Abstract
Study Design Retrospective case series.
Objective Diffuse idiopathic skeletal hyperostosis (DISH) or Forestier disease involves hyperostosis of the spinal column. Hyperostosis involving the anterior margin of the cervical vertebrae can cause dysphonia, dyspnea, and/or dysphagia. However, the natural history pertaining to the risk factors remain unknown. We present the surgical management of two cases of dysphagia secondary to cervical hyperostosis and discuss the etiology and management of DISH based on the literature review.
Methods This is a retrospective review of two patients with DISH and anterior cervical osteophytes. We reviewed the preoperative and postoperative images and clinical history.
Results Two patients underwent anterior cervical osteophytectomies due to severe dysphagia. At more than a year follow-up, both patients noted improvement in swallowing as well as their associated pain.
Conclusion The surgical removal of cervical osteophytes can be highly successful in treating dysphagia if refractory to prolonged conservative therapy.
Keywords: diffuse idiopathic skeletal hyperostosis (DISH), osteophytes, dysphagia, surgical management
Introduction
Diffuse idiopathic skeletal hyperostosis (DISH) or Forestier disease involves hyperostosis of the spine and may present in a variety of ways. The prevalence of DISH is quite variable and ranges between 2.9 and 28%.1 2 Some authors do not consider the condition a disease, but rather an incidental radiographic finding.3 4 The literature does not set a standard for diagnosing or treating DISH.
Cervical spinal osteophytes are estimated to affect 10 to 30% of the general population; however, the bony outgrowths tend to be largely asymptomatic.5 When hyperostosis involves the anterior margin of the cervical vertebrae, the osteophytes can cause dysphonia, dyspnea, and/or dysphagia. We report two cases of surgical treatment for dysphagia secondary to cervical hyperostosis and discuss the pertinent literature.
Case Reports
Case One
A 61-year-old man was referred for the evaluation of multilevel cervical spondylosis as well as a large anterior osteophyte impinging on the esophagus. His past medical history included appendicitis, gastroesophageal reflux disease, gout, and hyperlipidemia. The patient reported a 2.5-year history of dysphagia involving episodes of “choking” and aspiration that progressively increased in frequency. A speech pathologist initially diagnosed the patient with swallowing abnormalities. Concomitantly, the patient reported severe neck and shoulder pain, which prompted further workup. Noncontrast cervical computed tomography (CT) showed a large osteophyte formation, spanning from C3 to C6 anteriorly with the largest osteophyte at C4 impinging on the esophagus as well as C3–C4 mild anterior cord compression (Fig. 1). Because his neurologic examination was normal, only an anterior cervical osteophytectomy was performed. Bone wax was applied to the anterior walls of the vertebral bodies to reduce regrowth of the osteophytes. Three months postoperatively, there was no improvement in swallowing; however, at 6 months, his dysphagia was markedly improved with minor difficulty swallowing solid food. At 36 months, he reported complete resolution of his dysphagia, and the imaging revealed no evidence of osteophyte regrowth (Fig. 2).
Fig. 1.
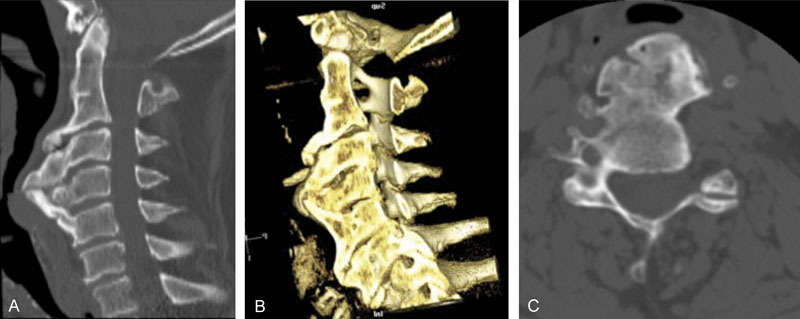
Case one. Sagittal (A) and three-dimensional sagittal reconstruction (B) computed tomography of the cervical spine obtained at the initial visit in a patient presenting with severe dysphagia with axial image at C4–C5 indicating severe hyperostosis (C).
