections, and morphometric procedures were performed on this sample and on an archived sample from a 22 year old man as an age- and gender-matched control. Embedded teased fiber electron microscopy was also performed.
Results
The biopsy revealed secondary demyelination and axonal degeneration. Under polarizing light, multiple bright hexagonal, rectangular, and starburst inclusions typical of calcium oxalate monohydrate crystals were seen1,2,3.
Discussion
Proposed mechanisms of nerve damage include disruption of axonal transport due to crystal deposition, toxic effect of oxalate, or nerve ischemia related to vessel occlusion from oxalate crystal deposition.
Keywords: Peripheral neuropathy, primary hyperoxaluria type 1, renal failure, nerve pathology, crystalline neuropathy
Primary hyperoxaluria type 1 (PH1) is a rare autosomal recessive disease caused by a mutation in the alanine-glyoxalate aminotransferase (AGXT) gene which encodes the hepatic enzyme alanine-glyoxylate aminotransferase (AGT). AGT is responsible for converting glyoxylate into glycine. When the enzyme is deficient or defective, glyoxylate accumulates and is converted to oxalate. Oxalate cannot be metabolized and is excreted by the kidneys 4. In PH1, high urinary levels of oxalate lead to kidney stones and deposition of calcium oxalate crystals within the renal tubules and parenchyma, which results in progressive renal fibrosis. As kidney function declines, oxalate production increasingly exceeds renal oxalate clearance. Plasma oxalate levels rise, and insoluble calcium oxalate crystals are deposited in tissues throughout the body, including the kidney, heart, bone, blood vessels, retina, muscle, skin, and nerve. Hemodialysis is needed to remove oxalate from the body, but removal is incomplete, and even when it is intensive, dialysis is often insufficient to prevent progressive systemic oxalate deposition 5,6,7. Because oxalate removal with dialysis is incomplete, the treatment of choice for patients with PH1 and poor kidney function, is combined liver/ kidney transplantation. Liver transplantation is required in the majority of PH type 1 patients to restore the hepatic enzyme defect and protect the renal allograft from recurrent oxalate nephropathy. Significant neurologic improvement in patients with neuropathy has been noted following combined liver and kidney transplantation 8.
Only a few case reports have described the nerve pathology associated with primary hyperoxaluria type 1. Demyelination (particularly at the internodes) and axonal degeneration have been reported,1 and some case reports describe crystals in nerve 1,3. The characterization and location of these crystals within nerve has not been well described, and their pathological significance has remained unclear 1,3. In each of these case reports, the patient had significant systemic oxalosis with deposition of oxalate into multiple tissues, whereas the patient we describe had oxalosis affecting peripheral nerve preferentially without other clinically discernible extrarenal manifestations.
Approval by our Institutional Review Board and Biospecimens Committee was received for this study.
CASE REPORT
A 24 year old man presented to our institution for evaluation of rapidly progressive numbness and weakness affecting the upper and lower limbs. His past medical history was significant for PH1 diagnosed at age 3 years when he presented with nephrolithiasis and marked hyperoxaluria. Subsequently he was treated for presumed PH1 with pyroxidine, 300 mg daily. Between ages 17 and 20 he had multiple kidney stones. At age 20, his pyridoxine dose was increased to 400 mg twice daily, given the finding that he had an AGXT mutation which can confer pyridoxine responsiveness. At that time, quantitative sensory testing was performed, which was normal with no evidence of peripheral neuropathy. He continued to develop kidney stones, and at age 21, he stopped his medications on his own initiative. Five months prior to presentation at age 24, the patient developed end stage renal disease and initiated hemodialysis. Within weeks of starting dialysis, the patient developed numbness of the hands and feet. Within 4 months of the onset of neurologic symptoms, he developed significant leg weakness which limited standing to brief periods of time. He described hand weakness causing difficulty twisting tops off bottles and holding his cell phone. At the time of presentation in the Neurology clinic, he was wheelchair bound with profound weakness and numbness of the upper and lower limbs. He also exhibited significant sensory ataxia. He described sharp shooting pain, dull aching pain, and severe muscle cramping.
On neurologic evaluation, he was areflexic with a stocking-glove distribution of sensory loss to all modalities (vibration, proprioception, touch/pressure, temperature, and pain). He had profound weakness distally [1/5 Medical Research Council (MRC) scale] with mild to moderate weakness proximally (3–4/5 MRC scale).
