Abstract
Plasmodium parasites belong to the Apicomplexan phylum, which consists mostly of obligate intracellular pathogens that vary dramatically in host cell tropism. Plasmodium sporozoites are highly hepatophilic. The specific molecular mechanisms, which facilitate sporozoite selection and successful infection of hepatocytes, remain poorly defined. Here, we discuss the parasite and host factors which are critical to hepatocyte infection. We derive a model where sporozoites initially select host cells that constitute a permissive environment and then further refine the chosen hepatocyte during liver stage development, ensuring life cycle progression. While many unknowns of pre-erythrocytic infection remain, advancing models and technologies that enable analysis of human malaria parasites and of single infected cells will catalyze a comprehensive understanding of the interaction between the malaria parasite and its hepatocyte host.
Introduction
Malaria-causing Plasmodium parasites are obligate intracellular pathogens within their mammalian host. Their first obligatory site of infection and replication occurs in hepatocytes, where the number of infected cells is low and the infection asymptomatic [1]. The second site of replication is the bloodstream, where parasites infect and multiply within red blood cells, ultimately destroying billions of them. It is blood stage infection that causes malaria and leads to disease and death. The mammalian host becomes initially infected when the bite of infected Anopheles mosquitoes deposits sporozoites into the skin. The highly motile sporozoites then move between and traverse through cells of the skin until they find a capillary, which they penetrate to access the blood circulation, thereby facilitating their transport to the liver. Once they reach the blood capillaries in the liver (called sinusoids), parasites traverse through liver sinusoidal endothelial cells (LSECs) [2] or Kupffer Cells (liver-resident macrophages) [3] to exit the blood stream, enter the parenchyma and infect hepatocytes. Sporozoites display an impressive protein armamentarium positioned on the surface and in specialized secretory organelles ([4], Figure 1), which they employ to travel to and invade hepatocytes and simultaneously evade host defenses. This includes active motility, the capacity to shed antibodies which impede their travel from the skin to the liver [5] and the ability to traverse cells by means of membrane wounding [6]. Once in the liver, each sporozoite invades a single hepatocyte, ensconcing itself within a protective parasitophorous vacuole (PV) for further life cycle progression as a liver stage (LS). Within the sheltered PV environment, the parasite establishes conduits to control and exploit the host hepatocyte and to protect it from untimely demise.
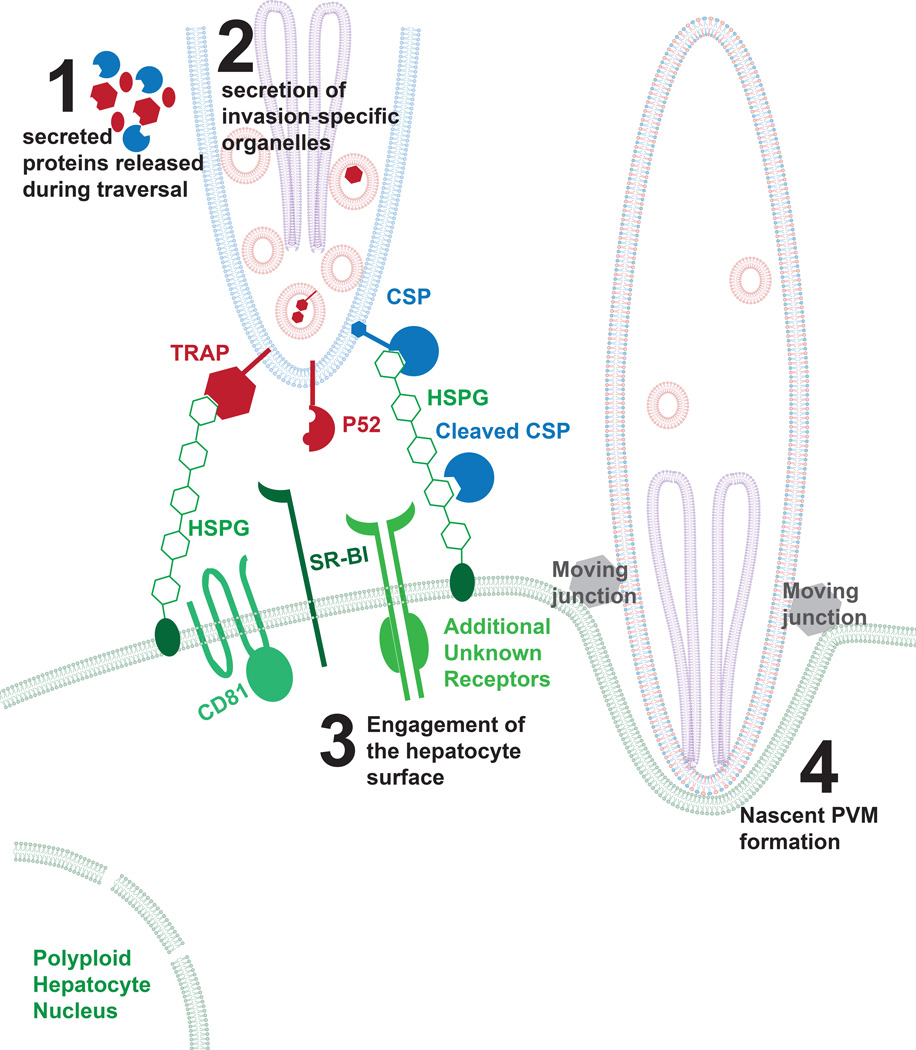
Figure 1. Model of initial attachment and invasion of the Plasmodium sporozoite.
