Abstract
Cilia are conserved subcellular organelles with diverse sensory and developmental roles. Recently, they have emerged as crucial organelles whose dysfunction causes a wide spectrum of disorders called ciliopathies. Recent studies on the pathological mechanisms underlying ciliopathies showed that the ciliary compartment is further divided into subdomains with specific roles in the biogenesis, maintenance and function of cilia. Several conserved sets of molecules that play specific roles in each subcompartment have been discovered. Here we review recent progress on our understanding of ciliary subcompartments, especially focusing on the molecules required for their structure and/or function. [BMB Reports 2015; 48(7): 380-387]
Keywords: Basal body, Ciliogenesis, Ciliopathies, Protein trafficking, Transition zone
INTRODUCTION
The cilium is a hair-like appendage that extends from the surface of many eukaryotic cells. Inside the cilium, a microtubule-based cytoskeleton called the axoneme is present. The axoneme of a motile cilium typically has a ring of nine outer microtubule doublets with two central microtubule singlets (9+2 axoneme), whereas the axoneme of non-motile or primary cilium usually lacks the central siglets (9+0 axoneme) (1). The loss of motility in primary cilia seems to facilitate the evolution of diverse primary cilia with distinct structures and functions. Due to this diversity, ciliary dysfunction causes a wide spectrum of disorders collectively known as ciliopathies. More than 30 human diseases are classified as ciliopathies. These include Alstrom syndrome, Bardet-Biedlle syndrome, Joubert syndrome, Meckel-Gruber syndrome, nephronophthisis, polycystic kidney disease, primary ciliary dyskinesia, retinitis pigmentosa, situs inversus and others (2). Identification of genes associated with ciliopathies provides a unique opportunity to understand the biogenesis, structure and function of cilia. To date, at least 80 such genes have been reported, including many conserved genes involved in vesicular and intraciliary trafficking pathways (2). In addition to common ciliogenic proteins, some membrane proteins involved in a wide range of cellular processes, from sensory transduction to signal transduction such as sonic hedgehog, have been also discovered (3). Interestingly, most proteins associated with ciliogenesis or ciliopathy are distributed in distinct regions within or near ciliary compartments, suggesting that these regions have specific roles in ciliary structure and/or function (4-6).
Protein localization and restriction to ciliary compartments are mainly driven by the cooperation between evolutionary conserved vesicular and intraciliary trafficking systems (7). The vesicular trafficking system involved in ciliary protein transport includes coat adaptors, small GTPases and membrane fusion components. The system delivers preciliary vesicles that contain cilia-destined proteins to the periciliary membrane at the early stage of ciliary protein targeting. This early event takes place at the ciliary base, which contains mother centriole-derived basal body and associated distal appendages. The intraciliary cargo-trafficking system, termed intraflagellar transport (IFT), then delivers and distributes the ciliary cargo proteins by operating bi-directionally along ciliary microtubules (8). The entry of proteins into the intraciliary compartment is gated by a modular component located in the most proximal region of the intraciliary compartment, termed the transition zone (TZ). Although ciliary entry of most proteins is dependent on IFT, IFT-independent pathways also exist (7, 9, 10). The structure and function of the intraciliary compartment distal to the TZ is very diverse depending on cell types. For example, many cells show different microtubule arrangements between proximal and distal intraciliary subcompartments (6, 11-14). Furthermore, many proteins including ciliopathy proteins, membrane receptors and channel proteins are restricted to specific intraciliary subcompartments (15-18). Here, we will review the ciliary subcompartments, focusing on the proteins specifically localized at the distinct subcompartments.
CILIARY BASE: THE BASAL BODY AND SURROUNDING PERICILIARY MEMBRANE REGION
The mother centriole-derived basal body and the surrounding periciliary membrane comprise the ciliary base region (see Fig. 2). In various mammalian cells, the surrounding periciliary membrane forms a ciliary pocket (19). The basal body, which contains triplet microtubules, is docked to the periciliary membrane via transition fibers or distal appendages (5) (see Fig. 2). This region is not only located at the base of the ciliary compartment, but is also the primary player in ciliogenesis and ciliary protein trafficking.
