Abstract
The 12 kDa FK506-binding protein (FK506BP12), an immunosuppressor, modulates T cell activation via calcineurin inhibition. In this study, we investigated the ability of PEP-1-FK506BP12, consisting of FK506BP12 fused to the protein transduction domain PEP-1 peptide, to suppress catabolic responses in primary human chondrocytes and in a mouse carrageenan-induced paw arthritis model. Western blotting and immunofluorescence analysis showed that PEP-1-FK506BP12 efficiently penetrated chondrocytes and cartilage explants. In interleukin-1β (IL-1β)-treated chondrocytes, PEP-1-FK506BP12 significantly suppressed the expression of catabolic enzymes, including matrix metalloproteinases (MMPs)-1, -3, and -13 in addition to cyclooxygenase-2, at both the mRNA and protein levels, whereas FK506BP12 alone did not. In addition, PEP-1-FK506BP12 decreased IL-1β-induced phosphorylation of the mitogen-activated protein kinase (MAPK) complex (p38, JNK, and ERK) and the inhibitor kappa B alpha. In the mouse model of carrageenan-induced paw arthritis, PEP-1-FK506BP12 suppressed both carrageenan-induced MMP-13 production and paw inflammation. PEP-1-FK506BP12 may have therapeutic potential in the alleviation of OA progression. [BMB Reports 2015; 48(7): 407-412]
Keywords: Chondrocyte, FK506-binding protein 12, Metalloproteinase, Osteoarthritis, Protein transduction domain
INTRODUCTION
Osteoarthritis (OA) is a degenerative disease of the joints characterized by an imbalance in cartilage homeostasis. OA is influenced by genetic, mechanical, and catabolic factors (1, 2). Chondrocytes are involved in remodeling the cartilage matrix and maintaining the structure and function of the matrix by responding to anabolic and catabolic factors (1). Although OA is not considered a classical inflammatory disease, a large body of evidence shows that overexpression of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and matrix metalloproteinases (MMPs), is associated with OA development (3, 4). Therefore, OA treatment must seek to control the balance of various catabolic and anabolic factors in cartilage tissues.
Among the therapeutic strategies considered useful in OA is targeting the activities of pro-inflammatory and catabolic mediators via gene delivery techniques, such as the ex vivo gene transfer of glucuronosyltransferase-1 (5). Although gene therapy is a promising method by which therapeutic molecules may be introduced into cells, applications are significantly constrained by the poor efficacy of gene delivery, the duration of gene expression, and problems associated with toxicity. Protein transduction technology, in which therapeutic proteins are delivered directly into target tissues across biological barriers, was recently investigated as a method to expand the potential therapeutic use of proteins in treating human diseases. In a previous study, we showed that a fusion protein consisting of superoxide dismutase (SOD) and the transactivator of transcription (Tat) was successfully delivered into both a cultured chondrocyte monolayer and cartilage explants. SOD enzyme activity levels in the two systems increased while nitric oxide (NO) production and inducible NO synthase (iNOS) expression in response to IL-1 stimulation were down-regulated (6).
FK506-binding proteins (FK506BPs) are peptidyl/prolyl cis/trans isomerases (PPIases) belonging to the group of proteins known as immunophilins, which bind immunosuppressive drugs such as rapamycin and FK506. The FK506BP12/FK506 complex inhibits the activities of calcineurin (CaN), which in turn induces dephosphorylation of the nuclear factor of activated T cell (NFATc) and the production of IL-2, sub sequently stimulating T cell activation (7). The anti-inflammatory properties of and the immunomodulatory role played by FK506BP12 have been demonstrated in several disease models independent of FK506 (8, 9). In this study, we explored the efficacy of transfecting a fusion protein consisting of the protein transduction domain (PTD) PEP-1 and the 12 kDa FK506-binding protein (PEP-1-FK506BP12). Subsequent expression of the protein in human articular chondrocytes and in cartilage explant cultures was then evaluated. The regulatory effects of FK506BP12 on the expression of cartilage catabolic factors and on inflammation were examined in IL-1β-treated chondrocytes and in a mouse carrageenan-induced paw arthritis model, respectively.
