Abstract
Sensory signals are processed in the brain by dedicated neuronal circuits to form perceptions used to guide behavior. Drosophila, with its compact brain, amenability to genetic manipulations and sophisticated behaviors has emerged as a powerful model for investigating the neuronal circuits responsible for sensory perception. Vision in particular has been examined in detail. Light is detected in the eye by photoreceptors, specialized neurons containing light sensing Rhodopsin proteins. These photoreceptor signals are relayed to the optic lobes where they are processed to gain perceptions about different properties of the visual scene. In this review we describe recent advances in the characterization of neuronal circuits underlying four visual modalities in the fly brain: motion vision, phototaxis, color and polarized light vision.
A fundamental goal in neuroscience is understanding how neuronal circuits interpret sensory signals in the brain to form behaviorally-relevant perception. The fly Drosophila melanogaster, a powerful model for developmental biologists, has recently emerged as a prolific system to elucidate complex problems in functional neuroscience, especially sensory perception. This “simple” organism is capable of many sophisticated behaviors and combines the advantages of a rather compact brain (only 200,000 neurons) with a large toolbox for genetic manipulation. These attributes make the fly an attractive model for reaching a complete understanding of microcircuits underlying a given sensory modality - to link specific cell types to a given behavior.
The visual system of the fly has been particularly well studied. While the development of the complex pattern of light-sensing photoreceptors in the eye has been elucidated in exquisite detail1, the role of these sensory receptors and their downstream circuits in visual perception is emerging. In this review we will focus on recent advances in the identification and characterization of microcircuits underlying four different visual modalities: motion vision, phototaxis, color and polarized light vision.
The eye and the optic lobe
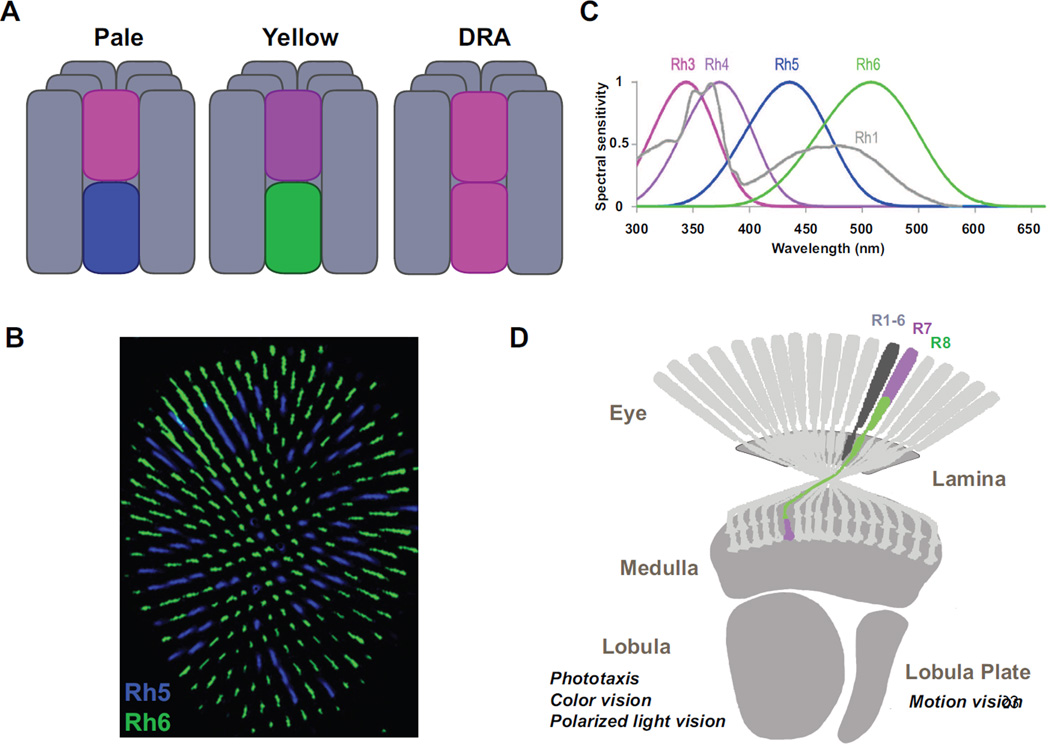
The fly eye contains about 800 independent unit eyes called ommatidia, corresponding to 800 pixels in the animal’s visual field. Each ommatidium is composed of eight photoreceptor cells: six outer (R1-6) and two inner (R7 and R8) (For review on this section see 1) (Fig1A). R1-6 photoreceptors, the equivalent of mammalian rods, all express the same broadband Rhodopsin Rh1 and are involved in dim light vision and the perception of motion2. Similar to mammalian cones, R7/R8 photoreceptors express different Rhodopsins in a pattern that defines two subtypes of stochastically distributed ommatidia (Fig1B). These are involved in color vision2–4: ‘Pale’ ommatidia have the UV-sensitive Rhodopsin Rh3 in R7 and blue Rh5 in R8, while ‘Yellow’ ommatidia have another UV Rhodopsin (Rh4) in R7 and the green-sensitive Rh6 in R8 (Fig1A, C). The rhabdomeres (i.e. light gathering structures made of microvilli containing the Rhodopsins) of R7/R8 are staked one on top of the other and hence share the same light-path, providing the ideal configuration to compare their outputs. A third type of ommatidia is found in a narrow band of ommatidia in the dorsal rim area (DRA) of the eye, where both R7/R8 photoreceptors express the same UV Rhodopsin Rh3 (Fig1A). These morphologically specialized ommatidia are involved in the detection of the e-vector of polarized skylight for navigation5. Finally, both UV-Rhodopsins are co-expressed in R7 cells of the ‘Yellow’ subset in the dorsal third of the eye, a region of the eye pointing toward the sky6. The function of these ommatidia remains elusive, although they have been proposed to be involved in the detection of solar vs. anti-solar orientations.