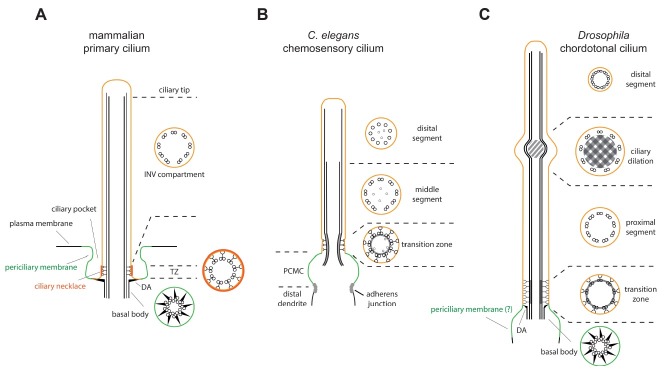
Fig. 2. Schematic drawing of cilia from different organisms. (A) A typical mammalian primary cilium. The basal body (BB) and the associated distal appendage (DA), the transition zone (TZ), the INV compartment, and the distal tip are shown. The BB microtubules show a typical triplet structure with attached transition fibers (= distal appendage (DA)). In the TZ, the characteristic Y linkers are shown. Some other structures, including the ciliary pocket and the ciliary necklace are also shown. (B) A canonical C. elegans amphid sensory cilium. Ciliary microtubules extend from a fully degenerated basal body at the ciliary base region called the periciliary membrane compartment (PCMC). In the transition zone (TZ), each microtubule doublet is connected to the ciliary membrane via Y-links. Some inner singlet microtubules are also seen. The TZ is followed by the middle segment consisting of 9 doublet microtubules. In the distal segment, the B-tubule of each doublet is missing. (C) A Drosophila chordotonal sensory cilium. The gross structure is similar to that of mammalian cilium. Three distinct features are shown. First, a prominent ciliary dilation (CD) is seen in the middle of the cilium. Second, the axoneme of the proximal segment located between the TZ and the CD contains outer dynein arms. Third, the distal segment contains doublet microtubules without attached outer dynein arms. Color coding: periciliary membrane in green, ciliary membrane in orange.
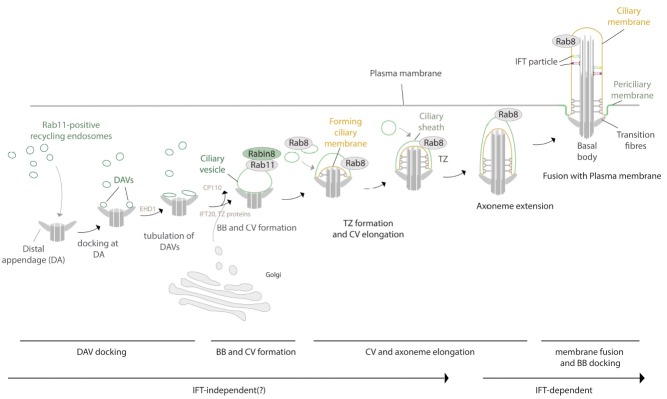
One of the major roles of this region is the initiation of ciliogenesis, as the basal body joined to the ciliary vesicle (CV) migrates to the membrane before axoneme elongation (20, 21) (Fig. 1). The joining of preciliary vesicles to the distal appendages of the mother centriole is a key event for the formation of the basal body and the CV (22). It was recently demonstrated that the membrane shaping proteins, EHD1 and EHD3, are essential in this step (22). The EHD proteins are localized in the small preciliary vesicles associated with the mother centriole distal appendages, termed the distal appendage vesicles (DAVs), and are required for the formation of CV from DAVs. DAV is considered to be a sort of recycling endosome mediated by a membrane trafficking regulator, the small GTPase Rab11. Rab11-positive vesicles may deliver EHD proteins as well as the guanine nucleotide exchange factor for Rab8 (Rabin8) to the distal appendages (22, 23). After EHD-dependent formation of CV, Rab8 is recruited to CV and plays a role in the elongation of CV and the progression of ciliogenesis. In addition, the EHD proteins are needed for CP110 loss from the mother centriole, which is essential for the maturation of the basal body from the mother centriole. After EHD-dependent CV formation, other ciliogenic proteins including IFT and TZ proteins are recruited into the CV, and Rab8-dependent CV elongation occurs. Thus, EHD protein is a key molecule for the initiation of ciliogenesis in the ciliary base (22). Tau tubulin kinase 2 (TTBK2) is also known to promote CP110 loss from the mother centriole in addition to the recruitment of IFTs, although the relationship between EHD proteins and TTBK2 is unclear (24).