RESULTS
PEP-1-FK506BP12 is efficiently transduced into primary human articular chondrocytes
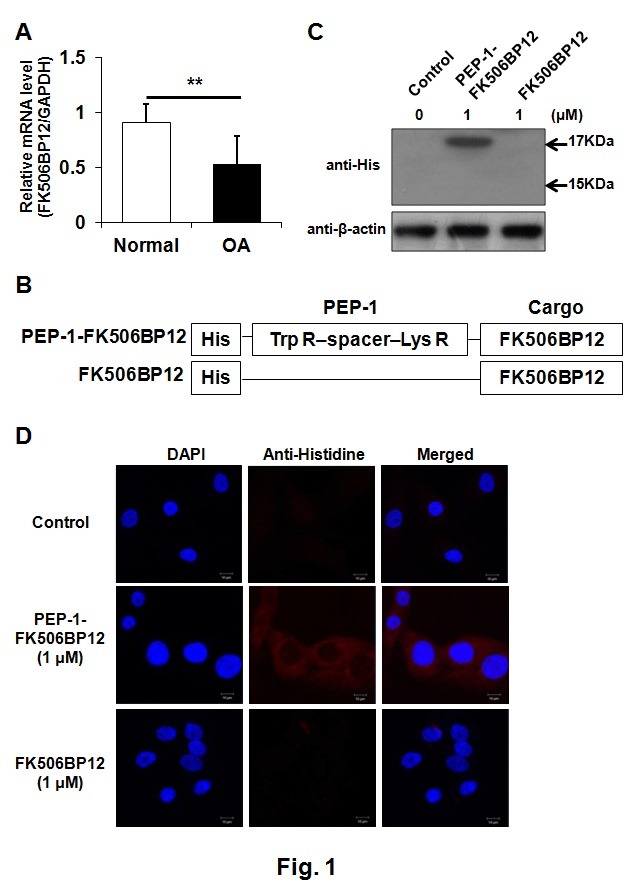
To determine whether cartilage damage was associated with decreased expression of FK506BP12, we measured the difference in FK506BP12 expression between normal and OA cartilages via real time-quantitative polymerase chain reaction (rt-qPCR). FK506BP12 expression in OA cartilage was significantly reduced (about 60%) compared to normal cartilage (Fig. 1A), suggesting a role for FK506BP12 in cartilage damage. We next explored whether PEP-1-FK506BP12, a PTD fusion protein, could be used to increase FK506BP12 levels in chondrocytes. The structure of PEP-1-FK506BP12 is schematically represented in Fig. 1B. First, to confirm the efficient transduction of PEP-1-FK506BP12 into primary human chondrocytes, monolayer-cultured chondrocytes were treated with PEP-1-FK506BP12 and FK506BP12 for 2 h and the levels of transduced PEP-1-FK506BP12 and FK506BP12 in the chondrocytes were evaluated by Western blotting and confocal microscopy. The results showed that PEP-1-FK506BP12 was efficiently transduced into the cytoplasm of primary chondrocytes (Fig. 1C and D).
Fig. 1. Transduction of PEP-1-FK506BP12 into human chondrocytes. (A) Expression of FK506BP12 in normal and OA cartilages. GAPDH served as an internal control. Data are expressed as the means ± SDs of data from triplicate experiments using cartilage from three different donors. **P < 0.01 vs. normal cartilage. (B) Schematic structure of PEP-1- FK506BP12 and FK506BP12. PEP-1-PTD, a 21-residue peptide carrier, consists of hydrophobic Trp R (tryptophan-rich), spacer, and hydrophilic Lys R (lysine-rich) domains. His, the 6-histidine motif used in the purification and detection of the two proteins. (C) Transduction of PEP-1-FK506BP12 into human primary chondrocytes. Chondrocytes were incubated with PEP-1-FK506BP12 or FK506BP12 (1 μM) for 2 h and then with trypsin-EDTA for 1 h. PEP-1-FK506BP12 and FK506BP12 levels in the cell lysates were measured by Western blotting. Control, untreated chondrocytes. (D) Distribution of transduced PEP-1-FK506BP12 in human primary chondrocytes. Chondrocytes were incubated for 2 h with PEP-1-FK506BP12 or FK506BP12 (1 μM). Control, untreated cells. Nuclei were stained with DAPI. Magnification, ×600.