Figure 1. The eye and the optic lobe of adult Drosophila.
A A single ommatidium contains eight photoreceptors, six outers R1-6 (grey) and two inners R7-8 (colors). Outers express the Rh1 opsin, R7s express either Rh3 or Rh4 while R8s express Rh5 or Rh6. Four types of ommatida are found in the eye. ‘Pale’ and ‘yellow’ ommatidia are distributed stochastically in the main part of the eye. In the dorsal third, pale and specialized dorsal third yellow subtypes are found (not shown). In one to two rows of ommatidia in the dorsal rim are (DRA) of the retina, the remaining DRA subtypes are found. B Stochastic distribution of Rh5 (blue) and Rh6 (green) expressing R8s in the main part of the eye. C Normalized spectral preference curves of the different rhodopsins expressed in the eye of the fly. Rh1 shows broad spectral sensitivity peaking in both the blue and the UV due to the presence of a sensitizing pigment (Modified from 3) D Photoreceptors project to the optic lobe. Outer photoreceptors send their axons to the lamina while R7/R8 photoreceptors send theirs to the medulla. The lobula is involved in spectral preference, color and polarized light vision. The lobula plate is a center for motion detection.
Photoreceptors are sensory neurons that send their axons to the optic lobe, which is organized retinotopically (Fig1D). The first level of neural integration of visual information is the lamina, where R1-6 photoreceptor axons terminate7 (Fig1D). Each pixel in the field of view of the fly corresponds to one column (or cartridge) in the lamina, as well as in the subsequent neuropil called the medulla, where R7/R8 photoreceptors terminate8. Each lamina cartridge contains 11 distinct classes of neurons7. The medulla is much more complex, with at least 70 different cell types being represented7. Lobula and lobula plate are higher level processing centers, with lobula thought to be involved in the processing of color vision9, spectral preference10 and polarized light vision11 while the lobula plate is the site of motion detection12 (Fig1D).
Motion detection
The perception of motion is by far the best-studied visual modality in the fly. It is critical for prey capture and mating, not to mention integration of the fly’s own movement in the world. Mechanisms describing how neurons compute direction-selective signals by interpreting spatiotemporal changes in luminance have been studied extensively, starting with seminal work in other insects. In the 1950s, Hassentein and Reichardt developed their now famous, eponymous correlator model (Hassentein and Reichardt Correlator: HRC) by examining the optomotor response of the beetle Chlorophanus, i.e. its tendency to rotate with the visual field to maintain a straight heading in its perceived environment13. Drosophila also displays a very robust optomotor behavior12, which has been demonstrated to rely largely on R1-6 photoreceptors2,14. This behavior is typically measured using tethered flies that are either flying in a flight simulator, or walking on an air-suspended ball, while a motion stimulus is presented15.
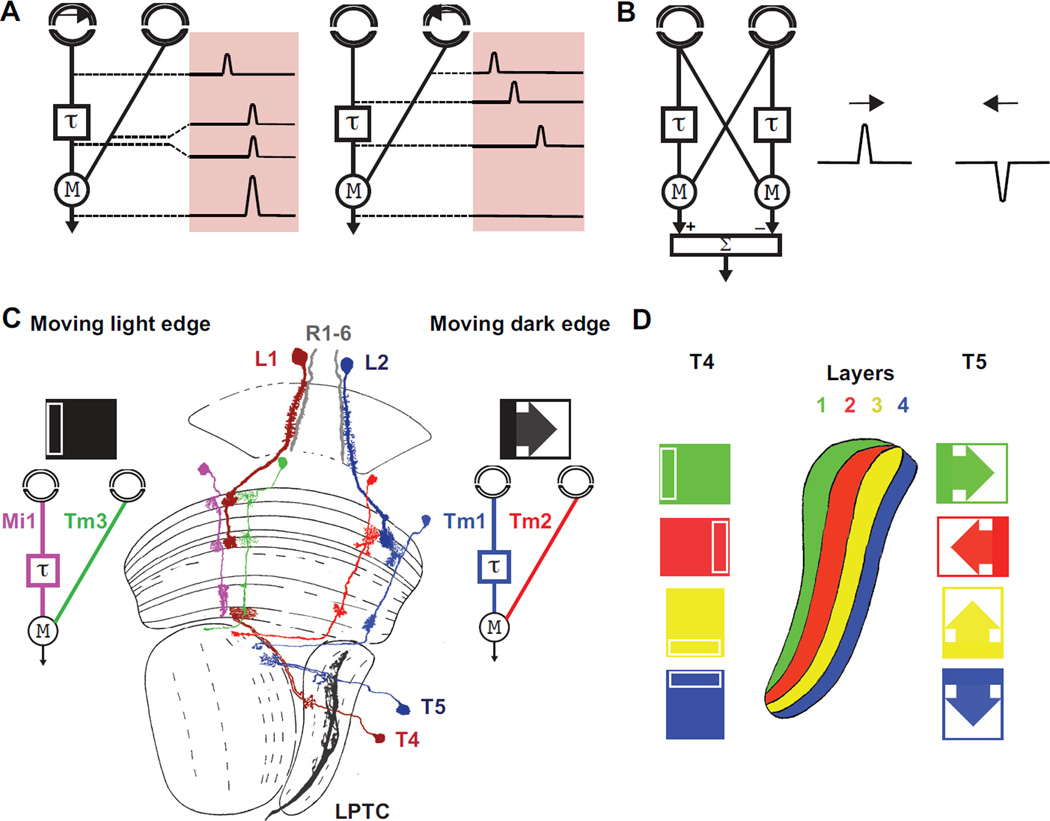
The HRC model consists of two spatially separated input channels, where the response of one channel is slower as compared to the other (Fig2A). This delay allows direction-selective amplification (multiplication) of signals generated by motion in front of the correlator only when the delayed and non-delayed signals coincide in time, indicating that an edge was moving in the preferred direction. In a subsequent step, the output of a correlator is subtracted from a mirror-imaged correlator, thereby producing responses that have different signs for opposite directions (Fig2B). A growing wealth of data has demonstrated that motion responses in Drosophila display the fundamental signatures predicted by the HRC16,17.
