Abstract
Objectives
To describe levels of fatigue and explore clinical factors that might contribute to fatigue in critically ill patients receiving mechanical ventilation.
Research Methodology/Design
Descriptive, correlational design. Sample was a sub-set of patients enrolled in a randomized clinical trial testing patient-directed music for anxiety self-management. Clinical factors included age, gender, length of ICU stay, length of ventilatory support, illness severity (APACHE III), and sedative exposure (sedation intensity and frequency). Descriptive statistics and mixed models were used to address the study objectives.
Setting
Medical and surgical intensive care units in the Midwestern U.S.A.
Main Outcome Measures
Fatigue was measured daily via a 100-mm Visual Analog Scale, up to 25 days.
Results
A sample of 80 patients (50% female) receiving ventilatory support for a median 7.9 days (range 1-46) with a mean age of 61.2 years (SD 14.8) provided daily fatigue ratings. ICU admission APACHE III was 61.5 (SD 19.8). Baseline mean fatigue ratings were 60.7 (SD 27.9), with fluctuations over time indicating a general trend upward. Mixed models analysis implicated illness severity (β(se(β)) = .27(.12)) and sedation frequency (β(se(β)) = 1.2(.52)) as significant contributors to fatigue ratings.
Conclusion
Illness severity and more frequent sedative administration were related to higher fatigue ratings in these mechanically ventilated patients.
INTRODUCTION
Fatigue can be defined as a general overall feeling of tiredness and/or decreased energy level, but not necessarily sleepy (http://www.npcrc.org/files/news/edmonton_symptom_assessment_scale.pdf). The symptom of fatigue and its contributors are well documented in persons with cancer, as are interventions to assist these patients with management of this common and debilitating symptom (Delgado-Guay, Parsons, Li, Palmer & Bruera, 2009; Henry, Viswanathan, Elkin, Traina, Wade & Celia, 2008; Poirier, 2013. Fatigue in cancer patients is subjective and multi-dimensional, related to the disease itself as well as the side-effects from medications and treatments. In addition, pain, emotional distress, and anaemia contribute to fatigue in cancer patients (Poirier, 2013). In patients who are critically ill, the literature documents that these patients often report feeling “tired” (Matthews, 2011; Puntillo, et al., 2010), however, little is known about the self-rating of fatigue and its potential clinical contributors, particularly in those ICU patients receiving mechanical ventilation. Sleep disturbances and fatigue can be intertwined in ICU patients, and fatigue can impact a patient’s ability to participate in one’s care (Matthews, 2011). One of the few studies reporting symptom assessment in critically ill patients revealed that 75% of participants reported being tired (Puntillo, et al., 2010). This descriptive study did not aim to determine the source of fatigue or any clinical covariates that might be associated with this symptom. Fatigue, conceptualised as tiredness, was the most frequently occurring, intense, and distressful symptom reported by the study participants (Puntillo, et al., 2010). However, a majority of the participants (65%) were not receiving mechanical ventilatory support. Screening, evaluation, and management of fatigue are thought to be suboptimal in the critical care setting (Matthews, 2011), with little information specifically in patients receiving the common supportive modality of mechanical ventilation. A description of fatigue and potential clinical factors that contribute to this vague, yet common symptom in critically ill patients is needed before interventions can be designed and tested to manage this common symptom. Thus, the following study was undertaken to begin to fill this knowledge gap by describing fatigue ratings and determining if any clinical variables are related to fatigue in critically ill patients receiving mechanical ventilatory support.
METHODS
Aims/Design
The aims of this descriptive, correlational study were to: 1) describe levels of fatigue over the course of mechanical ventilatory support, and 2) explore if selected clinical factors contribute to fatigue ratings in a sample of critically ill patients receiving mechanical ventilation. Participants in this study consisted of a sub-set of patients enrolled in a randomised clinical trial testing patient-directed music for anxiety self-management and sedative exposure reduction in patients receiving mechanical ventilatory support. Patients were randomised to one of three conditions: 1) experimental self-initiated music listening with preferred music whenever desired for as long as desired each day enrolled on protocol; 2) active control of noise-cancelling headphones that patients wore whenever quiet time was desired as frequently and for as long each day enrolled on protocol; and, 3) usual care for the respective ICU. Details on the findings from the parent study are reported elsewhere (Chlan, et al., 2013).