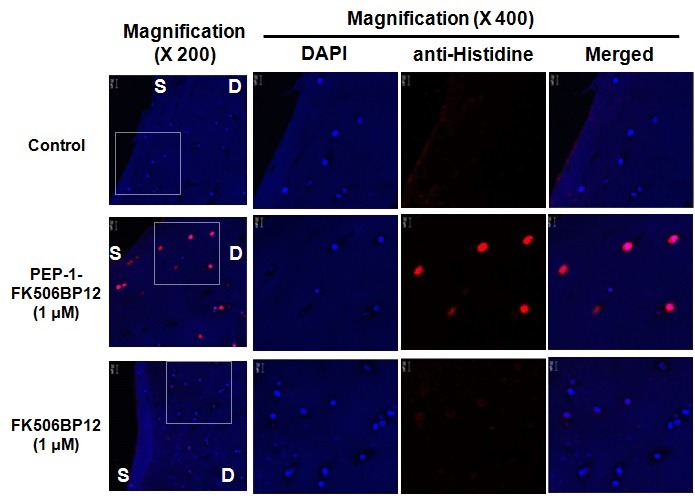
We next examined whether chondrocytes embedded in a cartilage matrix could be transduced with PEP-1-FK506BP12. Confocal microscopy revealed strong red fluorescence in the chondrocytes of PEP-1-FK506BP12-treated cartilage, indicating binding of the anti-His antibody to the histidine motif of PEP-1-FK506BP12 (Fig. 2). This confirmed that the fusion protein fully penetrated the cartilage tissues. The data showed that a construct of FK506BP12 fused with PEP-1 peptide effectively penetrated both cartilage tissues and cultured chondrocytes.
Fig. 2. Transduction of PEP-1-FK506BP12 into human-cartilage explant culture. Cartilage explants were incubated with 1 μM PEP-1- FK506BP12 and FK506BP12 for 24 h. Detection of the two proteins in cartilage explant culture was achieved using anti-His antibody and a Dylight594-conjugated secondary antibody. Magnification, ×200 and ×400. D, deep zone and S, superficial zone of cartilage.
PEP-1-FK506BP inhibits the expression of interleukin-1β-induced catabolic factors in human articular chondrocytes
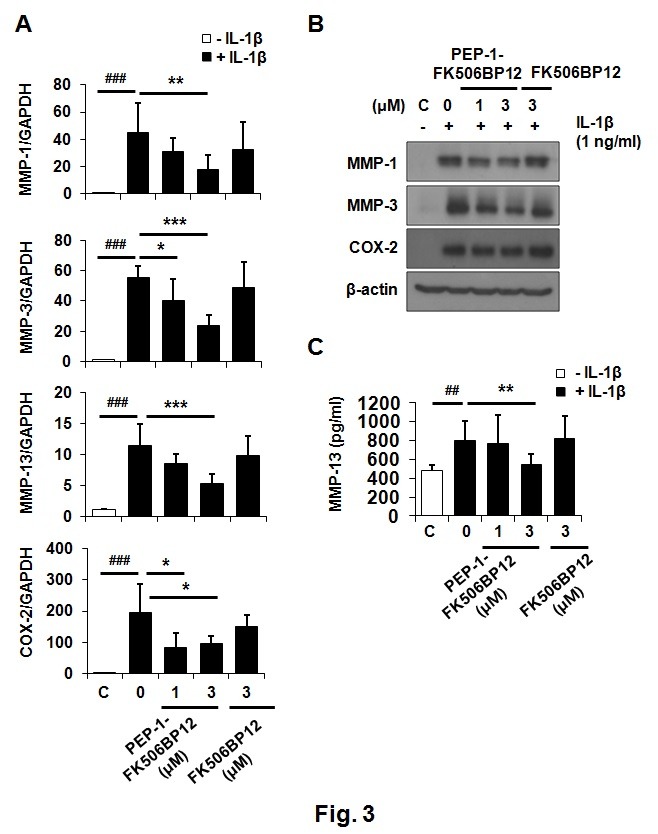
We next investigated whether PEP-1-FK506BP12 played a role in the IL-1β-triggered catabolic responses of primary chondrocytes. Chondrocytes were incubated with PEP-1-FK506BP12 for 2 h followed by incubation with IL-1β (1 ng/ml) for 6 h. IL-1β greatly increased the expression of MMP-1, -3, -13, and cyclooxygenase-2 (COX-2) mRNAs (Fig. 3A). The expression levels of all four genes in chondrocytes were inhibited by pre-treatment with PEP-1-FK506BP12 but not FK506BP12 (Fig. 3A). We then examined the effects of PEP-1-FK506BP12 on the protein-level expression of IL-1β-induced MMPs and COX-2. Transduced PEP-1-FK506BP12 (3 μM) significantly reduced IL-1β-induced MMP-1 and MMP-3 levels but not COX-2 protein expression (Fig. 3B). The enzyme-linked immunosorbent assay (ELISA) results showed that PEP-1-FK506BP12 (3 μM) suppressed IL-1β-induced MMP-13 expression (Fig. 3C). Our findings demonstrate that PEP-1-FK506BP12 suppresses IL-1β-stimulated MMPs and COX-2 expression in primary chondrocytes.
