Abstract
Macula densa cells in the distal nephron, according to the classic paradigm, are salt sensors that generate paracrine chemical signals in the juxtaglomerular apparatus to control vital kidney functions, including renal blood flow, glomerular filtration, and renin release. Renin is the rate-limiting step in the activation of the renin-angiotensin system, a key modulator of body fluid homeostasis. Here, we discuss recent advances in understanding macula densa sensing and suggest these cells, in addition to salt, also sense various chemical and metabolic signals in the tubular environment that directly trigger renin release.
The juxtaglomerular apparatus in the renal cortex represents a major structural component of the renin-angiotensin system and is one of the most important regulatory sites of renal salt and water conservation and BP maintenance. The juxtaglomerular apparatus consists of a tubular component, the macula densa, the extraglomerular mesangium, and a vascular element that involves the terminal parts of the afferent arteriole containing renin-producing juxtaglomerular cells. Two major regulatory functions are performed by the juxtaglomerular apparatus: the high distal tubular [NaCl]-induced afferent arteriolar vasoconstriction (tubuloglomerular feedback) and the low tubular [NaCl]-induced renin release.1 Macula densa cells are strategically positioned in the juxtaglomerular apparatus with their apical membrane exposed to the tubular fluid, whereas their basilar aspects are in contact with cells of the mesangium and the afferent arteriole (Figure 1A).
Figure 1.
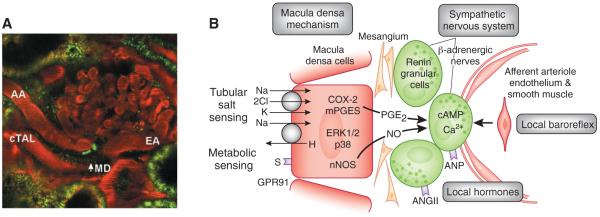
Fluorescence microscopic image (A) and schematic (B) of the juxtaglomerular apparatus (juxtaglomerular apparatus). (A) A multiphoton confocal fluorescence image of the juxtaglomerular apparatus in the intact rat kidney in vivo showing the afferent (AA) and efferent arterioles (EA) and cortical thick ascending limb (cTAL) containing the macula densa. Original magnification, ×250. Renin granular content in juxtaglomerular cells under the macula densa is labeled green using quinacrine as described before.34 (B) The main control mechanisms of renin release and elements of the macula densa sensing and signaling apparatus. Macula densa cells can sense variations in tubular fluid composition, including salt content and metabolites such as succinate. Salt is sensed via the NKCC2 and NHE2, whereas tubular succinate triggers the metabolic receptor GPR91 at the luminal plasma membrane. Signal transduction includes activation of MAP kinases p38 and pERK1/2, PGE2 synthesis through COX-2, and mPGES. PGE2 via paracrine signaling causes increased renin synthesis and release from adjacent juxtaglomerular cells and activation of the renin-angiotensin system (RAS). S, succinate; nNOS, neural nitric oxide synthase.
The macula densa plaque is a unique group of 15 to 20 cells located at the end of the cortical thick ascending limb forming a juxtaglomerular apparatus-glomerular complex. These cells play a pivotal role in sensing changes in tubular fluid composition, generating and sending signals to the juxtaglomerular apparatus that control renal blood flow and GFR through tubuloglomerular feedback and renin release.1–3 Tubular salt sensing by the macula densa involves apical NaCl transport mechanisms, including the furosemide-sensitive Na+:2Cl−:K+ cotransporter (NKCC2), which is the primary NaCl entry mechanism. In fact, a classic hallmark of tubuloglomerular feedback and renin release is their effective inhibition or stimulation, respectively, by furosemide or other loop diuretics.1–4
The downstream elements of macula densa-mediated signaling of renin release include, at least, the low tubular salt-induced and NKCC2-mediated activation of p38 and extracellular-regulated kinase 1/2 (ERK1/2) mitogen-activated protein (MAP) kinases, cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase (mPGES) in the macula densa,4–9 and the synthesis and release of PGE2.8 PGE2 acts on EP2 and EP4 receptors in juxtaglomerular cells and causes renin release (Figure 1B).10 In addition to COX-2-derived prostaglandins, the neural isoform of nitric oxide synthases, which is selectively expressed in macula densa cells,11 is critical in the tubuloglomerular feedback and renin signaling cascade.2,12,13 The paracrine chemical signals of macula densa-mediated inhibition of renin release include ATP and adenosine.1–3,14
Besides the well-known NKCC2 cotransporter, macula densa cells possess an apical Na+:H+ exchanger (NHE), identified as the NHE2 isoform,15 that participates in Na+ transport as well as the regulation of cell volume and intracellular pH.15,16 A recent study found that NHE2 is also involved in macula densa salt-sensing and renin control, and suggests that macula densa cell shrinkage is the likely cellular signal that activates renin release signaling.17 Renal tissue renin activity and plasma renin concentrations are both elevated 3-fold and 2-fold, respectively, in NHE2−/− mice compared with wild type.17 NHE2−/− mice also exhibit a significantly increased renal expression of cortical COX-2 and mPGES, indicating macula densa-specific mechanisms responsible for the increased renin content.17 Importantly, pharmacologic inhibition or genetic deletion of NHE2 activates MAP kinases ERK1/2, causing activation of the PGE2 synthetic enzymes COX-2 and mPGES. Hypertonicity-induced cell shrinkage, but not cell acidification, also triggers ERK1/2 activation in macula densa cells,17 suggesting it is the low macula densa cell volume rather than low intracellular pH that activates macula densa renin-release signal. Interestingly, macula densa cells also possess a basolateral Na+:H+ exchanger, NHE4.15 This polarized NHE2/NHE4 configuration in the macula densa is unique and distinct from the usual NHE3/NHE1 arrangement in other nephron segments, further suggesting that NHEs play a combined role in macula densa cell function.15
