Bacterial ACC deaminase affects ethylene production and plant growth under stress.
Abstract
A focus on the mechanisms by which ACC deaminase-containing bacteria facilitate plant growth.Bacteria that produce the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, when present either on the surface of plant roots (rhizospheric) or within plant tissues (endophytic), play an active role in modulating ethylene levels in plants. This enzyme activity facilitates plant growth especially in the presence of various environmental stresses. Thus, plant growth-promoting bacteria that express ACC deaminase activity protect plants from growth inhibition by flooding and anoxia, drought, high salt, the presence of fungal and bacterial pathogens, nematodes, and the presence of metals and organic contaminants. Bacteria that express ACC deaminase activity also decrease the rate of flower wilting, promote the rooting of cuttings, and facilitate the nodulation of legumes. Here, the mechanisms behind bacterial ACC deaminase facilitation of plant growth and development are discussed, and numerous examples of the use of bacteria with this activity are summarized.
Agricultural development policies and practices in the past sixty years have largely been based on external inputs (pesticides and fertilizers) to control soil-borne diseases and increase crop yields. Recently, stimulated by the awareness of potentially serious environmental and human health damage caused by the over use of agricultural chemicals (Alavanja et al., 2004; Leach and Mumford, 2008; Damalas and Eleftherohorinos, 2011), the controversy regarding the use of pesticides and fertilizers has gained prominence. Therefore, worldwide agricultural practice is moving toward a more sustainable and environmentally friendly approach.
In 2002, in the European Union, 5.7 million ha were designated as being cultivated organically, and by 2011, this number had increased to 9.6 million ha (http://ec.europa.eu/agriculture/markets-and-prices/more-reports/pdf/organic-2013_en.pdf). In other words, in 10 years, the area devoted to organic agriculture in the European Union increased by approximately 400,000 ha per year. This growth in organic agriculture notwithstanding, the total amount of organically cultivated land represents only 5.4% of the total agricultural land in Europe. In this context, the use of microbial inoculants instead of traditional chemicals is gaining popularity, and a number of new products have been formulated, marketed, and applied successfully.
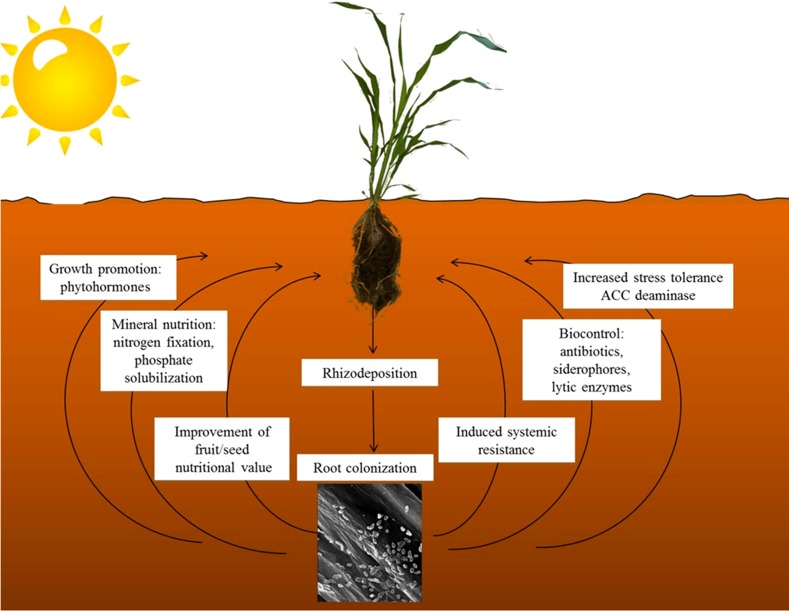
The soil surrounding plant roots (the rhizosphere) is one of the main sources of bacteria expressing plant-beneficial activities ( i.e. plant growth-promoting bacteria [PGPB]; Bashan and Holguin, 1998). Stimulation of growth and protection of different crops from pathogens and abiotic stressors by PGPB is well documented under both controlled conditions and in the field, and a large number of papers on this topic are available (Reed and Glick, 2005, 2013; Thakore, 2006). The positive effects induced by PGPB on plant growth are based on: (1) the improvement of mineral nutrition (nitrogen fixation, phosphate solubilization, and iron sequestration), (2) the enhancement of plant tolerance to biotic and abiotic stress (largely mediated by 1-aminocyclopropane-1-carboxylate [ACC] deaminase), (3) the modification of root development (via phytohormone synthesis), and (4) the suppression of phytopathogens (by antibiotics, competition, lytic enzymes, systemic resistance, etc.; Fig. 1). The current knowledge of microorganisms living in the rhizosphere, their role, and their biotechnological and environmental applications has been summarized in several reviews (Glick, 2012; Hirsch and Mauchline, 2012; Bakker et al., 2013; Mendes et al., 2013; Reed and Glick, 2013). This review focuses on the role of bacterial ACC deaminase in supporting the growth of plants exposed to environmental stress. In addition, the issues of the distribution and phylogeny of ACC deaminase, and the possible role of ACC as a signaling molecule, are addressed.
Figure 1.
Schematic overview of the main mechanisms used by PGPB. Following the release of root exudates, a variety of soil microorganisms are attracted to the root. Some of them can efficiently colonize the root surface while others (endophytes) can penetrate the root tissue and spread inside the plant. Plant growth promotion by beneficial microorganisms may occur by either direct or indirect mechanisms. Direct promotion of plant growth involves the improvement of mineral nutrition via nitrogen fixation, phosphate solubilization, and iron chelation, as well as the modulation of phytohormones levels (auxins, cytokinins, GAs, and ethylene). In addition to the increase of biomass, PGPB can positively affect the nutritional value of fruits and edible seeds. The indirect mechanisms are based on the improvement of plant health via suppression of soil-borne diseases by antibiotics, lytic enzymes, siderophore production, induced systemic resistance involving jasmonate and ethylene signaling within the plant, and other molecules (the O-antigenic side chain of the bacterial outer membrane protein lipopolysaccharide, flagellar fractions, pyoverdine, 2,4-diacetylphloroglucinol, cyclic lipopeptide, surfactants, and salicylic acid) that stimulate the host plant’s resistance to pathogens.