Fig. 3. PEP-1-FK506BP12 suppresses IL-1β-induced MMP expression in chondrocytes. (A) Relative expression levels of MMP-1, -3, and -13; and COX-2 mRNA, in chondrocytes treated with PEP-1-FK506BP12 or FK506BP12. Chondrocytes were pretreated with PEP-1-FK506BP12 (1 and 3 μM) or FK506BP12 (3 μM) for 2 h and exposed to IL-1β (1 ng/ml) for 6 h. mRNA levels were measured via rt-qPCR. (B), (C) Relative expression levels of MMP-1, -3, and -13; and COX-2 proteins, in chondrocytes treated with PEP-1-FK506BP12 (1 and 3 μM) or FK506BP12 (3 μM). Chondrocytes were incubated with PEP-1-FK506BP12 or FK506BP12 for 2 h and then stimulated with IL-1β (1 ng/ml) for 24 h. (B) MMP-1, and -3; and COX-2 protein levels, were measured by Western blotting. A representative blot from experiments performed using cartilage from three different donors is shown. (C) Relative levels of MMP-13 released into culture media. Data are the means ± SDs of data from duplicate experiments using cartilage from three different donors. ##P < 0.01, ###P < 0.005 vs. IL-1β-untreated chondrocytes. *P < 0.05, **P < 0.01, ***P < 0.005 vs. IL-1β-treated chondrocytes.
PEP-1-FK506BP12 suppresses IL-1β-induced catabolic responses via mitogen-activated protein kinase (MAPK) and the nuclear factor-kappaB (NF-κB) signaling pathway
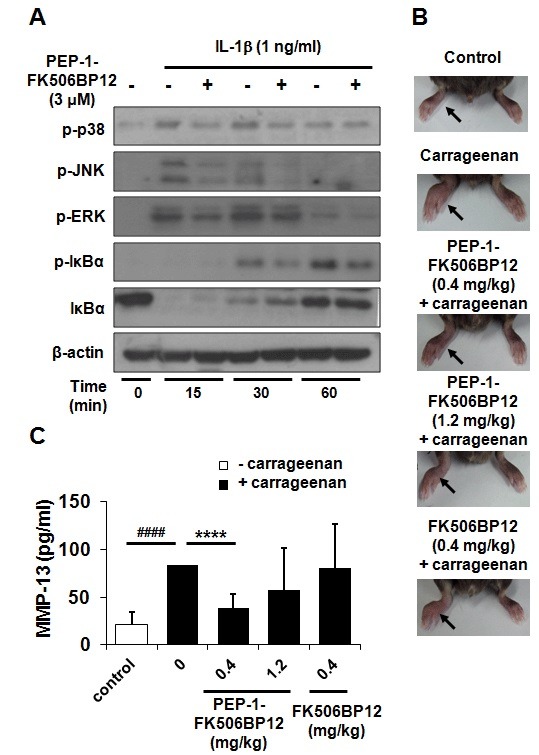
Signaling by the MAPK and NF-κB pathway plays major roles in the IL-1β- and TNF-α-stimulated expression of catabolic markers in articular chondrocytes (2, 10-12). Therefore, we examined the effect of PEP-1-FK506BP12 on this pathway. All three components of the MAPK complex (p38, JNK, and ERK1/2) were highly phosphorylated at 15 and 30 min after IL-1β treatment (Fig. 4A). The extent of phosphorylation was significantly less in PEP-1-FK506BP12-treated cells (Fig. 4A). PEP-1-FK506BP12 also reduced the phosphorylation of IκBα, a regulator of NF-κB, at 30 and 60 min after IL-1β treatment (Fig. 4A). These results demonstrated that PEP-1-FK506BP12 efficiently inhibits activation of the IL-1β-induced MAPK and NF-κB signaling pathway.
