Group VII ethylene response factors are key regulators of signal transduction at the interface of ethylene, oxygen, and nitric oxide signaling.
Abstract
The group VII ethylene response factors (ERFVIIs) are plant-specific transcription factors that have emerged as important regulators of abiotic and biotic stress responses, in particular, low-oxygen stress. A defining feature of ERFVIIs is their conserved N-terminal domain, which renders them oxygen- and nitric oxide (NO)-dependent substrates of the N-end rule pathway of targeted proteolysis. In the presence of these gases, ERFVIIs are destabilized, whereas an absence of either permits their accumulation; ERFVIIs therefore coordinate plant homeostatic responses to oxygen availability and control a wide range of NO-mediated processes. ERFVIIs have a variety of context-specific protein and gene interaction partners, and also modulate gibberellin and abscisic acid signaling to regulate diverse developmental processes and stress responses. This update discusses recent advances in our understanding of ERFVII regulation and function, highlighting their role as central regulators of gaseous signal transduction at the interface of ethylene, oxygen, and NO signaling.
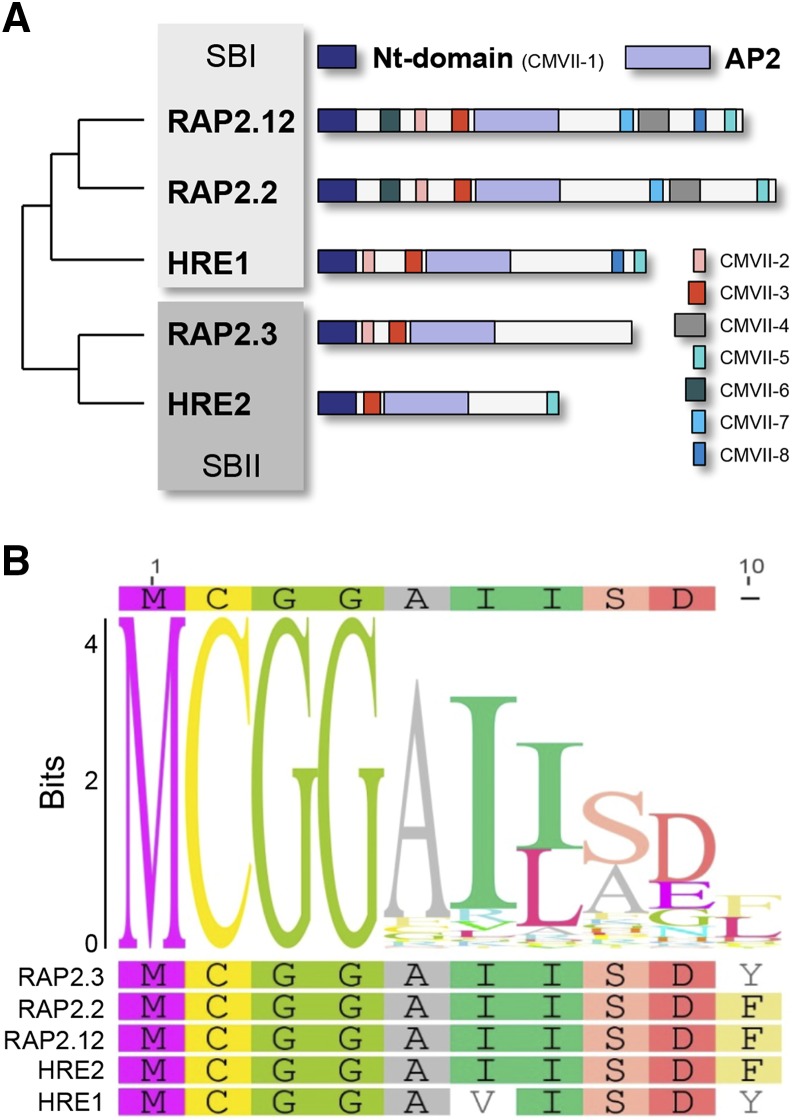
The ethylene response factor (ERF) transcription factors are plant-specific proteins characterized by a single DNA-binding APETALA2 (AP2)/ethylene-responsive element-binding protein domain (Nakano et al., 2006; Licausi et al., 2013). This domain was first discovered in ethylene-responsive element-binding proteins in tobacco (Nicotiana tabacum) and shown to bind to the GCC element of ethylene-responsive elements in promoters (Ohme-Takagi and Shinshi, 1995). ERFs constitute one of the largest transcription factor families in plants: a genome-wide analysis found that rice (Oryza sativa) has 139 and Arabidopsis (Arabidopsis thaliana) 122, clustered into 15 and 12 subgroups, respectively, based on the presence of conserved features (Nakano et al., 2006). One of these subgroups, the group VII ethylene response factors (ERFVIIs), has been heavily studied in recent years due to the roles its members play in orchestrating a wide range of plant growth, development, and stress responses. In addition to the AP2 domain, ERFVIIs are characterized by several other motifs, including, most importantly, N-terminal (Nt)-MCGGAII/L (Nakano et al., 2006; van Veen et al., 2014), which is a conserved feature of ERFVIIs