Fig. 2.
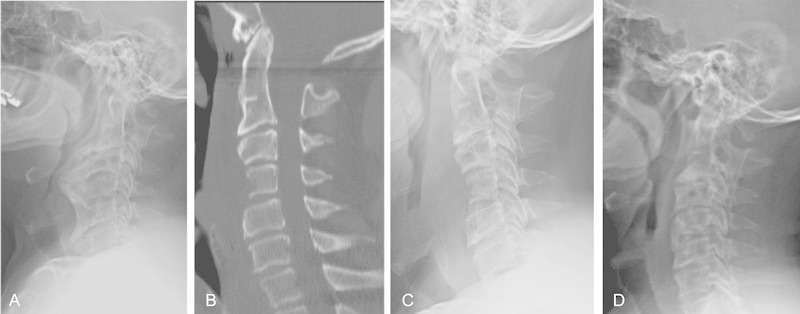
Case one. Lateral radiograph (A) obtained at the initial visit in a patient with severe hyperostosis. (B) Immediate postoperative sagittal computed tomography and (C) lateral radiograph. (D) Three-year postoperative lateral radiograph shows no significant regrowth of osteophytes.
Case Two
A 70-year-old man was referred for a surgical evaluation for progressive dysphagia, neck pain, right proximal and distal upper extremity weakness (4 + /5), and urinary retention. Past medical history included Parkinson disease, coronary artery disease, gastroesophageal reflux disease, and depression. He had chronic left vocal cord paralysis for 20 years, dysphagia associated with weight loss, and dysphonia. Strobovideolaryngoscopy and dynamic fluoroscopic swallow studies revealed cervical osteophytes causing mechanical dysphagia; from a neurologic standpoint, his swallowing was intact. The cervical radiographs and CT scans displayed anterior cervical osteophyte growths from C2 to C7 with the largest portions between C4 and C6 (Fig. 3). The magnetic resonance imaging showed multilevel cervical spinal stenosis with spinal cord and nerve root compression. He had a percutaneous endoscopic gastrostomy tube placed due to his severe dysphagia. He had anterior C3–C7 osteophytectomy, diskectomy, and instrumented arthrodesis. A thin layer of bone wax was applied to the anterior wall of the vertebral bodies from C3 to C7. A posterior decompressive laminectomy and C3–T3 instrumented arthrodesis was performed without aggressive measures to correct the sagittal balance due to the patient's comorbidities and underlying osteoporosis. Immediate postoperative X-rays showed no significant residual osteophyte, and 1-year postoperative X-rays showed no evidence for additional osteophyte formation (Fig. 4). Although this patient's dysphagia may have been secondary to Parkinson disease and extensive pharyngeal weakness, 8 months after surgery, the patient reported no difficulty swallowing; however, dysphonia was unchanged. At 13 months, the patient had improved arm strength with residual neck pain and bilateral hand numbness. Although there was no evidence of instability on flexion/extension films at 13 months, the persistent neck pain may have been due to positive sagittal balance seen on postoperative imaging (Fig. 4B).
Fig. 3.
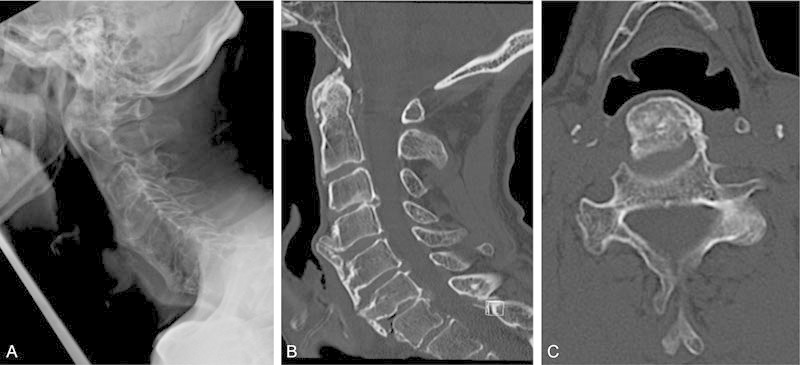
Case two. Lateral radiograph (A) and midsagittal computed tomography (CT; B) obtained at the initial visit in a patient with severe osteophyte formation extending from C4 to C7, most significant at C4 as illustrated on axial CT image (C).