RHIZOSPHERIC BACTERIA VERSUS ENDOPHYTES VERSUS RHIZOBIA
Thanks to carbon-rich exudates released from plant roots, bacteria in the rhizosphere establish themselves and proliferate along the roots, giving rise to a biofilm surrounding the roots’ surface (Danhorn and Fuqua, 2007). Following rhizosphere colonization, some of these microorganisms can penetrate the root tissue, therefore shifting their habitus from rhizospheric to endophytic. Endophytic bacteria include: (1) facultative endophytes living inside the plants as well as in other habitats, (2) obligate endophytes that can only live inside plant tissues, and (3) opportunistic endophytes that can occasionally enter plants and live inside their tissues. However, the fact that scientists can isolate and culture specific endophytic strains means that they are likely dealing exclusively with facultative endophytes that may be isolated from rhizosphere soil samples as well as from inside the plant.
According to Wilson (1995), endophytes are those microorganisms living inside plant tissues without harming the plant. Internal colonization typically starts in the zone of lateral root emergence or in root wounds and cracks; from there, endophytic bacteria proliferate, spread through xylematic vessels, and reach different plant compartments (Compant et al., 2008). Bacterial endophytes have been detected inside the endorhiza in stems, leaves, and flowers (Compant et al., 2010; Reinhold-Hurek and Hurek, 2011) of a number of plant species.
Inside plant tissues, endophytic bacteria express their physiological activities, synthesize secondary metabolites, and may, both directly and indirectly, facilitate plant development through phytopathogen suppression, mineral nutrition improvement, and enhancement of plant tolerance to stress. Consequently, a number of studies have focused on the application of bacterial endophytes as biofertilizers for phytostimulation and as biological control agents (Kuklinsky-Sobral et al., 2004; Gaiero et al., 2013).
Recently, based on genome sequences of 304 Proteobacteria, Bruto et al. (2014) analyzed the distribution of 23 genes that may contribute to the ability of these bacteria to promote plant growth. These authors suggest that gene transfers, predominantly ancient, resulted in characteristic gene combinations according to taxonomic subgroups of PGPB strains. In other words, genes associated with plant growth, such as the ACC deaminase structural gene (acdS), are found in rhizospheric bacteria as a consequence of ancient horizontal gene transfer, and are also present in endophytic bacteria. Thus, understanding the mechanisms utilized by rhizospheric bacteria also provides insight into the mechanisms used by endophytic bacteria.
Rhizobia represent a particular group of endophytic microorganisms able to improve plant mineral nutrition, primarily through nitrogen fixation. They colonize plant roots and establish a mutualistic symbiosis with compatible legume plants. The strict and highly specific relationship between these bacteria and the plant host induces physiological, genetic, and morphological changes in the plant. This includes the formation of root nodules containing bacteria (bacteroids), where nitrogen fixation occurs, under limited oxygen concentration via the action of the enzyme nitrogenase. However, rhizobia, moving from the root toward the shoot (Chi et al., 2005) can, to some extent, colonize internal root tissues of cereal crop plants, such as rice (Oryza sativa), maize (Zea mays), barley (Hordeum vulgare), and wheat (Triticum aestivum), increasing plant biomass and grain yield independently of root nodule formation and nitrogen fixation (Biswas et al., 2000; Gutiérrez-Zamora and Martínez-Romero, 2001; Lupway et al., 2004; García-Fraile et al., 2012).
Open-field application of rhizobia as biofertilizers for legume or cereal crop plants of agricultural importance facilitates plant development and high productivity when cultivated under low fertilization regimes. In this regard, rhizobia have been used to promote plant growth in the field for more than 100 years.
ACC DEAMINASE
Biochemistry of ACC Deaminase
The (largely bacterial) enzyme ACC deaminase (3.5.99.7) cleaves ACC, the immediate precursor of ethylene in plants, producing ammonia and α-ketobutyrate (Honma and Shimomura, 1978), reducing the amount of ethylene that the plant can synthesize (Glick et al., 1998). Ethylene is a gaseous hormone displaying a wide range of biological effects in plants at concentrations as low as 0.05 μL L–1 (Abeles et al., 1992). Ethylene is involved in seed germination, tissue differentiation, formation of root and shoot primordia, root branching and elongation, lateral bud development, flowering, flower senescence, fruit ripening and abscission, anthocyanin production, synthesis of volatile organic compounds responsible for aroma formation in fruits, storage product hydrolysis, leaf senescence, and abscission (Abeles et al., 1992; Glick, 2014). Local increases in the concentration of this hormone also occur during the establishment of symbioses between plants and microorganisms, including rhizobia and mycorrhizal fungi. In these cases, by locally lowering ethylene levels, ACC deaminase-producing bacteria can facilitate symbiosis development (Ma et al., 2003; Gamalero et al., 2008).
In all higher plants, ethylene is produced from S-adenosyl-Met via the action of the enzyme ACC synthase, both during normal plant development and when the plant is exposed to various environmental stresses (Abeles et al., 1992). By modulating ethylene levels, ACC deaminase represents one of the key bacterial physiological activities supporting plant growth under stressed conditions, where the ethylene concentration inside the plant might otherwise reach levels inhibitory to plant growth (Glick et al., 1998, 2007; Glick, 2014).
As a consequence of the wide range of potential applications of bacteria that produce ACC deaminase, there has been considerable interest in the biochemistry and functioning of this enzyme. Thus, a number of different ACC deaminases have now been characterized. ACC deaminase is a multimeric enzyme, cytoplasmically localized, that utilizes the coenzyme pyridoxal phosphate as a tightly bound cofactor. Its subunit mass is approximately 35 to 42 kD, while its native size is estimated to be approximately 100 to 112 kD (Sheehy et al., 1991; Jacobson et al., 1994; Hontzeas et al., 2004). Based on its protein fold, ACC deaminase has been classified as belonging to the Trp synthase β superfamily of pyridoxal phosphate-binding proteins (Glick et al., 2007). The affinity of this enzyme for the substrate is not particularly high (Km = 1.5–6.0 mm). Most organisms with ACC deaminase contain a basal level of enzyme activity. However, ACC deaminase synthesis is induced by ACC, at levels as low as 100 nm (Jacobson et al., 1994), with full induction requiring up to 10 h. The amino acids l-Ala, dl-Ala, and dl-Val can also induce enzyme activity to a small extent, and γ-aminoisobutyric acid can induce activity to almost the same level as ACC (Honma, 1983). Maximal enzyme activity typically occurs at 30°C and pH 8.5. The affinity for the substrate ACC and the competitive inhibitors l-Ala and l-Ser is also highest at pH 8.5 (Hontzeas et al., 2006).
Yoon and Kieber (2013) have recently posited a model in which, in addition to acting as the immediate precursor to ethylene, ACC may also act as a signaling molecule in several plant processes, including root-to-shoot communication. With this scenario, the interaction of plants with ACC deaminase-producing PGPB might be expected to decrease the extent of ACC signaling of specific plant functions such as the regulation of cell wall function. Unlike experiments that utilize chemical inhibitors of ethylene biosynthesis or ethylene perception, ACC deaminase specifically decreases ACC levels. Thus, to test the ability of ACC to act directly as a signaling molecule, one might repeat some of the experiments cited by Yoon and Kieber (2013) in the presence of ACC deaminase. In this regard, while ACC deaminase may not completely breakdown all of the available ACC, the resultant low levels of ACC may be readily quantified (Penrose et al., 2001).