Fig. 1. Model of early ciliogenesis. Rab11-positive recycling endosomes trafficked toward the mother centriole-associated distal appendages (DA). When the vesicles dock into the DA, they become distal appendage vesicles (DAVs). EHD1 included in the DAVs triggers SNARE-dependent membrane fusion to form the ciliary vesicle (CV). EHD1 also triggers transition of the mother centriole to the basal body (BB) by removing CP110 from the mother centriole. After the formation of CV and BB, small GTPase Rab8 is accumulated in the CV and is activated by Rabin8 from Rab11, facilitating elongation of the CV and axoneme. In addition, IFT20 and TZ proteins are delivered to the CV by Golgi-derived vesicles. During elongation of the CV and axoneme, the TZ is formed and the CV membranes are differentiated into the ciliary membrane (orange) and the ciliary sheath (green). Finally, the cilium is formed by the fusion of the ciliary sheath with the apical plasma membrane. The ciliary sheath becomes the periciliary membrane (green).
The other critical role of the ciliary base is the sorting and assembly of cargo proteins for delivery to intraciliary compartments. Protein trafficking to cilia is generally considered to have two separate steps: preciliary and intraciliary. The transition from preciliary to intraciliary trafficking takes place in the ciliary base region.
Preciliary trafficking is believed to be dependent on sorting at the trans-Golgi network (TGN). Before targeting to intraciliary compartments, all proteins are sorted at the TGN and delivered to the ciliary base region. Ciliary targeting signals (CTSs) required for apical sorting at the TGN have been identified from only a limited number of ciliary proteins. A VxPx motif present in the cytoplasmic C-terminus of rhodopsin is one of the most extensively characterized CTSs (25, 26). For the VxPx motif-dependent apical trafficking of rhodopsin, two small GTPase family proteins, Arf4 and Rab11, play a role in sorting and vesicle transport, respectively (25, 26).
There may be at least four different pathways for ciliary entry (7). One simple pathway is a direct pathway in which apical trafficking from TGN directly delivers vesicles containing cilia-destined proteins to the ciliary base region. The VxPx motif-dependent rhodopsin trafficking is an example of this pathway. In the pathway, the ciliary cargo protein-bearing vesicle usually contains adaptor proteins for ciliary entry (IFT/BBsome) as well as membrane fusion (SNARE). In the ciliary base region, the vesicle fuses with the periciliary membrane in a SNARE-dependent manner. An IFT protein, IFT20, contained in the vesicle serves as an adaptor to facilitate the assembly of IFT trains. Another pathway is mediated by the recycling endosome, which is a highly dynamic endocytic compartment located near the target membrane. The recycling endosome may serve as an intermediate sorting compartment in which proteins are segregated depending on their final destination (ciliary membrane or apical plasma membrane). As described earlier, proteins required for the initial stage of ciliogenesis, such as EHD protein, may be delivered to the ciliary base region through this pathway.
Some proteins can reach the periciliary membrane through a third pathway involving lateral diffusion (27). In this pathway, the protein is delivered to the apical plasma membrane just like other apical directed non-ciliary proteins, and lateral diffusion delivers the ciliary proteins to the periciliary membrane.
For most proteins that reach the ciliary base region via the above three pathways, entry into intraciliary compartments requires a switch from vesicular trafficking to intraflagellar trafficking. Thus, re-sorting of ciliary cargo proteins is necessary to assemble the intraflagellar trafficking-competent IFT trains. Several lines of evidence suggest that BBSome is an authentic sorting machinery for targeting GPCRs to the cilia (28, 29). One model of BBSome function in intraflagellar trafficking suggested that it decodes sorting signals from ciliary cargo proteins reached in the ciliary base region, and forms coats using the small GTPase Arf6 before assembling onto IFT trains (7). The assembled IFT trains deliver ciliary cargo proteins into the cilium.
Another pathway, which involves the ARL3-UNC119-RP2 GTPase cycle, is very discrete from the above three pathways (10). In this pathway, BBSome is not involved in the sorting of ciliary proteins. Instead UNC119, a GTP-specific effector of ARL3, selectively binds to the myristoylated cargo proteins (for example, NPHP3), and may deliver them to the intraciliary compartments. A ciliary small GTPase ARL3 also binds to UNC119 and to the membrane in a GTP-specific manner, unloading myristoylated proteins from UNC119. During this process, RP2, which is an ARL3-specific GAP, is required for the activation of ARL3.
In summary, the ciliary base serves as the organizing center for building the cilium by triggering ciliogenesis as well as for re-sorting and assembling cilia-destined cargo proteins. These processes are mediated by several evolutionary conserved modules that participate not only in general cellular functions such as vesicle trafficking, endocytosis, and membrane fusion, but also in cilium-specific functions such as intraflagellar transport.
