Abstract
A renewed global commitment to malaria elimination lends urgency to understanding the biology of Plasmodium transmission stages. Recent progress towards uncovering the mechanisms underlying P. falciparum sexual differentiation and maturation reveals potential targets for transmission-blocking drugs and vaccines. The identification of parasite factors that alter sexual differentiation, including extracellular vesicles and a master transcriptional regulator, suggest that parasites make epigenetically controlled developmental decisions based on environmental cues. New insights into sexual development, especially host cell remodeling and sequestration in the bone marrow, highlight open questions regarding parasite homing to the tissue, transmigration across the vascular endothelium, and maturation in the parenchyma. Novel molecular and translational tools will provide further opportunities to define host-parasite interactions and design effective transmission-blocking therapeutics.
I. Introduction
The parasite Plasmodium falciparum causes the most severe form of malaria with around 600,000 deaths annually, mostly young children and pregnant women in sub-Saharan Africa [1]. Resistance to current drug therapies, the absence of a licensed vaccine, and a large asymptomatic reservoir [2] make the development of effective transmission-blocking therapeutics particularly important to any malaria elimination or eradication program. Given the paucity of known transmission stage-specific biomarkers or drug and vaccine targets, a deepened understanding of the biology of transmissible parasite stages, including their interaction with the host, is essential [3].
P. falciparum has a complex life cycle, in which asexual replication and sexual development take place in red blood cells (RBCs) of the human host and sexual reproduction in the mosquito vector. Though the asexual stages are responsible for all morbidity and mortality, successful transmission is dependent on generation of the sexual stages, termed gametocytes. Gametocytes sequester in deep tissues during their development and once mature, are released back into circulation where they can be taken up by the mosquito vector. Once in the mosquito midgut, gametocytes emerge from host RBCs, develop into male and female gametes, and undergo fertilization and further development. Recent discoveries of factors in the human host microenvironment contributing to sexual differentiation and development raise exciting new questions about the biological mechanisms of these processes. In this review, we will examine recent advances in host-gametocyte interactions and discuss open questions and new tools to block malaria transmission.
II. Parasite and host factors drive commitment to gametocytogenesis
Blood stage parasites replicate asexually, with a small fraction diverting away from asexual multiplication and towards sexual development in each replication cycle. Although this process may have stochastic elements, it has long been thought that host environmental or secreted parasite factors may push cell fate decision from asexual to sexual differentiation. MicroRNAs from sickle cell erythrocytes have been associated with increased gametocyte numbers [4] while on the parasite side, genes such as P. falciparum gametocyte development gene 1 (Pfgdv1) have been implicated in control of sexual differentiation [5]. In addition, conditioned media (i.e. the supernatant of P. falciparum cultures) can stimulate sexual conversion in vitro [6,7], implying that the process is induced either by presence of parasite-secreted factors and/or by parasite depletion of nutrients present in the culture media. Several recent studies have identified parasite factors that contribute to the sexual conversion switch.
First, two groups showed that extracellular vesicles (EVs) secreted from malaria-infected red blood cells (iRBCs) can increase gametocytogenesis in vitro and hypothesized that EVs can transfer parasite and/or host factors that lead to sexual conversion (Figure 1b) [8,9]. Mantel et al observed that EVs purified from asexual parasite-conditioned media can be transferred between iRBCs and stimulate sexual conversion in a dose-dependent manner. Regev-Rudzki and colleagues demonstrated that drug pressure increased EV release and that EVs could transfer DNA between parasite lines, conferring drug resistance while also increasing sexual conversion in recipient cells. Both studies point towards EVs triggering downstream signaling that modulates the rate of commitment to the sexual pathway; however, given that EV characteristics, including size and timing of release, differed between these studies, further work is needed to validate this central finding. Additional next steps include identifying the EV component responsible for inducing sexual conversion and exploring how this signaling feeds into epigenetic control mechanisms that underlie sexual differentiation (discussed below).