Distribution and Phylogeny of ACC Deaminase
The bacterium Pseudomonas sp. ACP and the yeast Cyberlindnera saturnus (previously Hansenula saturnus) were the two first microorganisms reported to synthesize ACC deaminase (Honma and Shimomura, 1978; Minami et al., 1998). Subsequently, ACC deaminase activity has been found in numerous bacteria, both gram positive and negative with a variety of different types of metabolism (for review, see Gamalero and Glick, 2012; Glick, 2014). ACC deaminase genes (including both the structural gene acdS and the regulatory gene acdR) have been found in many different rhizobacteria (rhizospheric, endophytic, and rhizobia), including Azospirillum spp., Rhizobium spp., Agrobacterium spp., Achromobacter spp., Burkholderia spp., Ralstonia spp., Pseudomonas spp., and Enterobacter spp. (Blaha et al., 2006). More importantly, even if some strains of a particular genus and species have an acdS gene, not all strains do.
The frequency of ACC deaminase activity in various soil bacteria has been estimated, especially in rhizobia. Of 13 rhizobial strains tested, five (38%) isolates were able to synthesize ACC deaminase, while seven out of 13 (54%) possessed the acdS gene. This discrepancy was related to the fact that two strains, belonging to the genus Mesorhizobium are only able to produce the enzyme during the symbiotic phase, when localized inside a root nodule (Ma et al., 2003). It subsequently was shown that ACC deaminase genes in Mesorhizobium spp. were, unlike all other known ACC deaminases genes, under the transcriptional control of the nitrogen fixation positive regulatory gene nifA2 promoter and expressed only within root nodules (Nukui et al., 2006). In this regard, it has been suggested that the expression of ACC deaminase genes within nitrogen-fixing nodules may decrease the rate of nodule senescence, as nitrogen fixation with its high-energy demand could activate stress ethylene synthesis (Murset et al., 2012). Another study, including a much larger number of rhizobial isolates (233; Duan et al., 2009), revealed that 27 strains (12%) expressed ACC deaminase. These 27 strains were characterized for the presence of the acdS gene; while 17 of them had genes that were 99% identical to the previously characterized ACC deaminase structural gene (acdS) from Rhizobium leguminosarum bv viciae 128C53K, the other 10 strains were found to be 84% identical compared with the acdS gene from strain 128C53K (Duan et al., 2009). The observation that rhizobia strains with ACC deaminase activity from a wide geographic area showed very little diversity was somewhat surprising. It was then argued that given the harsh winters and lack of diverse vegetation in southern Saskatchewan (where these strains were isolated), there might be intrinsic limits to the diversity of these microorganisms (Duan et al., 2009).
Bacterial ACC deaminase activity is relatively common in rhizosphere bacteria, especially in soils that are often subjected to stressful conditions (Timmusk et al., 2011). Thus, rhizosphere bacteria that contain ACC deaminase may endow some plants with the ability to better withstand, and therefore survive in, harsh environmental conditions.
When analyzing the sequences of acdS genes, Blaha et al. (2006) found a high level of polymorphism. Consequently, they defined three acdS groups: groups I and II included sequences originating from the β- and γ-Proteobacteria, while group III was composed of α-Proteobacteria. Looking at their geographical origin and habitat, strains from a given acdS group originated from different plant hosts. Moreover, by comparing the sequences of 45 different acdS genes, from seven α-Proteobacteria, 35 β-Proteobacteria, and three γ-Proteobacteria, Prigent-Combaret et al. (2008) found a high similarity (62.1%–89.4%) with the acdS gene of the model strain Pseudomonas putida UW4 and 53.9% to 93.5% with the gene from Azospirillum lipoferum 4B.
A complete description of the phylogeny and evolution of the genes encoding acdS and its major regulatory gene, acdR, has been recently elaborated (Bruto et al., 2014; Nascimento et al., 2014). Information regarding acdS/acdR sequences must be considered together with the habitat, the origin, and the enzymatic activity of completely sequenced bacterial strains to obtain a comprehensive view. Overall, the data show that ACC deaminase activity is prevalent in some bacteria, fungi, and members of stramenopiles. Stramenopiles are a monophyletic eukaryotic group of organisms bearing an immature flagellum with tripartite hairs comprising more than 100,000 species and including a variety of life forms (single cells, large plasmodia, and complex multicellular thalli). The best known members of the group are the colorless oomycetes (aquatic fungi, including plant pathogens for cultivated crops), diatoms, chrysophyte algae, and giant kelp seaweeds. Stramenopiles able to perform photosynthesis are the predominant eukaryotes in most aquatic environments, where they are major primary producers (Yoon et al., 2009). In parallel, through multiple searches of the National Center for Biotechnology Information database, acdS genes have been found in Actinobacteria, members from the Deinococcus-Thermus phylum (Meiothermus spp.), α-, β- and, γ-Proteobacteria, various fungi (Ascomycota and Basidiomycota), and some stramenopiles (Nascimento et al., 2014).
Although ACC deaminase genes are mainly transmitted vertically in various microorganisms, occasional horizontal gene transfer, including interkingdom transfer events, occur. It is possible that acdS genes had an ancient origin in a eukaryote and bacterial common ancestor. Then, during vertical transmission, different constraints, such as adaptation to specific niches, induced acdS divergence or gene loss. The advantages conferred by ACC deaminase activity have been positively selected by evolution, leading to intragenomic transfers of acdS genes from primary chromosomes to plasmids and increased divergence of acdS genes. In fact, acdS genes in most Burkholderia and Cupriavidus spp. strains are located on a second smaller chromosome, while in other β-Proteobacteria (e.g. Ralstonia solanacearum), it is located on the primary chromosome or on megaplasmids (Nascimento et al., 2014). Here, it should be noted that some strains of Burkholderia spp. are exclusively rhizospheric, while others are facultative endophytes. Because plasmids are transmissible between bacteria via conjugation, it’s possible that some dispersion of acdS genes occurred. This is in agreement with work that previously reported the occurrence of horizontal acdS/acdR genes transfer in Proteobacteria and in many Mesorhizobium species (Hontzeas et al., 2005; Blaha et al., 2006; Nascimento et al., 2012). Moreover, due to intragenomic transfer events, many microorganisms may have lost acdS genes. Consistently, it has been reported that, during phenotypic variation events, A. lipoferum strain 4B readily loses the plasmid containing an acdS gene (Prigent-Combaret et al., 2008).