TRANSITION ZONE (TZ)
The transition zone (TZ) lies between the ciliary base region and the ciliary tip. Inside the TZ, the so-called Y-shaped linkers connect the outer doublets microtubules to the ciliary necklace, which is a membrane speciation found in the TZ. Although the composition of Y linkers and the necklace is largely unknown, it is generally accepted that the TZ functions as a ciliary gatekeeper.
The earliest evidence of TZ as a ciliary gatekeeper came from a study in which the distribution of rhodopsin from mechanically dissociated rods was investigated (30). The study showed that rhodopsin diffused freely from the outer to the inner segment without the TZ (connecting cilium), suggesting that the TZ acts as a diffusion barrier (30)
Recently, findings that multiple ciliopathy proteins are specifically localized in the TZ brought this subcompartment into the limelight. Among those ciliopathies, proteins associated with Joubert syndrome (JBTS,), Meckel-Gruber syndrome (MKS), and nephronophthisis (NPHP) are especially important for the structure and function of the TZ (4, 5, 31-33). A systematic protein network mapping found that proteins linked to those three ciliopathies comprise three distinct but heavily interconnected modules, called NPHP1-4-8, NPHP5-6, and MKS modules (31). The study also showed that many of the proteins comprising the NPHP-JBTS-MKS network are localized in the TZ (31). The NPHP/JBTS/MKS modules are also found in other model organisms including Chlamydomonas reinhardtii, Caenorhabditis elegans and Drosophila melanogaster (32, 34). They can be generally categorized into two functionally redundant modules: NPHP and MKS/JBTS modules (Table 1). In mammalian cells, the core NPHP module comprises three TZ-localized interconnected proteins: NPHP1, NPHP4 and NPHP8 (also known as RPGRIP1L or MKS5) (31)Two additional NPHP subcomplexes, NPHP2/3/9 and ATXN10/NPHP5, are preferentially localized in the nearby subcompartments for inversin and the ciliary base region, respectively (31, 33). Abrogation of NPHP1 by targeted knock-out in mice showed that transport of opsins to the outer segment was severely compromised, while the axoneme structure and IFT motor function appeared normal, suggesting that the NPHP module has a role in sorting or gatekeeping for intraciliary transport (35). The mammalian MKS/JBTS module contains at least 13 TZ-localized proteins, including MKS/MKSR proteins (MKS1, MKS3(=TMEM67), CEP290(=MKS4), CC2D2A(=MKS6), B9D1(=MKSR1), B9D2(=MKSR2)), Tectonic proteins (TCTN1, TCTN2, TCTN3), TMEM proteins (TMEM17, TMEM216, and TMEM 231), and AHI1(=JBTS3) (33). TCTN1 was originally identified as a regulator of mouse Hedgehog signaling (36), and Tectonic proteins were later found to be members of the MKS/JBTS module (33). Loss of MKS/JBTS components caused tissue-specific defects in ciliogenesis, and several ciliary proteins, including AC3, PKD2, SMO, and ARL13B, failed to localize in the ciliary compartments, suggesting that the MKS/JBTS module acts at the TZ to control ciliary membrane protein composition (33).
Table 1. List of proteins that localize and function in the ciliary transition zone.