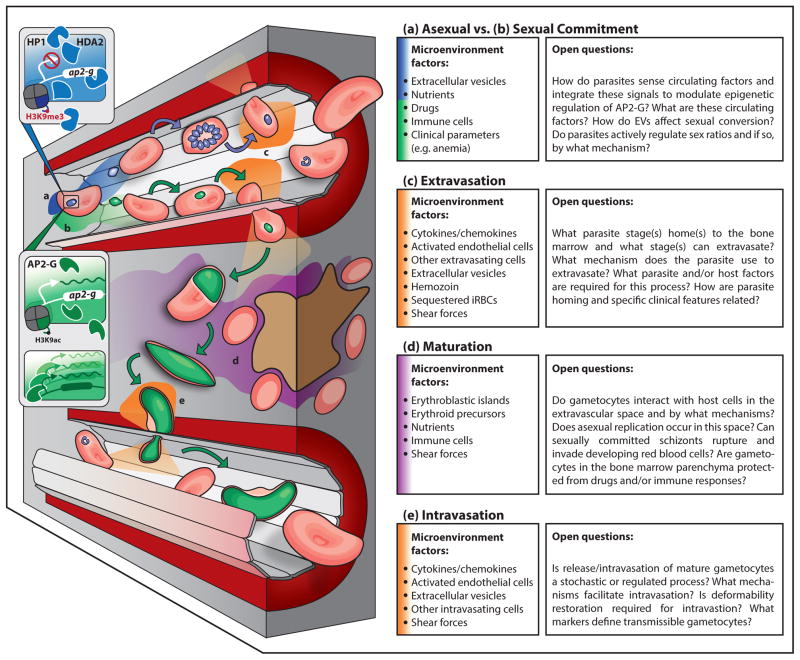
Figure 1. Model for gametocyte commitment and sequestration, including key host-parasite interactions and microenvironment characteristics for each step.
In the asexual development pathway (a) (blue), HP1 and HDA2 (and potentially other proteins) inhibit ap2-g, and therefore gametocyte gene transcription. Asexual parasites develop in RBCs and may extravasate into the bone marrow parenchyma (c) (orange). In a subset of asexual parasites, ap2-g is transcribed, leading to the expression of genes essential for gametocyte development (b) (green). There are multiple parasite stages that may be involved (asexual parasite, merozoite, early gametocyte) in homing to the bone marrow and extravasation through the endothelial lining into the bone marrow parenchyma (c) (orange). Various possible parasite and host factors likely determine homing and extravasation, which may occur in a trans- or para-cellular process, and may be guided by an active endothelial cell process. Local inflammation and endothelial activation stimulated by sequestered parasites, parasite EVs, or hemozoin may contribute to extravasation (c). Upon extravasation, parasite development or ability to remain in this microenvironment may depend on local interactions with host cells, including nurse macrophages or erythroid precursors, or soluble host factors, such as nutrients, that are also present (d) (purple). Finally, gametocytes must intravasate to return to circulation, with endothelial cells again likely mediating this process (e) (orange).
Four recent studies have uncovered a molecular framework by which parasites can integrate signals such as those provided by EVs to commit to sexual development (Figure 1a–b). A transcription factor, AP2-G, has been identified as a master transcriptional regulator for gametocytogenesis, as its deletion or disruption abolished sexual conversion in both P. falciparum and Plasmodium berghei, a murine malaria parasite. AP2-G expression during schizont stages was linked to upregulation of hundreds of genes, many of which have been implicated in gametocyte development. Positive feedback regulation may also play a role in commitment, as recombinant AP2-G in vitro binds two short recognition sequences frequently found upstream of gametocyte-specific genes including ap2-g itself [10,11]. Furthermore, two epigenetic regulators, histone deacetylase 2 (PfHda2) and heterochromatin protein 1 (PfHP1), have been shown to repress sexual development, with disruption of these proteins leading to increased gametocytogenesis and decreased asexual replication [12,13]. Conditional depletion of PfHP1 or PfHda2 in asexual parasites led to de-repression of the ap2-g locus and upregulation of gametocyte-specific genes [12,13], implying that epigenetic control of AP2-G regulates sexual commitment. Taken together, these findings support the hypothesis that epigenetic regulation allows P. falciparum to adjust developmental decisions promoting survival and transmission based on EVs, nutrients, drugs, and other host or environmental factors (illustrated in Figure 1a–b).
In the murine model, Sinha and colleagues identified an additional transcription factor from the AP2 family, AP2-G2, whose disruption completely blocks development of male gametocytes and reduces numbers of mature female gametocytes [10]. Analogous to regulation of AP2-G expression, it is hypothesized that there are environmental factors that can affect sex ratio by altering AP2-G2 expression. Indeed, mathematical and evolutionary models theorizing that plasticity in gametocyte investment enables parasites to maintain fitness in a changing host environment [14,15] highlight questions of how host factors and drugs interplay to alter male and female sexual commitment. Additional clinical and molecular studies are needed to probe mechanisms of male vs. female gametocyte formation and clearance.