in all flowering plant species (Fig. 1, A and B). A recent study investigated the phylogenetic origin of ERFVIIs using an analysis of synteny in 16 angiosperm species, revealing that ERFVIIs originated from two ancestral genes, and that subsequent gene duplication resulted in the appearance of five members before mono- and dicotyledonous plants divided (Fig. 1A). Of the five Arabidopsis ERFVIIs, HYPOXIA RESPONSIVE1 (HRE1), RELATED TO APETALA2.2 (RAP2.2), and RAP2.12 were shown to share syntenic origins, whereas HRE2 and RAP2.3 evolved from a separate ancestral protein (van Veen et al., 2014).
Figure 1.
Phylogeny and features of ERFVII transcription factors. A, Phylogenetic relationship of the five ERFVIIs in Arabidopsis, showing their groupings into two syntenic blocks (SBI and SBII; van Veen et al., 2014). A schematic representation of each family member is shown, including the Nt (conserved motif [CM]VII-1) and AP2 domains, which are defining features of the ERFVIIs found in all family members, and several other amino acid motifs that are conserved in some but not all ERFVIIs (Nakano et al., 2006). B, Sequence logo showing the first 10 amino acids of ERFVII protein sequences from Zea mays, Brachypodium distachyon, rice, Arabidopsis lyrata, Arabidopsis, Prunus persica, Medicago truncatula, and Populus trichocarpa. Logo was generated from 89 sequences using Geneious (Blosum 62 matrix, gap open penalty 15, gap extension penalty 3; Kearse et al., 2012). The observed frequency of amino acids at each position is measured in bits. The individual Arabidopsis ERFVII Nts are shown underneath.
ERFVIIs CONTROL FLOODING RESPONSES
A major role for ERFVIIs in controlling flooding and low oxygen (hypoxia) tolerance in plants has been discovered in recent years. For example, in Arabidopsis, hre1hre2 mutant seedlings showed drastically reduced survival in anoxia (0% oxygen), whereas ectopic overexpression of HRE1 enhanced survival by increasing the expression of core hypoxia-responsive genes, including ALCOHOL DEHYDROGENASE1 (ADH1; Licausi et al., 2010; Hess et al., 2011). A similar function was observed for RAP2.2, where ectopic expression increased ADH1 transcripts in hypoxia (5% oxygen), whereas rap2.2 mutants had reduced survival under this stress (Hinz et al., 2010). Other studies in Arabidopsis have also pointed to key roles for these proteins in controlling hypoxia and ethylene-regulated submergence responses (Papdi et al., 2008; Yang et al., 2011).