Fig. 4. The effect of PEP-1-FK506BP12 on MAPK and NF-κB activation in primary chondrocytes and MMP-13 expression in a mouse carrageenan-induced paw arthritis model. (A) PEP-1-FK506BP12 inhibits IL-1β-induced MAPK and NF-κB activation. Chondrocytes were incubated with PEP-1-FK506BP12 (3 μM) for 2 h and then with IL-1β for 15, 30, and 60 min. The levels of phosphorylated p38, JNK, ERK1/2, and IκBα were measured by Western blotting. β-actin served as the loading control. The blot is representative of the results of three independent experiments using cartilage from three different donors. (B, C) The anti-inflammatory effect of PEP-1-FK506BP12 in a mouse carrageenan-induced paw edema model. PEP-1-FK506BP12 (0.4 and 1.2 mg/kg) and FK506BP12 (0.4 mg/kg) was injected into the mouse hidpaw (n=5 mice/group) 2 h prior to injecting 2.5% carrageenan. The mice were euthanized 4 days later. (B) PEP-1-FK506BP12 suppresses carrageenan-stimulated inflammation in the mouse hindpaw. Representative image of a hindpaw from each group. The arrows indicate the carrageenan injection sites. (C) Carrageen-induced MMP-13 expression is inhibited by PEP-1-FK506BP12. Tissue homogenates were prepared as described in Materials and Methods. Data are expressed as the means ± SDs of duplicate experiments performed using tissue homogenates from three different animals. ####P < 0.001 vs. untreated control mouse. ****P < 0.001 vs. results from a carrageenan-injected mouse. − carrageenan, carrageen-non-injected group; + carrageenan, carrageenan-injected group.
PEP-1-FK506BP12 suppresses MMP-13 production and paw edema in the mouse carrageenan-induced paw arthritis model
The anti-inflammatory effect of PEP-1-FK506BP12 was examined in a mouse carrageenan-induced arthritis model. PEP-1-FK506BP12 and FK506BP12 were injected into the mouse right hindpaw at a dose of 0.4 or 1.2 mg/kg followed by the injection of 2.5% carrageenan into the same hindpaw. Representative photographic images from each group are shown in Fig. 4B. Carrageen-induced paw edema was suppressed by PEP-1-FK506BP12 (Fig. 4B).
To further investigate whether PEP-1-FK506BP12 could suppress catabolic responses in carrageenan-induced inflammation, MMP-13 expression levels were determined via ELISA. In the carrageenan-treated mice injected with 0.4 and 1.2 mg PEP-1-FK506BP12/kg, MMP-13 levels were reduced by 46% and 68%, respectively, although the decrease in MMP-13 level was significant only at the lower dose (Fig. 4C). In contrast, FK506BP12 did not inhibit carrageenan-induced MMP-13 expression.
DISCUSSION
We showed that the fusion construct PEP-1-FK506BP12, which traverses the cell membrane of chondrocytes, down-regulates IL-1β-triggered catabolic responses in primary human chondrocytes by modulating the phosphorylation of MAPK and IκBα. In addition, PEP-1-FK506BP12 reduced MMP-13 production in cultured chondrocytes and in a mouse carrageenan-induced paw arthritis model. These results suggest that PEP-1-FK506BP12 may be useful for treating arthritis and preventing cartilage catabolism.
