Abstract
Seaweed-origin electrophilic compounds are proposed as a class of neuroprotective compounds that provide neuroprotection through activation of the Nrf2/ARE pathway. Electrophilic hydroquinones are of particular interest due to their ability to become electrophilic quinones upon auto-oxidation. Although many marine plants produce a variety of electrophilic compounds, the detailed mechanism of action of these compounds remain unknown. Here, we focused on the neuroprotective effects of zonarol (ZO), a para-hydroquinone-type pro-electrophilic compound from the brown algae Dictyopteris undulata. We show that ZO activates the Nrf2/ARE pathway, induces phase-2 enzymes, and protects neuronal cells from oxidative stress. ZO is the first example of a neuroprotective pro-electrophilic compound obtained from brown algae.
Keywords: Zonarol, ARE, Nrf2, Pro-electrophilic drugs, Para-hydroquinone
1. Introduction
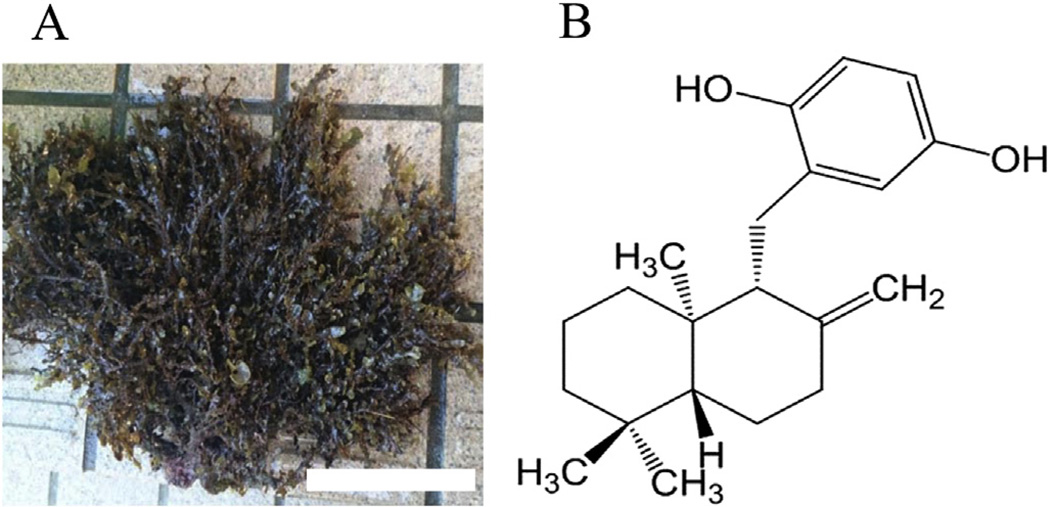
The marine biomass is critical for life, and different species of marine plants are important both culturally and nutritionally in disparate parts of the world. In northwest regions bordering the Pacific Ocean, various seaweeds have been used as foodstuffs and in folkloric medicines to maintain human health throughout the ages [1–3]. Some species of seaweeds and their ingredients have become a popular and recognized food around the world [1–3]. In order to obtain a marine biomass-based inhibitor of inflammation, e.g., from carrageenan-induced edema in mice, we screened extracts of 150 marine species from the seashore of the Japanese mainland, and found that a crude extract of Dictyopteris undulata (Fig. 1A) had the most potent inhibitory effect in this inflammation model [4]. Subsequent experiments revealed the active compound to be zonarol (ZO, Fig. 1B), based on analysis of nuclear magnetic resonance data after bioassay-guided purification from crude extract [4]. Originally, this sesquiterpene hydroquinone from Dictyopteris undulata had been reported to be a fungitoxic compound by Fenical and Cimino in the 1970's [5]. Subsequently, the chemical structure of the hydroquinone was elucidated in 1986 [6]. In a prior study, we described the protective role of ZO against dextran sodium sulfate-induced colon injury in young male Slc:ICR mice, representing a murine model of the inflammatory bowel disease, ulcerative colitis [7]. However, actions on the brain of ZO and its intracellular signaling pathways remain largely unknown.