At presentation, his plasma oxalate level was 107 µmol/L (normal <1.8 µmol/L). Genetic testing of the AGXT gene revealed 2 known pathologic mutations consistent with PH1. The first mutation involved exon 4 c. 454 T>A (TTC>ATC) resulting in an amino acid change (F152l), and the second mutation involved intron 8 c.847–3C→G.
The patient’s creatine kinase (CK) fluctuated between 800 and 900 U/L (normal: 52–336 U/L). His C-reactive protein was elevated at 11.8 mg/L (normal: <3.0 mg/L). Cerebrospinal fluid (CSF) protein was mildly elevated at 54 mg/dL (normal: <35 mg/dL). CSF glucose was 61 mg/dL with 1 red blood cell and 1 white blood cell, which are within normal limits. Other laboratory testing for treatable causes of peripheral neuropathy were unremarkable. Nerve conduction studies and electromyography revealed a severe, diffuse mixed axonal and demyelinating polyradiculoneuropathy (table 1). Fibrillation potentials and reduced recruitment of high amplitude, long duration, polyphasic motor unit potentials were noted at all locations examined, including the lumbar and thoracic paraspinal muscles. Neither conduction block nor temporal dispersion was observed. Only the ulnar F-wave was obtainable, and the latency was mildly prolonged compared with the F-estimate.
TABLE 1.
Electrodiagnostic Study
| Nerve Conduction Study | ||||||
|---|---|---|---|---|---|---|
| Nerve | Type | Recording | Side | Amplitude (mV) | Conduction velocity (m/s) | Distal Latency (ms) |
| Fibular | M | EDB | Left | NR (>2.0) | NR (>41) | NR (<6.6) |
| Fibular | M | TA | Left | 1.3 (>5.1) | 37 (>43) | 6.6 (<6.8) |
| Tibial | M | AH | Left | NR | NR | NR |
| Sural | S | Ankle | Left | NR | NR | NR |
| Median | M | APB | Left | 0.8 (>4.0) | 26 (>48) | 4.6 (<4.5) |
| Musculocutaneous | M | Biceps | Left | 1.9 (>4.0) | 3.5 (<3.4) | |
| Ulnar | M | ADM | Left | 1.0 (>6.0) | 26 ( >51) | 3.7 (<3.6) |
| Median | S | Index | Left | 3.0 uv (>15) | 3.9 (<3.6) | |
| Ulnar | S | Fifth | Left | 5.0 uv (>10 ) | 3.8 (<3.1) | |
| Electromyography | ||||||
|---|---|---|---|---|---|---|
| Muscle | Side | Insertional Activity |
Fibrillations | Reduced recruitment |
Long Duration |
High Amplitude |
| Thoracic PSP | Left | Increased | + | + | ||
| FDI | Right | Increased | +++ | ++ | + | + |
| PT | Right | Increased | +++ | +++ | ||
| Triceps | Right | Increased | ++ | +++ | + | + |
| Deltoid | Right | Increased | ++ | + | + | |
| Gmax | Left | Increased | + | + | ||
| Lumbar PSP | Left | Increased | + | |||
| TA | Left | Increased | +++ | +++ | ||
| MG | Left | Increased | +++ | +++ | + | + |
| VM | Left | Increased | +++ | +++ | + | + |
| TFL | Left | Increased | + | + | ||
All amplitudes are those obtained with distal stimulation. mV, millivolt; m/s, meters per second; ms, millisecond; M, motor; EDB, extensor digitorum brevis; NR, no response; TA, tibialis anterior; S, sensory; APB, abductor pollicis brevis; ADM, abductor digiti minimi; PSP, paraspinal, FDI, first dorsal interosseous; PT, pronator teres; Gmax, gluteus maximus; MG, medial gastrocnemius; VM, vastus medialis; TFL, tensor fasciae latae; L/R, left right.
The patient underwent sural nerve biopsy. Teased fiber, paraffin and epoxy sections, and morphometric procedures were performed by standard techniques9. Embedded teased fiber electron microscopy was performed by standard and published techiques10. Morphometry, teased fiber, and epoxy sections were also performed on an archived sample from a 22 year old man as an age and gender-matched control.
