Abstract
Actin dynamics is critical for the formation and sustainment of the immunological synapse (IS) during T cell interaction with antigen-presenting cells (APC). Thus, many actin regulating proteins are involved in spatial and temporal actin remodeling at the IS. However, little is known whether or how actin stabilizing protein controls IS and the consequent T cell functions. TAGLN2 − an actin-binding protein predominantly expressed in T cells − displays a novel function to stabilize cortical F-actin, thereby augmenting F-actin contents at the IS, and acquiring leukocyte function-associated antigen-1 activation following T cell activation. TAGLN2 also competes with cofilin to protect F-actin in vitro and in vivo. During cytotoxic T cell interaction with cancer cells, the expression level of TAGLN2 at the IS correlates with the T cell adhesion to target cancer cells and production of lytic granules such as granzyme B and perforin, thus expressing cytotoxic T cell function. These findings identify a novel function for TAGLN2 as an actin stabilizing protein that is essential for stable immunological synapse formation, thereby regulating T cell immunity. [BMB Reports 2015; 48(7): 369-370]
Keywords: TAGLN2, Actin stabilization, Immunological synapse, T cell activation
During adaptive immune responses, activated T cells play essential roles in elimination of pathogens through cytokine secretion or direct killing of infected cells. To perform these functions, the T cells require a physical contact with the antigen-bearing antigen-presenting cells (APCs). The specialized junctional structure, formed at the interface between a T cell and APC, is referred to as the immunological synapse (IS). Once formed, the IS leads to sustainment of TCR signaling and stabilization of adhesion, and also controls exocytosis and endocytosis, thereby allowing directed cytokine or granule release, and receptor internalizations. The actin cytoskeleton is essential for stable formation of the IS through regulation of integrin affinity and avidity, and the redistribution of TCR-mediated signaling molecules at the synaptic interface. Indeed, disruption of actin cytoskeleton results in impaired T cell immune responses.
The structure of the actin network is regulated through the actions of a variety of actin-regulating proteins, each of which participate in different aspects of T cell activation. Therefore, only a fully rearranged actin cytoskeleton is optimal for full T cell activation. Here, the selective expression of TAGLN2 in T cells and the specific localization at the distal region of supramolecular activation cluster (d-SMAC) in the IS, led us to investigate its role in IS formation and T cell effector functions. The TAGLN family comprises TAGLN 1, 2, and 3 isoforms, which share ∼80% homology, and have been identified as actin cross-linking/gelling proteins. TAGLN1 is exclusively expressed in smooth muscle cells and serves as an early marker of smooth muscle differentiation. TAGLN3 is specifically expressed in brain tissue and upregulated in chronic alcoholic humans and rats. However, in comparison with the other members, the biology of TAGLN2 is poorly understood.
We found that TAGLN2 localizes at the F-actin-rich region in d-SMAC, and controls the F-actin levels by stabilizing F-actin and, in part, antagonizing cofilin-mediated disassembly. This function was also connected to the up-regulation of LFA-1 activity in T cells. The inability of the actin-binding mutant (ΔAB) to protect F-actin and to promote T cell function suggested that TAGLN2 displays its biological function via binding to actin. Interestingly, although a previous report demonstrated the bundling activity of TAGLN1, we only observed this bundling activity at higher concentrations (1:8 ratio of actin to TAGLN2) of TAGLN2, implying that TAGLN2 may not act as a bundling protein in vivo. The actin stabilizing activity was further confirmed by TAGLN2-knockout experiments. TAGLN2-deficiency significantly diminished F-actin content and F-actin ring formation at the IS−resulting in decreased cell spreading−and showed attenuated cofilin activity in vivo.
We found that TAGLN2 controls T cell activation through two apparent mechanisms. First, it regulates the intracellular signaling events that are related to actin dynamics. We observed reduced phosphorylation of PI3K and ERK, an upstream Ras-signal-ing cascade of cofilin dephosphorylation, in TAGLN2-/- CD3+ T cells. The phosphorylation of PLC-γ was also attenuated in these cells. Attenuated signaling events result in reduced production of effector molecules in T cells, including IL-2, IFN-γ, granzyme B and perforin. Second, it controls T cell adhesion to target cells via modulating integrin LFA-1 activity. Knockout of TAGLN2 significantly reduced T cell adhesion to APC or target cancer cells. Reduced cell-to-cell adhesion may result in reduced delivery of toxic materials into the target cells. Similarly, we found decreased cytotoxicity of TAGLN2-/- CD8+ T cells. The summarized role of TAGLN2 is depicted in Fig. 1.