Fig. 1.
Dictyopteris undulata (A) and chemical structure of ZO (B). Note that ZO has a para-hydroquinone moiety, and shares the same basic chemical structure of sesquiterpene. Scale bar in “A” represents 10 cm.
Previously, we have shown that electrophilic compounds, such as the hydroquinone product of carnosic acid (CA) from the herb rosemary, manifest a significant advantage over other antioxidant molecules in mediating cytoprotection against oxidative damage because their actions are sustained and amplified by transcriptionmediated signaling pathways [7–12]. Electrophiles can induce the expression of a series of antioxidant enzymes, called “phase-2 enzymes”, including NADPH quinone oxidoreductase 1 (NQO1), glutathione S-tranferase, heme oxygenase-1 (HO-1), and peroxiredoxin 4 (PRDX4), all of which provide efficient cytoprotection by regulating intracellular redox state [13,14]. Given our experience with plant metabolites and the occurrence of structurally similar compounds in natural marine products, we have been searching for electrophiles in sea-based organisms as candidate neuroprotective compounds [15–17]. In this present study, among the vast number of substances produced by marine organisms, we have focused on ZO, because it is a para-hydroquinone, representing a pro-electrophilic compound. Here, we found that ZO activates the Nuclear factor (erythroid-derived 2)-like 2/antioxidantresponsive element (Nrf2/ARE) pathway and protects neuronal cells against oxidative stress. Our findings represent the first report of a pro-electrophilic compound from seaweed that provides neuroprotection through activation of the Nrf2/ARE pathway.
2. Materials and methods
2.1. Chemicals
Glutamate (Glu), Hoechst 33,258, rotenone, paraformaldehyde, Tween 20, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma. ZO was prepared as a 10 mM stock solution in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the culture medium was less than 0.1%. ZO was extracted from the brown algae Dictyopteris undulata collected in the Bousou Peninsula area in Chiba Prefecture in Japan, as described previously [4].
2.2. HT22 cultures and MTT assay
HT22 hippocampal neuronal cells were cultured as described previously [9]. The cells were maintained in 10-cm dishes (Invitrogen, Carlsbad, CA) containing 10 mL of Dulbecco's Modified Eagle medium supplemented with 10% (v/v) heat-inactivated (56 °C, 30 min) fetal calf serum (Invitrogen, Carlsbad, CA). The cells were seeded into 24-well plates at a density of 4 × 104 cells/cm2. After a 5-h incubation, the desired compounds were added to the cultures. Sixty minutes later, 5 mM Glu was added, and the cells were then incubated for an additional 24 h. To evaluate cell survival of the HT22 cells, we performed an MTT assay [8,9].
2.3. Nrf2/ARE reporter gene assay
HT22 cells were seeded into 48-well plates at a density of 4 × 104 cells/cm2 and incubated for 5 h in PBS containing 1000 ng of a reporter construct [ARE(GSTYa)-luciferase] plus Transfast (Promega, Madison, WI). Transfection efficiency was normalized to β-galactosidase activity after co-transfection with pSV-β-gal (Promega). For reporter gene assays, cells were transfected with the above reporter construct and 200 ng of pSV-β-gal for 1 h. The cells were then washed in PBS alone and incubated in the culture medium for another 24 h with or without ZO. Firefly luciferase activity and β-galactosidase activity in cell lysates were measured by using a luciferase system and β-Galactosidase enzyme assay system, (Promega), respectively [10,11]