| Modules | Mammal | Alga | Worm | Fly |
|---|---|---|---|---|
|
| ||||
| MKS/JBTS module | MKS1 | - | MKS-1 | Mks1 |
| MKS2/TMEM216 | 345082 | MKS-2 | CG8166 | |
| MKS3/TMEM67 | - | MKS-3 | Mks3 | |
| CEP290/MKS4/NPHP6 | CEP290 | - | Cep290 | |
| MKS6/CC2D2A | 176993 | MKS-6 | Mks6 | |
| B9D1/MKSR1 | 130473 | MKSR-1 | B9d1 | |
| B9D2/MKSR2 | 137074 | MKSR-2 | B9d2 | |
| TMEM17 | - | ZK418.3 | Tmem17 | |
| TMEM231 | 182890 | T26A8.2 | Tmem231 | |
| TCTN1, TCTN2, TCTN3 | 377718 | E04A4.6 | Tectonic | |
| AHI1/JBTS3 | - | - | - | |
| Core NPHP module | NPHP1 | - | NPHP-1 | - |
| NPHP4 | NPHP4 | NPHP-4 | - | |
| NPHP8/Rpgrip1L/MKS5 | - | MKS-5 | - | |
| NPHP-INV module | INV/NPHP2 | - | NPHP-2 | CG42534 |
| MKS7/ NPHP3 | - | - | - | |
| NEK8/NPHP9 | CNK2 | NEKL-1 | Niki | |
| NPHP-ATXN module | ATXN10 | - | - | Atxn10 |
| IQCB1/NPHP5 | - | - | - | |
Several lines of evidence from other model organisms also support the idea that NPHP and MKS/JBTS modules in the TZ act as ciliary gatekeepers. Interestingly, the MKS/JBTS module is well conserved throughout evolution, whereas the NPHP module is less conserved, suggesting that the two modules have some redundancy in their functions (Table 1). In C. elegans, most core components of both MKS/JBTS and NPHP modules are present, and many have been shown to possess conserved functions and localization in the TZ (32). Interestingly, functional studies using single mutant alleles showed no obvious defects in the structure and function of TZ for most of those proteins (32). However, double mutants for one MKS/JBTS and one NPHP component, but not two components from the same module, showed severe defects in the structure and function of cilia (32). These results again suggest that the two modules interact with each other, and have overlapping and redundant functions in the TZ. In Chlamydomonas, a MKS/JBTS component CEP290 is located in the TZ. Loss of CEP290 resulted in defects in the microtubule-membrane connectors, causing detachment of the flagellar membrane from the TZ microtubules as well as abnormal protein content (i.e., reduction of IFT-A and PKD2, accumulation of IFT-B and BBS4) in the isolated flagella, while showing little effect on IFT motility. The results suggested that CEP290 is required for tethering the flagellar membrane to the TZ microtubules, and for controlling flagellar protein composition without affecting intraflagellar transport (37). In addition, it has recently been shown that the Chlamydomonas homolog of NPHP4 also has similar functions as CEP290 (38). In Drosophila, it was recently shown that the composition, localization and function of the MKS/JBTS module are conserved, while most NPHP components are missing (34). Interestingly, the TZ can migrate away from, and function independent of the ciliary base during sperm maturation (34). This finding is consistent with the fact that the basal body degenerates after the initiation of ciliogenesis in C. elegans sensory cilia (6).
Although several studies in various organisms show that the mature TZ functions as a ciliary gatekeeper, it is still unclear how the TZ performs this function. The most attractive model portrays the TZ as a membrane diffusion barrier. In C. elegans, it has been shown that defects in MKS or NPHP modules caused abnormal accumulation of some proteins (MKS-3 and RPI-2) within cilia as well as the leakage of ciliary membrane-linked ARL-13 out of cilia, suggesting that they constitute a bidirectional membrane diffusion barrier at the TZ (32, 39). The barrier defect phenotype was also observed in single mutants of MKS or NPHP genes that have grossly normal TZ structures, and was enhanced in double mutants with defects in the structure of TZ. Moreover, the barrier function is independent of IFT (32). These findings suggested that MKS and NPHP modules in the TZ function as bona fide bidirectional diffusion barriers in the TZ. Another intriguing, but not mutually exclusive, model depicts the TZ as a modulator of IFT. In support of this hypothesis, some TZ proteins interact with IFT, and the interaction is essential for the intraciliary transport of opsins (35, 40).
In summary, the TZ residing at the base of the intraciliary compartments functions as a ciliary gatekeeper. Two functionally redundant and ciliopathy-related modules, MKS/JBTS and NPHP, have critical roles in establishing the TZ structure and function.
COMPARTMENTALIZATION IN THE CILIARY REGION DISTAL TO THE TZ
The structure and function of the ciliary region distal to the TZ are highly diverse, depending on cell types. In many cells, this region contains more than two distinct subcompartments at the level of the ultrastructure and protein localization. In this review, we will compare three different cilia: a typical mammalian primary cilium, C. elegans chemosensory amphid cilium, and Drosophila auditory chordotonal cilium (Fig. 2).