III. Parasites exploit host microenvironments: sequestration in bone marrow
Mature asexual stage parasites are known to avoid splenic clearance by cytoadhering to the endothelial lining of capillaries in many tissues. The extensively characterized remodeling mechanisms that mediate asexual sequestration involve specific ligand-receptor interactions, primarily mediated by binding of the parasite antigen PfEMP1 to host endothelial receptors such as ICAM-1 and CD36 [16]. In contrast to asexual stages, only limited binding of early gametocytes to human endothelial cell lines or to CD36 and ICAM-1 has been demonstrated [17,18]. Further evidence for a gametocyte-specific sequestration mechanism includes minimal levels of PfEMP1 on the surface of early gametocyte-iRBCs and downregulation of var genes (responsible for PfEMP1 expression) [18]. Both forward and reverse genetic studies support a role for gametocyte-specific proteins, including the PfGEXPs, which are expressed during early sexual differentiation [19], in gametocyte host cell remodeling [20,21]. Previous qualitative analyses established the presence of immature P. falciparum gametocytes in the bone marrow and spleen of infected individuals [22,23], but quantitative information about gametocyte sequestration and remodeling has only been obtained recently.
Gametocytes undergo a marked change in morphology during maturation. Beginning as a round form indistinguishable from asexual stages (termed Stage I), they then develop through several transition stages (Stages II/III) to an elongated spindle form (Stage IV) and finally, the curved sausage-like mature form seen in circulation (Stage V) [24,25]. Three groups have recently characterized the mechanical properties of these distinct morphological stages, using filtration through a bead matrix, micropipette aspiration and ektacytometry to show decreased gametocyte-iRBC deformability during Stage I–IV and restored deformability during or prior to Stage V [26–28]. Interestingly, the dissociation of polymorphic STEVOR proteins from the iRBC membrane correlates with the rigidity switch from Stage IV to V [28], suggesting a possible role for these proteins in gametocyte deformability. Fluorescence microscopy experiments probing the mechanism for gametocyte morphological and mechanical changes revealed that microtubules elongate from Stage I to IV and collapse from Stage IV to Stage V [29]. Further, an actin cytoskeleton present primarily at the gametocyte poles dissociates during the transition to Stage V [30]. Computational modeling based on iRBC deformability predicts that immature gametocytes cannot pass through sinusoidal slits during splenic filtration [26], agreeing with observations of circulating mature gametocytes vs. sequestering immature gametocytes.
Three recent studies, including a case study of a patient with subacute malaria [31], an autopsy study looking at different sequestration sites in children who died from cerebral malaria [32], and a study of bone marrow aspirates of children with nonfatal malarial anemia [33], together demonstrate by histology and transcript abundance that gametocytes are enriched in the bone marrow parenchyma. In the cerebral malaria study, the majority of bone marrow gametocytes in most patients localized at erythroblastic islands, specialized sites of erythropoiesis, and a minority of gametocytes appeared to be developing inside erythroid precursor cells [32]. These data suggest that gametocytes can develop in the bone marrow parenchyma before returning to circulation as deformable Stage V gametocytes, but they leave open which parasites (asexually or sexually committed) migrate to the bone marrow (illustrated in Figure 1c–e). Transcriptional profiling from malaria-infected patient blood demonstrates quantitative presence of a young gametocyte population in circulation, intimating that at least a subset of these stages are homing to the bone marrow [34]. However, presence of asexual stage parasites in the bone marrow parenchyma and formation of gametocytes in erythroid precursor cells in vitro [32,35] suggests that the bone marrow may also represent a reservoir for asexual replication and gametocyte formation. Figure 1c–e illustrates a hypothesized flow of events for parasite sequestration in human bone marrow.
Further research in this exciting new area of gametocyte biology should confirm gametocyte enrichment in the bone marrow parenchyma in other patient cohorts, develop phenotypic assays to characterize the binding and transmigration properties of different gametocyte stages, and replicate the bone marrow microenvironment under in vitro or ex vivo conditions. Severe anemia, dyserythropoiesis and the presence of the parasite byproduct hemozoin have independently been associated with a higher prevalence of mature gametocytes in the bone marrow [33,36], providing a compelling foundation for future studies on the impact of host pathology on gametocyte sequestration. In addition, advances in in vivo live imaging of Plasmodium infections in rodent and non-human primate models will enable the study of gametocyte sequestration in the context of the host organism (reviewed in [37,38]).