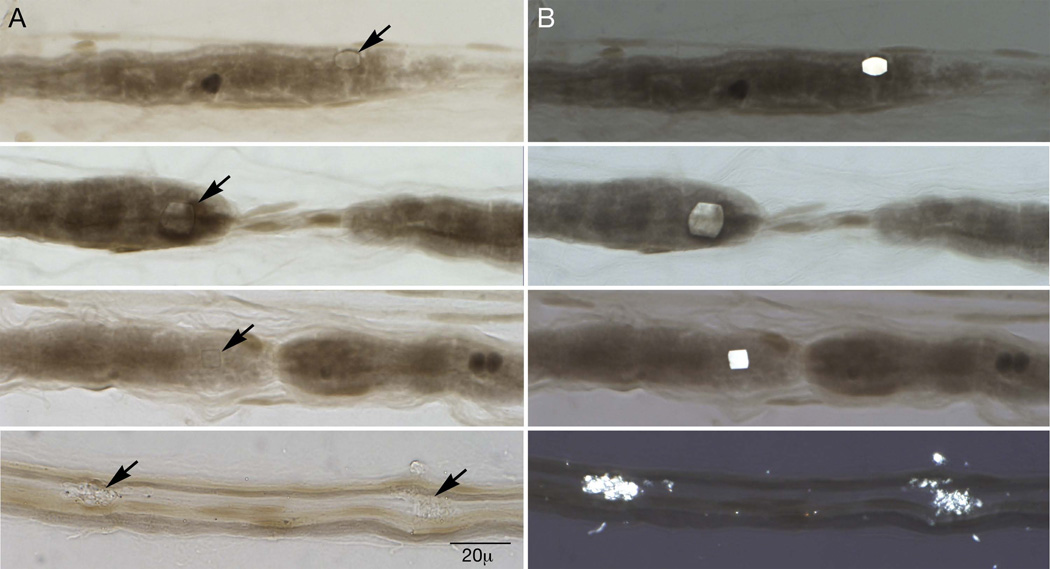
The teased fiber preparation (figure 1) revealed demyelination in 34 (37%) fibers. There were 24 (26%) fibers with multiple demyelinated segments, suggestive of secondary demyelination. There was significant axonal degeneration in 18 (20%) fibers. Under polarized light, 47 birefringent rectangular and hexagonal inclusions typical of calcium oxalate monohydrate crystals were found within teased fibers (figure 1). Twenty-three of the 47 crystals were perinodal. No crystals, demyelination, or axonal degeneration were noted in the control specimen. In the control specimen 96 (96%) fibers were normal; our patient had 39 (42%) normal fibers. Four empty strands were noted in the control, and 8 empty strands were seen in our patient.
Figure 1.
Teased fiber preparation of sural nerve: Calcium oxalate monohydrate crystals are noted by arrows on panel A under standard light and are clearly visible on panel B under polarized light. Note the associated perinodal demyelination.
Diameter histograms of the biopsied sural nerve showed mild to moderate loss of fibers with a shift of the large diameter peak to smaller sizes. The myelinated fiber density for the patient was 4103 nerve fibers/mm2 when compared with 8581 nerve fibers/mm2 for the control. The index of dispersion showed variability in the density of fibers, 2.32, which is indicative of multifocal fiber loss, compared with 1.30 for the control.