Model Including IAA Feedback Inhibition of Ethylene Action
In addition to being rich in sugars, root exudates contain high amounts of amino acids. Among them, Trp is released by the roots and may be taken up by bacterial cells in the rhizosphere. Bacteria use Trp to synthesize the phytohormone indole-3-acetic acid (IAA), some of which is then taken up by the plant. Production of IAA is widespread among soil bacteria; it has been estimated that approximately 80% of rhizosphere bacteria and a significant fraction of bacterial endophytes produce IAA (Patten and Glick, 1996).
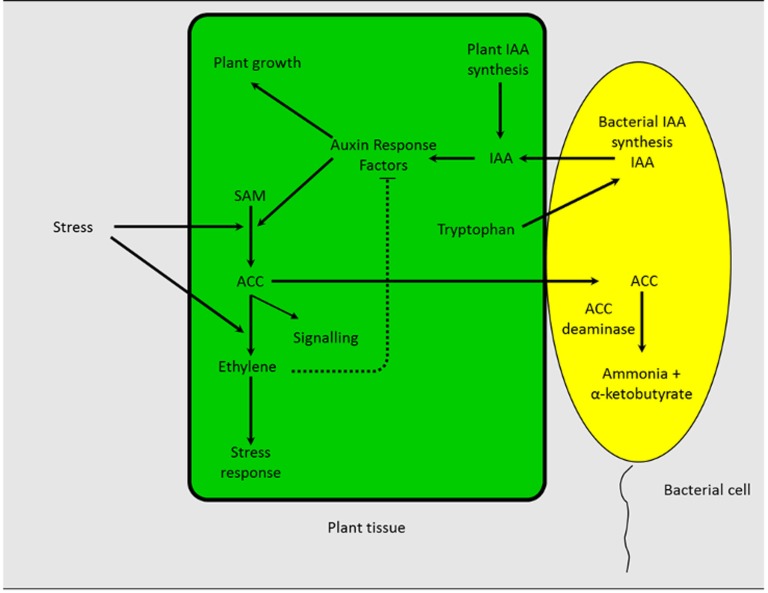
The bacterial IAA, together with endogenous plant IAA, can regulate several phases of plant development, such as seed and tuber germination, xylem formation, plant cell proliferation and elongation, vegetative growth, emergence of lateral and adventitious roots, plant responses to light and gravity, and florescence and fructification (Tsakelova et al., 2006). IAA can also affect the synthesis of ACC deaminase by activating the transcription of the plant enzyme ACC synthase (that catalyzes the conversion of ACC from S-adenosyl-Met). As a consequence of an increased amount of ACC, the ethylene level inside a plant is increased inducing a plant stress response. Bacteria that produce high levels of IAA often inhibit plant growth. However, this does not necessarily occur because as plant ethylene levels increase, the transcription of auxin response factors is inhibited (Pierik et al., 2006; Prayitno et al., 2006; Czarny et al., 2007; Glick et al., 2007; Stearns et al., 2012), thereby limiting the extent that IAA can activate ACC synthase transcription. Moreover, some ACC is released by the roots (Bayliss et al., 1997; Penrose and Glick, 2001), taken up by the bacteria, and through the action of ACC deaminase, converted to ammonia and α-ketobutyrate. As a result, the amount of ethylene produced by the plant is reduced. Therefore, root colonization by bacteria that synthesize ACC deaminase prevents a rise in ethylene levels that might otherwise become growth inhibitory (Glick, 1995). In plants inoculated with bacteria that produce both IAA and ACC deaminase, ethylene levels do not become elevated to the same extent as when plants interact with bacteria that synthesize IAA but not ACC deaminase. When bacterial ACC deaminase is induced and expressed, ethylene is synthesized at a relatively low level, and the bacterial IAA can continue to both stimulate plant growth and enhance the transcription of ACC synthase. However, a large portion of the ACC synthesized is released by the root, taken up by the bacterial cells and finally cleaved by ACC deaminase (Fig. 2). Consequently, the cross talk between IAA and ethylene enables ACC deaminase to effectively facilitate the stimulation of plant growth by IAA. Bacteria that synthesize both ACC deaminase and IAA may facilitate plant growth in the presence of several ethylene-producing environmental stresses (Gamalero and Glick, 2010).
Figure 2.
Schematic representation of how PGPB that produce both ACC deaminase and IAA facilitate plant growth. A detailed explanation is given in the text. SAM, S-Adenosyl Met.
Galland et al. (2012) reported that treatment of Arabidopsis (Arabidopsis thaliana) seedlings with the rhizospheric plant growth-promoting bacterium Phyllobacterium brassicacearum STM196 caused a significant increase in plant root hair elongation. Following this bacterial treatment, these workers were unable to detect any significant increase in ethylene biosynthesis. Moreover, this signaling pathway activation does not depend on local plant auxin biosynthesis. However, this bacterium also produces and secretes IAA so plant IAA biosynthesis is not needed to activate ACC synthase transcription. By using ethylene-insensitive mutants of Arabidopsis, Zamioudis et al. (2013) clarified which plant growth parameter is affected by the ethylene pathway. They concluded that the main impact of a PGPB strain, able to directly affect auxin signaling in plants, is on the length of the primary root; moreover, they demonstrated that other plant parameters such as lateral root and the root hair formation are affected by the strain independently by the ethylene pathways.
AMELIORATING PLANT STRESS VIA ACC DEAMINASE
In the past, stress ethylene has been suggested to both alleviate and exacerbate some of the effects of pathogen infection (Abeles et al., 1992). However, a simple model (originally developed to explain the effects of stress ethylene following biotic stress and later extended to include abiotic stress as well) was proposed to explain these seemingly contradictory results (Glick et al., 2007). That is, a short time following the onset of the stress, a small peak of ethylene is produced. This small peak of ethylene is thought to consume the existing pool of ACC within plant tissues and likely activates the synthesis of defensive genes within the plant (Stearns et al., 2012). Subsequently, following the synthesis of additional ACC within the plant, a second much larger peak of ethylene is typically observed. The second peak of ethylene occurs as a consequence of increased transcription of ACC synthase genes, mostly triggered by environmental cues, and acts as a signal to initiate processes such as senescence, chlorosis, and abscission, all of which are inhibitory to plant growth and survival. Thus, a significant fraction of the damage that occurs to a plant following a biotic or abiotic stress is due to the second (large) peak of ethylene that is synthesized by the plant rather than to the direct effects of the stress itself. Based on this model, it was predicted that bacteria, which produce an amount of ACC deaminase that can reduce the magnitude of the second ethylene peak, should decrease the damage to plants that occurs as a consequence of a wide range of biotic and abiotic stresses.