IV. Discussion
Recent advances in our understanding of P. falciparum gametocyte biology and development of molecular, imaging, and drug screening tools provide exciting opportunities to better define the parasite’s interaction with its host and design transmission-blocking therapeutics (summarized in Figure 2). Evidence for EV-mediated cellular communication and epigenetic/transcriptional machinery controlling commitment provides a rationale for the systematic dissection of the triggers and downstream targets involved in P. falciparum sexual differentiation. Similarly, research building on parasite sequestration in the bone marrow should define mechanisms of homing, transmigration across the vascular endothelium, and development in the parenchyma. Furthermore, it is still unknown whether other tissues of the reticulo-endothelial system, such as the spleen and liver, can also support extravascular parasite development. The application of molecular manipulation tools (most recently CRISPR-Cas-mediated genetic disruption [39,40]) to P. falciparum will enable targeted investigation of the developmental pathways involved in sexual commitment and sequestration.
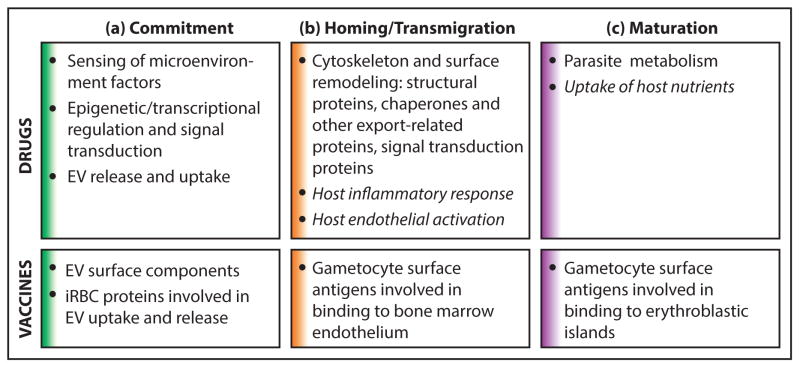
Figure 2. Points of transmission-blocking intervention.
Several recently elucidated aspects of gametocyte biology outlined in this review provide potential points of intervention for new clinical tools. Drugs or vaccines could block transmission by targeting (a) commitment to sexual development, (b) homing and transmigration (including extravasation and intravasation), and (c) gametocyte maturation. (a) During commitment, epigenetic regulation and signal transduction involved in sexual conversion and sex ratio determination could be targeted by transmission blocking drugs. Additionally, drugs could target machinery involved in the release or uptake of EVs from iRBCs. Vaccines could similarly target EV surface components or iRBC proteins involved in EV uptake and release. (b) During homing and transmigration, drugs could target parasite-encoded proteins that mediate cytoskeletal or surface remodeling involved in homing or transmigration in the bone marrow. Alternatively, host-targeted drugs could be used to modulate host inflammatory responses and endothelial activation that may drive sequestration. Vaccines could target gametocyte surface proteins required for bone marrow endothelium binding or transmigration. (c) During gametocyte maturation, drugs could target gametocyte metabolic enzymes or proteins involved in host nutrient uptake. Vaccines could target gametocyte surface proteins involved in binding to erythroblastic islands.
Host-targeted therapies are indicated by italics. Section colors in this figure correspond to microenvironment colors presented in Figure 1.
Though recent findings open up numerous possible avenues for drug development, a subset of gametocyte proteins, particularly those involved in epigenetic regulation, signal transduction, metabolism, and cytoskeletal remodeling, likely represent the most realistic points of intervention (Figure 2). Identified in a transposon mutagenesis screen and transcriptional analysis during gametocyte formation and development, putative genes involved in these processes may yield novel drug or vaccine targets [5,21,34]. Several possible drug targets have also emerged from metabolomics approaches indicating increased gametocyte sensitivity to TCA-cycle inhibitors [41] and work implicating the perforin-like protein PPLP2 and sex-specific organelles in membrane permeabilization during parasite egress [42–44]. Further upstream, recent work suggest that lipid metabolism differs between asexual stages and gametocytes [45] and that a female-specific ATP-binding cassette transporter is linked to the accumulation of lipids needed for membrane biogenesis [46]. Despite these advances, there remain many questions about parasite uptake of host nutrients and application of possible gametocyte vulnerabilities to transmission-blocking therapeutics.