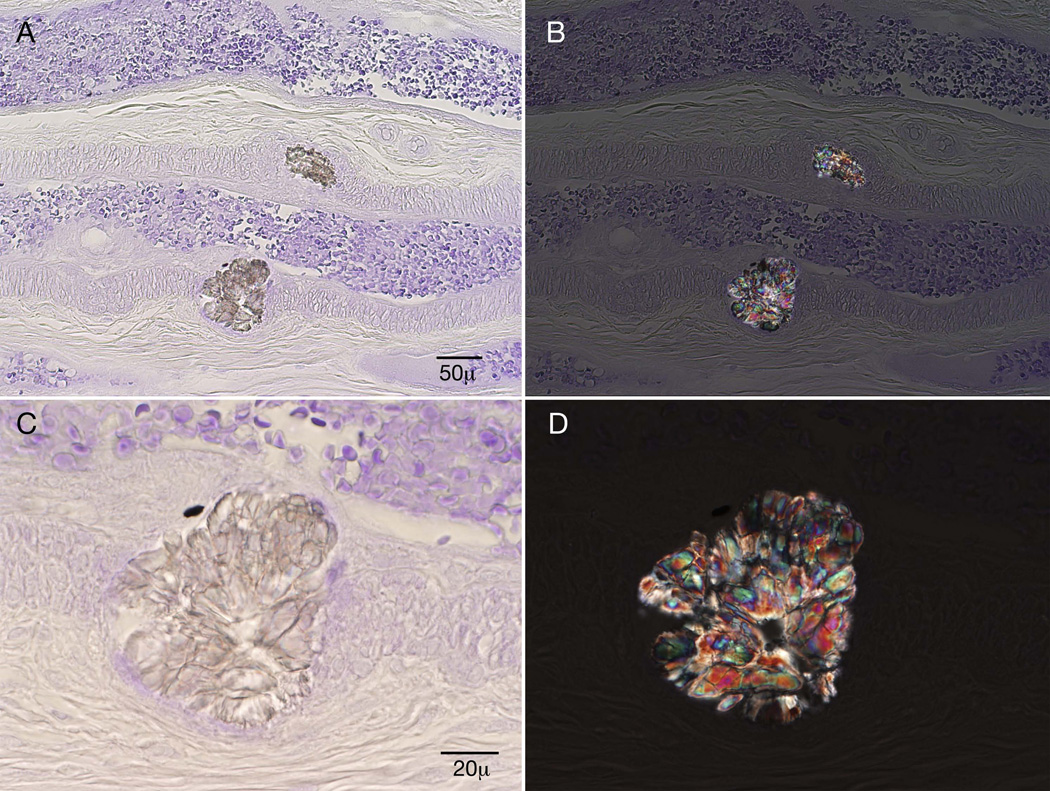
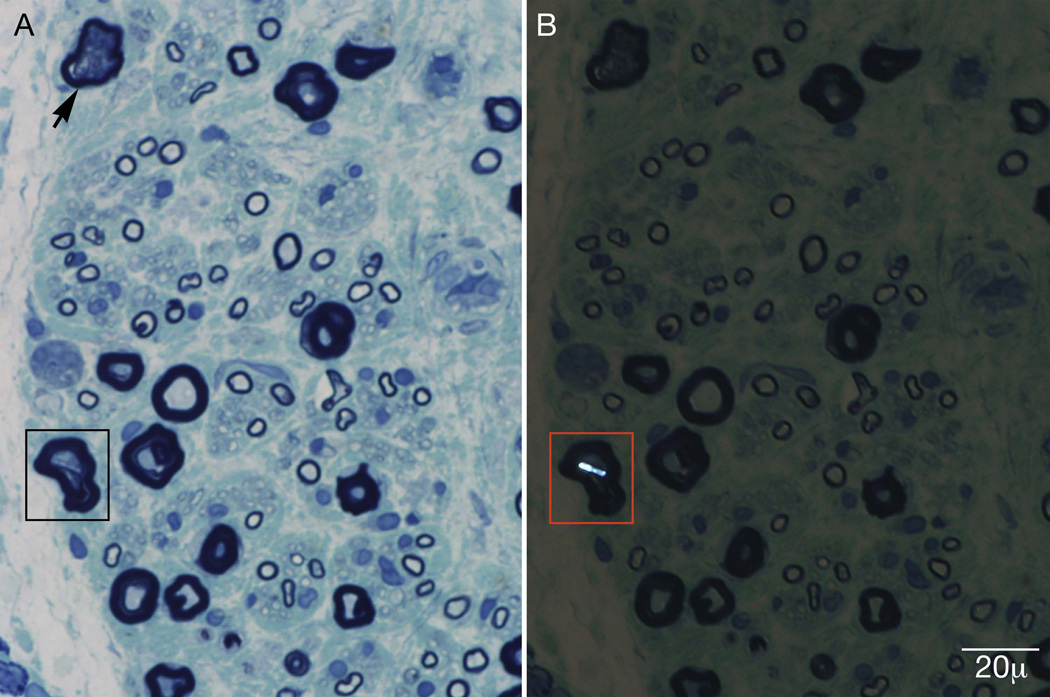
Two large starburst inclusions typical of calcium oxalate crystals were noted adjacent to an epineurial blood vessel (figure 2), on methyl violet preparation. Mild endoneurial and perivascular inflammation was noted, but inflammation was not a prominent feature. Epoxy sections revealed multifocal fiber loss, prominent axonal degeneration, and the presence of calcium oxalate crystals within individual nerve fibers (figure 3).