THE CLASSIC VIEW OF RENIN CONTROL IN THE JUXTAGLOMERULAR APPARATUS
Release of renin from juxtaglomerular cells in the terminal afferent arteriole is the first and, at least initially, the rate-limiting step of renin-angiotensin system activation that is precisely controlled by several mechanisms (Figure 1B). Reductions in extracellular fluid volume through four major mechanisms: low renal perfusion pressure (local baroreflex mechanism); activation of the sympathetic nervous system; reductions in macula densa salt transport; and reduced levels of locally acting hormones (such as angiotensin II and atrial naturetic peptide) ultimately increase circulating and interstitial renin levels that lead to enhanced generation of angiotensin peptides.18 Angiotensin II, one of the most potent vasoconstrictors and major products of the renin-angiotensin system, helps re-establish fluid balance and normal BP by actions on multiple organs providing blood vessel constriction, increased renal and gastrointestinal salt and water reabsorption, and aldosterone production by the adrenal gland.
On a cellular and molecular level, prostaglandins (mainly PGI2 and PGE2) and nitric oxide mediate paracrine renin-release signals in the juxtaglomerular apparatus (Figure 1B).18 These autacoids increase the production or block the degradation of juxtaglomerular cell cAMP, the key intracellular signaling molecule in juxtaglomerular cells that stimulates renin release.18 The most important inhibitory mechanism of renin synthesis and release is elevations in juxtaglomerular cell calcium concentration.19 This effect of calcium is rather unusual because calcium usually facilitates exocytosis in other cells and systems. Its inhibitory effect on renin secretion has been coined the “calcium paradox of renin release,” and this is because of the expression of calcium-inhibited adenylate cyclase, AC5, in juxtaglomerular cells.
COX-2, the source of macula densa-derived prostaglandins mediating renin expression and release by the juxtaglomerular apparatus, is present at low but detectable levels in the macula densa under normal homeostatic conditions.5 Induction of a high-renin state by imposition of a salt-deficient diet, angiotensin-converting enzyme inhibition, diuretic administration, or experimental renovascular hypertension all significantly increase COX-2 expression by the macula densa.20–23 Alterations in macula densa COX-2 expression also play an essential role in the tonic expression of juxtaglomerular cell renin rather than acting as an acute regulator of stimulated renin production and release in response to macula densa-derived signals as well as other signals for renin release, particularly β-adrenergic stimulation or renal perfusion pressure.24,25
Complex interactions exist between COX-2 expression by the macula densa and components of the renin-angiotensin system, with both positive and negative feedback mechanisms. Angiotensin II normally inhibits macula densa COX-2 expression, perhaps through a direct inhibition through macula densa AT1 receptors,26,27 suggesting an inhibitory feedback loop. However, in the presence of AT1 inhibition, angiotensin II actually stimulates macula densa COX-2 expression through AT2 receptors.27 To further complicate matters, transgenic rats overexpressing the prorenin receptor in all cells increase macula densa COX-2 expression and hyperfiltration,28 and immunoreactive prorenin receptor can be detected in the macula densa.29
The intrarenal dopaminergic system serves as a counter-regulatory mechanism to the renin-angiotensin system.30 Although studies in isolated cells from the juxtaglomerular apparatus indicate dopamine directly stimulates renin release by increasing cAMP, the net effect of activation of the intrarenal dopaminergic system is actually to decrease the expression and release of renin from juxtaglomerular cells through inhibition of macula densa COX-2 expression.31,32
MACULA DENSA CELLS AS METABOLIC DETECTORS AND CHEMOSENSORS
In addition to tubular salt sensing, macula densa cells also sense alterations in tissue metabolism through the accumulation of the Krebs cycle intermediate, succinate, in tubular fluid.33 At the onset of diabetes mellitus, tubular succinate activates a newly identified metabolic receptor (GPR91) localized to the apical membrane of macula densa cells.33 GPR91 activation in macula densa cells is connected to the same signaling cascade as salt sensing; namely, the activation of p38 and ERK1/2 MAP kinases, COX-2, and the synthesis and release of PGE2-triggered renin exocytosis.33 GPR91 expression is localized in the juxtaglomerular vasculature, where it plays a similar role in renin control,34 most likely acting in parallel with the macula densa mechanism. GPR91 signaling in the juxtaglomerular apparatus in early diabetes provides a new, direct link between high glucose levels and intrarenal renin-angiotensin activation. The importance of the renin-angiotensin system in the pathology of several metabolic diseases, including diabetes, metabolic syndrome, and hyperuricemia, is well established.35,36 Activated by alterations in local tissue metabolism and succinate accumulation, GPR91 signaling also regulates pathophysiological functions in other organs, such as the retina,37 another organ with common diabetic complications. It is interesting to speculate that GPR91 may be the molecular link between diabetic nephropathy and retinopathy.