After transmission, sporozoites glide and traverse through the skin into the blood stream, which involves the secretion of micronemal proteins. After sporozoites traverse the sinusoids, they directly engage a hepatocyte for invasion. This binding likely involves several factors including the interaction between CSP and highly sulfated proteoglycans. For most species of Plasmodium, this requires the expression of CD81 and SR-BI, although there has been no evidence of direct interaction between parasite proteins and these molecules. After attachment, sporozoites initiate the moving junction and the formation of the nascent parasitophorous vacuole membrane. The sporozoite plasma membrane is shown in blue, the micronemes in red, the rhoptries in purple and hepatocyte membranes in green. A red and blue co-colored membrane (shown in step 4) is indicative of the sporozoite membrane after it has been modified by micronemal proteins.
The sporozoite is exquisitely selective for infection of hepatocytes. This choice of host cell has likely evolved to support a nearly unparalleled magnitude of parasite replication and ensures further life cycle progression with the release of the first generation of red-blood cell infectious merozoites (exoerythrocytic merozoites). In the liver, some Plasmodium species also have the capacity for long-term persistence in the form of hypnozoites, which, when activated, initiate relapsing infection. Yet, the liver is a complex environment. Hepatocytes make up only ~60% of liver cells [7], and resident non-parenchymal cells are diverse including macrophages, other professional antigen presenting cells, endothelial cells, and a wide range of T cells [8], many of which are activated [9]. The liver is also the primary site for processing cellular toxins, and home to a variety of viral and bacterial pathogens [7]. Thus, the malaria parasite must ensure protection of its host cell in this tumultuous environment. Interestingly, the first line of defense innate immune responses elicited by primary parasite liver infection has only a modest negative impact on parasite survival [10, 11], although the impact of innate responses on survival of secondary liver infections is substantial, mediated by a type I interferon response [11, 12].
Here we highlight recent literature, which provides initial insights into how malaria parasites choose a hepatocyte and then modify their host cell to sustain intracellular growth and replication. While our understanding is based primarily on rodent models of malaria infection, new in vitro and in vivo models allow the analysis of hepatocyteparasite interactions directly with human-infecting malaria parasites. Furthermore, new approaches based on the analysis of few or single cells have already begun to enhance research on parasite hepatocyte infection. We emphasize data generated in the past two years, which provides insights into how the parasite navigates the challenges and exploits the riches of the liver microenvironment in which it thrives.
Point of invasion: a unique view of the hepatocyte surface and an important ‘choice’
Sporozoite selectivity for infection of hepatocytes was first described in 1948 by Short and Garnham [13]. Although recent reports show that a small fraction of exoerythrocytic forms develop in the skin of mice [14, 15], sporozoites are largely hepatotropic. Once sporozoites enter the parenchyma of the liver, they either traverse hepatocytes by membrane wounding [6] or invade hepatocytes and form a PV to establish residence (Reviewed in [16]). In each encounter with a potential host cell, sporozoites directly probe the hepatocyte surface. An intriguing but unproven hypothesis is that the sporozoite initially interacts with the hepatocyte via its surface but only secretes factors that commit it to invasion if the initial extracellular interaction signals a suitable host cell. One direct interaction between the sporozoite and the hepatocyte surface has been reported that triggers the cleavage of the main sporozoite surface protein circumsporozoite (CS) protein. This occurs when sporozoites contact the hepatocyte surface and CS interacts with highly sulfated proteoglycans (HSPGs) [17]. CS cleavage is mediated by a papain family cysteine protease of parasite origin [18]. Thrombospondin-Related Anonymous Protein (TRAP) also engages HSPGs but the functional consequences of this interaction during hepatocyte infection remain unclear [19] (Figure 1).
Several other hepatocyte receptors are also important for sporozoite invasion but none of their cognate parasite ligands have been identified. The tetraspanin CD81, which is involved in hepatocyte microdomain formation through its interaction with phospholipids [20], is critical for sporozoite invasion [21]. Cholesterol, often provided to the hepatocyte by Scavenger Receptor B1 (SR-BI) [22] also plays a significant role in microdomain formation, and is important for hepatocyte invasion [23, 24]. Interestingly, it has been recently demonstrated that monoclonal antibodies directed at CD-81 but not SR-BI block P. falciparum sporozoite infection in a liver-humanized mouse model [25]. However, the extent to which these antibodies disrupt micro-domain formation is unknown.