Flood-tolerant plants typically cope with submergence through two opposite survival strategies: quiescence during flash floods and escape during deep-water floods (Voesenek and Bailey-Serres, 2015). The quiescence strategy is characterized by reduced growth and respiration, metabolic changes that enhance use of carbohydrate reserves, induced responses against oxidative damage, and inhibition of floral initiation (Peña-Castro et al., 2011; Bailey-Serres et al., 2012). In contrast, the deep-water escape strategy requires rapid growth of petioles and stems, and vascular changes to facilitate gas diffusion (Bailey-Serres et al., 2012; Voesenek and Bailey-Serres, 2015). In rice, three different members of the ERFVII family control these antithetical survival strategies: SUBMERGENCE1A (SUB1A), one of three ERFVIIs located at the Sub1 locus, promotes quiescence, whereas SNORKEL1 and SNORKEL2 regulate escape (Xu et al., 2006; Hattori et al., 2009). These ERFVIIs are transcriptionally induced by ethylene, which rapidly accumulates in flooded tissues, but regulate their respective growth strategies by modifying GA signaling in opposite directions. Ethylene induction of SUB1A increases the accumulation of SLENDER RICE1 (SLR1) and SLENDER RICE1 LIKE1, two GA-labile signaling repressors that inhibit the transcription of GA-inducible genes, arresting elongation and promoting catabolism of carbohydrates (Fukao et al., 2006; Fukao and Bailey-Serres, 2008; Hirano et al., 2012). Furthermore, there is a strong up-regulation of GA-deactivating GA 2-oxidase in SUB1A-containing lines, which would reduce the levels of active GA and enhance SLR1 stability (Jung et al., 2010). In contrast, during flooding in deep-water rice varieties, the induction of SNORKEL1 and SNORKEL2 by ethylene triggers internode elongation by up-regulating GA 20-oxidase, which increases the accumulation of active GA (Raskin and Kende, 1984; Hattori et al., 2009; Ayano et al., 2014). Similar quiescent and escape-like flooding responses have also recently been identified in two related Rumex spp. species from contrasting hydrological niches, which may also be regulated by ERFVIIs (van Veen et al., 2013).
Together with ethylene and GA, abscisic acid (ABA) is also an important hormone involved in the signaling network regulated by SUB1A in rice (Hoffmann-Benning and Kende, 1992; Fukao and Bailey-Serres, 2008; Chen et al., 2010; Fukao et al., 2011). When flood waters subside, desubmergence can lead to rapid dehydration of the leaves (Setter et al., 2010; Fukao et al., 2011). ABA levels decline upon submergence (Fukao and Bailey-Serres, 2008), and SUB1A prevents leaf desiccation by increasing ABA responsiveness, which promotes the expression of several drought-associated DEHYDRATION RESPONSE ELEMENT-BINDING PROTEIN1 and LATE EMBRYOGENESIS ABUNDANT genes, limits the spread of reactive oxygen species (ROS), and induces enzymes (e.g. superoxide dismutases and catalases) that neutralize the oxidative damage associated with dehydration (Fukao et al., 2011). The role of SUB1A in fine tuning ROS levels seems to be also essential for flooding tolerance, specifically regulating the role of hydrogen peroxide in aerenchyma formation, but at the same time limiting its toxicity during the stress (Jung et al., 2010; Parlanti et al., 2011). Furthermore, there is evidence supporting a role for SUB1A in maintaining the levels of chlorophyll and carbohydrates during leaf senescence promoted by ethylene, a process associated with several stresses, including drought (Fukao et al., 2012). It has also been reported that GA 2-oxidase may inhibit leaf growth and promote root growth during drought (Wang et al., 2011). Intriguingly, recent data show a positive role in drought of GA-INSENSITIVE DWARF1, the GA receptor that counteracts SUB1A action by degrading SLR1 (Du et al., 2014); future studies will be required to unravel the interaction between these factors during dehydration.