Flooding and Anoxia
Plant roots typically respond to flooding by synthesizing a high level of ACC, and as a consequence of a lack of oxygen, the ACC is translocated to shoots, where it becomes a substrate for ACC oxidase and is converted to ethylene (Bradford and Yang, 1980; Else and Jackson, 1998). Ethylene synthesis in flooded plants induces the expression of various symptoms such as epinasty, leaf chlorosis, and necrosis (Li et al., 2013). Bacteria able to limit the increase of ethylene through the action of ACC deaminase can be useful in supporting plant growth in such adverse conditions (Grichko and Glick, 2001; Barnawal et al., 2012; Li et al., 2013)
The protein profile of cucumber (Cucumis sativus) roots, inoculated or not with P. putida UW4 and able to synthesize ACC deaminase, in normoxic (no oxygen limitation) and hypoxic conditions has been characterized (Li et al., 2013). In normoxic conditions, no significant change in protein expression occurred in cucumber seedling roots treated with P. putida UW4. However, expression of several root proteins changed following the plant’s inoculation with P. putida UW4 under hypoxic stress, including those involved in carbohydrate and nitrogen metabolism, defense stress, antioxidant activity, and binding to host plants (Li et al., 2013).
Drought
The first report of ACC deaminase-producing bacteria facilitating the growth of plants under drought stress was by Mayak et al. (2004a), who used Achromobacter piechaudii ARV8, from the rhizosphere of Lycium shawii from the Arava region of the Negev desert, to inoculate tomato (Solanum lycopersicum) and pepper (Capsicum annuum) plants exposed to drought stress. Plants inoculated with the bacterial strain had 4 times the biomass compared with noninoculated controls, concomitant with a significant reduction of the ethylene level.
Similar experiments (in the laboratory and in the field) with several plants (pea [Pisum sativum], maize, wheat, mung bean [Vigna radiata], and Trigonella foenum-graecum) and different ACC deaminase-producing bacteria have since demonstrated the efficacy of using bacteria able to synthesize ACC deaminase in protecting plants against yield loss induced by drought stress (Arshad et al., 2008; Belimov et al., 2009; Shakir et al., 2012; Barnawal et al., 2013; Sarma and Saikia, 2014; Zafarul-Hye et al., 2014).
Salt
Worldwide, the total area of salt-affected soil is about one billion ha, mainly in the arid-semiarid regions of Asia, Australia, and South America. In addition, salinity affects about 1 million ha in the European Union and is a major cause of desertification. In Spain, for example, 3% of the 3.5 million ha of irrigated land is severely affected, while another 15% of this land is considered to be under serious risk (Soil Atlas of Europe, European Soil Bureau Network European Commission 2005, http://eusoils.jrc.ec.europa.eu/projects/soil_atlas/pages/117.html).
Salt stress inhibits plant growth, inducing osmotic stress, Na+ and Cl– toxicity, ethylene production, plasmolysis, nutrient imbalance, production of reactive oxygen species, and interference with photosynthesis. Inhibition of seed germination, seedling growth and vigor, flowering, and fruit set occur as a consequence of these physiological changes (Sairam and Tyagi, 2004).
The initial responses of most plants to drought and salinity are very similar; both are attributed to water stress. When plants are exposed to high salt, a decrease in the growth rate followed by a slow recovery to a new reduced growth rate is the plant’s first response to the decrease in water potential caused by salt, rather than to any salt-specific toxicity. Subsequently, metabolic toxicity in plants caused by sodium ions is attributed to these ions competing with potassium ions for binding sites essential for cellular functioning (Gamalero et al., 2009a).
Mayak et al. (2004b) first reported on the ability of A. piechaudii ARV8 to promote tomato plant growth in the presence of up to 172 mm NaCl salt. This work has served as a model for other researchers employing similar bacterial strains to facilitate the growth of plants in the presence of inhibitory salt levels (Gamalero et al., 2010; Nadeem et al., 2010; Ahmad et al., 2011; Siddikee et al., 2011; Chookietwattana and Maneewan, 2012; Karthikeyan et al., 2012; Bal et al., 2013; Ramadoss et al., 2013; Akhgar et al., 2014; Ali et al., 2014; Barnawal et al., 2014; Chang et al., 2014).
Fungal and Bacterial Pathogens
Ethylene levels inside plants increase following pathogen infection, and this induces the appearance of specific symptoms (van Loon et al., 2006). In this context, seedling inoculation with bacteria expressing ACC deaminase may reduce pathogen-induced ethylene, e.g. for soil-borne disease caused by pathogenic bacteria such as Pseudomonas syringae pv tomato (Indiragandhi et al., 2008), Agrobacterium tumefaciens (Toklikishvili et al., 2010; Hao et al., 2011), Erwinia spp. (Wang et al., 2000), and fungi, including Pythium ultimum (Wang et al., 2000), Pythium aphanidermatum (El-Tarabily, 2013), and Pyricularia oryzae (Amutharaj et al., 2012).
Nematodes
Recently, bacterial ACC deaminase has been identified as a key trait in suppression of the pathogenic nematode Bursaphelenchus xylophilus causing pine wilt disease (Nascimento et al., 2013). Thus, seedling inoculation with bacteria able to synthesize ACC deaminase may lead to plant resistance to nematode-induced diseases.
Metals and Organic Contaminants
Phytoremediation is the use of plants, able to tolerate/accumulate/degrade organic or inorganic chemicals and/or producing high biomass, to clean up polluted soils (Pilon-Smits, 2005). However, plants tolerant to xenobiotics do not develop high biomass, often limiting the practical application of this technology (Khan et al., 2000). PGPB can often facilitate phytoremediation (Glick, 2010) by promoting plant growth, improving their health, enhancing root development, or increasing plant tolerance to the stress imposed by environmental toxicants (Burd et al., 1998; Huang et al., 2004; Reed and Glick, 2005; Gamalero et al., 2009b; Gurska et al., 2009; Glick, 2012).
Flower Wilting
To extend the shelf life of cut flowers, treatments with, potentially environmentally harmful, chemical ethylene inhibitors are routinely performed (Reid and Wu, 1991). The application of bacteria that produce ACC deaminase to lower the amount of ethylene in cut flowers represents a safer alternative. To prolong the lifetime of cut flowers, bacterial cells must be taken up by the cut flowers. In this context, the use of ACC deaminase-expressing endophytes, which are adapted to live inside plant tissues, may assure the efficacy of this treatment. Consistent with this hypothesis, Ali et al. (2012) demonstrated that two endophytic bacterial strains, Pseudomonas fluorescens YsS6 and Pseudomonas migulae 8R6, both of which internally colonize the stems of the cut flowers, lower the flower ethylene levels and delay flower senescence by 2 to 3 d.