In a more well-studied liver infection system, Hepatitis C Virus (HCV), hepatocyte surface molecules directly engage the virus (Occludin, CD81) [26, 27] and other molecules (e.g. Epidermal Growth Factor Receptor) are involved in regulating these host factors [28]. Current evidence suggests that CD81 likely regulates yet to be identified receptors since it does not appear to directly bind sporozoites [21] and some regions of CD81 that are critical in sporozoite invasion cannot eliminate sporozoite entry when blocked with a monoclonal antibody [29]. Another hepatocyte receptor, c- Met, has been implicated in hepatocyte infection [30]. However, its role appears to be specific for Plasmodium berghei and is not conserved in P. falciparum or Plasmodium yoelii infection [31]. Thus, the specific hepatocyte surface receptors, engaged by the sporozoite and downstream of HSPG binding to CS, remain to be uncovered.
The identity of key sporozoite entry factors might be extracted from the extensive presence of well-known eukaryotic adhesion domains in the sporozoite proteome such as EGF-like domains, TSR domains and IgG domains [4], each of which might have the structural capacity to engage hepatocyte surface proteins. Moreover, TRAP contains an A-domain with a von Willebrand factor (vWF) structure [32, 33] that is usually found in integrins, and might be an adhesion domain involved in engaging the hepatocyte surface during sporozoite entry. In addition, convergent evolution might have equipped the parasite with domains that lack sequence similarity to mammalian folds, yet share structural similarity, which might also allow the direct engagement of hepatocyte receptors. An emergent example is the Plasmodium-specific 6-Cys fold, which has structural similarity to metazoan Ephrin domains and is present in a family of secreted parasite proteins [34, 35]. Ephrin domains interact with their cognate receptors, the Eph receptor tyrosine kinases in cell-cell junction adhesion, suggesting that 6-Cys proteins might also have the potential in directly binding Eph receptors [36]. Considering the importance of sporozoite-specific 6-Cys proteins for productive hepatocyte invasion [34, 35], the hypothesis that they provide the sporozoite ligands to directly engage a hepatocyte receptor is worthy of further investigation. Future research should focus on detailed analysis of hepatocyte invasion. We should ask whether the principles of this process adheres to the standard invasion model derived from studies of Plasmodium merozoite invasion of red blood cells [37] and the Toxoplasma tachyzoite invasion of nucleated host cells [38].
Liver stage development: Dramatic changes require adaptation
During the intra-hepatocytic phase, the sporozoite transforms into a trophozoite, which then grows into a multinucleated schizont, replicating its genome between 104-105 times over the course of 2–10 days. Developmental phases of the parasite are likely accompanied by distinct interactions with the host hepatocyte. Before the parasite embarks on this rapid increase in cell mass and DNA replication, it spends approximately one third of its intrahepatocytic residence undergoing a process called de-differentiation. In this, the sporozoite transforms from its elongated state to a rounded, trophic stage. It disassembles the molecular and cellular components that are important for motility and invasion and jettisons some entire structures [39]. Host-parasite interactions during de-differentiation remain largely uncharacterized, yet the possibility that the parasite might use this time to mold and adapt its host environment to support subsequent expansion is an enticing possibility (Figure 2).
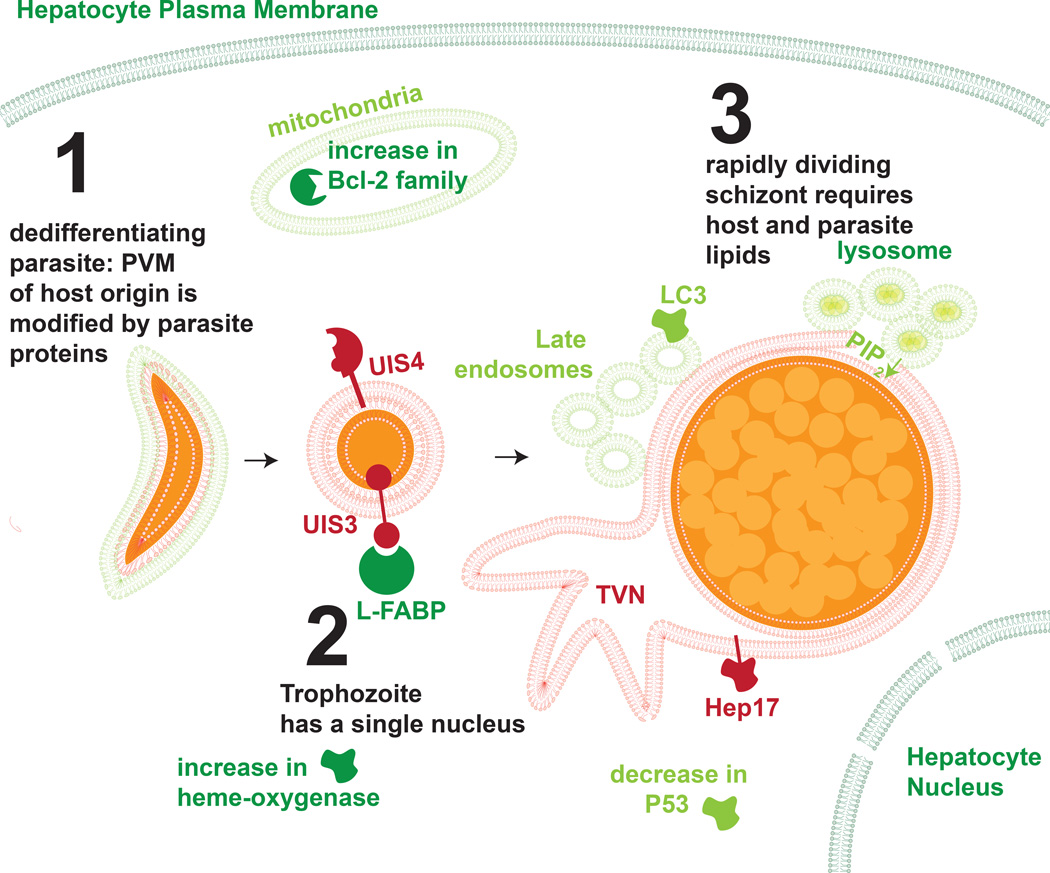
Figure 2. The current model of Plasmodium liver stage development.