Setting
Patients receiving mechanical ventilatory support for a pulmonary indication were enrolled from one of 12 ICUs in the urban Midwest of the United States. These ICUs were a mix of medical and medical-surgical ICUs where patient care was delivered by specially trained nurses in a one nurse to two patient ratio.
Ethical Approval
Approval for the use of human subjects in research was obtained from the parent study’s institutional review board and from the participating sites human subjects’ committees. Trained research nurses obtained all informed consent and collected all study data.
Participants
To be eligible for the parent study, patients receiving acute mechanical ventilatory support on the participating ICUs had to be alert, interacting appropriately with ICU nursing staff, and provide their own informed consent. Patients who were receiving aggressive ventilatory support, were haemodynamically unstable, or had documented mental incompetence (Alzheimer’s disease) were not approached for study participation. Participants remained enrolled on protocol in the parent study as long as they were receiving ventilatory support, up to 30 days, or until extubated, chose to withdraw, were transferred from the ICU, or died.
Data Collection
Demographic Characteristics
Demographic data were obtained from the enrolled patients and their medical records at study entry on age, gender, race, ethnicity, illness severity, ICU admission diagnosis, and indication for mechanical ventilation. Illness severity was determined by APACHE III (Knaus et al., 1991). Data were obtained from the patient’s medical record from the first 24 hours of ICU stay to determine the APACHE III score
Clinical Factors
Clinical factors considered in this study were limited to those data obtained for the parent study which included length of ICU stay, length of ventilatory support, and sedative exposure..
Sedative exposure
Each day while enrolled on protocol, data were abstracted on all sedative and opioid medications to determine sedative exposure. Sedative exposure is conceptualised as sedation intensity and sedative frequency throughout the time enrolled in the study, using a documented method to aggregate medications across disparate drug classes (Weinert & Calvin, 2007). Summed doses of intravenous (IV) midazolam, lorazepam, fentanyl, morphine, dexmedetomidine, hydromorphone, propofol, and haloperidol dosages (in milligrams) given during a 4-hour time block were converted to milligrams per kilogram per hour based on the ICU admission weight. Propofol doses were converted to micrograms per kilogram per minute.
A patient’s mean sedation intensity score (SIS) represents the average sedative exposure per 4-hour time block relative to all other patients in the study cohort. First, the weight-adjusted dose of each medication administered during a 4-hour time block was calculated. The dose was then categorised as 1–4 based on the quartile within the distribution of that drug for one 4-hour time block in the sample. For instance, if 0.1mg/kg of lorazepam and 0.2 mg/kg of morphine were given during a 4-hour time block and 0.1mg/kg fell into the second quartile of the distribution of all 4-hour lorazepam doses in the entire cohort and 0.2 mg/kg of morphine was in the third quartile, then the SIS for that time block was 2 + 3 = 5. A patient’s mean SIS score (quotient of sum of patient’s SIS values and number of 4-hour intervals on mechanical ventilation) represents the average sedative exposure per hour relative to all other patients.
Sedation frequency reflects the occurrence in which one or more of the eight sedative and opioid medications were administered at least once during a 4-hour time block, and are summed over 6, 4-hour time blocks that make up one day. For example, if a patient received one dose of fentanyl at 0400, and a dose of midazolam at 1200 during a 24-hour period, the sedation frequency for that specific study day would be = 2. Further information on methods to calculate sedative exposure are available elsewhere (Chlan, et al., 2013; Weinert & Calvin, 2007).
Fatigue
A simple, brief instrument that is easy to administer and score is necessary when measuring fatigue so as not to induce response burden (Lee, Hicks & Nino-Murcia, 1991). Given the high risk for response burden in non-verbal, mechanically ventilated patients, fatigue was measured for this study each day enrolled via a 100-mm visual analog scale-fatigue (VAS-F). The VAS-F was presented vertically to patients, like a thermometer, anchored at the bottom by 0 (not tired at all), to 100 at the top (the most tired I have ever been). Patients were asked to mark on the VAS-F how they were currently feeling in response to the question, “how tired are you feeling today?” Assistance was provided as needed by a member of the research team to aid patients in providing their daily fatigue ratings. All VAS-F ratings were scored using the same ruler from the bottom/zero anchor of the scale to the mark placed by the patient to indicate current level of fatigue from 0-100.