Several new platforms for high throughput screening of gametocytocidal drugs could be used to test drugs intervening in the processes mentioned above. Some of these assays rely on fluorescent or luminescent reporters, enabling monitoring of gametocyte-specific drug activity [47,48], while others use DNA dyes, viability dyes, or enzymatic assays to allow screening of all parasite lines including field isolates [49–52]. In addition, new readouts for transmission-blocking activity [53,54] will increase throughput of drug and vaccine testing while the sex-specific proteome of mature gametocytes [55] may help identify stage-specific biomarkers.
Finally, there is still much uncertainty about the nature and extent of transmission-blocking immunity. Numerous epidemiological studies have suggested that transmission-blocking antibodies are short-lived [56]; however, these studies have so far been in limited populations while transmission-blocking immunity likely varies by region. Furthermore, though model simulations suggest that antibodies attacking immature gametocytes would significantly lower the density of transmissible mature gametocytes [57], it is still unknown what role antibodies play compared to other immune components and if antibodies can target developing gametocytes in addition to mature gametocytes. Whatever gametocyte stage(s) is (are) ultimately targeted by a transmission-blocking drug or vaccine, promising results from a recent vaccine candidate combining the established gamete antigen Pfs48/45 with the asexual antigen GLURP [58] reinforce the value of targeting transmission stages together with asexual stages.
In conclusion, the malaria elimination agenda has driven recent discoveries with applications for novel biomarkers, drugs and vaccines. In particular, advances in illuminating the mechanisms of gametocyte commitment and sequestration uncover new parasite and host targets for transmission-blocking interventions. Further investigation of the knowledge gaps in these areas will both deepen our understanding of host-gametocyte biology and generate new tools to block malaria transmission.
Highlights.
P. falciparum sexual conversion is regulated by epigenetic control of AP2-G.
Extracellular vesicles and other environmental factors may alter sexual conversion.
Gametocytes are enriched in the bone marrow parenchyma.
Insights into sexual differentiation and development reveal potential drug targets.
New tools will enable better understanding of parasite-host interactions.
Acknowledgments
The authors wish to thank Ilana Goldowitz and Elamaran Meibalan for critical reading of the manuscript. Work in the Marti lab is funded by grants 5R01AI077558 and 1R21AI105328 from the National Institutes of Health and a career development award from the Burroughs Wellcome Fund to MM. KWD, DBR and NBMM are supported by a Herchel Smith graduate fellowship, a graduate fellowship (DGE1144152) from the U.S National Science Foundation and a postdoctoral fellowship (P2BSP3_151859) from the Swiss National Science Foundation, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. WHO Malaria Report 2014. 2014 [Google Scholar]
- 2.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 3.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, et al. A research agenda to underpin malaria eradication. PLoS medicine. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaMonte G, Philip N, Reardon J, Lacsina JR, Majoros W, Chapman L, Thornburg CD, Telen MJ, Ohler U, Nicchitta CV, et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell host & microbe. 2012;12:187–199. doi: 10.1016/j.chom.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eksi S, Morahan BJ, Haile Y, Furuya T, Jiang H, Ali O, Xu H, Kiattibutr K, Suri A, Czesny B, et al. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 2012;8:e1002964. doi: 10.1371/journal.ppat.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer M, Day KP. Regulation of the rate of asexual growth and commitment to sexual development by diffusible factors from in vitro cultures of Plasmodium falciparum. The American journal of tropical medicine and hygiene. 2003;68:403–409. [PubMed] [Google Scholar]
- 7.Williams JL. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am J Trop Med Hyg. 1999;60:7–13. doi: 10.4269/ajtmh.1999.60.7. [DOI] [PubMed] [Google Scholar]
- **8.Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13:521–534. doi: 10.1016/j.chom.2013.04.009. Characterizes EVs from infected RBCs, including their immunomodulatory effects on macrophages and neutrophils. Provides evidence for iRBCs internalizing EVs purified from asexual parasite-conditioned media and EVs stimulating sexual conversion in a dose-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. In agreement with [8], shows that exosome-like vesicles from iRBCs stimulate increased sexual differentiation and release more frequently with drug pressure. Suggests that EVs can transfer DNA between parasite strains, conferring drug resistance on plasmid-recipient parasites. [DOI] [PubMed] [Google Scholar]