Once the malaria parasite takes up residence in the hepatocyte, it transforms from its elongated sporozoite form to a rounded trophozite during a process called de-differentiation. After this process, the parasite undergoes rapid schizogony, replicating its DNA and producing tens of thousands of exo-erythrocytic merozoites within the confines of the PVM. Throughout this process, the parasite must regulate a variety of cellular processes, including the direct interaction with host structures and proteins. Host membranes and proteins are depicted in green, parasite structures and proteins in red and orange.
The PV membrane (PVM), which is modified by the parasite during dedifferentiation, enhances the parasites’ ability to complete LS development. Sporozoites, which enter hepatocytes without a PVM, are rapidly cleared by host cell apoptosis, whereas sporozoites that enter during PVM formation render host cells less susceptible to apoptosis [34, 40, 41]. Sensitivity to apoptosis is dependent on the Bcl-2 family of mitochondrial proteins [40], although no specific parasite molecules have been demonstrated to directly engage the hepatocyte mitochondria. The mitochondria of the host hepatocyte may also have additional importance as liver stages scavenge their lipoic acid through an undescribed mechanism [42]. There are conflicting reports of whether or not host mitochondria sequester around the LS PVM [39, 42], although it is clear that any direct interaction is far more subtle than in Toxoplasma, which decorates its PVM extensively with host organelles [39].
To generate a more comprehensive map of the hepatocyte responses to LS infection, analysis of both the transcriptome and signaling changes has been performed [43, 44]. Transcriptome data revealed that host hepatocytes exhibit an initial stress response to parasite infection and that resolves into a period of regulating cell viability and metabolic processes [43]. Probing hepatocyte protein levels and post-translational modification at 24 hours post-infection with rodent malaria parasites revealed a cohesive signaling network aimed at preventing host cell death. Interestingly, the tumor suppressor P53 is substantially suppressed in infected hepatocytes and this has functional importance as LS infection is nearly eliminated by boosting P53 levels [44]. This is however not linked to the parasite’s capacity to avoid host cell apoptosis [45]. Recent evidence demonstrates that the LS thrives in hypoxic conditions [46], which are often linked to elevated levels of P53 [47], suggesting that the parasite might lower P53 to survive one consequence of the hypoxic environment which it otherwise requires.
Beyond regulation of host cell signaling pathways, the liver stage likely needs to sustain their massive growth by importing host cell components [16]. Interestingly, it has been demonstrated that the PVM is porous [39] providing a clue to how small host factors might be transported to the vacuole-confined parasite. Furthermore, the PVM-resident protein Upregulated in Infectious Sporozoites 3 (UIS3) directly binds the Liver Fatty Acid Binding Protein (L-FABP) [48] which is hypothesized to facilitate fatty acid import, although the precise structure and function of this interaction remains poorly understood [49]. Itoe and colleagues have recently demonstrated that the developing liver stage scavenges phosphatidylcholine from their hepatocyte host [50]. Liver stages also scavenge PI(3,5)P2 from late endosomes which fuse with the tubovesicular vesicular network (TVN) during development [51]. Interestingly, liver stages sequester both late endosomes [52] and lysosomes [53], but not early endosomes [51] around their PVM. This process is required for optimal growth of liver stages as cells that have been treated with NH4Cl or Concanamycin A lose their capacity to acidify vesicles and harbor smaller parasites [52]. Interestingly, there is some evidence that these vesicles can cross the PVM [52] suggesting that their content might provide nutrients for parasite growth and development. In contrast to other parasites, such as Leishmania, the malaria parasite is not housed within a host cell phagosome. Yet, the recent reports suggest that the liver stages have a more entrenched interaction with the host cell endophagosomal system than previously understood.