Visual analog scales are appropriate for tracking a research participant's clinical course, are easily administered, easy for participants to see, and use few words to minimise the possibility of different interpretations (Gift, 1989; Wewers & Loew, 1990). Careful instructions and repeated use of a VAS can eliminate problems with conceptual understanding of the method (Wewers & Loew, 1990). Measures of reliability, such as test-retest, are usually not addressed with visual analog scales due to the dynamic nature of the phenomenon itself being measured (Lush et al., 1988; Wewers & Lowe, 1990). The fatigue visual analog scale and the Fatigue subscale of the Profile of Mood States have been found to be significantly correlated (r = .80) in a sample of haemodialysis patients (Brunier & Graydon, 1996). In a sample of cancer patients the VAS-F has been found to be sensitive to change in morning and evening fatigue levels (Meek, et al., 2000).
Data Analysis
Descriptive statistics and graphing of demographic and clinical factors were used to determine the distribution of the data. Graphing was done to explore the shape of change over time of the fatigue scores. In order to assess if significant change in the fatigue scores occurred over time and the influence of clinical and demographic measures on fatigue, mixed models were used. Mixed effects models accommodate correlated and non-homogeneous residuals, which would be expected in repeated measures. Mixed models are an ideal analysis for dealing with varying follow-up times and missing data points from patients being unable or unwilling to complete daily fatigue assessments. Spotty missing data occurred from patients being medically or mentally unable to complete assessments or being absent from their room due to medical procedures. Using the data as is, rather than imputing missing values, within a mixed model analysis has a lower type I error and higher power than any type of imputation method used for missing data, which would be required for a standard repeated measures ANCOVA (Glantz & Slinker, 2001). Also, imputation may result in biased estimates of effects and standard errors. After exploring the correlations of the fatigue scores over time, an autocorrelation covariance structure was chosen as most closely fitting the pattern of correlations that decreased with increasing lag time. Candidates included in the multivariate model were chosen based on measures identified in the literature, by clinical experience, and those available from the parent study to possibly be associated with fatigue. Analysis was performed using SPSS v.17 and Proc Mixed in SAS v.9.2 (Singer, 1998). Final parameter estimates were considered significant at p < .05.
RESULTS
Description of Patient Characteristics
A total of 80 patients enrolled in the parent study provided daily fatigue ratings, up to study day 25. Of these patients, gender was equivalent (50% male, 50% female); mean age was 61.2 (SD 14.8) years; 89% of the sample was Caucasian, 5% American Indian and 6% African American, 1% identified being Hispanic. The primary admission diagnosis category for 59% of the patients was pulmonary in origin, 13% had a diagnosis category of cardiac/vascular, 8% were identified as gastrointestinal and 6% trauma. The remaining had a primary diagnosis category of renal, oncology, neurological/neuromuscular, sepsis, other surgery and other. All documented indications for mechanical ventilation were respiratory in nature that included respiratory failure, respiratory distress, hypoxia/hypoxaemia, and pneumonia. Mean APACHE III scores (Illness severity) at ICU admission were 61.5 (SD 19.8), reflecting lower illness severity given the parent study’s requirement of alert and interactive to be eligible for participation. Patients received mechanical ventilatory support for a median of 7.9 days (range 1-46) and had ICU stays for a median of 14 days (range 1-85) over the course of parent study enrollment.