Several strategies are currently under development to block the pro-inflammatory and catabolic pathways of arthritis. However, very few compounds are known to specifically block well-defined target pathways, and nonspecific off-target effects often prohibit the clinical use of chemical drugs. Gene therapies in which the expression of pro-inflammatory genes is modulated by decoy oligonucleotides and RNA interference technology achieve more specific effects, but gene delivery poses many problems in terms of efficiency and safety, in addition to unwanted inflammatory and immunogenic effects elicited by the viral vectors. The potential advantages of protein transduction technology in regulating cellular functions are the specificity of targeting particular signaling pathways, direct high-efficiency regulation of intracellular protein levels, minimal side effects, and reversibility (13). The disadvantages of such methods include a lack of target-cell specificity, immunogenicity, and incomplete knowledge of the mechanisms underlying protein transduction. Among the applications of PTD examined thus far are apoptosis inhibition via the transduction of anti-apoptotic Bcl-XL protein into human islet cells (14) and chemosensitivity induction by transducing cytosine deaminase in human tumor cells (15). Chondrocytes are embedded in a thick extracellular matrix, which poses a challenge for the delivery of foreign genes or proteins to such cells. However, the inhibition of apoptosis upon the transduction of anti-apoptotic Bcl-XL protein into explant cultured human chondrocytes has been reported (16). In a previous study, we showed that SOD fused to the Tat domain could be successfully delivered into both monolayer and explanted cultured chondrocytes. Expression of the fusion construct in IL-1-stimulated cells resulted in the suppression of both NO production and iNOS mRNA expression (6). Such studies demonstrate the feasibility of protein delivery employing PTD as a therapeutic modality to regulate catabolic processes in cartilage. Protein transduction therapy has also shown promise in in vivo models, such as the reduction of brain damage caused by the PTD-anti-death FNK protein in a rat model of focal transient ischemia (17), and in the suppression of atopic dermatitis by PEP-1-FK506BP12 in NC/Nga mice (9). Phase I and II clinical trials are underway, with over 2,000 patients currently being treated with PTD-delivered peptides or protein cargos for a variety of indications (18).
The mechanism underlying the cellular uptake of PTDs has been studied extensively. PEP-1 peptide was shown to deliver cargo molecules in a biologically active form into various cell lines, without covalent coupling or denaturation (19). Cellular entry may be affected by several factors, including the type of PTD used, the nature and size of cargo molecules, the cell lines employed, the concentrations applied, and the incubation time. Complexes of PEP-1 and their cargos interact with membrane phospholipids and enter the cell by triggering transient membrane disorganization as well as conformational changes in the PEP-1 peptide, which together favor the rapid release of cargo molecules into the cytoplasm in the absence of degradation, suggesting that delivery is independent of the endosomal pathway (19, 20).
FK506BPs belong to a family of proteins termed immunophilins due to their ability to bind immunosuppressive drugs such as rapamycin and FK506. To date, 14 FK506BPs have been identified in humans; they are involved in biological processes, such as the DNA damage response in addition to exerting anti-tumor effects. FK506BP12 is a small immunophilin that contains a PPIase domain (21). The anti-inflammatory effect of FK506BP12 was recently demonstrated in animal models of atopic dermatitis and botulinum-toxin-A-induced dry eye diseases. In both models, the inhibition of cytokine/chemokine expression independent of the presence of FK506 was demonstrated (8, 9).
The CaN-NFAT pathway is blocked by FK506BP and activated by IL-1β. Previous reports suggested that FK506BP12 affects a number of biological processes by modulating the activity of CaN. Both the ryanodine receptor/calcium release channel (RyR1) of the sarcoplasmic reticulum (22) and the inositol 1,4,5-triphosphate receptor (23, 24) are stabilized via an association with FK506BP12 in the absence of FK506, thereby regulating Ca2+ flux through the channels. We also found that both the IL-1β-induced up-regulation of pro-inflammatory factors such as MMPs and COX-2 and activation of the NF-κB and MAPK pathway, crucial for regulating both cartilage catabolic responses and the immune system (2, 10-12), were significantly suppressed by an increase in the cellular level of PEP-1-FK506BP12 in chondrocytes (Figs. 3 and 4A). It is presumed that high-level expression of PEP-1-FK506BP12 results in the construct binding to and subsequently inhibiting CaN, which induces the dephosphorylation of NFAT and indirectly regulates the expression of NFAT-activation-related pro-inflammatory genes via MAPK and NF-κB signaling.