2.4. RT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen) from HT22 cells after 24 h of treatment with ZO (1 µM). All procedures were performed as described previously [10,11]. RNase-free conditions were used to prevent mRNA degradation. First-strand cDNA was synthesized with Superscript II RT (Invitrogen) using random primers, according to the manufacturer's instructions. One onehundredth of the cDNA was used for 1 PCR reaction. At the completion of the PCR, 10 µL of PCR products were mixed with 2 µL of loading buffer and electrophoresed in 1.5% agarose gel in the presence of 0.5 µg/mL ethidium bromide. PCR conditions were as follow: 50 °C for 2 min, 95 °C for 10 min followed by designated cycles at 95 °C for 15 s and 60 °C for 1 min. The following pairs of mouse primers, specific for β-actin, nqo1, ho-1, and prdx4, were used–
- β-Actin (β-actin: 287 bp):
- Forward 5′-ATC CGT AAA GAC CTC TAT GC-3′
- Reverse 5′-AAC GCA GCT CAG TAA CAG TC-3′
- NADP(H):quinone oxidoreductase1 (nqo1: 923 bp):
- Forward 5-ATC CTT CCG AGT CAT CTC TA-3′
- Reverse 5-CAA CGA ATC TTG AAT GGA GG-3′
- Heme oxygenase-1 (ho-1: 617 bp):
- Forward 5′-AGG TGT CCA GAG AAG GCT T-3′
- Reverse 5′-ATC TTG CAC CAG GCT AGC A-3′
- Peroxiredoxin 4 (prdx4: 322 bp):
- Forward 5′-CAA AGC CAA GAT CTC CAA GC-3′
- Reverse 5′-GAT CTG ATG GTT CAG GTC AG-3′
2.5. Primary cerebrocortical cultures
Cerebrocortical neurons were used as an in vitro system in order to investigate the cellular mechanism of neuronal death caused by glutamate, as previously described [8]. With increasing days in vitro (DIV), the expression level of glutamate receptors in cortical cultures increases. In immature cortical cultures, which do not yet express functional NMDA-type glutamate receptors, oxidative glutamate toxicity is predominates; under these conditions, high concentrations of Glu (e.g., 2 mM) induce cell death via oxidative stress [18]. To investigate this form of glutamate-induced oxidative stress in the present study, we prepared cerebrocortical cultures from embryonic day 17 (E17) Sprague–Dawley rats and examined them at DIV2. To assess the ability of ZO to block Glu-induced neuronal death in immature cortical neurons, we identified neurons by performing double-immunofluorescence labeling with anti-NeuN and anti-MAP-2 to specifically label neurons. Hoechst staining was used to assess nuclear morphology for apoptosis. Rotenone, a complex I inhibitor of the mitochondrial electrontransport chain, also induces oxidative stress in immature cortical cultures, and this model was also used to assess the neuroprotective potential of ZO.
2.6. Immunocytochemistry
Cultures were fixed with 3% paraformaldehyde at room temperature for 20 min. After 3 washes in PBS, the cells were permeabilized with 0.3% Triton X-100 for 5 min. After 3 additional washes in PBS, the cells were incubated at 4 °C overnight with primary antibodies. They were then washed 3 times in PBS containing 0.2% Tween 20, and next incubated with secondary antibodies for 1 h at room temperature. Thereafter, the cells were again washed, and their nuclei stained with Hoechst 33,258 (5 µg/ml) for 5 min. Stained preparations were mounted and examined by epifluorescence microscopy.
2.7. Antibodies
Primary antibodies included anti-Nrf2, anti-MAP2, and anti-NeuN antibodies (Sigma, St. Louis, MO), the latter two used to identify neuronal dendrites and nuclei, respectively. As secondary antibodies, we used rhodamine-conjugated anti-mouse IgG (Jackson Immuno Research Laboratories, Westgrove, PA) [8]. Protein A immobilized on Sepharose CL-4B (Sigma) was also used [8].
2.8. Statistical analysis
Experiments presented herein were repeated at least 3 times with each experiment performed in quadruplicate. Data are presented as mean ± SD (for in vitro experiments) or SEM (for in vivo experiments).
