Abstract
Growth factors, long studied for their involvement in neuronal development and plasticity, also regulate responses to drugs of abuse, including alcohol. This review details the intricate interaction between the Brain-Derived Neurotrophic Factor (BDNF) and alcohol, and provides evidence to suggest that corticostriatal BDNF signaling acts to keep alcohol drinking in moderation. Specifically, we describe studies in rodent models suggesting that moderate consumption of alcohol increases BDNF levels in the dorsal striatum, which in turn act to suppress alcohol intake by activating a Mitogen-Activated Protein Kinase (MAPK)-dependent genomic mechanism. We further provide data to suggest that alcohol intake levels escalate when the endogenous corticostriatal BDNF pathway becomes dysregulated. Finally, we summarize recent studies suggesting that specific microRNAs targeting BDNF mRNA in the medial prefrontal cortex (mPFC) regulate the breakdown of the protective corticostriatal BDNF pathway.
Introduction
Alcohol use disorders are pervasive problems characterized by increased alcohol consumption over time, loss of control over alcohol drinking and persistent alcohol use despite negative consequences. Alcohol use disorders afflict approximately 10% of the population worldwide, with a higher disease prevalence in developed countries (World Health Organization, 2014), and generate significant societal costs in the form of loss of productivity in the workplace, increased burden on the healthcare system and premature loss of life (McGinnis and Foege, 1999; Rehm, 2011; Whiteford et al., 2013). Thus determining the neuroadaptations underpinning the progression from casual to compulsive alcohol intake, which may lead to the development of improved treatments for alcohol-dependent individuals, is of great interest to alleviate the societal burden of alcohol use disorders. Even though alcohol is widely consumed, only 11.5% of consumers abuse alcohol and develop uncontrolled drinking behaviors, and 3.6% develop alcohol addiction (World Health Organization, 2014), suggesting the existence of mechanisms that protect the majority of social drinkers from the transition from moderate to excessive, uncontrolled alcohol intake. Here we present data suggesting that the neurotrophic factor BDNF is part of such an endogenous protective pathway.
Neurotrophic factors, originally discovered for their ability to support neuronal development, survival and differentiation (Davies et al., 1986; Lin et al., 1993), perform a variety of functions in the adult brain, including regulation of neuronal plasticity (Park and Poo, 2013; Zagrebelsky and Korte, 2014). Substance use disorders have been characterized as diseases of maladaptive plasticity (Feltenstein and See, 2013; Luscher and Malenka, 2011; Nestler, 2013), and thus neurotrophic factors present putative molecular mediators of the long-lasting effects of drugs of abuse, including alcohol (Ahmadiantehrani et al., 2014). Reduced hippocampal neurotrophic activity, assessed as the ability of hippocampal extracts to support the survival of dorsal root ganglion cultures following chronic, high levels of alcohol intake (Walker et al., 1992), first suggested an interaction between neurotrophic factor function and alcohol, and brain-derived neurotrophic factor (BDNF) has been particularly implicated in alcohol’s actions in the adult brain.
Brain-Derived Neurotrophic Factor and Regulation of Substance Use
BDNF, first discovered for its ability to support the survival and growth of sensory neurons (Barde et al., 1982), is a member of the nerve growth factor (NGF) family of neurotrophic factors (Barde, 1994). BDNF signals by binding to tropomyosin-related kinase B (TrkB), a receptor tyrosine kinase that autophosphorylates upon ligation of BDNF, initiating downstream signaling via the MAPK, phospholipase C γ (PLC γ) and phosphoinositol 3-kinase (PI3K) pathways (Huang and Reichardt, 2003). Both BDNF (Hofer et al., 1990) and TrkB (Klein et al., 1990) are widely expressed throughout the central nervous system (CNS), with particular enrichment in subdivisions of the cortex, hippocampus and cerebellum. BDNF regulates a variety of neuronal processes, including neuronal development, neuroprotection, synaptic plasticity and learning and memory (Castren, 2004; Cowansage et al., 2010; Lu et al., 2014; Lu et al., 2008; Minichiello, 2009). In contrast, dysregulation of BDNF function has been implicated in multiple neuropsychiatric disorders (Autry and Monteggia, 2012; Castren, 2014), including depression (Duman and Li, 2012), schizophrenia (Buckley et al., 2007) and anxiety disorders (Andero et al., 2014), as well as drug abuse (Ahmadiantehrani et al., 2014; Ghitza et al., 2010).