Rooting of Cuttings
The impact of inoculating plant cuttings with bacteria that are able to produce ACC deaminase was described by Mayak et al. (1999), who treated mung bean cuttings with P. putida GR12-2 or with its mutant lacking ACC deaminase activity. While the number of adventitious roots was similar in the two treatments, the length of the newly generated roots was significantly greater in mung bean cuttings inoculated with the wild-type strain. Similarly, carnation cuttings treated with a strain of Azospirillum brasilense engineered to synthesize ACC deaminase produced significantly more and longer roots than untreated cuttings (Li et al., 2005). Montero-Calasanz (2013) measured the rooting efficiency of olive (Olea europaea) cuttings following inoculation with five bacterial strains with different physiological traits: Pantoea spp. AG9, the only strain able to express ACC deaminase, was the most efficient strain in enhancing the rooting of these cuttings.
SUMMARY AND CONCLUSION
Plants that are grown in the field are subject to more or less continuous exposure to one stress after another, all of which can potentially inhibit plant growth and development. These stresses may be caused by biotic factors such as viruses, nematodes, insects, bacteria, or fungi or by abiotic factors such as extremes of temperature, high light, flooding, drought, the presence of toxic metals, and organic contaminants. While various plants may respond somewhat differently to stresses, nearly all plants respond to stress by producing ethylene. Lowering the amount of ethylene that is synthesized in response to stress through the application of ACC deaminase-producing bacteria can significantly decrease the extent of plant growth inhibition that accrues from the stress. From a practical perspective, as a consequence of the fundamental knowledge of plant growth-promoting bacterial modes of action that has been gained over the past 10 to 20 years, specifically emphasizing an understanding of the key role of ACC deaminase, this technology is currently accessible for use in agriculture, horticulture, and environmental cleanup technologies in both the developed and the developing world.
Glossary
- ACC
1-aminocyclopropane-1-carboxylate
- IAA
indole-3-acetic acid
- PGPB
plant growth-promoting bacteria
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, New York [Google Scholar]
- Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 57: 578–589 [DOI] [PubMed] [Google Scholar]
- Akhgar AR, Arzanlou M, Bakker PAHM, Hamidpour M (2014) Characterization of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-containing Pseudomonas spp. in the rhizosphere of salt-stressed canola. Pedosphere 24: 461–468 [Google Scholar]
- Alavanja MCR, Hoppin JA, Kamel F (2004) Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health 25: 155–197 [DOI] [PubMed] [Google Scholar]
- Ali S, Charles TC, Glick BR (2012) Delay of carnation flower senescence by bacterial endophytes expressing ACC deaminase. J Appl Microbiol 113: 1139–1144 [DOI] [PubMed] [Google Scholar]
- Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80: 160–167 [DOI] [PubMed] [Google Scholar]
- Amutharaj P, Sekar C, Natheer SE (2012) Intergeneric microbial coaggregates: bioinoculation effect of ACC deaminase positive wild type strains of Pseuodomonas and Paenibacillus, as coaggregates, on the maximization of ISR against Pyricularia oryzae in upland rice cv. ASD-19. CIBTech J Microbiol 1: 57–66 [Google Scholar]
- Arshad M, Shaharoona B, Mahmood T (2008) Inoculation with Pseudomonas spp. containing ACC deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere 18: 611–620 [Google Scholar]
- Bakker PA, Berendsen RL, Doornbos RF, Wintermans PC, Pieterse CM (2013) The rhizosphere revisited: root microbiomics. Front Plant Sci 4: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal HB, Nayak L, Das S, Adhya TK (2013) Isolation of ACC deaminase PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 366: 93–105 [Google Scholar]
- Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol Biochem 58: 227–235 [DOI] [PubMed] [Google Scholar]
- Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171: 884–894 [DOI] [PubMed] [Google Scholar]
- Barnawal D, Maji D, Bharti N, Chanotiya CS, Kalra A (2013) ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul 32: 809–822 [Google Scholar]
- Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (Plant Growth-Promoting Bacteria) and PGPB. Soil Biol Biochem 30: 1225–1228 [Google Scholar]
- Bayliss C, Bent E, Culham DE, MacLellan S, Clarke AJ, Brown GL, Wood JM (1997) Bacterial genetic loci implicated in the Pseudomonas putida GR12-2R3-anola mutualism: identification of an exudate-inducible sugar transporter. Can J Microbiol 43: 809–818 [DOI] [PubMed] [Google Scholar]
- Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181: 413–423 [DOI] [PubMed] [Google Scholar]
- Biswas JC, Ladha JK, Dazzo FB (2000) Rhizobia inoculation improves nutrient uptake and growth in lowland rice. Soil Sci Soc Am J 64: 1644–1650 [Google Scholar]
- Blaha D, Prigent-Combaret C, Mirza MS, Moënne-Loccoz Y (2006) Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol Ecol 56: 455–470 [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Yang SF (1980) Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol 65: 322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruto M, Prigen-Combaret C, Muller D, Moënne-Loccoz Y (2014) Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci Rpts 4: 6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64: 3663–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Gerhardt KE, Huang XD, Yu XM, Glick BR, Gerwing PD, Greenberg BM (2014) Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils. Int J Phytoremediation 16: 1133–1147 [DOI] [PubMed] [Google Scholar]
- Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol 71: 7271–7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chookietwattana K, Maneewan K (2012) Selection of efficient salt-tolerant bacteria containing ACC deaminase for promotion of tomato growth under salinity stress. Soil Environ 31: 30–36 [Google Scholar]
- Compant S, Clement C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42: 669–678 [Google Scholar]
- Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C (2008) Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiol Ecol 63: 84–93 [DOI] [PubMed] [Google Scholar]
- Czarny JC, Shah S, Glick BR (2007) Response of canola plants at the transcriptional level to expression of a bacterial ACC deaminase in the roots. In Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E, eds, Advances in Plant Ethylene Research. Springer, Dordrecht, Netherlands, pp 377–382 [Google Scholar]
- Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8: 1402–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhorn T, Fuqua C (2007) Biofilm formation by plant-associated bacteria. Annu Rev Microbiol 61: 401–422 [DOI] [PubMed] [Google Scholar]
- Duan J, Müller KM, Charles TC, Vesely S, Glick BR (2009) 1-Aminocyclopropane-1-carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb Ecol 57: 423–436 [DOI] [PubMed] [Google Scholar]
- Else MA, Jackson MB (1998) Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Aust J Plant Physiol 25: 453–458 [Google Scholar]
- El-Tarabily KA. (2013) Biocontrol of damping-off and root and crown rots of cucumber caused by Pythium aphanidermatum by ACC-deaminase producing endophytic actinomycetes. Phytopathology 103: 40 [Google Scholar]
- Gaiero JR, McCall CA, Thompson KA, Day NJ, Best AS, Dunfield KE (2013) Inside the root microbiome: bacterial root endophytes and plant growth promotion. Am J Bot 100: 1738–1750 [DOI] [PubMed] [Google Scholar]
- Galland M, Gamet L, Varoquaux F, Touraine B, Touraine B, Desbrosses G (2012) The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Sci 190: 74–81 [DOI] [PubMed] [Google Scholar]
- Gamalero E, Berta G, Glick BR (2009a) The use of microorganisms to facilitate the growth of plants in saline soils. In MS Khan, A Zaidi, J Musarrat, eds, Microbial Strategies for Crop Improvement. Springer-Verlag, Berlin, pp 1–22 [Google Scholar]
- Gamalero E, Berta G, Lingua G, Glick BR (2009b) Effects of plant growth-promoting bacteria and AM fungi on the response of plants to heavy metal stress. Can J Microbiol 55: 501–514 [DOI] [PubMed] [Google Scholar]
- Gamalero E, Berta G, Massa N, Glick BR, Lingua G (2008) Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiol Ecol 64: 459–467 [DOI] [PubMed] [Google Scholar]
- Gamalero E, Berta G, Massa N, Glick BR, Lingua G (2010) Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J Appl Microbiol 108: 236–245 [DOI] [PubMed] [Google Scholar]
- Gamalero E, Glick BR (2010) Bacterial ACC deaminase and IAA: interactions and consequences for plant growth in polluted environments. In Golubev IA, ed, Handbook of Phytoremediation. Nova Science Publishers, New York, pp 763–774 [Google Scholar]
- Gamalero E, Glick BR (2012) Ethylene and abiotic stress tolerance in plants. In P Ahmad, MNV Prasad, eds, Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. Springer-Verlag, Berlin, pp 395–412 [Google Scholar]
- García-Fraile P, Carro L, Robledo M, Ramírez-Bahena MH, Flores-Félix JD, Fernández MT, Mateos PF, Rivas R, Igual JM, Martínez-Molina E, et al. (2012) Rhizobium promotes non-legumes growth and quality in several production steps: towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 7: e38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41: 109–117 [Google Scholar]
- Glick BR. (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28: 367–374 [DOI] [PubMed] [Google Scholar]
- Glick BR (September 13, 2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica http://dx.doi.org/10.6064/2012/963401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169: 30–39 [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-containing soil bacteria. Eur J Plant Pathol 119: 329–339 [Google Scholar]
- Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190: 63–68 [DOI] [PubMed] [Google Scholar]
- Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39: 11–17 [Google Scholar]
- Gurska J, Wang W, Gerhardt KE, Khalid AM, Isherwood DM, Huang XD, Glick BR, Greenberg BM (2009) Field test of a multi-process phytoremediation system at a petroleum sludge contaminated land farm. Environ Sci Technol 43: 4472–4479 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Zamora ML, Martínez-Romero E (2001) Natural endophytic association between Rhizobium etli and maize (Zea mays L.). J Biotechnol 91: 117–126 [DOI] [PubMed] [Google Scholar]
- Hao Y, Charles TC, Glick BR (2011) An ACC deaminase containing A. tumefaciens strain D3 shows biocontrol activity to crown gall disease. Can J Microbiol 57: 278–286 [DOI] [PubMed] [Google Scholar]
- Hirsch PR, Mauchline TH (2012) Who’s who in the plant root microbiome? Nat Biotechnol 30: 961–962 [DOI] [PubMed] [Google Scholar]
- Honma M. (1983) Enzymatic determination of 1-aminocyclopropane-1-carboxylate deam-inase. Agric Biol Chem 47: 617–618 [Google Scholar]
- Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42: 1825–1831 [Google Scholar]
- Hontzeas N, Hontzeas CE, Glick BR (2006) Reaction mechanisms of the bacterial enzyme 1-aminocyclopropane-1-carboxylate deaminase. Biotechnol Adv 24: 420–426 [DOI] [PubMed] [Google Scholar]
- Hontzeas N, Richardson AO, Belimov AA, Safranova VI, Abu-Omar MM, Glick BR (2005) Evidence for horizontal gene transfer (HGT) of ACC deaminase genes. Appl Environ Microbiol 71: 7556–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontzeas N, Zoidakis J, Glick BR, Abu-Omar MM (2004) Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: a key enzyme in bacterial plant growth promotion. Biochim Biophys Acta 1703: 11–19 [DOI] [PubMed] [Google Scholar]
- Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130: 465–476 [DOI] [PubMed] [Google Scholar]
- Indiragandhi P, Anandham R, Kim K, Yim WJ, Madhaiyan M, Sa TM (2008) Induction of defense responses in tomato against Pseudomonas syringae pv. tomato by regulating the stress ethylene level with Methylobacterium oryzae CBMB20 containing 1-aminocyclopropane-1-carboxylate deaminase. World J Microbiol Biotechnol 24: 1037–1045 [Google Scholar]
- Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and characterization of ACC deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol 40: 1019–1025 [Google Scholar]
- Karthikeyan B, Joe MM, Islam MR, Sa T (2012) ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis 56: 77–86 [Google Scholar]
- Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41: 197–207 [DOI] [PubMed] [Google Scholar]
- Kuklinsky-Sobral J, Araújo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6: 1244–1251 [DOI] [PubMed] [Google Scholar]
- Leach AW, Mumford JD (2008) Pesticide Environmental Accounting: a method for assessing the external costs of individual pesticide applications. Environ Pollut 151: 139–147 [DOI] [PubMed] [Google Scholar]
- Li J, McConkey BJ, Cheng Z, Guo S, Glick BR (2013) Identification of plant growth-promoting bacteria-responsive proteins in cucumber roots under hypoxic stress using a proteomic approach. J Proteomics 84: 119–131 [DOI] [PubMed] [Google Scholar]
- Li Q, Saleh-Lakha S, Glick BR (2005) The effect of native and ACC deaminase-containing Azospirillum brasilense Cd1843 on the rooting of carnation cuttings. Can J Microbiol 51: 511–514 [DOI] [PubMed] [Google Scholar]
- Lupway N, Clayton G, Hanson K, Rice W, Bierderbeck V (2004) Endophytic rhizobia in barley, wheat, and canola roots. Can J Plant Sci 84: 37–45 [Google Scholar]
- Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69: 4396–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR (1999) Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18: 49–53 [DOI] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR (2004a) Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166: 525–530 [DOI] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR (2004b) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42: 565–572 [DOI] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37: 634–663 [DOI] [PubMed] [Google Scholar]
- Minami R, Uchiyama K, Murakami T, Kawai J, Mikami K, Yamada T, Yokoi D, Ito H, Matsui H, Honma M (1998) Properties, sequence, and synthesis in Escherichia coli of 1-aminocyclopropane-1-carboxylate deaminase from Hansenula saturnus. J Biochem 123: 1112–1118 [DOI] [PubMed] [Google Scholar]
- Montero-Calasanz MC, Santamaría C, Albareda M, Daza A, Duan J, Glick BR, Camacho M (2013) Alternative rooting induction of semi-hardwood olive cuttings by several auxin-producing bacteria for organic agriculture systems. Span J Agric Res 11: 146–154 [Google Scholar]
- Murset V, Hennecke H, Pessi G (2012) Disparate role of rhizobial ACC deaminase in root-nodule symbioses. Symbiosis 57: 43–50 [Google Scholar]
- Nadeem SM, Zahair ZA, Naveed M, Asghar HN, Asghar M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74: 533–542 [Google Scholar]
- Nascimento FX, Brígido C, Glick BR, Oliveira S (2012) ACC deaminase genes are conserved among Mesorhizobium species able to nodulate the same host plant. FEMS Microbiol Lett 336: 26–37 [DOI] [PubMed] [Google Scholar]
- Nascimento FX, Rossi MJ, Soares CRFS, McConkey BJ, Glick BR (2014) New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS ONE 9: e99168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento FX, Vicente CSL, Barbosa P, Espada M, Glick BR, Oliveira S, Mota M (2013) The use of the ACC deaminase producing bacterium Pseudomonas putida UW4 as a biocontrol agent for pine wilt disease. BioControl 58: 427–433 [Google Scholar]
- Nukui N, Minamisawa K, Ayabe S, Aoki T (2006) Expression of the 1-aminocyclopropane-1-carboxylic acid deaminase gene requires symbiotic nitrogen-fixing regulator gene nifA2 in Mesorhizobium loti MAFF303099. Appl Environ Microbiol 72: 4964–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42: 207–220 [DOI] [PubMed] [Google Scholar]
- Penrose DM, Glick BR (2001) Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can J Microbiol 47: 368–372 [DOI] [PubMed] [Google Scholar]
- Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocycopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47: 77–80 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E. (2005) Phytoremediation. Annu Rev Plant Biol 56: 15–39 [DOI] [PubMed] [Google Scholar]
- Prayitno J, Rolfe BG, Mathesius U (2006) The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol 142: 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent-Combaret C, Blaha D, Pothier JF, Vial L, Poirier MA, Wisniewski-Dyé F, Moënne-Loccoz Y (2008) Physical organization and phylogenetic analysis of acdR as leucine-responsive regulator of the 1-aminocyclopropane-1-carboxylate deaminase gene acdS in phytobeneficial Azospirillum lipoferum 4B and other Proteobacteria. FEMS Microbiol Ecol 65: 202–219 [DOI] [PubMed] [Google Scholar]
- Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K (2013) Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51: 1061–1069 [DOI] [PubMed] [Google Scholar]
- Reed MLE, Glick BR (2013) Applications of plant growth-promoting bacteria for plant and soil systems. In Gupta VK, Schmoll M, Maki M, Tuohy M, Mazutti MA, eds, Applications of Microbial Engineering. Taylor and Francis, Enfield, CT, pp 181–229 [Google Scholar]
- Reid MS, Wu MJ (1991) Ethylene in flower development and senescence. In Mattoo AK, Suttle JC, eds The Plant Hormone Ethylene. CRC Press, Boca Raton, FL, pp 215–234 [Google Scholar]
- Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14: 435–443 [DOI] [PubMed] [Google Scholar]
- Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86: 407–421 [Google Scholar]
- Sarma RK, Saikia R (2014) Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 377: 111–126 [Google Scholar]
- Shakir MA, Asghari B, Arshad M (2012) Rhizosphere bacteria containing ACC deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ 31: 108–112 [Google Scholar]
- Sheehy RE, Honma M, Yamada M, Sasaki T, Martineau B, Hiatt WR (1991) Isolation, sequence, and expression in Escherichia coli of the Pseudomonas sp. strain ACP gene encoding 1-aminocyclopropane-1-carboxylate deaminase. J Bacteriol 173: 5260–5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddikee MA, Glick BR, Chauhan PS, Yim WJ, Sa T (2011) Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing ACC deaminase activity. Plant Physiol Biochem 49: 427–434 [DOI] [PubMed] [Google Scholar]
- Stearns JC, Woody OZ, McConkey BJ, Glick BR (2012) Effects of bacterial ACC deaminase on Brassica napus gene expression. Mol Plant Microbe Interact 25: 668–676 [DOI] [PubMed] [Google Scholar]
- Thakore Y. (2006) The biopesticide market for global agricultural use. Ind Biotechnol (New Rochelle NY) 2: 194–208 [Google Scholar]
- Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS ONE 6: e17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toklikishvili N, Dandurishvili N, Vainstein A, Tediashvili M, Giorgobiani N, Lurie S, Szegedi E, Glick BR, Chernin L (2010) Inhibitory effect of ACC deaminase-producing bacteria on crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol 59: 1023–1030 [Google Scholar]
- Tsakelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol 42: 117–126 [PubMed] [Google Scholar]
- van Loon LC, Geraats BPJ, Linthorst HJM (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11: 184–191 [DOI] [PubMed] [Google Scholar]
- Wang C, Knill E, Glick BR, Défago G (2000) Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol 46: 898–907 [DOI] [PubMed] [Google Scholar]
- Wilson D. (1995) Endophyte: the evolution of a term and clarification of its use and definition. Oikos 72: 274–276 [Google Scholar]
- Yoon GM, Kieber JK (March 11, 2013) 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants http://dx.doi.org/10.1093/aobpla/plt017 [Google Scholar]
- Yoon HS, Andersen RA, Boo SM, Bhattacharya D (2009) Stramenopiles. In M Schaechter, ed, Encyclopedia of Microbiology, Ed 3. Academic Press, New York, pp 721–731 [Google Scholar]
- Zafarul Hye M, Farooq HM, Zahir ZA, Hussain M, Hussain A (2014) Application of ACC-deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int J Agric Biol 16: 591–596 [Google Scholar]
- Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162: 304–318 [DOI] [PMC free article] [PubMed] [Google Scholar]