Description of Fatigue
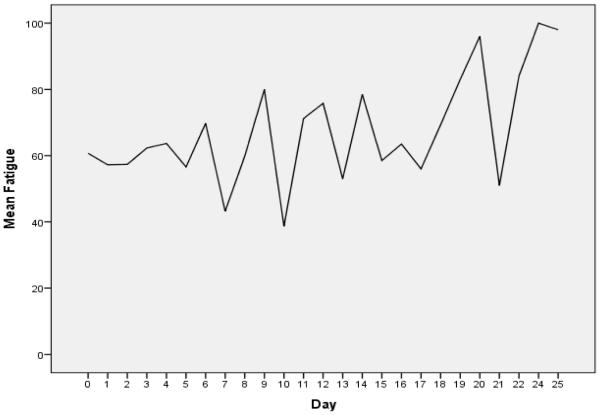
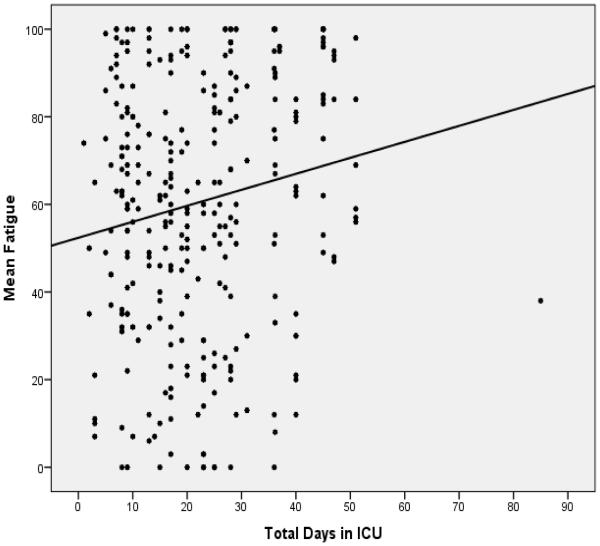
Mean fatigue ratings at study entry were 60.7 (SD 27.9). Figure 1 offers a graphic depiction of the mean VAS-F ratings over time, which represents the total sample over the course of study enrollment. The pattern of fatigue scores for study participants indicates a fluctuating level of fatigue, from a low of 40 upwards to 100. Figure 2 presents a scatterplot of all daily fatigue ratings provided by patients, with a best fitted line superimposed on the data points of mean fatigue ratings over time by total ICU days. The best fitted line shows an increase in fatigue from a baseline mean of 61 at study entry. These fatigue ratings depicted in Figure 2 indicate that the fatigue ratings increased over the course of study enrollment in this patient sample.
Figure 1.
Mean Fatigue Ratings over Time
Figure 2.
Scatterplot of Fatigue Ratings over Time with Best Fitted Line
regression line: fatigue = 52 + .36 (days in the ICU)
Patients start at an average of 52 in the fatigue scale as they enter the ICU.
For each consecutive day in the ICU, the fatigue score increases .36
Contribution of Clinical Factors to Fatigue Ratings: Mixed Models analysis
Variables included in the final models were total days enrolled in study, days in the ICU, gender, age, total ventilator hours, APACHE III score, and a measure of sedation. In one model, that measure was sedation frequency and in the other it was sedation intensity. Both could not be included in the same model due to their being highly correlated. In the model including sedation frequency, the APACHE III (β(se(β)) = .27(.12)) and sedation frequency (β(se(β)) = 1.2(.52)) were significantly associated with fatigue. In the model including sedation intensity, the APACHE III (β(se(β)) = .28(.12)) was the only measure significantly associated with fatigue, although sedation intensity approached significance (β(se(β)) = 1.4(.76)). The results of these final models are presented in table 1.
Table 1.
Results of Mixed Model Analysis of Contributors to Fatigue Ratings (n = 80)1
| Contributor | β(se(β)) | p-value | β(se(β)) | p-value |
|---|---|---|---|---|
| Total Days Enrolled in Study | .07(.43) | .86 | −.08(.42) | .85 |
| Days ICU | −.03(.30) | .92 | .006(.30) | .98 |
| Sex | −.89(4.7) | .85 | −1.2(4.8) | .81 |
| Age | −.11(.17) | .50 | −.11(.17) | .52 |
| Total Ventilator Hours | .01(.01) | .26 | .01(.01) | .33 |
| Illness Severity (APACHE III) | .27(.12) | .03* | .28(.12) | .03* |
| Sedation frequency | 1.2(.52) | .03* | ||
| Sedation Intensity | 1.4(.76) | .07 |
The table presents two multivariate models, one measuring sedation by frequency and the second measuring sedation by intensity.
DISCUSSION
The aims of this study were to describe fatigue ratings and to determine if select clinical factors contribute to this symptom in a sample of critically ill patients receiving mechanical ventilatory support.
To the best of our knowledge, this study is the first to report data on serial, daily fatigue ratings in critically ill patients receiving mechanical ventilatory support. The visual analog-fatigue scale represents fatigue from zero/none to 100/most ever. The middle of the scale, 50, would correspond to moderate fatigue. At study entry, our participants reported beginning fatigue ratings slightly higher than moderate or median levels of fatigue (50). While fatigue ratings in our patient sample demonstrated a fluctuating pattern (Figure 1), the trend overall was upward (figure 2). These findings indicate that fatigue does not diminish over the course of prolonged ventilatory support and lengthy ICU stays, and that management of this moderately intense symptom requires on-going assessment and intervention.