- **10.Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. References 10–11 independently identify PfAP2-G, a master transcriptional regulator for gametocytogenesis. AP2-G upregulates expression of hundreds of genes associated with gametocyte development and its disruption inhibits commitment to sexual conversion. Kafsack et al. show that loss-of-function mutations in AP2-G are absent in the genomes of 300 P. falciparum field isolates, while variation in ap2-g transcript levels correlate highly with level of gametocyte production in a reference lab strain. Sinha et al. also identify PbAP2-G2, which may be involved in determining gametocyte sex ratio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Brancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z, et al. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014;16:165–176. doi: 10.1016/j.chom.2014.07.004. Highlights the importance of epigenetic control of sexual commitment by characterizing the role of heterochromatin protein 1 (HP1) in regulating PfAP2-G (see references 10–11). Conditional depletion of HP1 in late asexual stage parasites causes 50% of progeny in the following cycle to become gametocytes and 50% to continue with asexual development and then arrest prior to schizogony. HP1-depleted gametocytes progress normally through stage V development, suggesting that the role of HP1 in gametocytogenesis is confined to commitment. [DOI] [PubMed] [Google Scholar]
- **13.Coleman BI, Skillman KM, Jiang RH, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BF, Marti M, et al. A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe. 2014;16:177–186. doi: 10.1016/j.chom.2014.06.014. Parallel to [12], demonstrates an epigenetic role for histone deacetylase 2 (Hda2) in controlling PfAP2-G (see references 7–8), and therefore repressing commitment to sexual commitment. Conditional depletion of Hda2 in asexual stage parasites causes a threefold increase in sexual commitment as well as a decrease in asexual replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron A, Reece SE, Drew DR, Haydon DT, Yates AJ. Plasticity in transmission strategies of the malaria parasite, Plasmodium chabaudi: environmental and genetic effects. Evol Appl. 2013;6:365–376. doi: 10.1111/eva.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Carter LM, Kafsack BF, Llinas M, Mideo N, Pollitt LC, Reece SE. Stress and sex in malaria parasites: Why does commitment vary? Evol Med Public Health. 2013;2013:135–147. doi: 10.1093/emph/eot011. Presents an evolutionary theory-based model that aims to understand why gametocyte densities are usually low compared to asexual densities and examines why parasites adjust investment in gametocytes according to changing host conditions. Provides mathematical support for plasticity in gametocyte investment enabling parasites to optimize fitness in a changing host environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 17.Silvestrini F, Tiburcio M, Bertuccini L, Alano P. Differential adhesive properties of sequestered asexual and sexual stages of Plasmodium falciparum on human endothelial cells are tissue independent. PLoS One. 2012;7:e31567. doi: 10.1371/journal.pone.0031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiburcio M, Silvestrini F, Bertuccini L, Sander AF, Turner L, Lavstsen T, Alano P. Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol. 2012 doi: 10.1111/cmi.12062. [DOI] [PubMed] [Google Scholar]
- 19.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morahan BJ, Strobel C, Hasan U, Czesny B, Mantel PY, Marti M, Eksi S, Williamson KC. Functional analysis of the exported type IV HSP40 protein PfGECO in Plasmodium falciparum gametocytes. Eukaryotic cell. 2011;10:1492–1503. doi: 10.1128/EC.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Ikadai H, Shaw Saliba K, Kanzok SM, McLean KJ, Tanaka TQ, Cao J, Williamson KC, Jacobs-Lorena M. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc Natl Acad Sci U S A. 2013;110:E1676–1684. doi: 10.1073/pnas.1217712110. Uses piggyBac transposon-mediated insertional mutagenesis to identify parasite clones that no longer form mature gametocytes. Ultimately identified 16 genes putatively responsible for the loss of gametocytogenesis; some appear to arrest gametocyte maturation while others may be involved in commitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smalley ME, Abdalla S, Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg. 1981;75:103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- 23.Thomson JG, Robertson A. The Structure and development of Plasmodium falciparum gametocytes in the internal organs and peripheral circulation. Trans R Soc Trop Med Hyg. 1935;14:31–40. [Google Scholar]