ERFVIIs ARE SUBSTRATES OF THE N-END RULE PATHWAY OF PROTEOLYSIS
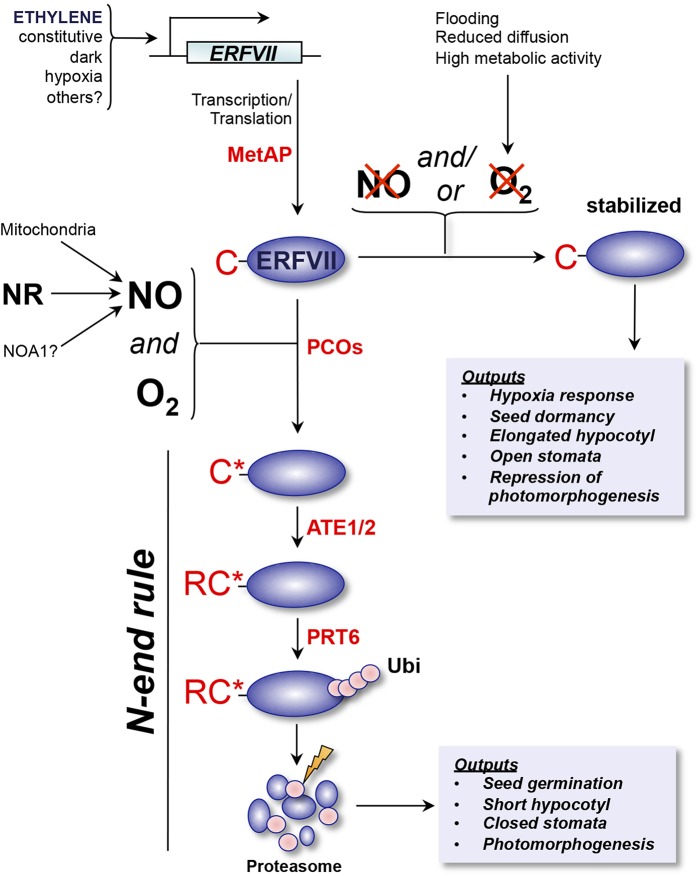
In addition to ethylene accumulation, another key signal associated with flooding is reduced oxygen availability (van Dongen and Licausi, 2015). It has been shown that ERFVIIs function as homeostatic sensors of hypoxia via the N-end rule pathway of targeted proteolysis (Gibbs et al., 2011; Licausi et al., 2011), an ancient and conserved branch of the ubiquitin proteasome system that relates the stability of a protein to the nature of its N terminus (Fig. 2; Bachmair et al., 1986; Varshavsky, 2011; Gibbs et al., 2014a). Moreover, this ERFVII-based system has also emerged as a critical mechanism for NO sensing (Gibbs et al., 2014b), highlighting a central role for ERFVIIs and their proteolytic control by the N-end rule pathway in gaseous signal transduction.
Figure 2.
Oxygen (O2) and nitric oxide (NO) signal transduction via N-end rule regulation of ERFVII stability. Arabidopsis ERFVII transcription factors are transcribed either constitutively and/or in response to several upstream signals, including ethylene. MET AMINOPEPTIDASE (MetAP) enzymes cleave Nt-Met cotranslationally to reveal Nt-Cys (C). In the presence of oxygen and NO (which may be derived from a variety of cellular sources: NR, nitrate reductase; NOA1, nitric oxide associated protein1), Nt-Cys is oxidized to Cys-sulfinic or Cys-sulfonic acid (C*). This oxidation is facilitated by PLANT CYS OXIDASE (PCO) enzymes (Weits et al., 2014). Oxidized Nt-Cys is then arginylated (R) by ARGINYL tRNA TRANSFERASES (ATE1/2). Nt-Arg-ERFVII is recognized by the N-end rule E3 ligase (N-recognin) PROTEOLYSIS6 (PRT6), which targets the protein for proteasomal degradation via polyubiquitination (Ubi; Gibbs et al., 2011; Licausi et al., 2011). ERFVII degradation initiates germination, inhibits hypocotyl growth, stimulates stomatal closure, represses the hypoxia transcriptional response, and promotes photomorphogenesis. In conditions in which either oxygen or NO (or both) become limiting, Nt-Cys oxidation is inhibited, and ERFVIIs are stabilized (Gibbs et al., 2014b). Stable ERFVIIs promote anaerobic gene expression, maintain seed dormancy and open stomata, promote hypocotyl growth, and repress photomorphogenesis. Regulation of ERFVII stability by the N-end rule pathway therefore controls the homeostatic response to oxygen and NO availability.