Fig. 4.
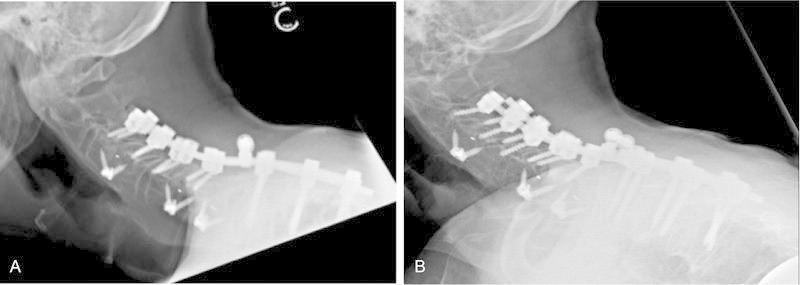
Case two. Lateral radiographs obtained immediately postoperative (A) with anterior C3–C4, C5–C6, and C6–C7 diskectomy and posterior decompressive laminectomy, C3 to T3 instrumented arthrodesis at 1-year follow-up visit (B) without regrowth of osteophytes.
Discussion
Symptomatic degenerative changes of the cervical spine affect ∼75% of the population above 60 years of age.6 The reported prevalence of DISH varies, ranging from 3 to 30%.5 7 8 9 The radiographic diagnosis for DISH proposed by Resnick and Niwayama10 (also known as the Resnick criteria) is comprised of the following three criteria: (1) the presentation of flowing calcification and ossification of the anterolateral aspect of four contiguous vertebral bodies, (2) preservation of intervertebral disk height in the involved segments without radiographic changes of degenerative disk disease, and (3) absence of intervertebral or apophyseal joint ankylosis and degeneration, sclerosis, or intra-articular osseous fusion. Despite the Resnick criteria, the precise diagnosis of DISH continues to be debated.11 One obvious problem with the Resnick criteria is that most of the older patients have significant degenerative changes involving the disks.
Some authors refer to the dysphagia secondary to DISH as DISHphagia,12 13 which typically begins with difficulty swallowing solids and progressing to liquids.5 This condition has the potential to be detrimental to one's health and quality of life, especially in cases of undesired weight loss, malnutrition, and/or aspiration.5 There are several theories to explain dysphagia: (1) physical obstruction of the esophageal lumen; (2) growth where the esophagus is anatomically anchored; (3) stenosis of the esophageal lumen from inflammation or muscular spasms due to friction between the bone spurs and soft tissue; (4) restricted motion of the epiglottis and larynx; (5) narrowing of the pharynx wall resulting in food retention.14 Dysphagia from hyperostosis is most commonly associated with anterior osteophyte formation of C3–C5, likely due to the fact that the normal epiglottic tilt lies over the laryngeal inlet at this level.9 13 15 However, cases of dysphagia have been reported to occur due to protrusion in the thoracic regions as well.16 17 18
Because most cases of Forestier disease remain asymptomatic, some authors consider the skeletal abnormalities as radiographic findings rather than a disease.19 20 Thus, it is difficult to confirm the actual prevalence of DISHphagia. Despite the variable prevalence, there are increasing numbers of case reports in the literature demonstrating dysphagia secondary to DISH.6 9 13 21
Etiology
The first systematic review of the literature for DISHphagia by Verlaan et al analyzed 204 cases.9 Two important trends were elucidated: (1) the major affected vertebrae were C3, C4, and C5; (2) the male-to-female ratio was 2:1. Of note, patients treated for dysphagia have been primarily male (Table 1). The natural history pertaining to the risk factors remains unknown.