Figure 2.
Methyl violet preparation: Two starburst crystals are noted adjacent to an epineurial blood vessel. Panels B and D are under polarized light.
Figure 3.
Epoxy sections stained with methylene blue reveal multifocal fiber loss, prominent axonal degeneration (arrow), and calcium oxalate crystals within nerve fibers (box). Panel B is under polarized light.
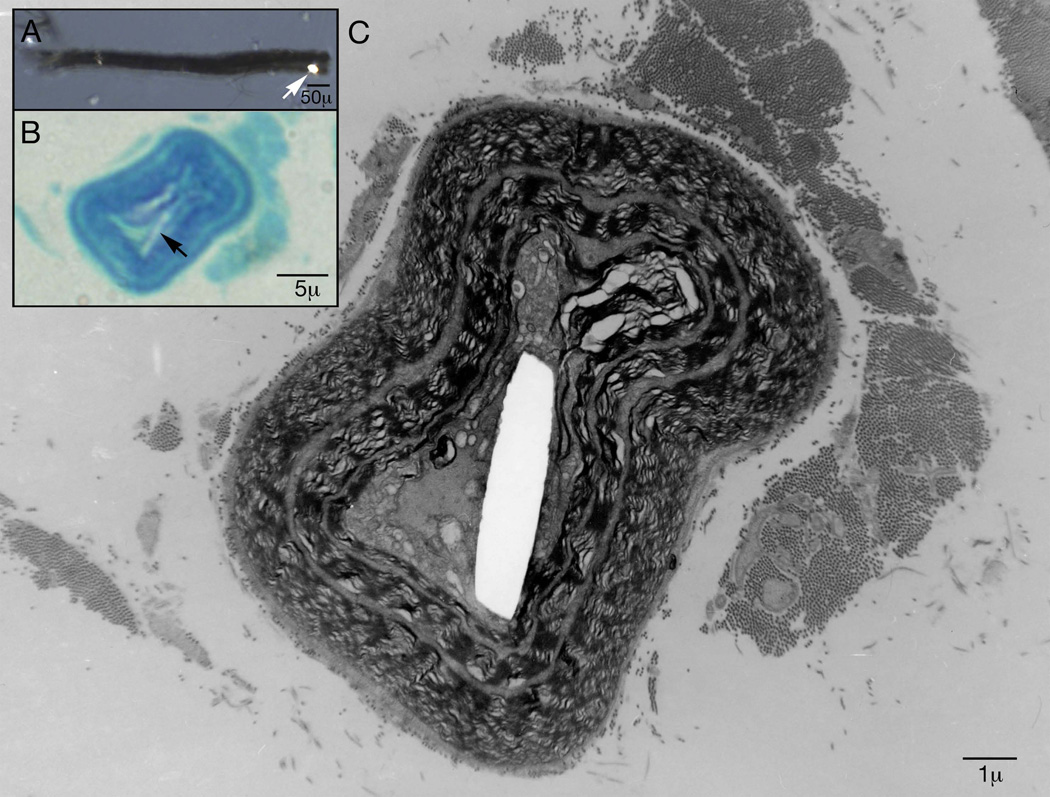
Electron microscopy (EM) was utilized to investigate the location of these abnormalities within nerve. The EM along the length of individual plastic-embedded teased fibers revealed a calcium oxalate crystal within the nerve myelin, just outside of the axolemma (figure 4).
Figure 4.
Electron microscopy along the course of an individual plastic embedded teased nerve fiber reveals a calcium oxalate monohydrate crystal within nerve myelin just outside of the axolemma. In box A the arrow points to a crystal under polarized light on a single teased fiber. In box B the arrow points to that calcium oxalate crystal on an epoxy section stained with methylene blue.
Pyridoxine was reinitiated at a dose of 600 mg daily, and the patient continued on hemodialysis 6 days per week. His weakness progressed to the point he was unable to feed himself. He was admitted to the hospital for initiation of continuous veno-venous hemodialysis (CVVH) in an effort to remove the greatest amount of oxalate. At admission, his plasma oxalate level was 114.8 µmol/L. After 4 days of CVVH, the plasma oxalate level fell to 27 µmol/L. His dialysis regimen was subsequently transitioned to daily intermittent hemodialysis. On daily hemodialysis, the predialysis plasma oxalate concentration remained in the range of 16.6 to 54.8 µmol/L. His pyridoxine was decreased to 500 mg daily due to the concern that pyridoxine toxicity could be contributing to the oxalate-induced nerve injury. Additionally, he was given 60 mg of methylprednisolone over 3 consecutive days as treatment for inflammation that can accompany oxalate deposition and contribute to tissue injury.