The first compelling suggestion that a typical mammalian primary cilium has a distinct subcompartment in the body of cilium distal to the TZ was from the finding that an NPHP protein INV (Inversin, also known as NPHP2) was localized within a confined ciliary region termed the INV compartment (15). Removal of 60 C-terminal amino acids distributed the protein evenly along the cilium, suggesting that protein localization in the INV compartment is actively regulated (15). Other proteins that show restricted distribution in the INV compartment include a JBTS-associated small GTPase ARL13B, and two NPHP submodule components NPHP3, and NEK8 (39, 41). Many mammalian primary cilia have a distinct microtubule structure at the distal most region, where singlet microtubules comprise the axoneme (Fig. 2A). Some proteins have been reported to be specifically localized at the singlet microtubule containing the distal tip. For example, the odor-detecting CNG channels are preferentially localized at the distal tip in the olfactory cilia (42). The Sonic hedgehog signaling mediators GLI2, GLI3, and SUFU are also actively targeted in the distal tip (17, 43). Although increasing evidence indicate that differential localization of proteins within the ciliary region beyond the TZ is common, the relationship between function and protein localization is poorly understood.
The sensory cilia in C. elegans have been used as a good model system to study ciliogenesis and the function of cilia. They have some distinct structural features compared to mammalian primary cilia (Fig. 2B). First, the mature cilium lacks the basal body and associated accessory structures such as distal appendages. Second, the ciliary base region called the periciliary membrane compartment (PCMC) contains an asymmetric swelling of the dendrite tip. Third, the ciliary axoneme contains bipartite microtubule arrangements: doublet microtubules in the proximal or middle segment, and singlet microtubules in the distal segment (Fig. 2B) (6). The middle segment is somewhat analogous to the INV compartment of mammalian primary cilia. Some C. elegans homologs of mammalian proteins localized in the INV compartment are also localized specifically in the middle segment, including NPHP-2 and ARL-13 (39, 44, 45). Various cyclic nucleotide-gated (CNG) ion channel subunits TAX-2, TAX-4, and CNG-3 are also localized in this segment (46-48). Although the distal segment clearly has a distinct structure from the middle segment, proteins with specific localization and function in this region have not been found yet. It is also true that the significance of restricted protein localization remains to be discovered.
Drosophila ch cilium has a unique structure in the post-TZ region, with three notable distinctions. First, a prominent swelling called the ciliary dilation (CD) is located in the middle of a cilium. The CD divides the ch cilium into two distinct subcompartments (Fig. 2C). The molecular composition and function of the CD is not yet known (49). Second, unlike other primary cilia, the axonemal microtubule contains dynein arms that can only be seen in the motile cilia (49). This suggests that the ch cilium may be motile. Third, unlike other bipartite cilia, the axoneme of the distal segment contains doublet microtubules, but lacks the dynein arms present in the proximal segment (49). Several proteins have been shown to be enriched or restricted in the specific subcompartments. Proteins localized in the CD include a Drosophila homolog of IFT140 RempA, a doublecortin containing microtubule-associated protein DCX- EMAP, a ciliary rootlet protein Rootletin, a Drosophila homolog of TbCMF46, and an uncharacterized protein encoded by CG31036 (50-52). The exact roles of these proteins in the CD remain to be clarified however. The CD is severely disorganized in the loss of function mutant of btv, which encodes a subunit of cellular dynein motor, although the localization of the Btv protein is not yet determined (53). There is also an example of differential protein localization in the proximal and distal segments. A Drosophila ch neuron has two different TRP ion channels, TRPV and TRPN (also known as NompC), which have distinct roles in auditory transduction (54-56). Interestingly, their localization is segregated from each other in the ch cilium; TRPV is restricted to the proximal segment, while TRPN is in the distal segment (18). This suggests that the ciliary subcompartments may be able to perform distinct functions by segregating proteins with different roles into proper subcompartments. Although it is unclear how the two TRP channels segregate into different subcompartments, the finding that both channels were evenly distributed along the cilium following btv mutation, which disrupts the CD, suggested that the CD may act as a structural barrier separating the two subcompartments (18, 50). Recently, another interesting feature about the specific localization of TRP channels was discovered. Loss of a Drosophila homolog of TULP3 Ktub (also known as dTULP) caused distinctive phenotypes in the ciliary localizations of TRPV and TRPN (57). In ktub mutants, ciliary localization of TRPV was completely abolished, while TRPN was still targeted into the cilium but leaked from the distal segment into the proximal segment. This suggests that the two TRP channels may have distinct mechanisms for ciliary targeting, and that Ktub may have a role in establishing the proximal segment identity (57).