Plasmodium blood stages extensively remodel their erythrocyte host cell, including the establishment of endomembrane structures, such as the Maurer’s clefts [54, 55], Schueffner’s dots [56, 57], J-dots [58] and the TVN [59]. Yet, only the TVN has been described in liver stage-infected hepatocytes [53], suggesting that parasites might have a comparatively limited need to remodel the endomembrane system of the hepatocyte. This might differ because one of the major functions of the intraerythrocytic endomembrane system is export of virulence factors, such as the Plasmodium falciparum erythrocyte membrane protein 1 family, that are exported to the infected erythrocyte surface and mediate adhesion of the infected cell to the vascular endothelium [60], allowing the parasite to avoid clearance by the spleen [60]. There is currently little evidence of parasite protein export beyond the confines of the PVM during liver stage development and no evidence that the parasite modifies the infected hepatocyte surface. Such modifications might not be needed as infected hepatocytes reside within a solid tissue, and export to the hepatocyte surface might expose parasite antigens to the array of non-parenchymal immune cells in the liver.
Interrogating liver stages of human malaria parasite species
Most research on sporozoite invasion and liver stage infection has been conducted with rodent malaria parasites. However, the extent to which host-pathogen interactions are conserved between human malaria parasites and rodent malaria parasites remains unclear. Humanized mouse models [61–63] and increasingly robust in vitro models [64, 65] provide an opportunity to directly interrogate the impact of P. falciparum and P. vivax parasites on their host hepatocytes. Recently, the FAH(−/−) Rag2(−/−) IL2γ receptor(−/−) FRG human hepatocyte (HuHep) model has been used to demonstrate that, as with rodent-malaria-infected hepatocytes, inhibiting the Bcl-2 family of proteins or boosting levels of P53 eliminates P. falciparum LS-infected human hepatocytes [45]. The Micro-patterned Cell Culture primary hepatocyte in vitro co-culture model combines a pattern of primary hepatocytes co-cultured with fibroblasts. It has been used to demonstrate that P. falciparum parasites develop better in hypoxic conditions [46] and scavenge host phospholipids [50]. More recently, it has been demonstrated that hepatocytes derived from induced pluripotent stem cells can also support liver stage parasites [65], facilitating an investigation of whether or not differences in human genetic background are linked to the capacity to support LS development.
While new models have facilitated early discoveries about host responses to P. falciparum LS infection, host-parasite interactions during P. vivax LS infection remain unstudied. Unlike P. falciparumP. vivax LS can remain dormant in the liver in the form of hypnozoites for months or even years [66]. Hepatocytes often die as a result of liver damage and are subsequently regenerated [67], thus P. vivax hypnozoites might have a specialized machinery to deal with this unique challenge. Recently, the FRG HuHep mouse model has been used to analyze P. vivax hypnozoites in more depth [68], providing a platform to interrogate host-parasite interactions unique to hypnozoites.
Conclusions
Malaria parasites are obligate intracellular pathogens that require a host cell throughout their lifetime in the liver. During first encounter, sporozoites have a unique view of the hepatocyte surface, which expresses surface proteins that may provide a molecular signature for the metabolic, proteomic and lipidomic properties within. Current evidence supports a model where the sporozoite ‘selects’ its optimal hepatocyte host at entry, and then further molds it surroundings to ensure liver stage survival. Recent findings that describe substantial differences in mouse hepatocyte susceptibility to rodent malaria sporozoite infection might provide experimental systems to further elucidate the molecular mechanisms of hepatocyte selection [69, 70]. Furthermore, there is building evidence that liver stages import host material, which sustains their developmental progress. The machinery and specific parasite proteins which mediate this process remain a major point for future investigation.
While the last several years have generated substantial advances describing the changes in the hepatocyte after infection, the direct interactions between host and parasite proteins remain largely uncharacterized. Research in this area remains limited by technical hurdles associated with collection of material from the relatively rare liver stage-infected hepatocytes that is compatible with traditional biochemical techniques. The current technological advances in animal models (humanized mice, CRISPR-Cas9-based knockout generation) and analysis techniques (correlative microscopy, highly multiplexed and imaging flow cytometry and microfluidic-based platforms) are well-suited for the challenges of studying malaria parasite infection in the liver. These technologies provide a platform not only to analyze single-infected cells but also to link host cell perturbations to parasite survival outcomes. If fully exploited, the study of hepatocyte-parasite interactions could be a major beneficiary of recent technological advances.
Highlights.