ERFVIIs and Oxygen Sensing
A defining feature of the ERFVIIs is the presence of an Nt motif that initiates with the residues Met-Cys (Fig. 1, A and B; Gibbs et al., 2011; Licausi et al., 2011). This motif is highly conserved across ERFVIIs in flowering plants and functions as an N-degron, rendering these proteins substrates of the N-end rule pathway. The Nt-Met of ERFVIIs is cotranslationally cleaved by cytosolic MET AMINOPEPTIDASEs to reveal a tertiary destabilizing Nt-Cys residue that is susceptible to oxidative modifications to produce an oxidized Cys (Cys sulfinic or sulfonic acid; Hu et al., 2005), which is then arginylated by ATEs. Nt-Arg-Cys is then recognized by the N-end rule pathway E3 ligase (N-recognin) PRT6, which targets the ERFVII for destruction via polyubiquitination (Fig. 2). In the presence of oxygen (and NO; see next section), oxidation of Nt-Cys therefore catalyzes protein degradation, whereas under hypoxia, ERFVIIs accumulate to coordinate the transcriptional response to oxygen limitation (Gibbs et al., 2011; Licausi et al., 2011). In Arabidopsis, RAP2.12, RAP2.2, and RAP2.3 are all constitutively expressed, whereas HRE1 and HRE2 are hypoxia inducible, suggesting that there is a cascade of transcription and stabilization in response to declining oxygen levels, and that individual ERFVIIs have different contributions to the response (Licausi et al., 2010; Bui et al., 2015).
It was hypothesized that reliance upon spontaneous Nt-Cys oxidation alone would not allow plants to fine tune their response to hypoxia, and would instead expose ERFVIIs to unregulated fluctuations in cell redox status. The stability of the mammalian hypoxia sensor protein HYPOXIA INDUCIBLE FACTOR1α is regulated in an oxygen-dependent manner by prolyl hydroxylases (Kaelin and Ratcliffe, 2008). A survey of hypoxia-responsive genes in Arabidopsis identified several PCO enzymes, which were shown to oxidize Nt-Cys using oxygen as a cosubstrate (Weits et al., 2014). These enzymes promote degradation of RAP2.12 in the presence of oxygen, and therefore play a similar regulatory role to mammalian prolyl hydroxylases (Weits et al., 2014). PCOs were shown to counteract RAP2.12-mediated induction of hypoxia-responsive reporter genes, and hypoxic induction of PCO1 and PCO2 indicates that they are important for dampening anaerobic gene transcription through negative regulation of RAP2.12, and likely the other ERFVIIs (Weits et al., 2014).
Interestingly, analysis of rice SUB1A-1 demonstrated that it is not an N-end rule substrate in vitro, in contrast to all five of the Arabidopsis ERFVIIs and at least one barley (Hordeum vulgare) ERFVII (Gibbs et al., 2011; Mendiondo et al., 2015). Enhanced tolerance of rice varieties carrying the SUB1A-1 gene might therefore be due to the increased stability of the protein, although more research is needed to fully support this hypothesis. Perhaps the semiaquatic nature of rice has placed evolutionary pressure on ERFVII dynamics to enhance survival in fluctuating water schemes; it will be interesting to see whether ERFVIIs from other wetland species, for example, those from Rumex spp. (van Veen et al., 2014), are also uncoupled from N-end rule regulation.
Arabidopsis N-end rule pathway mutants have altered responses to hypoxia or flooding, either enhancing or negatively impacting survival rates, depending upon the context of the stress and recovery conditions (Gibbs et al., 2011; Licausi et al., 2011; Riber et al., 2015). This indicates that the N-end rule pathway is a promising target for manipulating flooding tolerance in crops. In barley, the ERFVII BARLEY ERF1 was shown to be a putative N-end rule substrate in vitro, and posttranscriptional accumulation of an artificial N-end rule reporter protein consisting of the ERFVII Nt domain fused to GUS (MCGGAIL-GUS) was observed under waterlogged conditions, indicating that ERFVIIs are also stabilized by low oxygen in monocots (Mendiondo et al., 2015). Barley PRT6 (HvPRT6) RNA interference lines with reduced HvPRT6 expression had increased levels of anaerobic response gene transcripts (including ADHs), similar to what is observed in Arabidopsis N-end rule mutants (Gibbs et al., 2011; Licausi et al., 2011). Furthermore, RNA interference lines performed better than null controls under waterlogging stress, as evidenced by retention of chlorophyll, increased biomass, and sustained yield poststress (Mendiondo et al., 2015). This translational study highlights the value of targeting ERFVIIs and the N-end rule pathway for engineering flooding tolerance in agronomically important species.