Table 1. Reported literature on surgical resection of osteophytes for dysphagia.
| Author and year | Cases (n) | Age/gender | Surgical resection level |
|---|---|---|---|
| Miyamoto et al 200921 | 7 | 55/M | C4–C5, C5–C6 |
| 57/F | C3–C4, C4–C5, C5–C6, C6–C7 | ||
| 63/M | C4–C5, C5–C6, C6–C7 | ||
| 64/M | C4–C5, C5–C6, C7–T1 | ||
| 66/M | C4–C5 | ||
| 70/M | C5–C6 | ||
| 78/M | C2–C3, C3–C4, C4–C5, C5–C6, C6–C7 | ||
| Goh et al 201037 | 1 | 55/M | C4–C5, C5–C6 |
| Lecerf and Malard 20105 | 2 | 79/M | C3–C4, C4–C5 |
| 80/M | C3–C4 | ||
| Hwang et al 201332 | 1 | 56/M | C4–C5 |
| Urrutia et al 201336 | 1 | 45/M | C3–C4, C4–C5, C5–C6 |
| von der Hoeh et al 201435 | 6 | 67 ± 5/M | C3–C4 C3–C4 C3–C4, C4–C5, C5–C6 C3–C4 C5–C6 C4–C5, C5–C6 |
A wide range of factors for DISHphagia has been proposed, but definitive associations have yet to be made. These include trauma, vitamin A exposure, and metabolic and endocrine factors.4 In the recent literature, the strongest correlation with DISH is sex and age.9 It is interesting to note that there is no conclusive correlation between the osteophyte size and symptoms; however, older age has been correlated with more severe symptoms.6 Because life expectancy is increasing, the prevalence of DISHphagia may also increase in the coming decades.9 22 23 24
It has been suggested that type 2 diabetes mellitus, hyperuricemia, and dyslipidemia are all risk factors for DISH.25 Obesity and type 2 diabetes mellitus have been hypothesized to increase several growth factors and inflammatory mediators, which lead to proliferation of osteoblasts and eventually bone deposition.9 In a study by Littlejohn and Hall, males over the age of 45 with gout were more likely to have DISH when compared with a control group (43% versus 8%).26 One potential explanation is that hyperinsulinemia has been demonstrated in patients with DISH and gout. Insulin is thought to be a potential anabolic agent in bone.27 As a result, endocrine imbalance and subsequent osteoblast proliferation may be altered.
Osteoblast proliferation is maintained by several growth factors and not limited to those in bone. Growth hormone induces local production of insulin-like growth factor-1, which in turn stimulates alkaline phosphatase and type-2 collagen activity in osteoblasts. Denko and Malemud found that DISH patients tend to have higher insulin and growth hormone levels.25 The osteoblast growth may be due to the actions and elevation of these hormones.28
Hypervitaminosis A has also been commonly suspected as an underlying risk factor for hyperostosis. Wendling et al reported three cases of DISHphagia in which high levels of vitamin A were the only observable abnormalities.29 Our patients did not have hypervitaminosis A. Nonetheless, retinoids and other exogenous supplements of vitamin A are well known to cause bone anomalies, including skeletal hyperostosis.30
Although DISH is not believed to be a genetic disease, a recent study suggested that alterations in gene expression might play a role in this type of hyperostosis. The Dickkopf-1 (DDK) gene is involved with the Wnt pathway that regulates bone formation and regeneration. Recently, low levels of DKK-1 have been linked to cases of severe ossification.31 This link is substantiated by our current understanding that DKK-1 plays an inhibitory role on osteoblast proliferation and osteoclast suppression.11 In cases of low DKK-1 status, unobstructed osteoblastogenesis may contribute to skeletal hyperostosis.