Malaria sporozoites are highly selective for their hepatocyte host
Malaria parasites refine the host during their liver stage of development
Novel approaches and models will deepen our understanding of interactions between host and parasite
Acknowledgments
AK is a recipient of a K99/R00 Transition to Independence Award (1K99AI111785-01A1) which has partially funded this work. This work has also been funded by an R01 to SHIK (1R01GM101183-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science. 2010;328:862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- 2.Tavares J, Formaglio P, Thiberge S, Mordelet E, Van Rooijen N, Medvinsky A, Menard R, Amino R. Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med. 2013;210:905–915. doi: 10.1084/jem.20121130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer K, Roosevelt M, Clarkson AB, Jr, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol. 2007;9:397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 4. Lindner SE, Swearingen KE, Harupa A, Vaughan AM, Sinnis P, Moritz RL, Kappe SH. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol Cell Proteomics. 2013;12:1127–1143. doi: 10.1074/mcp.M112.024505. * This paper is the most complete published report of the Plasmodium sporozoite proteome and also includes information about the proteins localized to the sporozoite surface.
- 5.Cochrane AH, Aikawa M, Jeng M, Nussenzweig RS. Antibody-induced ultrastructural changes of malarial sporozoites. J Immunol. 1976;116:859–867. [PubMed] [Google Scholar]
- 6.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 7.Arias HJA Irwin M, Boyer James L, Cohen David E, Fausto Nelson, Shafritz David A, Wolkoff Allan W. The liver biology and pathobiology. edn 5th. Wiley-Blackwell; 2009. [Google Scholar]
- 8.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 9.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- 10.Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, Carapau D, Konert M, Hanson KK, Carret C, Lassnig C, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014;20:47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SH. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Liehl P, Meireles P, Albuquerque IS, Pinkevych M, Baptista F, Mota MM, Davenport MP, Prudencio M. Innate immunity induced by Plasmodium liver infection inhibits malaria reinfections. Infect Immun. 2015 doi: 10.1128/IAI.02796-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shortt HE, Garnham PC. Pre-erythrocytic stage in mammalian malaria parasites. Nature. 1948;161:126. doi: 10.1038/161126a0. [DOI] [PubMed] [Google Scholar]
- 14.Gueirard P, Tavares J, Thiberge S, Bernex F, Ishino T, Milon G, Franke-Fayard B, Janse CJ, Menard R, Amino R. Development of the malaria parasite in the skin of the mammalian host. Proc Natl Acad Sci U S A. 2010;107:18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voza T, Miller JL, Kappe SH, Sinnis P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infect Immun. 2012;80:2158–2164. doi: 10.1128/IAI.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppi A, Natarajan R, Pradel G, Bennett BL, James ER, Roggero MA, Corradin G, Persson C, Tewari R, Sinnis P. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med. 2011;208:341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matuschewski K, Nunes AC, Nussenzweig V, Menard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002;21:1597–1606. doi: 10.1093/emboj/21.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 21.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 22.Valacchi G, Sticozzi C, Lim Y, Pecorelli A. Scavenger receptor class B type I: a multifunctional receptor. Ann N Y Acad Sci. 2011;1229:E1–E7. doi: 10.1111/j.1749-6632.2011.06205.x. [DOI] [PubMed] [Google Scholar]
- 23.Yalaoui S, Huby T, Franetich JF, Gego A, Rametti A, Moreau M, Collet X, Siau A, van Gemert GJ, Sauerwein RW, et al. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe. 2008;4:283–292. doi: 10.1016/j.chom.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues CD, Hannus M, Prudencio M, Martin C, Goncalves LA, Portugal S, Epiphanio S, Akinc A, Hadwiger P, Jahn-Hofmann K, et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe. 2008;4:271–282. doi: 10.1016/j.chom.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Foquet L, Hermsen CC, Verhoye L, van Gemert GJ, Cortese R, Nicosia A, Sauerwein RW, Leroux-Roels G, Meuleman P. Anti-CD81 but not anti-SR-BI blocks Plasmodium falciparum liver infection in a humanized mouse model. J Antimicrob Chemother. 2015 doi: 10.1093/jac/dkv019. [DOI] [PubMed] [Google Scholar]
- 26.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 28.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalaoui S, Zougbede S, Charrin S, Silvie O, Arduise C, Farhati K, Boucheix C, Mazier D, Rubinstein E, Froissard P. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog. 2008;4:e1000010. doi: 10.1371/journal.ppat.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario AM, Silva S, Leiriao P, Carapau D, Armas-Portela R, Comoglio PM, et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nat Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- 31.Kaushansky A, Kappe SH. The crucial role of hepatocyte growth factor receptor during liver-stage infection is not conserved among Plasmodium species. Nat Med. 2011;17:1180–1181. doi: 10.1038/nm.2456. author reply 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson KJ, Hall JR, Jennings MW, Harris TJ, Marsh K, Newbold CI, Tate VE, Weatherall DJ. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 33.Pihlajamaa T, Kajander T, Knuuti J, Horkka K, Sharma A, Permi P. Structure of Plasmodium falciparum TRAP (thrombospondin-related anonymous protein) A domain highlights distinct features in apicomplexan von Willebrand factor A homologues. Biochem J. 2013;450:469–476. doi: 10.1042/BJ20121058. [DOI] [PubMed] [Google Scholar]
- 34.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arredondo SA, Cai M, Takayama Y, MacDonald NJ, Anderson DE, Aravind L, Clore GM, Miller LH. Structure of the Plasmodium 6-cysteine s48/45 domain. Proc Natl Acad Sci U S A. 2012;109:6692–6697. doi: 10.1073/pnas.1204363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright GJ, Rayner JC. Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog. 2014;10:e1003943. doi: 10.1371/journal.ppat.1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci. 2014;71:1245–1263. doi: 10.1007/s00018-013-1491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bano N, Romano JD, Jayabalasingham B, Coppens I. Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int J Parasitol. 2007;37:1329–1341. doi: 10.1016/j.ijpara.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 40. Kaushansky A, Metzger PG, Douglass AN, Mikolajczak SA, Lakshmanan V, Kain HS, Kappe SH. Malaria parasite liver stages render host hepatocytes susceptible to mitochondria-initiated apoptosis. Cell Death Dis. 2013;4:e762. doi: 10.1038/cddis.2013.286. * This paper describes the significance of the mitochondrial Bcl-2 proteins and the parasite PVM in preventing apoptosis during the malaria liver stage.