3. Results
3.1. Neuroprotective effect of ZO in HT22 cells
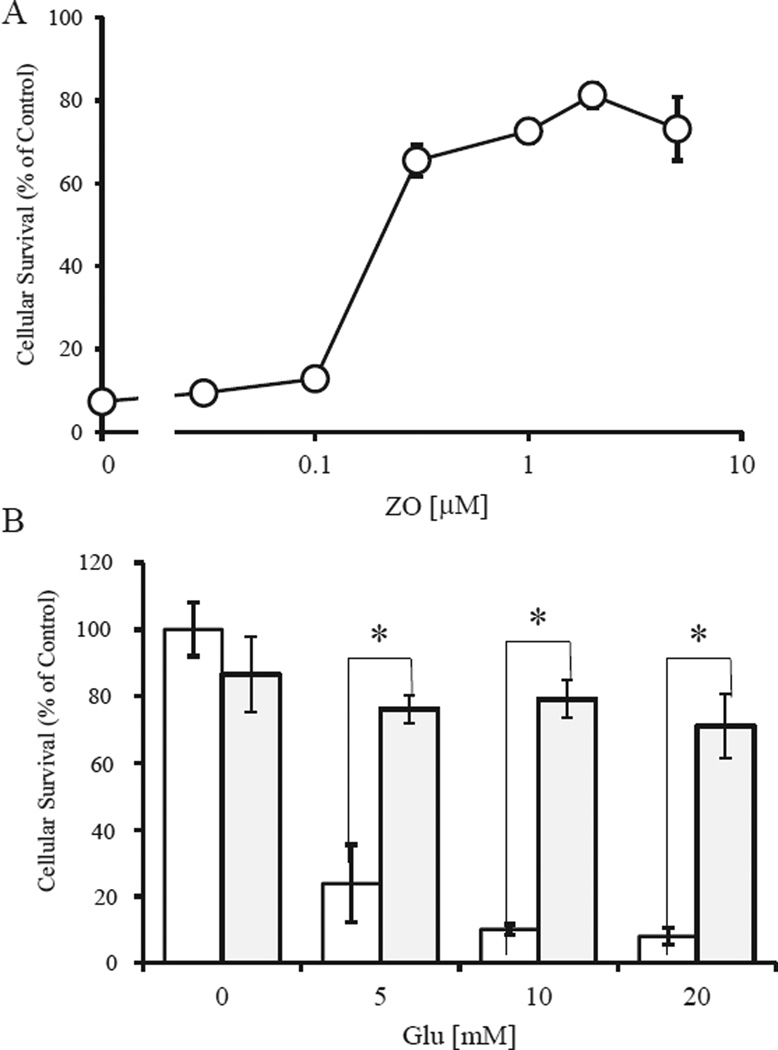
ZO contains a para-hydroquinone moiety that is converted to a quinone by autoxidation; such quinones are capable of activating the Nrf2/ARE pathway [8]. We used HT22 cells, a neuronal cell line from mouse hippocampus, as a model for oxidative cell damage. In HT22 cells, high concentrations (mM levels) of Glu can induce cell death by depleting intracellular glutathione (GSH) through inhibition of cystine influx [18,19]. Cell survival was quantified by using the MTT assay (Fig. 2A). Treatment of cells with 5 mM Glu induced cell death by oxidative stress within 24 h. Co-treatment of cells with ZO led to dose-dependent protection from the effects of Glu (Fig. 2A). Next, we assessed the protective effects of 1 µMZO against oxidative glutamate toxicity caused by various concentrations of Glu (Fig. 2B). At 1 µM, ZO almost completely prevented death induced by 5–20mMGlu in HT22 cells.We also found that the ED50 (protective effect) of ZO was 0.22 µM. Thus, the therapeutic index, defined as the ratio of ED50 to LD50, for ZO is 14.2 fold. These results suggest that ZO, a para-hydroquinone sesquiterpene, can protect neuronal cells against oxidative stress with a reasonable therapeutic index.
Fig. 2.