The regulation of drug self-administration by BDNF depends both on the drug of abuse and on the neural circuitry under investigation. BDNF in the mesolimbic dopamine system promotes drug sensitization and self-administration, particularly for cocaine (Graham et al., 2007; Graham et al., 2009; Horger et al., 1999; Lu et al., 2004) but also for opiates (Vargas-Perez et al., 2009; Wan et al., 2011). BDNF infusion into the ventral tegmental area (VTA) (Horger et al., 1999; Lu et al., 2004) or nucleus accumbens (NAc) (Graham et al., 2007; Horger et al., 1999) increased cocaine sensitization (Horger et al., 1999), self-administration (Graham et al., 2007) and reinstatement of cocaine-seeking (Lu et al., 2004). Even a single VTA BDNF infusion generated a long-lasting increase in cocaine-seeking (Lu et al., 2004) and, in previously naïve rats, a shift in opiate reward that mimicked dependence (Vargas-Perez et al., 2009). Drug withdrawal elevated BDNF expression in the VTA and NAc over several weeks for both cocaine (Grimm et al., 2003) and nicotine (Kivinummi et al., 2011), possibly underlying the incubation of craving observed across withdrawal (Grimm et al., 2003; Pickens et al., 2011). Unlike the potentiating effect of BDNF on stimulant drug self-administration in the mesolimbic dopamine system, elevating BDNF levels in the medial prefrontal cortex (mPFC) can reverse molecular adaptations underlying stimulant drug-seeking. Infusion of BDNF into the mPFC immediately after the final cocaine self-administration session reduced later cocaine-seeking, even in the presence of drug cues or priming injections (Berglind et al., 2007; Hearing et al., 2008; Sadri-Vakili et al., 2010). This post-self-administration infusion of BDNF, which acts via TrkB-induced MAPK signaling (Whitfield et al., 2011), may reduce later cocaine-seeking by correcting cocaine-induced maladaptations, including decreased MAPK signaling pathway activation in the mPFC (Sun et al., 2013; Whitfield et al., 2011) and disruption of glutamatergic input to the NAc (Berglind et al., 2009). Surprisingly, elevated BDNF levels were observed during abstinence (Hearing et al., 2008; Sadri-Vakili et al., 2010) and following reinstatement of cocaine-seeking (Hearing et al., 2008), times of elevated drug craving, yet reduction in BDNF levels by RNA interference (RNAi) increased motivation to work for cocaine (Sadri-Vakili et al., 2010). Together these studies implicate BDNF in the mPFC as a negative regulator of cocaine seeking, similar to the important protective role BDNF plays in alcohol use disorders.
Alcohol regulation of cotricostriatal BDNF expression
Multiple lines of evidence demonstrate elevated BDNF mRNA expression in the dorsal striatum in response to acute alcohol exposure or repeated, moderate alcohol consumption. Acute alcohol administration (2 g/kg) significantly increased BDNF mRNA levels in the dorsal striatum of C57BL/6J mice (McGough et al., 2004). Importantly, the increase in BDNF mRNA in the dorsal striatum (McGough et al., 2004) was observed in C57BL/6J mice after 4 weeks of voluntary 10% alcohol consumption in an unlimited 2-bottle choice (2-BC) access paradigm that generates moderate levels (10 g/kg/24 hrs) of alcohol intake(McGough et al., 2004), as well as after a single 4-hr session of 2-BC access (5.6 g/kg intake) under a modified drinking in the dark paradigm (Logrip et al., 2009). A similar increase in BDNF mRNA expression was observed in the dorsal striatum following rat operant alcohol self-administration of 10% alcohol, with substantially greater alcohol-induced BDNF levels in the lateral subdivision of the dorsal striatum (dorsolateral striatum, or DLS), as compared to the medial subdivision (dorsomedial striatum, or DMS) (Jeanblanc et al., 2009). Importantly, these elevations in BDNF expression observed after acute and low-dose alcohol drinking were regionally restricted to the dorsal striatum, as a similar upregulation of BDNF was not observed in the neighboring NAc (Logrip et al., 2009; McGough et al., 2004). The effects also showed reinforcer specificity, as sucrose consumption did not alter dorsal striatal BDNF expression (Logrip et al., 2009). Acute application of alcohol onto striatal primary neurons also increased BDNF expression, resulting in the translation and secretion of the polypeptide and in the activation of the BDNF receptor, TrkB (Logrip et al., 2008). Together these data demonstrate that acute alcohol exposure and, importantly, chronic alcohol consumption at moderate levels insufficient to generate sustained elevations in blood alcohol levels associated with intoxication (>80 mg/dl) (Dole and Gentry, 1984), increase striatal BDNF, likely resulting in BDNF release and TrkB-mediated activation of downstream signaling pathways.
Contrary to the elevated dorsal striatal BDNF expression observed subsequent to alcohol exposure under conditions of limited experience or moderate alcohol intake, escalated alcohol intake following 6 weeks of daily, 4-hr limited access, when mice consume sufficient alcohol to become intoxicated (BAL > 80 mg/dl) (Rhodes et al., 2007), generated no alteration in BDNF mRNA expression (Logrip et al., 2009). In light of the sustained ability of alcohol consumption to elevate BDNF mRNA levels after 4 or more weeks of moderate drinking under free access or operant conditions, the breakdown of alcohol’s ability to upregulate BDNF expression following repeated limited access to alcohol intake suggests the possible loss of a protective mechanism, such that a lack of alcohol-induced expression of BDNF in the dorsal striatum contributes to heightened drinking. This breakdown of dorsal striatal BDNF expression in response to alcohol was accompanied by a significant, long-lasting reduction in BDNF mRNA levels in cortical regions (Logrip et al., 2009). In line with these findings, prolonged voluntary intake of excessive levels (20%) of alcohol produced a robust reduction in BDNF expression specifically in the mPFC; a reduction which was directly correlated with the amount of alcohol the mice consumed (Darcq et al., 2014).