The exact mechanism underlying segregating proteins into distinct subcompartments within the ciliary body is unclear. There may be a diffusion barrier between those subcompartments, similar to the TZ that segregates ciliary proteins from non-ciliary proteins. The CD in the insect chordotonal cilia could be a candidate structural barrier. Another intriguing possibility is that the ciliary membrane of subcompartments may have distinct lipid compositions that enable the retention of membrane proteins with specific lipid modifications such as myristoylation, or palmitoylation. Indeed, it has been shown that such lipid-modifications facilitated the localization of some proteins to the INV compartment, including NPHP3, ARL13B, and C. elegans ARL-13 (44, 45, 58). Another possible model is that distinct motors are used to transport proteins into specific ciliary subcompartments. In supporting this, two functionally redundant anterograde IFT motors, heterotrimeric (Kinesin-II) and homodimeric (OSM-3) Kinesin-2, are involved in the establishment of bipartite sensory cilia in C. elegans (59). Among them, only OSM-3 passes beyond the INV sub-compartment (59).
CONCLUDING REMARKS
Growing evidence indicates that the compartmentalized cilium can be further divided into subcompartments, in terms of structure and protein localization. It is reasonable to speculate that distinct subcompartments with unique protein compositions and structures have distinct functions. The function and molecular composition of the two most proximal subcompartments of the cilium, the ciliary base region and the TZ, have been extensively explored over the last five or so years. Thanks to those efforts, we now know much about the molecular mechanisms underlying their functions. The ciliary base region functions as the organizing center for ciliogenesis as well as a sorting and assembly station for cilia-destined protein cargos. Several evolutionary conserved cellular components participate in this process, including vesicle trafficking, endocytosis, and membrane fusion components. The IFT/BBSome complex is a specialized trafficking system for delivering proteins into the intraciliary subcompartments. The TZ acts as a ciliary gatekeeper. Two conserved and redundant modules, NPHP and MKS/JBTS modules, have been discovered to perform this function. But the exact mechanisms underlying their functions remain to be clarified.
The ciliary region distal to the TZ is highly variable in terms of structure and function. The discovery of many proteins with discrete localization pattern in this region suggested that it can be subdivided into functionally distinct subcompartments. But the functional significance of discrete protein localization is mostly unknown. The molecular mechanisms underlying discrete protein localization within the narrow compartment is also a very challenging but intriguing question that remains to be solved.
Acknowledgments
We thank Young-Tae Kwon for assisting us with graphic art work. This work was supported by the grant to Y.D.C. from the University of Seoul (#201404301116).
References
- 1.Haimo LT, Rosenbaum JL. Cilia, flagella, and microtubules. J Cell Biol. (1981);91:125s–130s. doi: 10.1083/jcb.91.3.125s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. (2011);26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. (2010);11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymanska K, Johnson CA. The transition zone: an essential functional compartment of cilia. Cilia. (2012);1:10. doi: 10.1186/2046-2530-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. (2012);13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blacque OE, Sanders AA. Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease. Organogenesis. (2014);10:126–137. doi: 10.4161/org.28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol. (2013);15:1387–1397. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blacque OE, Cevik S, Kaplan OI. Intraflagellar transport: from molecular characterisation to mechanism. Front Biosci. (2008);13:2633–2652. doi: 10.2741/2871. [DOI] [PubMed] [Google Scholar]
- 9.Belzile O, Hernandez-Lara CI, Wang Q, Snell WJ. Regulated membrane protein entry into flagella is facilitated by cytoplasmic microtubules and does not require IFT. Curr Biol. (2013);23:1460–1465. doi: 10.1016/j.cub.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright KJ, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. (2011);25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber WA, Lee J. Fine structure of mammalian renal cilia. Anat Rec. (1975);182:339–343. doi: 10.1002/ar.1091820307. [DOI] [PubMed] [Google Scholar]
- 12.Mesland DA, Hoffman JL, Caligor E, Goodenough UW. Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J Cell Biol. (1980);84:599–617. doi: 10.1083/jcb.84.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidaka K, Ashizawa N, Endoh H, Watanabe M, Fukumoto S. Fine structure of the cilia in the pancreatic duct of WBN/Kob rat. Int J Pancreatol. (1995);18:207–213. doi: 10.1007/BF02784943. [DOI] [PubMed] [Google Scholar]
- 14.Field L, Matheson T. Chordotonal organs of insects. Adv Insect Physiol. (1998);27:1–228. [Google Scholar]
- 15.Shiba D, Yamaoka Y, Hagiwara H, Takamatsu T, Hamada H, Yokoyama T. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein- homolog-containing region. J Cell Sci. (2009);122:44–54. doi: 10.1242/jcs.037408. [DOI] [PubMed] [Google Scholar]