Diagnosis
Initial evaluation of dysphagia involves imaging studies (X-ray, CT, and/or magnetic resonance imaging) to study anatomic abnormalities. Although the larynx is noted to be C3–C4, dysphagia from cervical osteophytes has ranged from C3 to C6 (Table 1). Although clinical symptoms have been to some extent dependent on the size of the osteophytes, there does not appear to be a direct correlation between the size and the severity of dysphagia.6 Further evaluation by specialists in neurology, otolaryngology, and/or speech therapy is helpful to rule out other pathologies. The literature does not set a standard for diagnosis. Therefore, more invasive diagnostic tests such as barium swallow, nasal endoscopy, esophagram, and videofluoroscopic studies are also useful for visualizing potential mechanical obstruction.7 14 Functional endoscopic evaluation of swallowing should be performed to rule out other potential causes.32 33 If the radiographic images confirm severe anterior cervical osteophyte formation and there does not appear to be any other explanation for the dysphagia, the patient may be a candidate for conservative treatment or surgical resection.10
Treatment
Initially, the dysphagia may be treated nonsurgically with dietary restrictions, speech and swallow therapy, anti-inflammatory medication, steroids, muscle relaxants, and antireflux medication.14 34 These methods are most effective in patients with osteophyte-induced inflammation, pain, or muscle spasms.14 Although conservative methods may offer temporary relief in patients affected by dysphagia, long-term treatment often is difficult due to its poorly understood etiology. Severe cases of dysphagia may lead to unintentional weight loss, dyspnea, dysphonia, and/or aspiration.5
There has yet to be a study that compares the efficacy of conservative and surgical treatments9; however, the literature suggests that osteophyte resection is considered to be highly successful when conservative methods fail.14 21 35 36 37 A significant improvement in symptoms was more frequently seen with surgical treatment than with conservative treatment.9 It should be noted that in ∼10% of surgical cases, serious complications such as vocal cord paralysis, stroke, hematoma, Horner syndrome, infection, and pharyngocutaneous fistula can occur.34 Interestingly, postsurgical recurrence of osteophytes in DISH has also been reported. Miyamoto et al showed that 10 to 11 years after the initial surgery for anterior cervical osteophytes, the osteophytes recurred and dysphagia developed in two of the seven patients.21 Nevertheless, surgical intervention should be considered if a patient experiences clinical and/or radiographic progression despite conservative measures.
In both cases, osteophytectomy was completed after they had failed nonoperative measures. In both cases, the patient had delayed resolution of dysphagia without significant regrowth of the osteophytes at the latest follow-up. Application of a thin layer of bone wax over the osteophytectomy sites may impede osteophyte formation.
Conclusion
DISH is a generally asymptomatic phenomenon; however, in severe cases of dysphagia, surgical osteophyte resection may be appropriate. Although multiple risk factors have been addressed in the literature, the sample size for many reports is too small to be conclusive. Further natural history and prospective surgical and conservative treatment studies are necessary to determine the etiology as well as efficacy and longevity of treatment.
Footnotes
Disclosures Alexander C. Egerter, none Eric S. Kim, none Darrin J. Lee, none Jonathan J. Liu, none Gilbert Cadena, none Ripul R. Panchal, none Kee D. Kim, Royalties: Precision Spine, LDR, Globus; Stock/stock options: Molecular Matrix, Inc.