- 24.Hawking F, Wilson ME, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 25.Sinden RE, Canning EU, Bray RS, Smalley ME. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978;201:375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- 26.Aingaran M, Zhang R, Law SK, Peng Z, Undisz A, Meyer E, Diez-Silva M, Burke TA, Spielmann T, Lim CT, et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell Microbiol. 2012;14:983–993. doi: 10.1111/j.1462-5822.2012.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dearnley MK, Yeoman JA, Hanssen E, Kenny S, Turnbull L, Whitchurch CB, Tilley L, Dixon MW. Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. Journal of cell science. 2012 doi: 10.1242/jcs.099002. [DOI] [PubMed] [Google Scholar]
- 28.Tiburcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, Silvestrini F, Khattab A, Milon G, David PH, et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood. 2012;119:e172–180. doi: 10.1182/blood-2012-03-414557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon MW, Dearnley MK, Hanssen E, Gilberger T, Tilley L. Shape-shifting gametocytes: how and why does P. falciparum go banana-shaped? Trends Parasitol. 2012;28:471–478. doi: 10.1016/j.pt.2012.07.007. [DOI] [PubMed] [Google Scholar]
- *30.Hliscs M, Millet C, Dixon MW, Siden-Kiamos I, McMillan P, Tilley L. Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell Microbiol. 2015;17:207–225. doi: 10.1111/cmi.12359. Demonstrates that an F-actin cytoskeleton is present at the ends of maturing gametocytes under the inner membrane complex, and that this actin cytoskeleton is dismantled in stage V gametocytes at the same time as microtubule cytoskeleton disassembly. Also shows the presence of Formin-1 at gametocyte ends. [DOI] [PubMed] [Google Scholar]
- 31.Farfour E, Charlotte F, Settegrana C, Miyara M, Buffet P. The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar J. 2012;11:285. doi: 10.1186/1475-2875-11-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6:244re245. doi: 10.1126/scitranslmed.3008882. Using autopsy tissue samples from children who died from cerebral malaria, reveals gametocyte enrichment in the bone marrow, particularly in the bone marrow parenchyma. Specific localization of gametocytes at the erythroblastic island, the site of RBC development, raises new questions about which parasite stage homes to the bone marrow and how gametocytes develop in the bone marrow microenvironment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cistero P, Li Wai Suen CS, Nhabomba A, Macete E, Mueller I, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123:959–966. doi: 10.1182/blood-2013-08-520767. Using bone marrow aspirates from children with nonfatal malarial anemia, shows higher prevalence and transcript levels of immature gametocytes in the bone marrow than in the peripheral blood. Severe anemia and dyserythropoiesis were associated with a higher prevalence of mature gametocytes in bone marrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelle K, Oh K, Buchholz K, Narasimhan V, Milner D, Ketman K, Seydel K, Taylor T, Barteneva N, Huttenhower C, et al. Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med. doi: 10.1186/s13073-015-0133-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peatey CL, Watson JA, Trenholme KR, Brown CL, Nielson L, Guenther M, Timmins N, Watson GS, Gardiner DL. Enhanced gametocyte formation in erythrocyte progenitor cells: a site-specific adaptation by Plasmodium falciparum. J Infect Dis. 2013;208:1170–1174. doi: 10.1093/infdis/jit309. [DOI] [PubMed] [Google Scholar]
- *36.Aguilar R, Moraleda C, Achtman AH, Mayor A, Quinto L, Cistero P, Nhabomba A, Macete E, Schofield L, Alonso PL, et al. Severity of anaemia is associated with bone marrow haemozoin in children exposed to Plasmodium falciparum. Br J Haematol. 2014;164:877–887. doi: 10.1111/bjh.12716. Analyzes the presence of malaria parasites and hemozoin in bone marrow aspirates from anemic children. An association of hemozoin presence with decreased hemoglobin and increased dyserythropoiesis implies that hemozoin is involved in the pathogenesis of malarial anemia. [DOI] [PubMed] [Google Scholar]
- 37.Beignon AS, Le Grand R, Chapon C. In vivo imaging in NHP models of malaria: challenges, progress and outlooks. Parasitol Int. 2014;63:206–215. doi: 10.1016/j.parint.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claser C, Malleret B, Peng K, Bakocevic N, Gun SY, Russell B, Ng LG, Renia L. Rodent Plasmodium-infected red blood cells: imaging their fates and interactions within their hosts. Parasitol Int. 2014;63:187–194. doi: 10.1016/j.parint.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Ghorbal M, Gorman M, Macpherson CR, Martins RM, Scherf A, Lopez-Rubio JJ. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 40.Wagner JC, Platt RJ, Goldfless SJ, Zhang F, Niles JC. Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nat Methods. 2014;11:915–918. doi: 10.1038/nmeth.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacRae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, Kenny S, Bottova I, Tilley L, McConville MJ. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013;11:67. doi: 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deligianni E, Morgan RN, Bertuccini L, Wirth CC, Silmon de Monerri NC, Spanos L, Blackman MJ, Louis C, Pradel G, Siden-Kiamos I. A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell Microbiol. 2013;15:1438–1455. doi: 10.1111/cmi.12131. [DOI] [PubMed] [Google Scholar]