Mixed models analyses were used to determine if selected clinical factors contributed to fatigue ratings. Fatigue ratings in this sample of patients were not influenced by gender, length of ventilatory support or length of ICU stay when controlling for illness intensity and sedative exposure. Illness severity (APACHE III scores) at ICU admission and sedation frequency explained a significant amount of variance over time in the fatigue ratings in these patients. For every increase of 1 in the illness severity score, fatigue rose .27 points when controlling for sedation frequency, and .28 points when controlling for sedation intensity. For example, the average illness severity score was 64, which would add 17 - 18 points to the mean fatigue score of 61.5 for a total fatigue score of 78.5, the highest illness severity score of 133 would be expected to add another 36-37 points for a fatigue score of 97.5. Every increase of 1 in the sedation frequency would add 1.2 points to fatigue and every increase of 1 in the sedation intensity score would add 1.4 points to the fatigue rating. At the mean sedation frequency of 4.6, an increase of 5.5 points on the fatigue scale could be expected. At the mean sedation intensity of 3.2, an increase of 4.5 points on the fatigue scale could be expected. A maximum score of 14 for sedation frequency and 12 for sedation intensity would be expected to increase the fatigue score by 17 points.
There is a paucity of data reported in the literature on fatigue ratings in mechanically ventilated patients on which to compare our findings. In one of the few studies to obtain more than cross-sectional fatigue ratings, Puntillo and colleagues (2010) used a 0-10 numeric rating scale to obtain ICU patients’ fatigue ratings (n = 171), including 58 (35% of sample) receiving ventilatory support at some time during the symptom assessments; mean fatigue ratings were 1.94. Our study entry fatigue ratings were 60.7, which suggest that the fatigue ratings may be higher in our sample which consisted entirely of patients receiving mechanical ventilatory support. However, the VAS-fatigue and the numeric rating scale used in the Puntillo study (2010) are not comparable instruments as the rating scales are different. A full 95% of ICU patients receiving palliative care consultations reported severe symptom distress with fatigue (Delgado-Guay, et al., 2009). However, screening, evaluation, and management of fatigue are suboptimal in the critical care setting (Matthews, 2011). Clearly, the paucity of data on serial fatigue measurements and lack of effective management strategies for critically ill patients indicates a need for attention to this common ICU symptom.
Implications for Future Research
The descriptive nature of this study can provide directions for future research. One area of need is to validate the VAS-fatigue in mechanically ventilated patients, and to identify clinically meaningful levels of fatigue among ICU patients. For example, given the limited clinical factors available for inclusion in the analysis, prospective studies are needed that examine sleep and metabolic factors such as electrolyte imbalance and nutritional status and how those important factors might contribute to fatigue. Another area for research is examining clinical interventions that focus on directly modifiable factors that contribute to fatigue, such as the frequency of sedative administration and prolonged immobility, require further evaluation. Research to determine if progressive mobility/activity interventions decrease fatigue ratings are warranted. Further study is needed to evaluate if fatigue influences salient clinical outcomes such as time to weaning and successful weaning in ventilated patients. While fatigue may impact recovery from critical illness, this is not known and requires further study. Likewise, it is not known how sleep quality and sleep disturbance while in the ICU may contribute to fatigue ratings. Further, it is unknown if fatigue improves after extubation or if it lingers.
Implications for Clinical Practice
The clinical importance of the findings from this study are that fatigue is a moderately intense symptom in ventilated patients, and that it is an important symptom to assess in ventilated patients. There is the potential that fatigue could impact weaning trials, mobility interventions and other ICU care processes. Patients who are too fatigued may not be willing or physically able to safely participate in progressive mobility interventions. Our findings suggest that sedative administration regimens may be one directly modifiable area for reducing fatigue ratings. This warrants carefully examining the need for sedative medications and administering the lowest possible amount of medication for the shortest amount of time to manage patient symptoms of anxiety and distress from receiving mechanical ventilatory support.