References
- 1.Weinfeld R M, Olson P N, Maki D D, Griffiths H J. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 1997;26(4):222–225. doi: 10.1007/s002560050225. [DOI] [PubMed] [Google Scholar]
- 2.Kim S K, Choi B R, Kim C G. et al. The prevalence of diffuse idiopathic skeletal hyperostosis in Korea. J Rheumatol. 2004;31(10):2032–2035. [PubMed] [Google Scholar]
- 3.Hutton C. DISH . . . a state not a disease? Br J Rheumatol. 1989;28(4):277–278. doi: 10.1093/rheumatology/28.4.277. [DOI] [PubMed] [Google Scholar]
- 4.Mata S, Fortin P R, Fitzcharles M A. et al. A controlled study of diffuse idiopathic skeletal hyperostosis. Clinical features and functional status. Medicine (Baltimore) 1997;76(2):104–117. doi: 10.1097/00005792-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lecerf P, Malard O. How to diagnose and treat symptomatic anterior cervical osteophytes? Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(3):111–116. doi: 10.1016/j.anorl.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Seidler T O, Pèrez Alvarez J C, Wonneberger K, Hacki T. Dysphagia caused by ventral osteophytes of the cervical spine: clinical and radiographic findings. Eur Arch Otorhinolaryngol. 2009;266(2):285–291. doi: 10.1007/s00405-008-0735-4. [DOI] [PubMed] [Google Scholar]
- 7.Carlson M L, Archibald D J, Graner D E, Kasperbauer J L. Surgical management of dysphagia and airway obstruction in patients with prominent ventral cervical osteophytes. Dysphagia. 2011;26(1):34–40. doi: 10.1007/s00455-009-9264-6. [DOI] [PubMed] [Google Scholar]
- 8.Epstein N E. Simultaneous cervical diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament resulting in dysphagia or myelopathy in two geriatric North Americans. Surg Neurol. 2000;53(5):427–431, discussion 431. doi: 10.1016/s0090-3019(00)00227-5. [DOI] [PubMed] [Google Scholar]
- 9.Verlaan J J, Boswijk P F, de Ru J A, Dhert W J, Oner F C. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11(11):1058–1067. doi: 10.1016/j.spinee.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119(3):559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 11.Mader R, Buskila D, Verlaan J J. et al. Developing new classification criteria for diffuse idiopathic skeletal hyperostosis: back to square one. Rheumatology (Oxford) 2013;52(2):326–330. doi: 10.1093/rheumatology/kes257. [DOI] [PubMed] [Google Scholar]
- 12.De Jesus-Monge W E, Cruz-Cuevas E I. Dysphagia and lung aspiration secondary to anterior cervical osteophytes: a case report and review of the literature. Ethn Dis. 2008;18(2) 02:S2–S137, 40. [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis J R, Lander P H, Moreland L W. Swallowing difficulties from “DISH-phagia.”. J Rheumatol. 2004;31(12):2526–2527. [PubMed] [Google Scholar]
- 14.Oppenlander M E, Orringer D A, La Marca F. et al. Dysphagia due to anterior cervical hyperosteophytosis. Surg Neurol. 2009;72(3):266–270, discussion 270–271. doi: 10.1016/j.surneu.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 15.Granville L J, Musson N, Altman R, Silverman M. Anterior cervical osteophytes as a cause of pharyngeal stage dysphagia. J Am Geriatr Soc. 1998;46(8):1003–1007. doi: 10.1111/j.1532-5415.1998.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 16.Rana S S Bhasin D K Rao C Gupta R Nagi B Singh K Thoracic spine osteophyte causing dysphagia Endoscopy 201244(Suppl 2 UCTN):E19–E20. [DOI] [PubMed] [Google Scholar]
- 17.Underberg-Davis S, Levine M S. Giant thoracic osteophyte causing esophageal food impaction. AJR Am J Roentgenol. 1991;157(2):319–320. doi: 10.2214/ajr.157.2.1853815. [DOI] [PubMed] [Google Scholar]
- 18.Willing S, El Gammal T. Thoracic osteophyte producing dysphagia in a case of diffuse idiopathic skeletal hypertrophy. Am J Gastroenterol. 1983;78(6):381–383. [PubMed] [Google Scholar]
- 19.Beyeler C, Schlapbach P, Gerber N J. et al. Diffuse idiopathic skeletal hyperostosis (DISH) of the elbow: a cause of elbow pain? A controlled study. Br J Rheumatol. 1992;31(5):319–323. doi: 10.1093/rheumatology/31.5.319. [DOI] [PubMed] [Google Scholar]