Over the next 2 weeks, his neurologic status improved, and he regained the ability to shake hands, feed himself, and ambulate with a walker. Five months after his original presentation, the patient received a combined liver/kidney transplant. He had prompt and sustained good function of both the kidney and liver allografts with no rejection episodes. Renal allograft function was stable with an estimated glomerular filtration rate of 42 ml/min/1.73 m2 (normal: >60 ml/min/1.73m2), and his plasma oxalate level was 3.5 µmol/L. His serum creatinine stabilized at 1.9 mg/dL (normal: 0.8–1.3 mg/dL). Post-transplant, his neurologic status continued to improve. At last neurologic follow-up, 3 months post-transplant, physical examination demonstrated significant improvement in proximal muscle strength, and he was no longer dependent on a wheelchair or gait aid for ambulation. However, he had persistent distal weakness and sensory loss.
Discussion
PH1 polyneuropathy is characterized by axonal and demyelinating features on nerve conduction studies and electromyography. The pathologic features include significant axonal degeneration and demyelination1 with associated multifocal fiber loss and a shift toward smaller diameter fibers. Inflammation is present but is not a prominent feature. The hexagonal and starburst crystals of oxalate polyneuropathy are distinctive, and in a patient with primary hyperoxaluria, they are diagnostic of calcium oxalate monohydrate deposition within nerve 1,2,3. Oxalate crystals are often perinodal and within the Schwann cell cytoplasm.
The mechanism of nerve injury in primary hyperoxaluria type 1 is unclear. Soluble oxalic acid likely enters the Schwann cell or neuron, combines with calcium and forms insoluble calcium oxalate crystals. A direct toxic effect of calcium oxalate crystals is possible; they have been reported to incite inflammatory reactions in the kidney11. Inflammation was noted on our biopsy, and this may have contributed to nerve injury. Calcium oxalate also has a toxic effect on mitochondria in animal models,12 and the oxalate ion is believed to have injurious effects on cells13. Additionally, the crystals may cause physical disruption of axonal transport, leading to secondary demyelination and axonal injury3, although the crystal seen on electron microscopy was outside the axolemma. The multifocal fiber loss and crystal deposition seen in endoneurial blood vessels raises the possibility of ischemic nerve injury. Acrocyanosis, ulceration, and peripheral gangrene related to crystal deposition have been reported in PH18. Although our patient’s biopsy did not reveal vasculitis or vascular occlusion, the multifocal fiber loss observed is typical of ischemic nerve injury.
The elevated CK seen in this patient may have been the result of either muscle atrophy due to fulminant denervation or to calcium oxalate deposition within muscle.
Other contributing factors in this case include potential pyridoxine toxicity and uremia. Pyridoxine in doses as low as 200 mg per day can cause neuropathy, although neuropathy associated with pyridoxine toxicity typically presents as a symmetric sensory neuropathy14,15. Profound weakness, as seen in our case would be unusual14,15. In addition, the patient had not taken pyridoxine in the 2 years prior to the onset of renal failure. Uremia may have also played a role in the neuropathy. This presentation is similar to uremic neuropathy. The crystals could simply represent cellular sequestration of toxic material16, although the patient began developing weakness and numbness weeks to months after the onset of regular hemodialysis. This would be atypical of uremia and more in keeping with nerve injury associated with oxalosis.
Acknowledgments
This work was supported by a grant from the Mayo Foundation to the Peripheral Nerve Lab. The work was also supported by The Rare Kidney Stone Consortium which is a part of NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54-DK083908 from the NIDDK and the NIH National Center for Advancing Translational Sciences (NCATS). Support for the Mayo Clinic Hyperoxaluria Center is also provided by the Oxalosis and Hyperoxaluria Foundation.