One mechanism for regulating mRNA expression involves microRNAs (miRs), short, noncoding RNA sequences that block mRNA translation by binding to cytoplasmic mRNA and targeting them for degradation (Bartel, 2004). Recent data suggest that the attenuation of BDNF levels in the mPFC is associated with an increase in the levels of two microRNAs (miRs) that specifically target BDNF mRNA resulting in the degradation of the message. Specifically, a history of excessive alcohol intake in mice led to an increase in miR30a-5p (Darcq et al., 2014) and protracted withdrawal from alcohol in dependent rats resulted in increased expression of miR-206 (Tapocik et al., 2014), with an inverse association between these miRs and BDNF levels observed in both paradigms (Darcq et al., 2014; Tapocik et al., 2014). Alcohol may also regulate striatal BDNF via altered miR expression, as miR124a decreased, whereas BDNF expression increased, in the DLS following moderate alcohol intake, and changing miR124a expression levels inversely altered alcohol intake (Bahi and Dreyer, 2013). Innately lower BDNF expression may predispose rats to higher alcohol intake, as alcohol-preferring P rats, genetically selected for high alcohol intake and preference (Li et al., 1987), display reduced BDNF protein levels in the nucleus accumbens (Yan et al., 2005) and the central and medial nuclei of the amygdala and bed nucleus of the stria terminalis (Prakash et al., 2008) relative to the low-drinking NP rats. Together, these data indicate that while acute or moderate alcohol exposure increases BDNF expression in the dorsal striatum, particularly in the lateral subdivision, chronic alcohol does not alter striatal BDNF expression, but rather reduces BDNF in the cortex and hippocampus, and lower BDNF expression levels are associated with heightened alcohol intake.
Corticostriatal BDNF axis and the regulation of alcohol intake
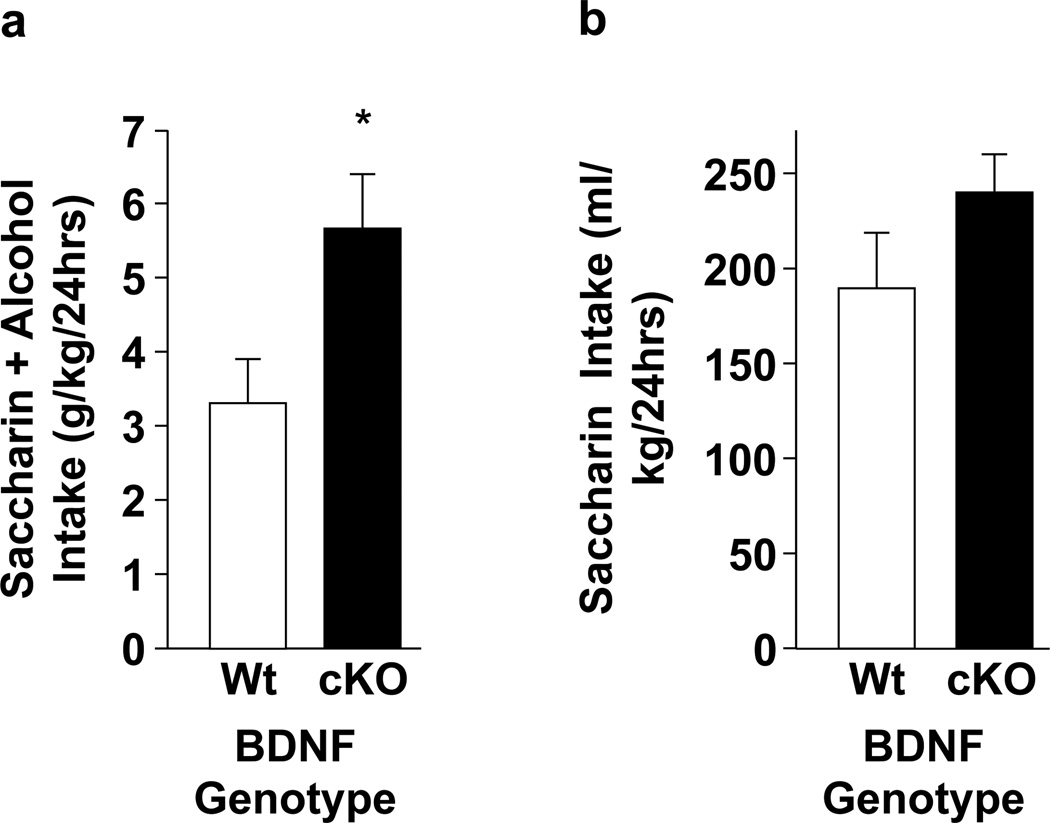
Modulation of alcohol intake under conditions of increased and decreased BDNF expression has elucidated a role for BDNF in the suppression of alcohol intake. For example, mice expressing approximately half the normal level of BDNF in the brain display elevated 2-BC alcohol intake both under baseline conditions (Hensler et al., 2003) and after a period of deprivation (McGough et al., 2004), as well as elevated alcohol-induced sensitization and increased preference for an alcohol-paired location, relative to wildtype mice (McGough et al., 2004). Similarly, mice heterozygous for the CREB transcription factor express reduced levels of BDNF and demonstrate higher alcohol preference than wildtype mice (Pandey et al., 2004). Furthermore, postnatal conditional deletion of BDNF in mice (Rios et al., 2001) led to an increase in saccharin-sweetened alcohol intake, relative to wildtype littermates (Figure 1A, t11=2.56, p<0.05), with no significant genotype difference observed for consumption of an alcohol-free saccharin solution in the same mice (Figure 1B, t11=1.39, p=0.19). Importantly, systemic or intra-dorsal striatum administration of RACK1, a protein that increases BDNF levels (He et