Protection of HT22 cells by ZO. Dose-dependent protective effects of ZO (A) and protection by 1 µM ZO against various concentrations of Glu (B). HT22 cells were seeded into 24-well plates at a density of 4 × 104 cells/cm2. After a 5-h incubation, test compounds were added. One hour later the cells were treated with glutamate for 24 h and then were subjected to MTT assay. Values, representing the percentage of the control MTT value (obtained in the absence of glutamate), are mean ± SD (n = 4). *Significantly different (p < 0.01) from control cells as determined by ANOVA. In B, white bars are Glu alone, gray bars represent Glu + ZO.
3.2. ARE activation and phase 2 enzyme induction
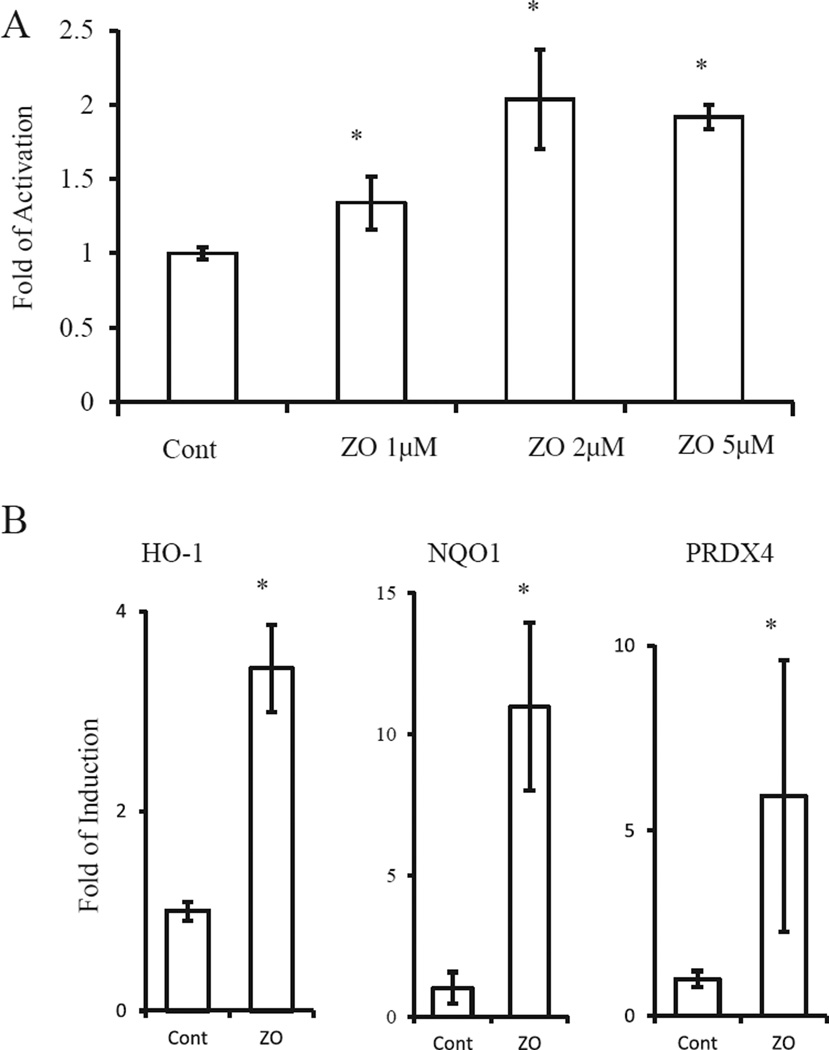
As the neuroprotective effects of [8] electrophilic compounds may be due to activation of the Nrf2/ARE pathway, we next examined whether ZO could activate the ARE in HT22 cells (Fig. 3A). We quantified activation of GSTYaARE [8,9], a transcriptional element that responds to Nrf2, by conducting a luciferase assay using HT22 cells transfected with a ARE(GSTYa)-luciferase expression vector (Fig. 3A). Based on luciferase activity, ZO (1–5 µM) stimulated the expression of ARE-regulated genes by >2-fold. Because the Nrf2/ARE pathway leads to induction of phase-2 enzymes, we performed RT-PCR analysis using primers for nqo1, ho-1, and prdx4. The expression of these genes was significantly induced by ZO (Fig. 3B). These results suggest that ZO effectively activates the Nrf2/ARE pathway in neural cells.