Figure 2. Motion vision in the Drosophila optic lobe.
A. The Hassentein and Reichard Correlator (HRC) relies on differential temporal filtering of two spatially separated input channels, delaying one signal with respect to the other. Motion from left to right in this case causes these delayed and non-delayed luminance signals to arrive simultaneously at a subsequent processing step where they are multiplied and amplified (multiplication) as a motion signal. Motion in the opposite direction where the delay separates the signals in time, leads to a null output. B The subtraction of the output of a correlator from that of a mirror-imaged correlator produces responses that have different signs for opposite directions. (A and B modified from 17) C Two pathways lead from photoreceptors in the eyes to LPTCs. In the moving light-edge-specific pathway, L1 neurons, postsynaptic to photoreceptors, act as inputs, while direction selective T4 neurons, presynaptic to LPTCs, act as outputs. L2 and T5 have equivalent roles in the pathway that detects moving dark edges. Medulla neurons Mi1/Tm3, Tm1/Tm2 have been proposed to be the delayed and the non-delayed lines of an HRC for moving light edges respectively. The dendrites of T4/T5 neurons define potential sites for HRC signal multiplication in these two pathways. D T4 cells respond preferentially to moving bright edges, T5 cells respond to moving dark edges. Dendrites responding to different cardinal directions are located to fours different layers of the lobula plate.
Recent anatomical and functional work focusing on the different cell types in the optic lobes of Drosophila has defined a precise neuronal circuit for motion detection. Lobula plate tangential cells (LPTCs) (Fig2C) are the outputs of the motion pathways and respond to wide-field motion in a direction-selective manner12: they depolarize to their preferred direction and hyperpolarize to the opposite direction18 (Fig2C). Two parallel pathways lead from photoreceptors to LPTCs19–22, one for the detection of moving light edges and one for dark edges (Fig2C) as all visual motion can be described as a combination of these two. In the moving light edge-specific pathway, lamina monopolar L1 neurons, postsynaptic to photoreceptors, act as inputs, while direction selective lobula plate T4 neurons, presynaptic to LPTCs, act as outputs. Similarly, different lamina monopolar cells called L2 and lobula plate neurons T5 have equivalent roles in the pathway that detects moving dark edges. These findings suggest that two ON/OFF HRCs act in parallel in the optic lobes. Recently, two pairs of medulla neurons were identified in each of the pathways that process motion signals between the lamina inputs and the lobula plate outputs23,24. In the moving light edge pathway, L1 cells connect specifically to medulla cell types Mi1 and Tm3, which then both synapse onto T423. In the moving dark edge pathway, L2 cells specifically connect to medulla cell types Tm1 and Tm2, which then synapse onto T525,26 (Fig2C). Both activity imaging using the genetically encoded calcium sensor Gcamp5 and electrophysiological recordings confirmed the segregation of light ‘ON’ information into Mi1/Tm3 cells, and light ‘OFF’ into Tm1/Tm224,27,28 (Fig2C). Moreover, recordings show that within each pair of neurons, there is a differential temporal processing where Mi1 responds slower than Tm3, and Tm1 responds slower than Tm224. Therefore, Mi1/Tm3, appear to act as the delayed and non-delayed arms of an ‘ON’ HRC for moving light edges and Tm1/Tm2 play equivalent roles in an ‘OFF’ HRC for moving dark edges (Fig2C). Nonetheless, the synapses between Mi1/Tm3 and T4, and those between Tm1/Tm2 and T5 could impose additional delays to either input channel before a correlation operation.