al., 2010; McGough et al., 2004; Neasta et al., 2012; Yaka et al., 2003), expressed recombinantly with a Tat sequence (Tat-RACK1) that allows for transduction across the blood-brain barrier (Schwarze et al., 2000), significantly reduced 2-BC alcohol intake in mice (Jeanblanc et al., 2006; McGough et al., 2004). Conversely, both BDNF haploinsufficiency (McGough et al., 2004) and the Trk inhibitor K252a (Jeanblanc et al., 2006) prevented the effect of Tat-RACK1 on alcohol drinking. Importantly, direct infusion of Tat-RACK1 into the dorsal striatum also reduced operant alcohol self-administration (Jeanblanc et al., 2006). Together, these data demonstrate an inverse relationship between BDNF expression levels and alcohol consumption, likely via BDNF action in the dorsal striatum.
Figure 1. Conditional deletion of BDNF increases sweetened alcohol intake.
Conditional, postnatal deletion of the BDNF gene (cKO) was generated by crossing mice in which the BDNF gene was surrounded by LoxP sites with mice expressing Cre recombinase under the control of the αCaMKII promoter. cKO mice and their wildtype littermates (Wt) were provided 24-h continuous access to 2 bottles on the home cage, with one containing water and the other containing a sweetened alcohol solution [10% (v/v) alcohol with 0.2% (w/v) saccharin], or a sweetened, alcohol-free solution [0.2% (w/v) saccharin in water], as specified. Bottles were weighed every other day to determine the amount of fluid intake, and positions on the cage rotated to prevent side bias. (a) Weight-normalized amount of sweetened alcohol (g/kg) consumed during 24 hours, averaged across 1 week of access. cKO mice consumed significantly more alcohol solution than their Wt counterparts, t11 = 2.56, *p < 0.05 vs Wt control. (b) Weight-normalized amount of sweetened saccharin solution (ml/kg) consumed during 24 hrs, averaged across 1 week of access. No significant difference was observed by genotype, t11 = 1.39, p = 0.19. Results are expressed as mean ± SEM. n = 6, cKO; n=7, Wt.
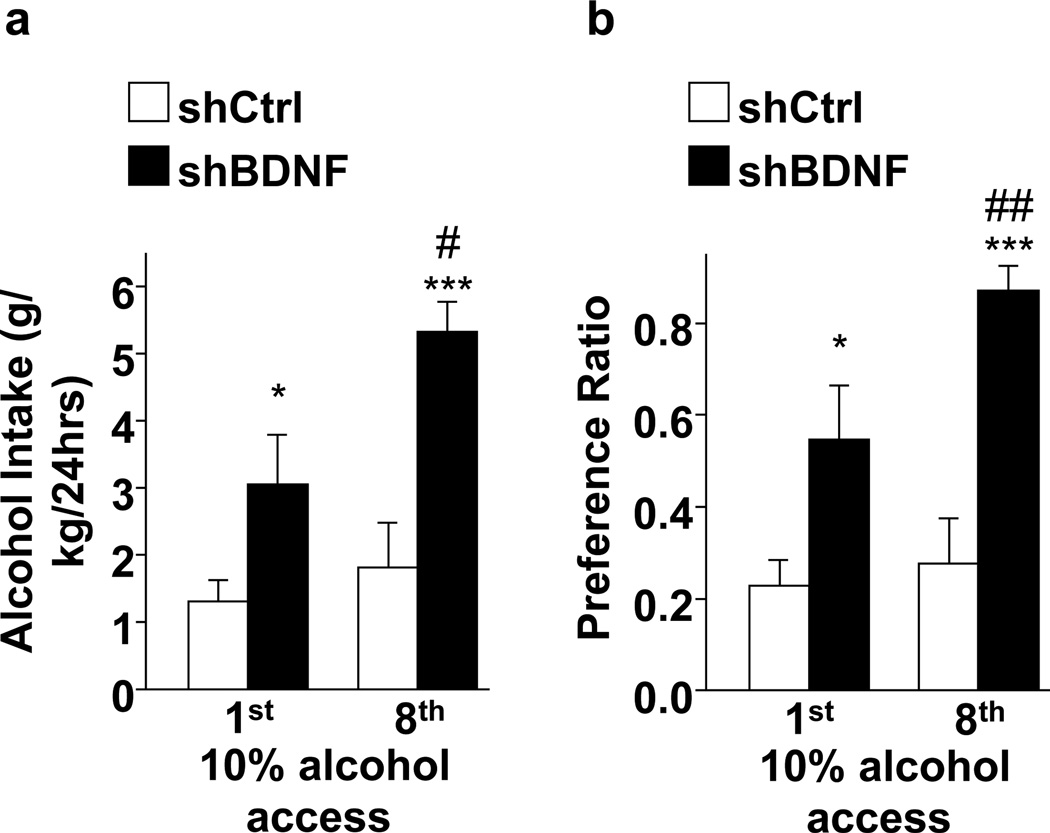
Because acute and moderate, but not escalated, alcohol intake increased BDNF expression in the dorsal striatum (Jeanblanc et al., 2013; Logrip et al., 2009; McGough et al., 2004), and Tat-RACK1 induced central increases in BDNF expression, including in the dorsal striatum, accompanied by reductions in alcohol intake (Jeanblanc et al., 2006; McGough et al., 2004), we hypothesized that BDNF in the dorsal striatum and specifically in the DLS functions as a negative regulator of alcohol intake. To test this possibility, we investigated the consequences of upregulation or downregulation of BDNF in the DLS on rat operant self-administration of moderate (10%) levels of alcohol (Jeanblanc et al., 2009). We found that exogenous BDNF infusion into the DLS significantly decreased alcohol self-administration, whereas reduction of endogenous BDNF levels in the DLS via adenoviral delivery of RNAi targeting the BDNF gene significantly elevated alcohol self-administration (Jeanblanc et al., 2009). Interestingly, intra-NAc infusion of BDNF did not alter alcohol intake, and