Further supporting the ability of macula densa cells to directly sense certain chemical compounds in the tubular microenvironment is the recent discovery of the olfactory signaling system along the apical surface of these cells.38 At least one olfactory receptor, the olfactory G protein, and the olfactory isoform of adenylate cyclase, AC3, are found in macula densa cells.38 Consistent with macula densa function, AC3−/− mice have significantly reduced glomerular filtration and plasma renin levels,38 suggesting the physiologic importance of this tubular chemosensory machinery. It remains to be established, however, exactly which chemical compounds macula densa cells can “smell” in addition to succinate. Nevertheless, the idea that certain chemical signals in the tubular fluid can directly regulate GFR and renin-angiotensin activity is exciting and will undoubtedly open new areas in renal research.
ACKNOWLEDGMENTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants 64324 (to J.P.-P.), DK62795 (to R.C.H.), DK74754 (to J.P.-P.), and DK79341 (to R.C.H.), funds from the Veterans’ Administration (to R.C.H.), and the American Heart Association Established Investigator Award 0640056N (to J.P.-P.).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: Control of glomerular hemodynamics and renin secretion. In: Alpern RJ, Hebert SC, editors. The Kidney Physiology and Pathophysiology. Elsevier Academic Press; Burlington/San Diego/London: 2008. pp. 589–626. [Google Scholar]
- 2.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: Adenosine ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 3.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiolv. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 5.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN. Breyer macula densa: Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J Clin Invest. 2000;106:681–688. doi: 10.1172/JCI10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Parks JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 8.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. Breyer macula densa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol. 2003;285:F558–64. doi: 10.1152/ajprenal.00433.2002. [DOI] [PubMed] [Google Scholar]
- 10.Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol. 2872004:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 11.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992;42:1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- 12.Kovács G, Komlósi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase: Its role and regulation in macula densa cells. J Am Soc Nephrol. 2003;14:2475–2483. doi: 10.1097/01.asn.0000088737.05283.2b. [DOI] [PubMed] [Google Scholar]
- 13.Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. Am J Physiol. 1997;272:R1359–R1364. doi: 10.1152/ajpregu.1997.272.5.R1359. [DOI] [PubMed] [Google Scholar]
- 14.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- 15.Peti-Peterdi J, Chambrey R, Bebok Z, Biemesderfer D, St John PL, Abrahamson DR, Warnock DG, Bell PD. Macula densa Na(+)/H(+) exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am J Physiol Renal Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- 16.Peti-Peterdi J, Bebok Z, Lapointe JY, Bell PD. Novel regulation of cell [Na(+)] in macula densa cells: Apical Na(+) recycling by H-K-ATPase. Am J Physiol Renal Physiol. 2002;282:F324–9. doi: 10.1152/ajprenal.00251.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, Shull GE, Lorenz JN, Eladari D, Peti-Peterdi J. Increased renal renin content in mice lacking the Na+/H+ exchanger NHE2. Am J Physiol Renal Physiol. 2008;294:F937–F944. doi: 10.1152/ajprenal.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology (Bethesda) 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension. 2007;49:162–169. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 21.Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 22.Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding P, Carretero OA, Beierwaltes WH. Chronic cyclooxygenase-2 inhibition blunts low sodium-stimulated renin without changing renal haemodynamics. J Hypertens. 2000;18:1107–1113. doi: 10.1097/00004872-200018080-00016. [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 25.Matzdorf C, Kurtz A, Hocherl K. COX-2 activity determines the level of renin expression but is dispensable for acute upregulation of renin expression in rat kidneys. Am J Physiol Renal Physiol. 2007;292:F1782–F1790. doi: 10.1152/ajprenal.00513.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs G, Peti-Peterdi J, Rosivall L, Bell PD. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol. 2002;282:F301–F306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103:6045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–646. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen G. Increased cyclooxygenase-2, hyperfiltration, glomerulosclerosis, and diabetic nephropathy: Put the blame on the (pro)renin receptor? Kidney Int. 2006;70:618–62028. doi: 10.1038/sj.ki.5001723. [DOI] [PubMed] [Google Scholar]
- 30.Jose PA, Eisner GM, Felder RA. Dopamine and the kidney: A role in hypertension? Curr Opin Nephrol Hypertens. 2003;12:189–194. doi: 10.1097/00041552-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–F845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 32.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–567. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas SL, Toma I, Kang JJ, Meer E, Peti-Peterdi J. Succinate receptor (GPR91) activation in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20:1002–1011. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118:2526–2534. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honoré JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beauséjour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 38.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A. 2009;106:2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]