Each medulla column is surrounded by not one but four different morphological subtypes of T4 cells (T4a,b,c,d) and four subtypes of T5 cells (T5a,b,c,d), which terminate in four different layers of the lobula plate26 (Fig 2D). Each of these four subtypes of T4 and T5 neurons responds specifically to one of the four cardinal directions of motion (up, down, left, right)21, making each layer of the lobula plate specifically sensitive to one direction of motion (Fig2D). In every layer, T4 and T5 subtypes make excitatory synapses along the dendrite of wide field motion detectors LPTCs29 that each responds to one preferred direction. Finally, the subtraction is implemented through a push-pull mechanism, where excitatory cholinergic inputs from T4 and T5 and inhibitory GABAergic inputs from interneurons are spatially integrated on the LPTC dendrites30.
Motion circuits are however, likely much more complex than this simple circuit. For example, in addition to aforementioned Tm1 and Tm2 medulla columnar cells, two other cell types (Tm9 and Tm4) also synapse specifically onto T5 cells26. The role of these later two cell types in dark edge detection remains to be defined. Moreover, lamina neurons L3 and L4 have also been implicated in the detection of dark edges27,31 but the nature of their contribution remains to be clarified. Finally, the biophysical implementation of the multiplication (or other non-linearity) step in the HRC, remains undefined. A model invoking the interplay of different metabotropic and ionotropic channels on T526 has been put forward.
Phototaxis
Drosophila exhibits positive phototaxis32, i.e. when given a choice between light and darkness, it moves towards the light. Moreover, Drosophila exhibits positive phototactic behavior towards short wavelengths of light: In a Blue vs. Green choice assay, flies choose Blue, and in a UV vs. Blue choice, they choose UV33. Importantly, this choice can be reversed if the intensity of the preferred color is reduced, which makes this behavior dependent on both wavelength and intensity and not true color vision (see next paragraph). Ethologically, this choice behavior likely allows the fly to perceive UV light as a sign of open space and hence an escape route. Since most objects in nature absorb rather than reflect UV radiation, the main source of natural UV light is the open sky.
All PR types contribute to phototaxis33. As might be expected from their wavelength sensitivity (Fig1C), R8 cells play a major role in the Blue vs Green choice, while R7s are necessary for UV choice. Additionally, R1-6 photoreceptors have also been implicated in UV attraction (see peak of Rh1 in the UV (Fig1C) due to a sensitizing pigment34).
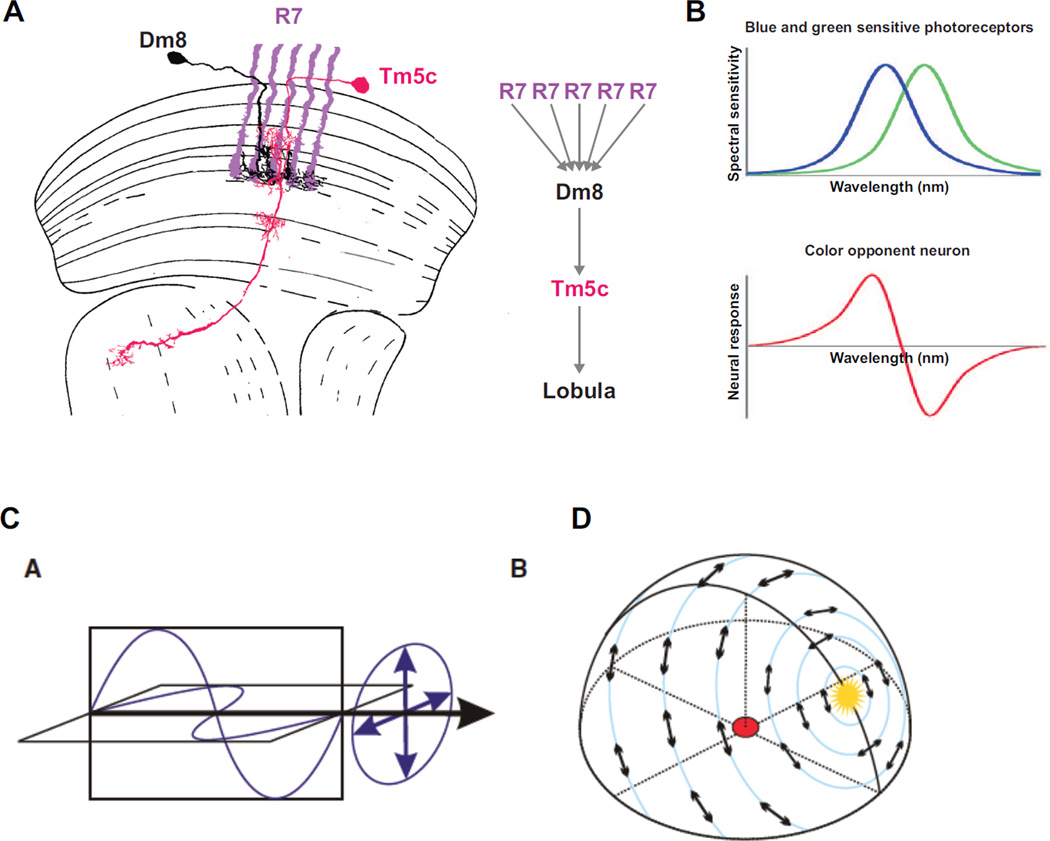
R7 photoreceptors connect to a multi-columnar (i.e. connecting to multiple columns) local neuron, the wide-field amacrine cell Dm8, which is necessary for UV spectral preference35 (Fig3A). Each Dm8 cell receives inputs from approximately 16 R7s10 (Fig3A), which suggests a neural pooling mechanism across many points in space for enhanced UV phototaxis. The output of Dm8 neurons is, in turn, passed through excitatory synapses to the columnar (i.e. present in each column) neuron Tm5c in the medulla10, which then projects to the lobula. Tm5c cells are also necessary for UV preference in a Green vs. UV phototaxis assay (Fig3A). Overall, this connectivity increases UV sensitivity at the expense of spatial resolution. Interestingly, Tm5c also receives additional inhibitory columnar inputs from R8 neurons, which are required for phototaxis towards green light in a dark vs. Green choice assay, but not towards UV light is a dark vs. UV assay10. The role of this double inhibitory input (from R7, through Dm8, and from R8) on Tm5c is unclear.