BDNF’s actions in the DLS were specific for alcohol as no changes in sucrose self-administration were observed in response to BDNF application (Jeanblanc et al., 2009). Furthermore, knockdown of BDNF in the DMS did not alter self-administration of alcohol, although intra-DMS infusion of BDNF reduced both alcohol self-administration and sucrose self-administration (Jeanblanc et al., 2009). In line with these findings, more recently we showed that RNA interference-mediated knockdown of BDNF in the DLS also promoted the development of excessive alcohol drinking (Figure 2). In fact, in rats receiving intermittent access to 10% alcohol on alternate days, knockdown of BDNF expression in the DLS elevated alcohol drinking (Figure 2A, 1st) and preference (Figure 2B, 1st), even on the first day of intermittent access. In addition, infection of the DLS with virus targeting BDNF produced an escalation of alcohol intake (Figure 2A, 8th) and preference (Figure 2B, 8th) after only a few access sessions, which was not observed in control rats infected with a control virus in the DLS (Figure 2). These data support a role for endogenous BDNF in the DLS in maintaining moderate levels of alcohol intake, as reduced levels of DLS BDNF impart a vulnerability towards developing harmful alcohol drinking.
Figure 2. BDNF knockdown in the DLS promotes excessive alcohol drinking in rats.
Rats received intra-DLS infusion of AdV-sh BDNF or AdV-sh control (Ctrl, Jeanblanc et al., 2009). After 5 days of recovery, rats had access to 10% (v/v) alcohol every other day for 2 weeks. (a) Weight-normalized amount of alcohol consumed (g/kg) during 24 hours of alcohol access. Two-way RM-ANOVA showed a main effect of BDNF knockdown on the level of intake (F(1,12) = 15.6, p = 0.002) and session (F(1,12) = 9.50, p = 0.01), without significant interaction (F(1,12) = 3.82, p = 0.07). For specific between-group comparisons, subsequent analysis using the method of contrasts revealed that the knockdown of BDNF in the DLS promoted high alcohol drinking in the first and eighth sessions of alcohol drinking, as compared to Ctrl (p’s <0.05). Rats with BDNF knockdown in the DLS also showed an escalation in the level of alcohol drinking between the first and the eighth sessions (p <0.05). (b) Alcohol preference, calculated as the volume of alcohol solution intake relative to the volume of total fluid intake during 24 hours of alcohol access. Two-way RM-ANOVA showed a main effect of BDNF knockdown on the level of intake (F(1,12) = 19.1, p < 0.002) and session (F(1,12) = 8.85, p < 0.05), with significant interaction (F(1,12) = 4.80, p < 0.05). Post hoc analysis via the Student-Newman-Keuls test indicated that the preference for 10% alcohol was significantly higher for both the first and the eighth sessions of alcohol drinking in rats with DLS BDNF knockdown relative to Ctrl rats (ps <0.05). Furthermore, alcohol preference was significantly increased between the first and eighth drinking sessions in rats with DLS BDNF knockdown (p <0.05). Results are expressed as mean ± SEM. *p<0.05, ***p<0.001 vs shCtrl; #p<0.05, ##p<0.01 vs. shBDNF Day 1. n = 7.