Dr. Dyck- Peter J. Dyck is receiving financial support directed to research activity in the Peripheral Neuropathy Laboratory at Mayo Clinic, Rochester, MN from Pfizer, Inc.; Pfizer Japan, Inc.; ISIS, Inc.; Alnylam, Inc. and other pharmaceutical houses. Dr. Peter J. Dyck serves as an Associate Editor for Diabetes and receives an honorarium. He also receives financial support from Mayo Foundation.
Abbreviations
- AGT
alanine-glyoxalate aminotransferase enzyme
- AGXT
alanine-glyoxalate aminotransferase gene
- CK
creatinine kinase
- CVVH
continuous veno-venous hemodialysis
- EM
electron microscopy
- MRC
Medical Research Council
- PH1
Primary hyperoxaluria type 1
Footnotes
Disclosures:
Dr. Berini- No disclosures
Dr. Tracy- No disclosures
Ms. Engelstad- No disclosures
Dr. Lorenz- No disclosures
Dr. Milliner- No disclosures
Contributions:
Dr Berini- design, acquisition and analysis of data along with drafting and editing the manuscript
Ms. Engelstad- embedded single fiber electron microscopy, creation of the figures, editing the figures, and critical edits
Dr. Tracy- acquisition of data, analysis and review of the manuscript
Dr. Milliner- acquisition of data, review of manuscript
Dr. Lorenz- acquisition of data, review of manuscript
Dr. Dyck- concept and design, acquisition and analysis of data, critical editing of the manuscript and figures
References
- 1.Bilbao JM, Berry H, Merotta J, Ross RC. Peripheral neuropathy in oxalosis. A case report with electron microscopic observations. Le Journal Canadien Des Sciences Neurologiques. 1976;3(1):63–67. doi: 10.1017/s0317167100026020. [DOI] [PubMed] [Google Scholar]
- 2.Daudon M, Jungers P, Bazin D. Peculiar morphology of stones in primary hyperoxaluria. New England Journal of Medicine. 2008;359(1):100–102. doi: 10.1056/NEJMc0800990. [DOI] [PubMed] [Google Scholar]
- 3.Moorhead PJ, Cooper DJ, Timperley WR. Progressive peripheral neuropathy in patient with primary hyperoxaluria. British Medical Journal. 1975;2:312–313. doi: 10.1136/bmj.2.5966.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009 Jun;75(12):1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006 Nov;70(9):1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012 Jun 12;8(8):467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 7.Tang X, Voskoboev N, Wannarka SL, Olson JB, Milliner DS, Lieske JC. Oxalate quantification in hemodiasylate to assess dialysis quality in primary hyperoxaluria. J Am Soc Nephrol. 2013;24:587A. doi: 10.1159/000360624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulter-Mackie MB, White CT, Hurley RM, Chew BH, Lange D. Primary hyperoxaluria type 1. GeneReviews. 2011 [Google Scholar]
- 9.Dyck PJ, Dyck PJ. Pathologic alterations of nerves. In: PJ D, Thomas PK, editors. Peripheral Neuropathy. 4th ed. 2005. pp. 737–780. [Google Scholar]
- 10.Dyck PJ, Lais AC. Electron microscopy of teased nerve fibers: method permitting examination of repeating structures of same fiber. Brain Res. 1970;23:418–424. doi: 10.1016/0006-8993(70)90067-3. [DOI] [PubMed] [Google Scholar]
- 11.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest. 2013 Jan 2;23(1):236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMartin KE, Wallace KB. Calcium oxalate monohydrate, a metabolite of ethylene glycol is toxic for rat renal mitochondrial function. Toxicological Sciences. 2005 Mar;84(1):195–200. doi: 10.1093/toxsci/kfi062. [DOI] [PubMed] [Google Scholar]
- 13.Khan SR. Reactive oxygen species at the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. Journal of Urology. 2013;189:803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schamburg H, Kaplan J, Windebank A, Vick N, Rasmus S, Pleasure D, et al. Sensory neuropathy from pyridoxine abuse: a new megavitamin syndrome. N Eng J Med. 1983 Aug 25;309(8):445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- 15.Parry GJ, Bredesen DE. Sensory neuropathy with low-dose pyridoxine. Neurology. 1985;35:1466–1468. doi: 10.1212/wnl.35.10.1466. [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Johnson WJ, Lambert EH, Bushek W, Pollock M. Detection and evaluation of uremic peripheral neuropathy in patients on hemodialysis. Kidney International Supplement. 1975;2:201–205. [PubMed] [Google Scholar]