A recent study has shown that hypoxia can also act as an important environmental positional cue (Abbas et al., 2015). It was found that low oxygen levels repress photomorphogenesis in dicot species, promoting the maintenance of a skotomorphogenic developmental program. This response was linked to stabilized ERFVIIs, which actively maintained a closed apical hook, and repressed chlorophyll biosynthesis and cotyledon greening. Counterintuitively, hypoxic conditions were beneficial to seedlings, helping to protect them from photooxidative damage following extended darkness and dramatically enhancing survival rates once light was perceived (Abbas et al., 2015). Remarkably, hypocotyl elongation still occurred under hypoxia, demonstrating an active role for oxygen sensing by the ERFVIIs in protecting the stem cell niche, as opposed to inducing a quiescent-like state, as can occur during flooding stress. This study indicates that oxygen availability may have a wider role in regulating general plant growth and development than has been previously considered.
NO Signal Transduction via Proteolytic Control of ERFVIIs
An important signal associated with flood-induced hypoxia is the accumulation of NO, which alongside the activity of class-1 nonsymbiotic hemoglobins plays a role in balancing the antioxidant status of the cell (van Dongen and Licausi, 2015). NO is a highly reactive gaseous signaling molecule that is known to regulate a diverse range of processes in plants (Yu et al., 2014). In contrast to animals, which produce NO through the action of NO synthases, the origins of NO in plants are less well defined, and production can occur through several different reductive and oxidative pathways (Yu et al., 2014). NO typically induces effects through covalent modification of proteins, thereby altering function, such as via S-nitrosylation, Tyr-nitration, and metal nitrosylation (Besson-Bard et al., 2008). Cys S-nitrosylation has been shown to control a number of key regulatory proteins during plant stress responses. For example, in Arabidopsis, S-nitrosylation of the NADPH oxidase RESPIRATORY BURST OXIDASE HOMOLOG D regulates the salicylic acid-induced hypersensitive response (Yun et al., 2011), whereas S-nitrosylation of SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6/OPEN STOMATA1 negatively regulates ABA signaling in guard cells (Wang et al., 2015). However, in these instances, the downstream effect of this modification is process or stress specific, and no unifying sensing mechanism for coordinating multiple transcriptional, developmental, and physiological responses to NO had been identified until recently.
Destabilization of Nt-Cys-initiating REGULATOR OF G PROTEIN SIGNALING (RGS) substrates during cardiovasculature development in mammals requires NO in addition to oxygen (Hu et al., 2005; Jaba et al., 2013). It was hypothesized that Nt-Cys of RGS proteins is first S-nitrosylated, and then subsequently further oxidized to permit arginylation and degradation (Hu et al., 2005). An analysis of ERFVII stability revealed that ERFVII degradation is also dependent on NO, indicating that a similar mechanism occurs in plants (Fig. 2; Gibbs et al., 2014b). Under NO-limited conditions, such as in the nitrate reductase1 (nia1)nia2 double mutant or using pharmacological NO scavengers, ERFVII proteins are stabilized. Remarkably, prt6 and ate1ate2 N-end rule mutants, in which ERFVIIs constitutively accumulate, were completely insensitive to exogenous NO for a wide range of responses, including induction of germination, inhibition of hypocotyl elongation in the dark, and stomatal closure, suggesting that N-end rule-mediated proteolysis is essential for NO signal transduction. Furthermore, the NO insensitivity of N-end rule mutants was genetically linked to ERFVIIs for each of the processes investigated, revealing that ERFVIIs play a key role in regulating plant NO responses (Gibbs et al., 2014b). It will be important to elucidate the exact mechanism by which NO controls the stability of these transcription factors and the relationship between NO and oxygen during this process; for example, it is not yet known if NO spontaneously modifies ERFVIIs, or whether the effect of NO on their stability is dependent on enzymatic activity or occurs indirectly.