- 41.van de Sand C, Horstmann S, Schmidt A, Sturm A, Bolte S, Krueger A, Lutgehetmann M, Pollok JM, Libert C, Heussler VT. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol. 2005;58:731–742. doi: 10.1111/j.1365-2958.2005.04888.x. [DOI] [PubMed] [Google Scholar]
- 42.Deschermeier C, Hecht LS, Bach F, Rutzel K, Stanway RR, Nagel A, Seeber F, Heussler VT. Mitochondrial lipoic acid scavenging is essential for Plasmodium berghei liver stage development. Cell Microbiol. 2012;14:416–430. doi: 10.1111/j.1462-5822.2011.01729.x. [DOI] [PubMed] [Google Scholar]
- 43.Albuquerque SS, Carret C, Grosso AR, Tarun AS, Peng X, Kappe SH, Prudencio M, Mota MM. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, Metzger PG, Douglass AN, MacBeath G, Kappe SH. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 2013;3:630–637. doi: 10.1016/j.celrep.2013.02.010. * This paper describes changes in host proteins and post-translational modifications during liver stage development. It also highlights that parasites require suppression of host p53 for optimal LS progression.
- 45.Douglass AN, Kain HS, Abdullahi M, Arang N, Austin LS, Mikolajczak SA, Billman ZP, Hume JC, Murphy SC, Kappe SH, et al. Host-based prophylaxis successfully targets liver stage malaria parasites. Mol Ther. 2015 doi: 10.1038/mt.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ng S, March S, Galstian A, Hanson K, Carvalho T, Mota MM, Bhatia SN. Hypoxia promotes liver-stage malaria infection in primary human hepatocytes in vitro. Dis Model Mech. 2014;7:215–224. doi: 10.1242/dmm.013490. * This paper highlights the important of the hepatocyte factor HIF1-alpha and hypoxia in maintainance of P. falciparum infection in vitro.
- 47.Pflaum J, Schlosser S, Muller M. p53 Family and Cellular Stress Responses in Cancer. Front Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikolajczak SA, Jacobs-Lorena V, MacKellar DC, Camargo N, Kappe SH. L-FABP is a critical host factor for successful malaria liver stage development. Int J Parasitol. 2007;37:483–489. doi: 10.1016/j.ijpara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Favretto F, Assfalg M, Molinari H, D'Onofrio M. Evidence from NMR interaction studies challenges the hypothesis of direct lipid transfer from L-FABP to malaria sporozoite protein UIS3. Protein Sci. 2013;22:133–138. doi: 10.1002/pro.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Itoe MA, Sampaio JL, Cabal GG, Real E, Zuzarte-Luis V, March S, Bhatia SN, Frischknecht F, Thiele C, Shevchenko A, et al. Host Cell Phosphatidylcholine Is a Key Mediator of Malaria Parasite Survival during Liver Stage Infection. Cell Host Microbe. 2014;16:778–786. doi: 10.1016/j.chom.2014.11.006. ** This paper describes the requirement of host-derived fatty acids for liver stage development.
- 51. Thieleke-Matos C, da Silva ML, Cabrita-Santos L, Pires CF, Ramalho JS, Ikonomov O, Seixas E, Shisheva A, Seabra MC, Barral DC. Host PI(3,5)P2 activity is required for Plasmodium berghei growth during liver stage infection. Traffic. 2014;15:1066–1082. doi: 10.1111/tra.12190. ** This paper demonstrates that PIP2 from the host is critical during liver stage infection, and proposes that the developing liver stage parasite is able to scavenge this phosphoinositol from endosomes in the host.