In summary, we show that FK506BP12 is down-regulated in OA cartilage compared to normal cartilage tissues. High-level cellular expression of FK506BP12 after fusion with the PEP-1 peptide significantly enhanced FK506BP12 levels in primary human chondrocytes. PEP-1-FK506BP12 prevented the IL-1β-induced catabolic responses of chondrocytes by suppressing the phosphorylation of MAPK and IκBα. The expression of MMP-13, a type II collagen-degrading enzyme, was significantly inhibited by PEP-1-FK506BP12 in a carrageenan-induced mouse arthritis model. Thus, PEP-1- FK506BP12 may have therapeutic potential in the alleviation of cartilage degradation.
MATERIALS AND METHODS
Materials, Expression and purification of PEP-1-FK506BP12, Cell culture and cartilage explant culture, Transduction of PEP-1-FK506BP12 into primary chondrocytes, Confocal fluorescence microscopy for verification of protein transduction, Carrageenan-induced paw arthritis, Quantification of MMP-13 levels via ELISA, and Data analysis are described in the Supplementary Materials, available at http://www.bmbreports.org/.
Real time-quantitative polymerase chain reaction
Total RNA was isolated from cartilage explants or chondrocytes using Trizol (Invitrogen, Carlsbad, CA, USA) according to a previous report (25). First-strand cDNA synthesis was performed with 2 μg of RNA using a reverse transcription Go Script kit (Promega, Fitchburg, WI, USA). Rt-qPCR was performed using a QuantiFast SYBR Green PCR kit (Qiagen, Hilden, Germany) and the StepOnePlus real-time PCR system (Applied Biosystems, Foster, CA, USA). The primer sequences of specific genes used in real-time qPCR were as follows; FK506BP forward 5’-ACT ACA CCG GGA TGC TTG AA-3’, reverse 5’-TCA GTT TGG CTC TCT GAC CC-3’, MMP-1 forward 5’-AGT GAC TGG GAA ACC AGA TGC TGA-3’, reverse 5’-GCT CTT GGC AAA TCT GGC GTG TAA-3’, MMP-3 forward 5’-GCG TGG ATG CCG CAT ATG AAG TTA-3’, reverse 5’-AAA CCT AGG GTG TGG ATG CCT CTT-3’, MMP-13 forward 5’-AAG GAC CCT GGA GCA CTC ATG TTT-3’, reverse 5’-TGG CAT CAA GGG ATA AGG AAG GGT-3’, COX-2 forward 5’-AGC TGG GAA GCC TTC TCT AAC-3’, reverse 5’-AGA TCA TCT CTG CCT GAG TAT CTT-3’, GAPDH forward 5’-TGA TGA CAT CAA GAA GGT GGT GAA G-3’, reverse 5’-TCC TTG GAG GCC ATG TGG GCC AT-3’.
Western blot analysis
Chondrocytes were lysed with RIPA buffer (50 mM sodium acetate pH 5.8, 10% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 μg aprotinin/ml) at 4℃ according to a method previously described (26). Equal amounts of proteins were resolved via 10% SDS polyacrylamide gel electrophoresis and electrotranferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MS, USA). The membranes were blocked with 5% nonfat milk in TBS-T buffer (25 mM Tris-HCl, 140 mM NaCl, 0.1% Tween 20, pH 7.5) and next incubated with primary and horseradish peroxidase-conjugated secondary antibodies. Each membrane was developed using an enhanced chemiluminescence kit (Santa Cruz).
Acknowledgments
This work was supported by a grant (A120960) from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea, by the National Research Foundation of Korea; the Mid-career Research program grant (NRF-2009-0084569), and by the Hallym University Research Fund.