REGULATION OF PLANT RESPONSES TO OTHER ENVIRONMENTAL STRESSES BY ERFVIIs
In addition to hypoxia, ERFVIIs from a range of flowering plant species enhance tolerance to other abiotic and biotic stresses. For example, a recent study of Arabidopsis ERFVIIs found that the constitutively expressed group members also regulate responses to oxidative and osmotic stresses, which are both also associated with submergence (Papdi et al., 2015). ERFVII genes are up-regulated in response to phytohormones and stresses, including ethylene, ABA, sodium chloride, salicylic acid, cold and heat, drought, and osmotic stress (Yi et al., 2004; Jung et al., 2007; Xu et al., 2007; Zhang et al., 2009, 2010; Park et al., 2011; Chen et al., 2012; Zhu et al., 2013; Yang et al., 2014). Pathogen infection was shown to increase expression of RAP2.2 in Arabidopsis in response to Botrytis cinerea (Zhao et al., 2012), BENZOTHIADIAZOLE (bth)-INDUCED ERF1 (OsBIERF1) and OsBIERF4 in rice in response to Magnaporthe grisea (Cao et al., 2006), PATHOGEN FREEZING TOLERANCE PROTEIN1 (CaPF1) in Capsicum annuum in response to Xanthomonas axonopodis (Yi et al., 2004), GmERF3 in soybean (Glycine max) in response to soybean mosaic virus (Zhang et al., 2009), and wheat (Triticum aestivum) TaERF1 in response to infection with Blumeria graminis (Xu et al., 2007). Ectopic expression of ERFVIIs in transgenic plants increased cross tolerance to multiple stresses. Arabidopsis HRE2 overexpression increased tolerance to salt and mannitol, whereas the hre2 mutant showed higher sensitivity to these stresses (Park et al., 2011). CaPF1 overexpressed in Arabidopsis and tobacco increased tolerance to freezing and Pseudomonas syringae (Yi et al., 2004), and in Pinus virginiana (Virginia pine), to the heavy metals cadmium, copper, and zinc; to heat; and to the pathogens Bacillus thuringiensis and Staphylococcus epidermidis (Tang et al., 2005). Ectopic expression of GmERF3 in transgenic tobacco enhanced resistance to Ralstonia solanacearum, Alternaria alternata, and tobacco mosaic virus, and improved tolerance to salt and dehydration (Zhang et al., 2009). Expression of tomato (Solanum lycopersicum) JASMONATE AND ETHYLENE RESPONSE FACTOR1 in tobacco and rice led to increased tolerance to salt and drought (Zhang et al., 2004, 2010), and in rice, resistance to Rhizoctonia solani (Pan et al., 2014). Ectopic expression of TaERF1 and barley ROOT ABUNDANT FACTOR in Arabidopsis led to increased tolerance, respectively, to salt, drought, cold, B. cinerea, and R. solanacearum (Jung et al., 2007; Xu et al., 2007). Transgenic tobacco expressing the Jatropha curcas ERFVII JcERF1 showed increased salt tolerance (Yang et al., 2014).