It is important to note that the beneficial actions of BDNF on alcohol intake are also mediated by brain regions outside of the striatum. For example, BDNF in the amygdala concurrently represses both anxiety-like behavior and alcohol intake (Pandey et al., 2006), suggesting a function for amygdala BDNF in regulating anxiety-modulated alcohol consumption. Indeed, knockdown of BDNF expression via antisense oligonucleotide infusion in the medial and central nuclei of the amygdala, the same regions with decreased BDNF levels in alcohol-preferring rats (Prakash et al., 2008), significantly increased both anxiety-like behavior and alcohol intake, which could be rescued by BDNF infusion (Pandey et al., 2006). In the amygdala, BDNF likely reduces anxiety-like behavior and alcohol intake via increasing dendritic spine density (Moonat et al., 2011), a function which remains to be determined for the DLS. Nonetheless, both DLS and amygdala BDNF serve to reduce alcohol intake via downstream effectors that participate in the modulation of alcohol self-administration. Whereas BDNF in the DLS performs a beneficial function to reduce drinking, excessive alcohol reduces BDNF levels in the PFC, the main source of BDNF in the striatum (Kokaia et al., 1993), possibly via a miRNA-mediated mechanism. Specifically, overexpression of miR-206 in the mPFC of rats (Tapocik et al., 2014) or miR30a-5p in the mPFC of mice (Darcq et al., 2014) produced a robust increase in alcohol drinking as compared with animals in which the mPFC was infected with a scrambled virus. Conversely, intra-mPFC infusion of an inhibitor of miR30a-5p function led to a significant reduction in excessive alcohol drinking and preference (Darcq et al., 2014).
Downstream effectors mediating BDNF effects
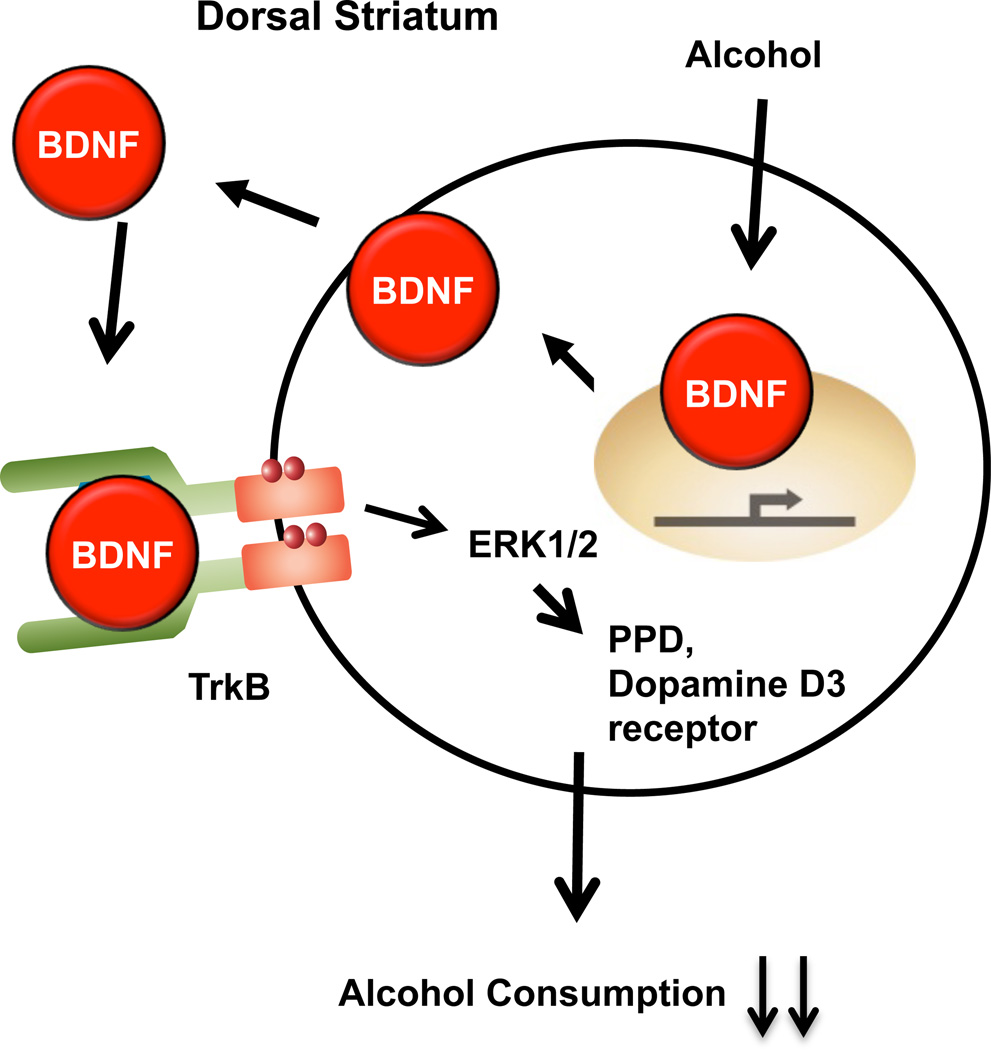
As described above, BDNF exerts its function in neurons via binding to the TrkB receptor, resulting in autophosphorylation and subsequent activation of the MAPK, PLC and PI3K signaling pathways, thereby recruiting transcriptional and translational mechanisms to generate downstream effector proteins (Huang and Reichardt, 2003). Reduction of alcohol self-administration by BDNF did not occur immediately after infusion, but rather subsequent to a 3-h delay (Jeanblanc et al., 2009), indicative of a mechanism requiring downstream signaling and transcription/translation. Indeed, blockade of protein synthesis by cyclohexamide prevented the reduction of alcohol self-administration normally observed after BDNF infusion into the DLS (Jeanblanc et al., 2013), providing further support for the requirement of downstream signaling events in the BDNF regulation of alcohol intake. Furthermore, acute exposure of striatal neurons to alcohol led to the activation of the BDNF receptor TrkB and alcohol-induced BDNF, which in turn caused the downstream activation of the MAPK extracellular signal-regulated kinase 1/2 (ERK1/2) (Logrip et al., 2008). In line with these findings, pretreatment of rats in the DLS with an inhibitor of MAPK Kinase (MEK), the enzyme that activates ERK1/2, prevented BDNF-mediated decreases in alcohol self-administration (Jeanblanc et al., 2013). In contrast, inhibiting PLCγor PI3K signaling did not alter alcohol drinking (Jeanblanc et al., 2013), suggesting that activation of the TrkB/ERK1/2 signaling pathway is necessary for the beneficial actions of BDNF on drinking. Activated ERK1/2 translocates to the nucleus, where it can interact with transcription factors to modulate gene expression (Roskoski, 2012). Among the genes that are known to be induced by the activation of the BDNF pathway in the striatum are those for the neuropeptides dynorphin, enkephalin, neuropeptide Y, substance P and cholecystokinin (Croll et al., 1994), as well as the dopamine D3 receptor (Guillin et al., 2001), and BDNF haploinsufficient mice display constitutively reduced striatal dynorphin, enkephalin and D3 receptor expression (Saylor and McGinty, 2010). We found that dynorphin (Logrip et al., 2008) and the dopamine D3 receptor (Jeanblanc et al., 2006) act as downstream effectors of BDNF in the regulation of alcohol