Figure 3. Spectral preference, color vision and polarized light vision in the Drosophila optic lobe.
A Dm8 and Tm5c neurons are necessary for UV spectral preference. Each Dm8 gets inhibitory inputs from approximately 16 R7s. The information from Dm8 is in then passed on through excitatory synapses to Tm5c which projects to the lobula (modified from 7). B Color vision cannot be achieved by single photoreceptors that respond to light over a broad range of wavelengths. Instead, it necessitates the comparison of the output of photoreceptors with different spectral sensitivity (top, showing hypothetical blue and green Rhodopsin-expressing photoreceptor sensitivity). Neural response of a color opponent cell which gets excitatory input from the blue sensitive photoreceptor and inhibitory input from the green sensitive photoreceptor shown above. The response of this neuron increases for wavelengths below the x-axis crossing point and decreases above it, no mater the intensity. C Light is an electromagnetic wave. Its electric and magnetic fields vibrate in planes perpendicular to each other and to the direction of wave travel (black arrow). When all the electric field vectors lie in a plane, the wave is linearly polarized. The orientation of this plane is the direction of the e-vector of polarization. D Pattern of polarized light of the sky. E-vectors are arranged along concentric circles around the sun shown in yellow (B and C from 53).
Color vision
Color vision is the ability of most diurnal animals to distinguish between lights of different spectral composition independently of intensity. It increases the salience of objects in complex visual fields and can be used to identify important features such as sources of food (i.e. fruit or flower among foliage or ripe vs. unripe fruit), as well as suitable mates. Color vision cannot be achieved with a single photoreceptor class, as this would make it impossible to distinguish between wavelength and intensity36. Instead, color vision requires comparing the output of at least two photoreceptor classes with different spectral sensitivity (i.e. different Rhodopsin expression)37(Fig3B). In the mammalian brain, this comparison is processed by color-opponent neurons, which exhibit an excitatory response to a certain range of wavelengths and an inhibitory response to wavelengths from the opponent part of the spectrum38–40 (Fig3B). Drosophila has the neuronal hardware necessary for wavelength comparison, with four distinct types of R7/R8 photoreceptors: ‘pale’/’yellow’ R7 and ‘pale’/’yellow’ R8 photoreceptors (Fig1A). Two recent behavioral studies using color learning have shown that the fly is capable of color vision, although with limited discriminatory power, at least under the conditions tested. A population assay3 relies on gustatory reward association with color while a single-fly flight simulator assay4 relies on aversive heat association. Importantly, in both cases color learning is independent of the intensity of the stimulus.
Flies with only their R7/R8 photoreceptors intact retain color vision whereas those with only functional R1-6 photoreceptors can no longer distinguish blue from green3,4. However, the population assay also revealed a contribution of R1-6 photoreceptors in blue vs. green discrimination in combination with ‘yellow’, Rh4-expressing R73. This was unexpected since Rh1, which is expressed in all R1-6 photoreceptors, manifests a broad excitation spectrum (Fig 1C), contrary to the Rhodopsins expressed in R7/R8 photoreceptors, which together form a separate morphological subsystem projecting longer axon fibers to the medulla neuropil and thus have long been considered to be the sole inputs for color vision2.
Several medulla neurons have emerged as candidate elements of the neuronal circuits processing chromatic information, mostly through the identification of postsynaptic partners for R7/R8 photoreceptors4. Tangential cell Tm20 is postsynaptic to both R8 and lamina monopolar cell L3. Two very similar tangential cells, Tm5a and b are postsynaptic to R7 while a third (Tm5c) is postsynaptic to both R7 and R8 (see phototaxis section). Tm5a shows an interesting connectivity as it is specifically found postsynaptic to Rh4-expressing ‘yellow’ R7 cells10. From behavioral experiments, it appears that Tm20 and Tm5a,b,c act in parallel, redundant pathways for color vision4. Finally, four morphologically distinct classes of so-called TmY medulla neurons, which send bifurcated axons to both the lobula and lobula plate neuropiles, are specifically activated post-synaptically to ‘pale’ or ‘yellow’ R7 or R841 but their function has not been tested in detail.