Fig. 3.
Activation of the Nrf2/ARE pathway. (A) ARE activation by ZO. HT22 cells transfected with an ARE (GST-Ya)-luciferase vector were treated with various concentrations of ZO for 24 h and then subjected to the reporter gene assay. (B) Induction of phase-2 genes by ZO. Total RNA was extracted from HT22 cells treated with 5 µM ZO for 24 h. RT-PCR was performed to detect β-actin, nqo1, ho-1, and prdx4 mRNA. Aliquots of the reaction products were subjected to electrophoresis after PCR amplification for 24–28 cycles. *Significantly different (p < 0.01) from control cells as determined by ANOVA.
3.3. Neuroprotective effects of ZO on cortical cultures
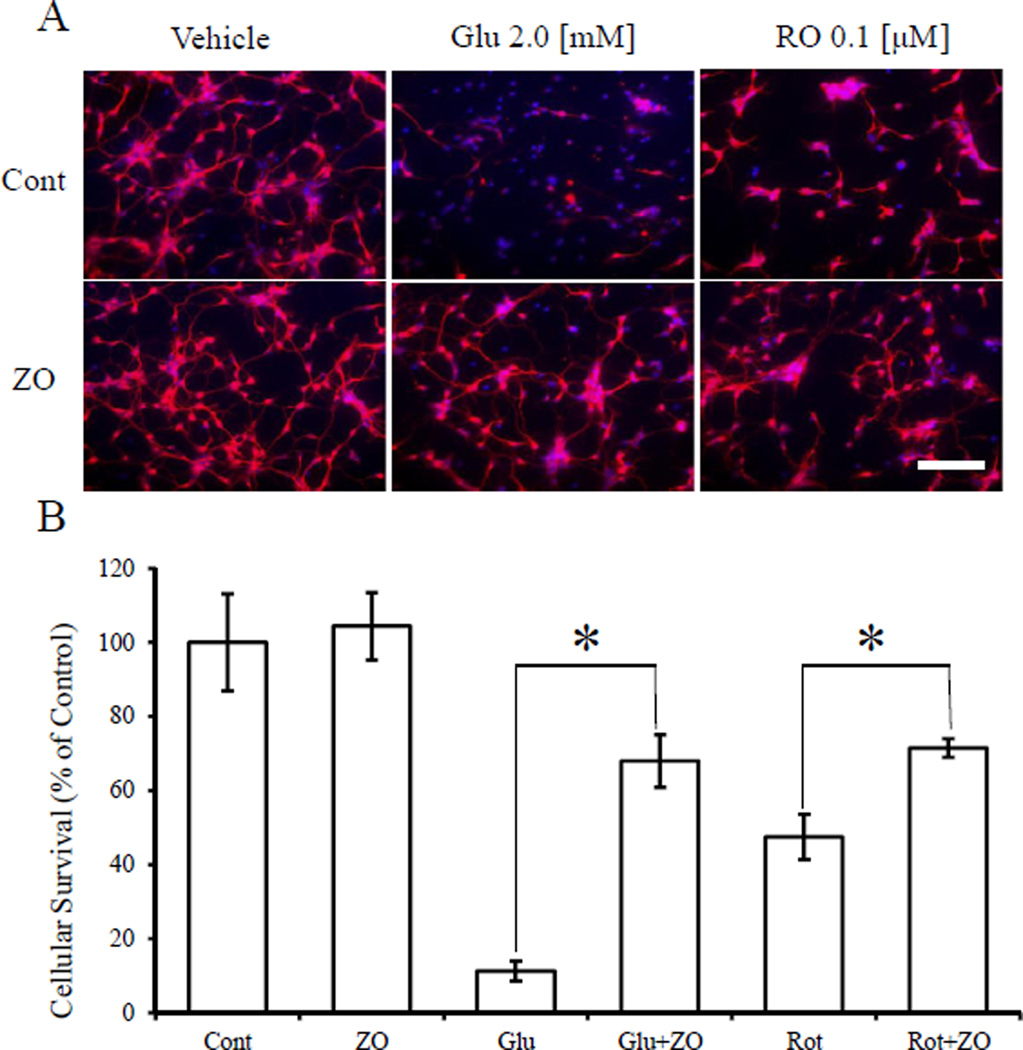
Next, we assessed whether ZO could protect immature cerebrocortical neurons in primary culture from an oxidative insult (Fig. 4). In this system, oxidative stress due to GSH depletion plays a major role in cell death [18]. We found that ZO protected cortical neurons exposed to glutamate or rotenone. Glu (2 mM) and rotenone (0.1 µM) reduced the number of MAP2 and NeuN-positive cells to 11.2 ± 2.7% and 47.5 ± 6.1% of the control value, respectively. Strikingly,1 µMZO increased cell survival in the face of these insults to 67.9 ± 7.1% and 71.4 ± 2.5%, respectively. These results thus suggest that ZO can protect primary CNS neurons against oxidative stress.
Fig. 4.
Protective effects of ZO against Glu- or rotenone-induced oxidative toxicity in cultures of immature cortical neurons (A) Representative images of immunoreactive neuronal staining for MAP2 and NeuN (red) and Hoechst 33,258 dye (blue). (B) Quantitative analysis of cell survival after oxidative insult and ZO treatment. ZO (1 µM) or vehicle was added to cerebrocortical cultures (E17, DIV2) 60 min prior to exposure to glutamate or rotenone. The cultures were then incubated for 20 h and thereafter stained with anti-MAP-2 and anti-NeuN (red) as well as with Hoechst 33,258 dye (blue). *significantly different (p < 0.01) from control cells as determined by ANOVA. Scale bar in A represents 50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge, this is the first report to show that an electrophilic compound from brown algae can protect neurons against oxidative stress. Moreover, our results suggest that