intake. Specifically, preprodynorphin (Logrip et al., 2008) and dopamine D3 receptor (Jeanblanc et al., 2006) mRNA expression are increased following BDNF or Tat-RACK1 treatment, and inhibition of either the dopamine D3 receptor (Jeanblanc et al., 2006) or dynorphin’s receptor, the kappa opioid receptor (Logrip et al., 2008), reduced the ability of Tat-RACK1 to decrease alcohol consumption. In fact, genetic deletion of the dopamine D3 receptor in mice significantly reduced voluntary alcohol consumption relative to wildtype mice, despite similar elevations in RACK1 and BDNF mRNA expression after forced alcohol intake (Leggio et al., 2014). These data support a prominent role for BDNF, via downstream activation of the MAPK pathway and generation of effector proteins like dynorphin and the dopamine D3 receptor, in the maintenance of moderate levels of alcohol intake. Together these data demonstrate that DLS BDNF gates alcohol intake via TrkB-mediated activation of MAPK signaling, resulting in the upregulation of the downstream effectors preprodynorphin and the dopamine D3 receptor that function to dampen alcohol consumption (Figure 3).
Figure 3. A model depicting the molecular mechanisms underlying BDNF’s actions in the DLS.
(a) Moderate levels of alcohol produce an increase in BDNF mRNA, which is then translated to the polypeptide. Secreted BDNF activates its receptor TrkB, resulting in receptor autophosphorylation and the activation of ERK1/2. ERK1/2 translocates to the nucleus where it participates in the transcription of preprodynorphin and the dopamine D3 receptor mRNA resulting in their translation and processing. Activation of the dopamine D3 receptor and the kappa opioid receptor in turn control the level of alcohol drinking. (b) After alcohol intake has escalated, as observed in limited access paradigms, the corticostriatal BDNF supply is reduced due to elevated expression of miRs in the medial prefrontal cortex. While alcohol may still increase BDNF expression, miRs target BDNF transcripts for degradation, resulting in decreased BDNF mRNA expression in the medial prefrontal cortex and thus less release of BDNF by cortical inputs in the dorsal striatum. This breakdown of corticostriatal BDNF yields elevated levels of alcohol intake.
Summary
Together, the studies presented in this review suggest that the corticostriatal BDNF pathway plays a crucial role in the regulation of alcohol consumption, keeping it in moderation via activation of the BDNF signaling pathway within the DLS and driving the transition from moderate to high intake when BDNF levels in the mPFC and plausibly other cortical regions are reduced. Yet many open questions remain to be explored. For example, as the DLS is a major contributor to habitual behavior (Everitt et al., 2008), does BDNF in the DLS gate the level of intake by preventing habit learning and/or compulsive behaviors? Conversely, hypofunction of the mPFC contributes to the transition from social to excessive drinking (George et al., 2012) as well as to craving and relapse (Seo et al., 2013). Thus, it would be of great interest to examine the specific role of BDNF in the mPFC and other cortical regions in generating these alcohol-related behaviors. It would also be of interest to determine the mechanism by which the expression of BDNF is altered by moderate levels of alcohol in the DLS but not in other striatal regions and is reduced by excessive alcohol intake in the mPFC but not in the hippocampus (Darcq et al., 2014). Furthermore, the corticostriatal BDNF pathway is specific for alcohol and does not generalize to other consummatory behaviors, as saccharin and sucrose consumption are unaltered (Darcq et al., 2014; Jeanblanc et al., 2009) (Figure 1). Thus the mechanisms underlying the selective interactions between BDNF and alcohol should be further explored. Finally, it is highly likely that additional downstream effector genes in the DLS mediate the beneficial actions of the BDNF/ERK1/2 pathway, and these should be explored as they may lead to the identification of new leads for drug development for the treatment of alcohol use disorders.