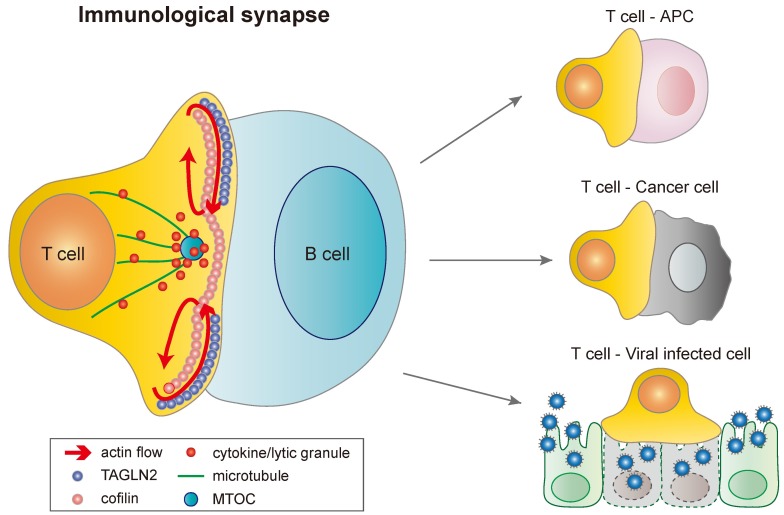
Fig. 1. IS formation between T cells and various cells. TAGLN2 is recruited and localized at the d-SMAC during IS formation. Cofilin is also recruited at the contact site, but located at the upper region of d-SMAC as well as the central region of SMAC (c-SMAC) in which TAGLN2 is almost excluded. Differential positioning of two proteins may correspond to regulate retrograde actin flow at the IS. MTOC (microtubule-organizing center) is polarized at the region just beneath the contact site. Once MTOC is reoriented, cytokines or lytic granules move along the microtubules to the MTOC and are secreted to the surface of target cells through the cofilin-rich actin hole in c-SMAC. T cells make various types of IS, depending on the counter cells (which include APCs, cancer cells and viral infected cells).
The mechanism how TAGLN2 does stabilize and, further, protect F-actin from cofilin attack is currently unclear but predictable. We ruled out capping of barbed ends, as the proteins in this category significantly affect the spontaneous polymerization. Instead, we are convinced that TAGLN2 is a filament-side binding protein, and may stabilize the filaments by altering their structure. In accordance with this idea, we found that F-actin-TAGLN2 complex shows a much straighter morphology than the F-actin itself, as determined by EM analysis. Thus, we suggest a “molecular staple” model for TAGLN2-mediated F-actin stabilization. In this sense, some side-binding proteins display an interesting feature. For example, epithelial protein lost in neoplasm (EPLN) inhibits branching nucleation of actin filaments by Arp2/3 complex and promotes the formation of stable actin filament structures such as stress fibers. Bacterial protein SipA, which is required for efficient S. typhimurium entry into host cells, also stabilizes F-actin. Interestingly, this protein does not nucleate actin at the high ionic conditions, but induces extensive polymerization under low salt conditions where spontaneous nucleation and polymerization do not occur. Therefore, further studies are now in progress to elucidate whether TAGLN2 participates in the process of Arp2/3-mediated actin branching, or directly in actin nucleation and polymerization.
Actin is involved in many different cellular processes that are essential for cell growth, differentiation, division, membrane organization, and motility. Mutations or deficiencies in actin-regulating genes encoding WASp, cofilin, actinin, and Vav proteins cause various human diseases. Disruption of TAGLN promotes inflammation after arterial injury via NF-kB activity, and TAGLN modulates the phenotype of vascular smooth muscle cells during atherogenesis. Interestingly, TAGLN2 is upregulated in certain tumors, and is suggested to be involved in tumor development. Accordingly, our lab is currently in the process of developing agents that can control the activity or expression of TAGLN2, thereby controlling tumor development in vivo. Because overexpression of TAGLN2 significantly increases T cell adhesion to cancer target cells, we are also applying TAGLN2 peptides for cell therapy against cancer cells.
Acknowledgments
This work was supported by the Basic Science Research Program (2012R1A2A1A03002115), Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (2013R1A6A3A04 064259), and Cell Dynamics Research Center Program (2007-0056244) through the NRF grants funded by the Ministry of Science, ICT and Future Planning, Korea. This work was also partially supported by the Bio-Imaging Research Center at GIST.