the Nrf2/ARE pathway appears to mediate, at least in part, the protective action [15–17]. Although electrophiles are capable of inducing neuroprotective pathways in astrocytic and neuronal cells, one of the disadvantages that must be overcome is non-specific reaction of the electrophile with cellular thiols such as GSH [15–17]. We have proposed that one solution to this problem is the use of proelectrophilic compounds [17]. Such compounds are not directly electrophilic but can be converted to electrophiles by the very oxidative insult that they are designed to then counter [17]. We designated this type of compound a “pro-electrophilic drug (PED)” [17]. One of the best examples of a PED is a hydroquinone that can be oxidized to a quinone [17]. ZO possesses a para-hydroquinone ring and thus represents a pro-electrophilic compound that can be converted to a quinone-type electrophile by oxidation [8,12]. We have focused on hydroquinone-type pro-electrophilic compounds because of their distinctively “prodrug”-like properties [17]. The current results in conjunction with our prior studies suggest that activation of PEDs can lead to effective neuroprotection against oxidative insults, mitochondrial dysfunction, and ER stress [17]. Given its excellent therapeutic index and long history of human consumption, ZO represents a candidate lead compound for treatment of chronic neurodegenerative diseases associated with oxidative stress.
Acknowledgments
The authors thank Dr. Larry D. Frye for editorial help with the manuscript. This work was supported in part by grants-in-aid for scientific research (No. 22500282, 2011-2014 and No. 25350985, 2014-2017) from the JSPS and the NIH (R01 NS086890 and R21 NS080799).
Abbreviations
- ARE
antioxidant-responsive element
- CA
carnosic acid
- Cont
control
- DIV2
2 days in vitro culture
- DMSO
dimethyl sulfoxide
- E17
embryonic day 17
- Glu
glutamate
- GSH
reduced glutathione
- HO-1
heme oxygenase-1
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NQO-1
NADPH quinone oxidoreductase 1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PED
pro-electrophilic drug
- PRDX4
peroxiredoxin 4
- RT-PCR
reverse transcription-polymerase chain reaction
- SD
standard deviation
- ZO
zonarol
Footnotes
Conflict of interest
None.