- 20.Fahrer H, Barandum R, Gerber N J, Friederich N F, Burkhardt B, Weisman M H. Pelvic manifestations of diffuse idiopathic skeletal hyperostosis (DISH): are they clinically relevant? Rheumatol Int. 1989;8(6):257–261. doi: 10.1007/BF00270981. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto K, Sugiyama S, Hosoe H, Iinuma N, Suzuki Y, Shimizu K. Postsurgical recurrence of osteophytes causing dysphagia in patients with diffuse idiopathic skeletal hyperostosis. Eur Spine J. 2009;18(11):1652–1658. doi: 10.1007/s00586-009-1133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mader R, Lavi I. Diabetes mellitus and hypertension as risk factors for early diffuse idiopathic skeletal hyperostosis (DISH) Osteoarthritis Cartilage. 2009;17(6):825–828. doi: 10.1016/j.joca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Mader R, Novofestovski I, Adawi M, Lavi I. Metabolic syndrome and cardiovascular risk in patients with diffuse idiopathic skeletal hyperostosis. Semin Arthritis Rheum. 2009;38(5):361–365. doi: 10.1016/j.semarthrit.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Bray G A, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29(1):109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 25.Denko C W, Malemud C J. Body mass index and blood glucose: correlations with serum insulin, growth hormone, and insulin-like growth factor-1 levels in patients with diffuse idiopathic skeletal hyperostosis (DISH) Rheumatol Int. 2006;26(4):292–297. doi: 10.1007/s00296-005-0588-8. [DOI] [PubMed] [Google Scholar]
- 26.Littlejohn G O, Hall S. Diffuse idiopathic skeletal hyperostosis and new bone formation in male gouty subjects. A radiologic study. Rheumatol Int. 1982;2(2):83–86. doi: 10.1007/BF00541250. [DOI] [PubMed] [Google Scholar]
- 27.Thrailkill K M, Lumpkin C K Jr, Bunn R C, Kemp S F, Fowlkes J L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735–E745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarzi-Puttini P, Atzeni F. New developments in our understanding of DISH (diffuse idiopathic skeletal hyperostosis) Curr Opin Rheumatol. 2004;16(3):287–292. doi: 10.1097/00002281-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Wendling D, Hafsaoui C, Laurain J M, Runge M, Magy-Bertrand N, Prati C. Dysphagia and hypervitaminosis A: cervical hyperostosis. Joint Bone Spine. 2009;76(4):409–411. doi: 10.1016/j.jbspin.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Nesher G, Zuckner J. Rheumatologic complications of vitamin A and retinoids. Semin Arthritis Rheum. 1995;24(4):291–296. doi: 10.1016/s0049-0172(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 31.Senolt L, Hulejova H, Krystufkova O. et al. Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis. 2012;71(1):71–74. doi: 10.1136/annrheumdis-2011-200357. [DOI] [PubMed] [Google Scholar]
- 32.Hwang J S, Chough C K, Joo W I. Giant anterior cervical osteophyte leading to Dysphagia. Korean J Spine. 2013;10(3):200–202. doi: 10.14245/kjs.2013.10.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozgocmen S, Kiris A, Kocakoc E, Ardicoglu O. Osteophyte-induced dysphagia: report of three cases. Joint Bone Spine. 2002;69(2):226–229. doi: 10.1016/s1297-319x(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 34.McGarrah P D, Teller D. Posttraumatic cervical osteophytosis causing progressive dysphagia. South Med J. 1997;90(8):858–860. doi: 10.1097/00007611-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 35.von der Hoeh N H, Voelker A, Jarvers J S, Gulow J, Heyde C E. Results after the surgical treatment of anterior cervical hyperostosis causing dysphagia. Eur Spine J. 2014 doi: 10.1007/s00586-014-3507-4. [DOI] [PubMed] [Google Scholar]
- 36.Urrutia J, Bernardín A, Morales C, Millán R. Diffuse idiopathic skeletal hyperostosis causing dysphagia in a young patient. Rev Med Chil. 2013;141(6):803–806. doi: 10.4067/S0034-98872013000600017. [DOI] [PubMed] [Google Scholar]
- 37.Goh P Y, Dobson M, Iseli T, Maartens N F. Forestier's disease presenting with dysphagia and dysphonia. J Clin Neurosci. 2010;17(10):1336–1338. doi: 10.1016/j.jocn.2010.04.002. [DOI] [PubMed] [Google Scholar]