All of the above-mentioned neurons are likely to relay chromatic information and represent pre-processing elements in potential color opponent pathways, comparing the output of R7/R8 photoreceptors from the same ommatidum or ‘pale’ and ‘yellow’ R7/R8 photoreceptors. Color opponent neurons have been found in other insects such as bees, and are located in the medulla42, lobula43 and optic tubercule44, but neurons with such properties remain to be identified in Drosophila.
Polarized light detection
Light can be described as an electromagnetic wave. Its electric and magnetic fields vibrate in planes perpendicular to each other and to the direction of propagation. The e-vector of light describes the orientation of these fields (Fig3C). Direct sunlight is not polarized with all e-vector orientations represented equally. However, it becomes polarized when it is scattered by particles in the atmosphere. This creates concentric bands of linearly polarized light around the sun, which, even on a cloudy day, can allow for an estimation of the position of the sun (Fig3D). Drosophila can sense the pattern of linearly polarized light5, like many other insects that use it to improve their navigational skills45.
A population assay where the movement of walking flies exposed to linearly polarized light is monitored shows that flies exhibit a spontaneous tendency to align their body axis parallel to the incident e-vector of polarized light (polarotaxis)5. This assay showed that R7 and R8 in the DRA (which both express the same UV-Rh3 and thus cannot be involved in color vision) are both necessary and sufficient for polarotaxis. Only in the DRA, the rhabdomeric microvilli of R7 and R8 are untwisted and oriented orthogonally to each other (R7 vs. R8), resulting in high polarization-sensitivity that allows these R7 and R8 to act as potent polarization detectors. In contrast, non-DRA R7 and R8 cells involved in color vision have twisted rhabdomeres to disrupt the detection of polarized light that could interfere with color vision. In addition to dorsal polarotaxis mediated by the DRA, Drosophila can also detect polarized light presented ventrally5,46. Such ventral polarotaxis remains poorly understood but it might be useful for the animal to detect or avoid water surfaces, which reflect polarized light. This behavior is mediated by ommatidia located in in the ventral third of the eye, in which a combination of R1-6 and R7/R8 photoreceptors also manifest partially untwisted rhabdomeres5.
The neuronal circuits processing polarization downstream of R7/R8 photoreceptors have yet to be identified. Polarization opponent interneurons receiving inputs from R7 and R8 of DRA ommatidia have only been described in the medulla of crickets and so-called ‘compass’ neurons with maximal sensitivity to discrete e-vector orientations were described in desert locusts47. It remains unknown whether cells with similar properties exist in Drosophila, or whether different cell types are recruited for polarization vision. Due to the dominant role of R7 and R8 as input channels in both color and polarization vision, it will be interesting to compare the mechanisms for processing these two stimuli. Indeed, similar neuronal configurations could be used for both color and polarized light discrimination48 in different regions of the optic lobes, downstream of a pair of R7/R8 photoreceptors in the main part of the eye for color or in the DRA for polarization.
From the optic lobe, visual information is further processed in the central brain. The connections between these two brain areas are relatively unknown49. In the central brain, the central complex in particular has been involved in other high level visual modalities such as feature detection (object size, contour, length, orientation of edges, or elevation in the panorama) and spatial memory50–52. The role of these central structures in the processing of the modalities discussed in this review as well as their role in visually guided behaviors needs to be addressed. There is no doubt that, in the next few years, this and many of the unknowns we have discussed will be mapped to specific neuronal circuits, leading to a better understanding of how the visual world is represented in the brain.
Highlights.
We describe recent advances in the field of visual processing in Drosophila.
We present in detail the neuronal circuits underlying four visual modalities.
Motion vision, phototaxis, color and polarized light vision are addressed.
Acknowledgements
We thank Jens Rister, Brent Wells and Mathias Wernet for helpful comments on the manuscript. This work was supported by a grant from NIH to C.D. R01 EY017916. R.B. was supported by fellowships from EMBO and HFSP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston RJ. Lessons about terminal differentiation from the specification of color-detecting photoreceptors in the Drosophila retina. Annals of the New York Academy of Sciences. 2013;1293:33–44. doi: 10.1111/nyas.12178. [DOI] [PubMed] [Google Scholar]
- 2.Heisenberg M, Buchner E. The role of retinula cell types in visual behavior of Drosophila melanogaster. Journal of comparative physiology. 1977;117:127–162. [Google Scholar]
- 3.Schnaitmann C, Garbers C, Wachtler T, Tanimoto H. Color Discrimination with Broadband Photoreceptors. Current Biology. 2013;23:2375–2382. doi: 10.1016/j.cub.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Melnattur KV, et al. Multiple Redundant Medulla Projection Neurons Mediate Color Vision in Drosophila. Journal of neurogenetics. 2014:1–15. doi: 10.3109/01677063.2014.891590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wernet MF, et al. Genetic Dissection Reveals Two Separate Retinal Substrates for Polarization Vision in Drosophila. Current Biology. 2012;22:12–20. doi: 10.1016/j.cub.2011.11.028. The authors of this article describe a novel behavioral paradigm to dissect the neuronal circuits for polarization vision. They show that flies exhibit detection of ventral as well as dorsal polarization and dissect the contribution of different photoreceptor types in the eye of the fly to these two polarization driven behaviors.