References
- 1.Brown ES, Allsopp PJ, Magee PJ, Gill CI, Nitecki S, Strain CR, McSorley EM. Seaweed and human health. Nutr. Rev. 2014;72:205–216. doi: 10.1111/nure.12091. [DOI] [PubMed] [Google Scholar]
- 2.Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2014;31:160–256. doi: 10.1039/c3np70117d. [DOI] [PubMed] [Google Scholar]
- 3.Rosen JE, Gardiner P, Saper RB, Pearce EN, Hammer K, Gupta-Lawrence RL, Lee SL. Kelp use in patients with thyroid cancer. Endocrine. 2014;46:123–130. doi: 10.1007/s12020-013-0048-2. [DOI] [PubMed] [Google Scholar]
- 4.Koyama T, Shirosaki M, Ishii M, Hirose T, Yazawa K. New value and functionality found in undeveloped food materials. J. Jpn. Soc. Med. Use Func. Foods. 2010;6:109–114. (In Japanese) [Google Scholar]
- 5.Fenical W, Sims JJ, Squatrito D, Wing RM, Radlick P. Zonarol and isozonarol, fungitoxic hydroquinones from the brown seaweed Dictyopteris zonarioides. J. Org. Chem. 1973;38:2383–2386. doi: 10.1021/jo00953a022. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Komatsu M. Synthesis and absolute configuration of zonarol, a fungitoxic hydroquinone from the brown seaweed Dictyopteris zonarioides. Bull. Soc. Chim. Belg. 1986;95:771–781. [Google Scholar]
- 7.Yamada S, Koyama T, Noguchi H, Ueda Y, Kitsuyama R, Shimizu H, Tanimoto A, Wang KY, Nawata A, Nakayama T, Sasaguri Y, Satoh T. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS One. 2014;19:e113509. doi: 10.1371/journal.pone.0113509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of specific cysteines. J. Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh T, Izumi M, Inukai Y, Tsutumi Y, Nakayama N, Kosaka K, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci. Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Tabuchi T, Tamaki Y, Kosaka K, Takikawa Y, Satoh T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase 2 enzymes and activation of glutathione metabolism. Biochem. Biophys. Res. Commun. 2009;382:549–554. doi: 10.1016/j.bbrc.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Yanagitai M, Kitagwa T, Itoh S, Takenouchi T, Kitani H, Satoh T. Carnosic acid, a pro-electrophilic compound, inhibits LPS-induced activation of microglia. Biochem. Biophys. Res. Commun. 2012;418:22–26. doi: 10.1016/j.bbrc.2011.12.087. [DOI] [PubMed] [Google Scholar]
- 12.Satoh T, Raraie T, Seki M, Sunico CR, Tabuchi T, Kitagawa T, Yanagitai M, Senzaki M, Kosegawa C, Taira H, Mckercher SR, Hoffman JK, Roth GP, Lipton SA. Dual neuroprotective pathways of a pro-electrophilic compound via HSF-1-activated heat-shock proteins and Nrf2-activated phase 2 antioxidant response enzymes. J. Neurochem. 2011;19:569–578. doi: 10.1111/j.1471-4159.2011.07449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 15.Satoh T, Okamoto S, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc. Nat. Acad. Sci. U. S. A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh T, Lipton SA. Redox regulation of neuronal survival by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Satoh T, McKercher SR, Lipton SA. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013;65:645–657. doi: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 19.Sagara Y, Dargusch R, Chambers D, Davis J, Schubert D, Maher P. Cellular mechanisms of resistance to chronic oxidative stress. Free Radic. Biol. Med. 1989;24:1375–1389. doi: 10.1016/s0891-5849(97)00457-7. [DOI] [PubMed] [Google Scholar]