References
- 1.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. (2000);43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. (2007);213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. (2004);427 Suppl:S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 4.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. (2004);427 Suppl:S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan N, Barre L, Benani A, et al. Stimulation of proteoglycan synthesis by glucuronosyltransferase-I gene delivery: a strategy to promote cartilage repair. Proc Natl Acad Sci U S A. (2004);101:18087–18092. doi: 10.1073/pnas.0404504102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HA, Kim DW, Park J, Choi SY. Transduction of Cu, Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic domain into human chondrocytes. Arthritis Res Ther. (2006);8:R96. doi: 10.1186/ar1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiene-Fischer C, Yu C. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. (2001);495:1–6. doi: 10.1016/S0014-5793(01)02326-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim DW, Lee SH, Ku SK, et al. Transduced PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin A-induced dry eye mouse model. BMB Rep. (2013);46:124–129. doi: 10.5483/BMBRep.2013.46.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Sohn EJ, Kim DW, et al. Transduced PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga mice. J Invest Dermatol. (2013);131:1477–1485. doi: 10.1038/jid.2011.49. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor- 2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. (2010);16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 11.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. (2000);43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Fujii T, Onohara N, Maruyama Y, et al. Galpha12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J Biol Chem. (2005);280:23041–23047. doi: 10.1074/jbc.M409397200. [DOI] [PubMed] [Google Scholar]
- 13.Kabouridis PS, Hasan M, Newson J, Gilroy DW, Lawrence T. Inhibition of NF-kappa B activity by a membrane-transducing mutant of I kappa B alpha. J Immunol. (2002);169:2587–2593. doi: 10.4049/jimmunol.169.5.2587. [DOI] [PubMed] [Google Scholar]
- 14.Klein D, Ribeiro MM, Mendoza V, et al. Delivery of Bcl-XL or its BH4 domain by protein transduction inhibits apoptosis in human islets. Biochem Biophys Res Commun. (2004);323:473–478. doi: 10.1016/j.bbrc.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Ryu J, Kim KA, et al. Transduction of yeast cytosine deaminase mediated by HIV-1 Tat basic domain into tumor cells induces chemosensitivity to 5-fluorocytosine. Exp Mol Med. (2004);36:43–51. doi: 10.1038/emm.2004.6. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki D, Sudo K, Asoh S, Yamagata K, Ito H, Ohta S. Transduction of anti-apoptotic proteins into chondrocytes in cartilage slice culture. Biochem Biophys Res Commun. (2004);313:522–527. doi: 10.1016/j.bbrc.2003.11.144. [DOI] [PubMed] [Google Scholar]
- 17.Katsura K, Takahashi K, Asoh S, et al. Combination therapy with transductive anti-death FNK protein and FK506 ameliorates brain damage with focal transient ischemia in rat. J Neurochem. (2008);106:258–270. doi: 10.1111/j.1471-4159.2008.05360.x. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. (2011);22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. (2001);19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 20.Gros E, Deshayes S, Morris MC, et al. A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochim Biophys Acta. (2006);1758:384–393. doi: 10.1016/j.bbamem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Kang CB, Hong Y, Dhe-Paganon S, Yoon HS. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals. (2008);16:318–325. doi: 10.1159/000123041. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y, Ogunbunmi EM, Freund EA, Timerman AP, Fleischer S. FK-binding protein is associated with the ryanodine receptor of skeletal muscle in vertebrate animals. J Biol Chem. (1998);273:34813–34819. doi: 10.1074/jbc.273.52.34813. [DOI] [PubMed] [Google Scholar]
- 23.Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. (1995);83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 24.Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD, Snyder SH. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci U S A. (1995);92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee NH, Jung YS, Lee HY, et al. Mouse neutrophils express functional umami taste receptor T1R1/T1R3. BMB Rep. (2014);47:649–654. doi: 10.5483/BMBRep.2014.47.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Jiang K, Qiu X, et al. Overexpression of CXCR4 is significantly associated with cisplatin-based chemotherapy resistance and can be a prognostic factor in epithelial ovarian cancer. BMB Rep. (2014);47:33–38. doi: 10.5483/BMBRep.2014.47.1.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