- 6.Mazzoni EO, et al. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS biology. 2008;6:e97. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischbach K-F, Dittrich A. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell and tissue research. 1989;258:441–475. [Google Scholar]
- 8.Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. Journal of Comparative Neurology. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Current Biology. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karuppudurai T, et al. A Hard-Wired Glutamatergic Circuit Pools and Relays UV Signals to Mediate Spectral Preference in Drosophila. Neuron. 2014;81:603–615. doi: 10.1016/j.neuron.2013.12.010. This article describes the neuronal circuit underlying phototaxis toward UV. Using anatomical studies, as well as a combination of silencing and behavioral experiments, the authors show that two neuronal types in the optic lobe of the fly, the multicolumnar Dm8 and the columnar Tm5c, are together necessary for UV phototaxis.
- 11.Homberg U, Heinze S, Pfeiffer K, Kinoshita M, El Jundi B. Central neural coding of sky polarization in insects. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:680–687. doi: 10.1098/rstb.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borst A, Haag J, Reiff DF. Fly motion vision. Annual review of neuroscience. 2010;33:49–70. doi: 10.1146/annurev-neuro-060909-153155. [DOI] [PubMed] [Google Scholar]
- 13.Hassenstein V, Reichardt W. System theoretical analysis of time, sequence and sign analysis of the motion perception of the snout-beetle Chlorophanus. Z Naturforsch B. 1956;11:513–524. [Google Scholar]
- 14.Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proceedings of the National Academy of Sciences. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silies M, Gohl DM, Clandinin TR. Motion-Detecting Circuits in Flies: Coming into View. Annual Review of Neuroscience. 2014;37 doi: 10.1146/annurev-neuro-071013-013931. [DOI] [PubMed] [Google Scholar]
- 16.Buchner E. Elementary movement detectors in an insect visual system. Biological Cybernetics. 1976;24:85–101. [Google Scholar]
- 17.Borst A, Egelhaaf M. Principles of visual motion detection. Trends in neurosciences. 1989;12:297–306. doi: 10.1016/0166-2236(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 18.Joesch M, Plett J, Borst A, Reiff DF. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Current Biology. 2008;18:368–374. doi: 10.1016/j.cub.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 19. Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–304. doi: 10.1038/nature09545. This article combines silencing techniques and electrophysiology to provide proof of the separation of processing of light and dark edges in the optic lobe of the fly and define the lamina neurons L1 and L2 respectively as inputs of these two pathways.
- 20. Clark DA, Bursztyn L, Horowitz MA, Schnitzer MJ, Clandinin TR. Defining the computational structure of the motion detector in Drosophila. Neuron. 2011;70:1165–1177. doi: 10.1016/j.neuron.2011.05.023. Together with 19, using both calcium imaging and silencing combined with optomotor behavioral experiments, this article shows that L1 and L2 provide different inputs to motion detection for moving light and dark edges.
- 21. Maisak MS, et al. A directional tuning map of Drosophila elementary motion detectors. Nature. 2013;500:212–216. doi: 10.1038/nature12320. This article defines elementary motion detectors T4 and T5 using elegant calcium imaging experiments. The authors show that these neurons are direction selective and tuned to one of the four cardinal directions (front-to-back, back-to-front, upwards and downwards). Moreover, T4 cells respond to moving light while and T5 cells respond to dark edges. Their activity is necessary to the response of LPTCs to light and dark edges respectively.
- 22.Rister J, et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 23. Takemura S-y, et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature. 2013;500:175–181. doi: 10.1038/nature12450. This article provides a dense reconstruction of one medulla column using ssTEM. It is particularly interesting for the detailed reconstruction of Mi1, Tm3 and their inputs on T4, relating displacement of receptive fields in the eye to the preferred direction of each T4 cell. The authors propose a candidate pathway that implements the elementary motion detector.
- 24. Behnia R, Clark DA, Carter AG, Clandinin TR, Desplan C. Processing properties of ON and OFF pathways for Drosophila motion detection. Nature. 2014;512:427–430. doi: 10.1038/nature13427. The authors performed whole-cell recordings to show that Mi1/Tm3 and Tm1/Tm2 have processing properties (rectification and delay) that make them candidates for being the delayed and non-delayed lines of an HRC for moving light and dark edges respectively.