Limitations
Limitations of this study include a small sample of patients readily available from the parent study. This small sample size limits the generalisability of the findings yet provides a first glimpse of fatigue ratings over time in mechanically ventilated patients. Further, the selected clinical factors examined in this study with the daily fatigue ratings were limited to those data obtained for the parent study. In addition, nutritional status, metabolic status, and sleep quality and quantity were not assessed, which are all known contributors to fatigue in persons with cancer (Poirer, 2013). Another limitation to our sample is that it was unknown if patients were debilitated prior to ICU admission, which could have impacted the daily fatigue ratings. We only measured fatigue intensity, one aspect of symptom assessment, once daily. We did not want to induce unnecessary response burden in our mechanically ventilated patients by administering lengthy fatigue instruments with a Likert scale response format. Instrument selection is very important as it is undesirable to induce or amplify fatigue that could artificially increase fatigue ratings.
CONCLUSION
Patients in this study with higher illness severity scores at ICU admission and more frequent sedation administration contributed to higher fatigue ratings that increase over time. Further study is needed to determine how fatigue ratings may impact clinical outcomes such as successful weaning trials and engagement in progressive mobility programs.
Implications for Clinical Practice.
Patients receiving prolonged periods of mechanical ventilatory support report moderate levels of fatigue
Illness severity and frequent receipt of sedative medications are clinical factors that contribute to fatigue
Interventions need to be designed and tested that target clinical factors amenable to treatment to manage fatigue
It is not known if fatigue ratings influence salient patient outcomes
Acknowledgement of funding
The parent study was supported by a grant from the National Institute of Nursing Research, National Institutes of Health 1R01 NR009295. The findings reported here are the authors’ own and the funder had no part in the analysis or the interpretation of the results. The funder had no direct role or input in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brunier G, Graydon J. A comparison of two methods of measuring fatigue in patients on chronic haemodialysis: visual analogue vs Likert scale. International Journal of Nursing Studies. 1996;33(3):338–348. doi: 10.1016/0020-7489(95)00065-8. [DOI] [PubMed] [Google Scholar]
- Chlan L, Weinert C, Heiderscheit A, Tracy MF, Skaar D, Guttormson J, et al. Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: A randomized clinical trial. JAMA: The Journal of the American Medical Association. 2013;309(12):2335–2344. doi: 10.1001/jama.2013.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Guay M, Parsons H, Li Z, Palmer L, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115:437–445. doi: 10.1002/cncr.24017. [DOI] [PubMed] [Google Scholar]
- Edmonton Symptom Assessment Scale. http://www.npcrc.org/files/news/edmonton_symptom_assessment_scale.pdf. Acceesed 11/21/14.
- Gift AG. Visual analogue scales: measurement of subjective phenomena. Nursing Research. 1989;38(5):286–288. [PubMed] [Google Scholar]
- Glantz S, Slinker B. Primer of Applied Regression and Analysis of Variance. 2nd McGraw-Hill; New York: 2001. [Google Scholar]
- Henry DH, Viswanathan HN, Elkin LP, Traina S, Wade S, Cella D. Symptoms and treatments burden assicated with cancer treatment: Results from a cross-sectional national survey in the U.S. Supportive Cancer Care. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- Knaus W, Wagner D, Draper E, Zimmerman J, Bergner M, Bastos P, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. CHEST. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Lee K, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- Lush MT, Janson-Bjerklie S, Carrieri VK, Lovejoy N. Dyspnea in the ventilator-assisted patient. Heart and Lung. 1988;17(5):528–535. [PubMed] [Google Scholar]
- Matthews E. Sleep disturbances and fatigue in critically ill patients. AACN Advanced Critical Care. 2011;22(3):204–224. doi: 10.1097/NCI.0b013e31822052cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek P, Nail L, Barsevick A, Schwartz A, Stephen S, Whitmer K, et al. Psychometric testing of fatigue instruments for use with cancer patients. Nursing Research. 2000;49:181–90. doi: 10.1097/00006199-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Poirier P. Nursing-led management of side effects of radiation: evidence-based recommendations for practice. Nursing: Research and Reviews. 2013;3:47–57. [Google Scholar]
- Puntillo K, Arai S, Cohen N, Gropper M, Neuhaus J, Paul S, et al. Symptoms experienced by intensive care unit patients at high risk of dying. Critical Care Medicine. 2010;38(11):2155–2160. doi: 10.1097/CCM.0b013e3181f267ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert C, Calvin A. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Critical Care Medicine. 2007;35(2):393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- Wewers M, Loew N. A critical review of visual analog scales in the measurement of clinical phenomena. Research in Nursing & Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]