HIGHLIGHTS.
Corticostriatal BDNF keeps alcohol drinking in check
DLS BDNF reduces alcohol intake through MAPK induction of downstream effectors
Excessive alcohol consumption blunts corticostriatal BDNF
Cortical BDNF expression is blunted with high alcohol consumption via miRNAs
Acknowledgements
Research described in, and writing of, the review were supported by NIAAA R01 AA016848 (DR), NIAAA P50 AA017072 (DR), NIAAA R01 AA016848 (DR), RO1 AA014366 (DR). ISF 968-13 (S.B.), ISF 1916-13 (S.B.), Brain and Behavior Research Foundation NARSAD 19114 (S.B.), and NIAAA K99 AA021802 (M.L.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadiantehrani S, et al. Neurobiology of Alcohol Dependence. Book Chapter; 2014. From Signaling Pathways to Behavior: The Light and Dark Sides of Alcohol. [Google Scholar]
- Andero R, Choi DC, Ressler KJ. BDNF-TrkB Receptor Regulation of Distributed Adult Neural Plasticity, Memory Formation, and Psychiatric Disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38:2328–2337. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, et al. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, et al. Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol Neurobiol. 2004;29:289–302. doi: 10.1385/MN:29:3:289. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophins and psychiatric disorders. Handb Exp Pharmacol. 2014;220:461–479. doi: 10.1007/978-3-642-45106-5_17. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Croll SD, et al. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Darcq E, et al. MicroRNA-30a-5p in the Prefrontal Cortex Controls the Transition from Moderate to Excessive Alcohol Consumption. Molecular Psychiatry. 2014 doi: 10.1038/mp.2014.155. In Press. [DOI] [PubMed] [Google Scholar]
- Davies AM, Thoenen H, Barde YA. Different factors from the central nervous system and periphery regulate the survival of sensory neurones. Nature. 1986;319:497–499. doi: 10.1038/319497a0. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci U S A. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Systems level neuroplasticity in drug addiction. Cold Spring Harb Perspect Med. 2013;3:a011916. doi: 10.1101/cshperspect.a011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, et al. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, et al. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285:19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, et al. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem. 2003;85:1139–1147. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Hofer M, et al. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, et al. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, et al. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, et al. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2013;37:607–612. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivinummi T, et al. Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. Neurosci Lett. 2011;491:108–112. doi: 10.1016/j.neulet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Klein R, et al. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, et al. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci U S A. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, et al. Dopamine D Receptor is Necessary for Ethanol Consumption: An Approach with Buspirone. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, et al. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109:1459–1468. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Mortality and morbidity attributable to use of addictive substances in the United States. Proc Assoc Am Physicians. 1999;111:109–118. doi: 10.1046/j.1525-1381.1999.09256.x. [DOI] [PubMed] [Google Scholar]
- McGough NN, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Moonat S, et al. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, et al. Direct interaction between scaffolding proteins RACK1 and 14-3-3zeta regulates brain-derived neurotrophic factor (BDNF) transcription. J Biol Chem. 2012;287:322–336. doi: 10.1074/jbc.M111.272195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–443. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, et al. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, et al. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135–143. [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF. An intrastriatal brain-derived neurotrophic factor infusion restores striatal gene expression in Bdnf heterozygous mice. Brain Struct Funct. 2010;215:97–104. doi: 10.1007/s00429-010-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- Seo D, et al. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Zelek-Molik A, McGinty JF. Short and long access to cocaine self-administration activates tyrosine phosphatase STEP and attenuates GluN expression but differentially regulates GluA expression in the prefrontal cortex. Psychopharmacology (Berl) 2013;229:603–613. doi: 10.1007/s00213-013-3118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, et al. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, et al. Chronic ethanol consumption reduces the neurotrophic activity in rat hippocampus. Neurosci Lett. 1992;147:77–80. doi: 10.1016/0304-3940(92)90778-6. [DOI] [PubMed] [Google Scholar]
- Wan L, et al. RACK1 affects morphine reward via BDNF. Brain Res. 2011;1416:26–34. doi: 10.1016/j.brainres.2011.07.045. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Jr, et al. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health. 2014 ed.^eds.
- Yaka R, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yan QS, Feng MJ, Yan SE. Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and -nonpreferring (NP) rats. Brain Res. 2005;1035:215–218. doi: 10.1016/j.brainres.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]