- 25.Takemura S-y, et al. Cholinergic circuits integrate neighboring visual signals in a Drosophila motion detection pathway. Current Biology. 2011;21:2077–2084. doi: 10.1016/j.cub.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinomiya K, Karuppudurai T, Lin T-Y, Lu Z, Lee C-H, Meinertzhagen IA. Candidate neural substrates for off-edge motion detection in Drosophila. Curr. Biol. 2014;24:1–9. doi: 10.1016/j.cub.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier M, et al. Neural circuit components of the Drosophila OFF motion vision pathway. Current biology : CB. 2014 doi: 10.1016/j.cub.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Strother JA, Nern A, Reiser MB. Direct Observation of ON and OFF Pathways in the Drosophila Visual System. Current Biology. 2014;24:976–983. doi: 10.1016/j.cub.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Schnell B, Raghu SV, Nern A, Borst A. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. Journal of Comparative Physiology A. 2012;198:389–395. doi: 10.1007/s00359-012-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brotz TM, Borst A. Cholinergic and GABAergic receptors on fly tangential cells and their role in visual motion detection. Journal of neurophysiology. 1996;76:1786–1799. doi: 10.1152/jn.1996.76.3.1786. [DOI] [PubMed] [Google Scholar]
- 31.Silies M, et al. Modular use of peripheral input channels tunes motion-detecting circuitry. Neuron. 2013;79:111–127. doi: 10.1016/j.neuron.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertholf LM. The extent of the spectrum for Drosophila and the distribution of stimulative efficiency in it. Zeitschrift für vergleichende Physiologie. 1932;18:32–64. [Google Scholar]
- 33.Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proceedings of the National Academy of Sciences. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschfeld K, Franceschini N. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 35. Gao S, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. This article used the dissection of the ort promoter, the gene encoding the histamine gated chloride channel present only in neurons postsynaptic to histaminergic PRs in Drosophila , as a way to identify the neurons and mark them specifically. This led to the identification of Dm8 as necessary for UV spectral preference downstream of R7s.
- 36.Rushton W. Review lecture. Pigments and signals in colour vision. The Journal of physiology. 1972;220:1P. doi: 10.1113/jphysiol.1972.sp009719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurvich LM, Jameson D. An opponent-process theory of color vision. Psychological review. 1957;64:384. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- 38. Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. The authors examine the orientation behavior of fruit flies under outdoor conditions and find that they actively orient using the sky’s polarization pattern.
- 39.Masland RH. The fundamental plan of the retina. Nature neuroscience. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 40.Gegenfurtner KR, Kiper DC. Color vision. Neuroscience. 2003;26:181. doi: 10.1146/annurev.neuro.26.041002.131116. [DOI] [PubMed] [Google Scholar]
- 41.Jagadish S, Barnea G, Clandinin TR, Axel R. Identifying Functional Connections of the Inner Photoreceptors in Drosophila using Tango-Trace. Neuron. 2014;83:630–644. doi: 10.1016/j.neuron.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kien J, Menzel R. Chromatic properties of interneurons in the optic lobes of the bee. Journal of comparative physiology. 1977;113:17–34. [Google Scholar]
- 43.Yang E-C, Lin H-C, Hung Y-S. Patterns of chromatic information processing in the lobula of the honeybee. Apis mellifera Journal of insect physiology. 2004;50:913–925. doi: 10.1016/j.jinsphys.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Mota T, Gronenberg W, Giurfa M, Sandoz J-C. Chromatic processing in the anterior optic tubercle of the honey bee brain. The Journal of Neuroscience. 2013;33:4–16. doi: 10.1523/JNEUROSCI.1412-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weir PT, Dickinson MH. Flying Drosophila Orient to Sky Polarization. Current Biology. 2012;22:21–27. doi: 10.1016/j.cub.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf R, Gebhardt B, Gademann R, Heisenberg M. Polarization sensitivity of course control in Drosophila melanogaster. Journal of comparative physiology. 1980;139:177–191. [Google Scholar]
- 47.el Jundi B, Pfeiffer K, Heinze S, Homberg U. Integration of polarization and chromatic cues in the insect sky compass. Journal of Comparative Physiology A. 2014;200:575–589. doi: 10.1007/s00359-014-0890-6. [DOI] [PubMed] [Google Scholar]
- 48.Bernard GD, Wehner R. Functional similarities between polarization vision and color vision. Vision research. 1977;17:1019–1028. doi: 10.1016/0042-6989(77)90005-0. [DOI] [PubMed] [Google Scholar]
- 49.Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster . I. Lobula - specific pathways. Journal of Comparative Neurology. 2006;497:928–958. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 51. Seelig JD, Jayaraman V. Feature detection and orientation tuning in the Drosophila central complex. Nature. 2013 doi: 10.1038/nature12601. Using calcium imaging in walking and flying flies, the authors describe the receptive field properties of ring neurons, part of the central complex, a structure in the central brain previously known to be required for drosophila’s innate responses to vertical visual features and for the formation of memory regarding visual patterns.
- 52. Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–207. doi: 10.1038/nature10131. The authors describe a behavioral paradigm, inspired by the Morris water maze used in mice, to investigate the neuronal circuits underlying visual place learning in Drosophila . They show that neurons in the ellipsoid body, and not the mushroom body, are required for visual place learning.
- 53.Hardie RC. Polarization Vision: Drosophila Enters the Arena. Current Biology. 2012;2274:R12–R14. doi: 10.1016/j.cub.2011.11.016. [DOI] [PubMed] [Google Scholar]