- 52. Lopes da Silva M, Thieleke-Matos C, Cabrita-Santos L, Ramalho JS, Wavre-Shapton ST, Futter CE, Barral DC, Seabra MC. The host endocytic pathway is essential for Plasmodium berghei late liver stage development. Traffic. 2012;13:1351–1363. doi: 10.1111/j.1600-0854.2012.01398.x. ** This paper demonstrates that late endosomes sequester around the parasite PVM during liver stage development.
- 53. Grutzke J, Rindte K, Goosmann C, Silvie O, Rauch C, Heuer D, Lehmann MJ, Mueller AK, Brinkmann V, Matuschewski K, et al. The spatiotemporal dynamics and membranous features of the Plasmodium liver stage tubovesicular network. Traffic. 2014;15:362–382. doi: 10.1111/tra.12151. * This paper is a beatiful description of the liver stage TVN.
- 54.Trager W, Rudzinska MA, Bradbury PC. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35:883–885. [PMC free article] [PubMed] [Google Scholar]
- 55.Maurer G. Die malaria perniciosa. Zentralbl Bakteriol Parasitenkunde. 1902;23:695–719. [Google Scholar]
- 56.Akinyi S, Hanssen E, Meyer EV, Jiang J, Korir CC, Singh B, Lapp S, Barnwell JW, Tilley L, Galinski MR. A 95 kDa protein of Plasmodium vivax and P. cynomolgi visualized by three-dimensional tomography in the caveola-vesicle complexes (Schuffner's dots) of infected erythrocytes is a member of the PHIST family. Mol Microbiol. 2012;84:816–831. doi: 10.1111/j.1365-2958.2012.08060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schüffner W. Beitrag zur Kenntniss der Malaria. Deutsch Archiv Klin Med. 1899;64:428–449. [Google Scholar]
- 58.Kulzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol. 2010;12:1398–1420. doi: 10.1111/j.1462-5822.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 59.Tamez PA, Bhattacharjee S, van Ooij C, Hiller NL, Llinas M, Balu B, Adams JH, Haldar K. An erythrocyte vesicle protein exported by the malaria parasite promotes tubovesicular lipid import from the host cell surface. PLoS Pathog. 2008;4:e1000118. doi: 10.1371/journal.ppat.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JD. The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research. Mol Biochem Parasitol. 2014;195:82–87. doi: 10.1016/j.molbiopara.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–3628. doi: 10.1172/JCI62684. * This paper describes a major advance in the ability to monitor Plasmodium LS development in vivo in humanized mice.
- 62.Morosan S, Hez-Deroubaix S, Lunel F, Renia L, Giannini C, Van Rooijen N, Battaglia S, Blanc C, Eling W, Sauerwein R, et al. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis. 2006;193:996–1004. doi: 10.1086/500840. [DOI] [PubMed] [Google Scholar]
- 63.Sacci JB, Jr, Schriefer ME, Resau JH, Wirtz RA, Detolla LJ, Jr, Markham RB, Azad AF. Mouse model for exoerythrocytic stages of Plasmodium falciparum malaria parasite. Proc Natl Acad Sci U S A. 1992;89:3701–3705. doi: 10.1073/pnas.89.9.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, Carpenter AE, Thomas D, Sim BK, Mota MM, et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. * This paper describes a major advance in the ability to monitor Plasmodium LS development in vitro.
- 65. Ng S, Schwartz RE, March S, Galstian A, Gural N, Shan J, Prabhu M, Mota MM, Bhatia SN. Human iPSC-derived hepatocyte-like cells support Plasmodium liver-stage infection in vitro. Stem Cell Reports. 2015;4:348–359. doi: 10.1016/j.stemcr.2015.01.002. * This paper describes a major advance in the ability to monitor Plasmodium LS development in vitro using iPS cells.
- 66.Shanks GD, White NJ. The activation of vivax malaria hypnozoites by infectious diseases. Lancet Infect Dis. 2013;13:900–906. doi: 10.1016/S1473-3099(13)70095-1. [DOI] [PubMed] [Google Scholar]
- 67.Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26(Suppl 1):203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mikolajczak SA, Vaughan AM, Kangwanrangsan N, Roobsoong W, Fishbaugher M, Yimamnuaychok N, Rezakhani N, Lakshmanan V, Singh N, Kaushansky A, et al. Plasmodium vivax Liver Stage Development and Hypnozoite Persistence in Human Liver-Chimeric Mice. Cell Host Microbe. 2015;17:526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Austin LS, Kaushansky A, Kappe SH. Susceptibility to Plasmodium liver stage infection is altered by hepatocyte polyploidy. Cell Microbiol. 2014;16:784–795. doi: 10.1111/cmi.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaushansky A, Austin LS, Mikolajczak SA, Lo FY, Miller JL, Douglass AN, Arang N, Vaughan AM, Gardner MJ, Kappe SH. Susceptibility to Plasmodium yoelii preerythrocytic infection in BALB/c substrains is determined at the point of hepatocyte invasion. Infect Immun. 2015;83:39–47. doi: 10.1128/IAI.02230-14. [DOI] [PMC free article] [PubMed] [Google Scholar]