- 43.Olivieri A, Bertuccini L, Deligianni E, Franke-Fayard B, Curra C, Siden-Kiamos I, Hanssen E, Grasso F, Superti F, Pace T, et al. Distinct properties of the egress-related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei. Cell Microbiol. 2014 doi: 10.1111/cmi.12370. [DOI] [PubMed] [Google Scholar]
- 44.Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, Kachman MM, Weissbach T, Zimmerberg J, Dandekar T, et al. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol. 2014;16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Lamour SD, Straschil U, Saric J, Delves MJ. Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar J. 2014;13:468. doi: 10.1186/1475-2875-13-468. Based on analysis of parasite uptake and release of nutrients in culture medium, proposes that energy metabolism and lipid utilization are different in gametocytes than in asexual stages. Provides support for future investigations of acetate and fatty acid metabolism in gametocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Tran PN, Brown SH, Mitchell TW, Matuschewski K, McMillan PJ, Kirk K, Dixon MW, Maier AG. A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat Commun. 2014;5:4773. doi: 10.1038/ncomms5773. Identifies a role for a female gametocyte-specific ATP-binding cassette (ABC) transporter in the regulation of gametocyte numbers and in the aggregation of lipids needed for membrane biogenesis. The structure containing these lipids may function to prepare the parasite for the transition from the human host to the mosquito host and may also play a role in gametocyte commitment. [DOI] [PubMed] [Google Scholar]
- 47.Cevenini L, Camarda G, Michelini E, Siciliano G, Calabretta MM, Bona R, Kumar TR, Cara A, Branchini BR, Fidock DA, et al. Multicolor bioluminescence boosts malaria research: quantitative dual-color assay and single-cell imaging in Plasmodium falciparum parasites. Anal Chem. 2014;86:8814–8821. doi: 10.1021/ac502098w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Liu M, Liang X, Siriwat S, Li X, Chen X, Parker DM, Miao J, Cui L. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS One. 2014;9:e93825. doi: 10.1371/journal.pone.0093825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemother. 2013;68:2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 50.Duffy S, Avery VM. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J. 2013;12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One. 2014;9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka TQ, Dehdashti SJ, Nguyen DT, McKew JC, Zheng W, Williamson KC. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol Biochem Parasitol. 2013;188:20–25. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother. 2014;58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone WJ, Churcher TS, Graumans W, van Gemert GJ, Vos MW, Lanke KH, van de Vegte-Bolmer MG, Siebelink-Stoter R, Dechering KJ, Vaughan AM, et al. A scalable assessment of Plasmodium falciparum transmission in the standard membrane-feeding assay, using transgenic parasites expressing green fluorescent protein-luciferase. J Infect Dis. 2014;210:1456–1463. doi: 10.1093/infdis/jiu271. [DOI] [PubMed] [Google Scholar]
- 55.Tao D, Ubaida-Mohien C, Mathias DK, King JG, Pastrana-Mena R, Tripathi A, Goldowitz I, Graham DR, Moss E, Marti M, et al. Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol Cell Proteomics. 2014;13:2705–2724. doi: 10.1074/mcp.M114.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bousema T, Sutherland CJ, Churcher TS, Mulder B, Gouagna LC, Riley EM, Targett GA, Drakeley CJ. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int J Parasitol. 2011;41:293–300. doi: 10.1016/j.ijpara.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McQueen PG, Williamson KC, McKenzie FE. Host immune constraints on malaria transmission: insights from population biology of within-host parasites. Malar J. 2013;12:206. doi: 10.1186/1475-2875-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theisen M, Roeffen W, Singh SK, Andersen G, Amoah L, van de Vegte-Bolmer M, Arens T, Tiendrebeogo RW, Jones S, Bousema T, et al. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine. 2014;32:2623–2630. doi: 10.1016/j.vaccine.2014.03.020. [DOI] [PubMed] [Google Scholar]