- 16.Flannery RJ, French DA, Kleene SJ. Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophys J. (2006);91:179–188. doi: 10.1529/biophysj.105.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. (2005);1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One. (2010);5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molla-Herman A, Ghossoub R, Blisnick T. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. (2010);123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 20.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. (1962);15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. (1968);3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q, Insinna C, Ott C. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. (2015);17:228–240. doi: 10.1038/ncb3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westlake CJ, Baye LM, Nachury MV. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. (2011);108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz SC, Liem KF, Jr, Anderson KV. The spinocerebellar ataxia-associated gene tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. (2012);151:847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. (1999);97:877–887. doi: 10.1016/S0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 26.Mazelova J, Astuto-Gribble L, Inoue H. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. (2009);28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. (2009);187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. (2008);105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, White SR, Shida T. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. (2010);141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. Invest Ophthalmol Vis Sci. (1988);29:1012–1020. [PubMed] [Google Scholar]
- 31.Sang L, Miller JJ, Corbit KC. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. (2011);145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CL, Li C, Kida K. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. (2011);192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. (2011);43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basiri ML, Ha A, Chadha A. A migrating ciliary gate compartmentalizes the site of axoneme assembly in Drosophila spermatids. Curr Biol. (2014);24:2622–2631. doi: 10.1016/j.cub.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang ST, Chiou YY, Wang E. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum Mol Genet. (2009);18:1566–1577. doi: 10.1093/hmg/ddp068. [DOI] [PubMed] [Google Scholar]
- 36.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. (2006);20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craige B, Tsao CC, Diener DR. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. (2010);190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awata J, Takada S, Standley C. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J Cell Sci. (2014);127:4714–4727. doi: 10.1242/jcs.155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cevik S, Sanders AA, Van Wijk E. Active transport and diffusion barriers restrict Joubert Syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain. PLoS Genet. (2013);9:e1003977. doi: 10.1371/journal.pgen.1003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. (2011);30:2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) (2010);67:112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuzaki O, Bakin RE, Cai X, Menco BP, Ronnett GV. Localization of the olfactory cyclic nucleotide-gated channel subunit 1 in normal, embryonic and regenerating olfactory epithelium. Neuroscience. (1999);94:131–140. doi: 10.1016/S0306-4522(99)00228-6. [DOI] [PubMed] [Google Scholar]
- 43.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. (2013);14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 44.Cevik S, Hori Y, Kaplan OI. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. (2010);188:953–969. doi: 10.1083/jcb.200908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. (2010);189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. (2008);14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wojtyniak M, Brear AG, O'Halloran DM, Sengupta P. Cell- and subunit-specific mechanisms of CNG channel ciliary trafficking and localization in C. elegans. J Cell Sci. (2013);126:4381–4395. doi: 10.1242/jcs.127274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen PA, Liou W, Hall DH, Leroux MR. Ciliopathy proteins establish a bipartite signaling compartment in a C. elegans thermosensory neuron. J Cell Sci. (2014);127:5317–5330. doi: 10.1242/jcs.157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yack JE. The Structure and Function of Auditory Chordotonal Organs in Insects. Microsc Res Tech. (2004);63:315–334. doi: 10.1002/jemt.20051. [DOI] [PubMed] [Google Scholar]
- 50.Lee E, Sivan-Loukianova E, Eberl DF, Kernan MJ. An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr Biol. (2008);18:1899–1906. doi: 10.1016/j.cub.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bechstedt S, Albert JT, Kreil DP, Müller-Reichert T, Göpfert MC, Howard J. A doublecortin containing microtubule- associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat Commun. (2010);1:11. doi: 10.1038/ncomms1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurençon A, Dubruille R, Efimenko E. Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol. (2007);8:R195. doi: 10.1186/gb-2007-8-9-r195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. (2000);20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gopfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. (2006);9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 55.Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. (2013);77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamikouchi A, Inagaki HK, Effertz T. The neural basis of Drosophila gravity-sensing and hearing. Nature. (2009);458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Lee J, Shim J. dTULP, the Drosophila melanogaster homolog of tubby, regulates transient receptor potential channel localization in cilia. PLoS Genet. (2013);9:e1003814. doi: 10.1371/journal.pgen.1003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakata K, Shiba D, Kobayashi D, Yokoyama T. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled-coil domains. Cytoskeleton (Hoboken) (2012);69:221–234. doi: 10.1002/cm.21014. [DOI] [PubMed] [Google Scholar]
- 59.Snow JJ, Ou G, Gunnarson AL. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. (2004);6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]