In many cases, similar alterations associated with ectopic expression hint at the downstream molecular and biochemical mechanisms that are enhanced by overexpressing ERFVIIs. There is certainly evidence of repression of ROS production by ERFVIIs. Tobacco BRIGHT YELLOW-2 cells expressing Arabidopsis RAP2.3 were more resistant to hydrogen peroxide (H2O2), and showed increased expression of the H2O2-induced GLUTATHIONE S-TRANSFERASE6 gene (Ogawa et al., 2005), whereas transgenic Arabidopsis expressing HRE2 showed increased tolerance to methyl viologen (MV)-induced oxidative stress and lower levels of ROS in response to high salt (Park et al., 2011). Levels of several antioxidant enzymes were increased in Virginia pine overexpressing CaPF1 (Tang et al., 2005). It was also previously shown that the Sub1 locus increases seedling tolerance to MV and H2O2 due to enhanced transcript levels of genes encoding ascorbate peroxidase, superoxide dismutase, and catalase (Fukao et al., 2011). In fact, transcripts for SUB1A and several other ERFVIIs increase in response to MV treatment (Jung et al., 2007; Fukao et al., 2011; Park et al., 2011). In addition, in several cases, overexpression of ERFVIIs from a variety of species resulted in increased expression of pathogenesis-related genes, many of which contain GCC boxes in their promoters, suggesting the mechanism through which ERFVIIs may increase tolerance to pathogens (Yi et al., 2004; Ogawa et al., 2005; Jung et al., 2007; Xu et al., 2007; Zhang et al., 2009).
To understand how ectopic expression of ERFVIIs regulates plant responses to diverse stresses, it will be essential to determine the mechanism controlling N-end rule (and potentially other)-mediated protein stabilization and destabilization. None of the published studies of ectopically expressed ERFVIIs from non-Arabidopsis species have analyzed levels of transgenic ERFVII protein, but as similar phenotypes are invariably observed, it is very likely that their overexpression overrides the destabilizing function of the N-end rule pathway. As it has been shown (at present, only in vitro) that SUB1A is not a substrate of the N-end rule pathway (Gibbs et al., 2011), it is likely that this protein is constitutively stable, thus mimicking the phenotypes of ectopically expressed ERFVIIs. It is unlikely that oxygen levels vary under the nonhypoxic abiotic and biotic stresses analyzed, suggesting that either NO (Gibbs et al., 2014b) or protection of the N terminus (Shemorry et al., 2013) may be involved in modulating stability.
KEY INTERACTIONS MEDIATING ERFVII FUNCTION
How do the ERFVII transcription factors control such a diverse range of plant developmental and stress responses, and how do they distinguish between oxygen and NO signals to appropriately regulate gene expression? It is probable that several factors are involved, including diversity in gene targets, differences in temporal and tissue-specific expression or subcellular localization, and context-specific protein-protein interactions. Variation in gene targets and interaction partners is conceivable, since comparative analysis of ERFVIIs reveals that they are highly variable in sequence length and identity outside of the N-terminal and AP2 domains (Fig. 1A; Nakano et al., 2006). For example, Arabidopsis RAP2.3 was shown to associate with OCTOPINE SYNTHASE GENE ELEMENT BINDING FACTOR4 (Büttner and Singh, 1997), and RAP2.2 with SEVEN IN ABSENTIA OF ARABIDOPSIS2 (SINAT2; Welsch et al., 2007). SINAT2 is a REALLY INTERESTING NEW GENE E3 ligase, and a recent study showed that GFP-RAP2.12, in which the N-degron is removed, was stabilized in SINAT1/2-silenced Arabidopsis lines (Papdi et al., 2015). Furthermore, an N-terminal YELLOW FLUORESCENT PROTEIN-RAP2.3 fusion was also shown to be degraded in response to light independently of the Cys-2 N-degron (Abbas et al., 2015). Both of these findings indicate that ERFVII stability is regulated by more than one proteolytic mechanism, suggesting that complex posttranslational control of ERFVIIs occurs in plants. ERFVIIs have previously been shown to associate with a range of promoter DNA motifs, including GCC boxes (Büttner and Singh, 1997; Zhang et al., 2004; Gibbs et al., 2014b) and the ATCTA sequence (Welsch et al., 2007), suggesting they have diverse gene targets. Furthermore, there is evidence that posttranslational phosphorylation by kinases might affect ERFVII activity (Cheong et al., 2003; Xu et al., 2007). Combined with these previous reports, recent studies indicate that ERFVIIs function as promiscuous transcription factors, providing mechanistic insight into how they regulate diverse signal- and context-specific responses.
ERFVII-Protein Interactions during the Low-Oxygen Response
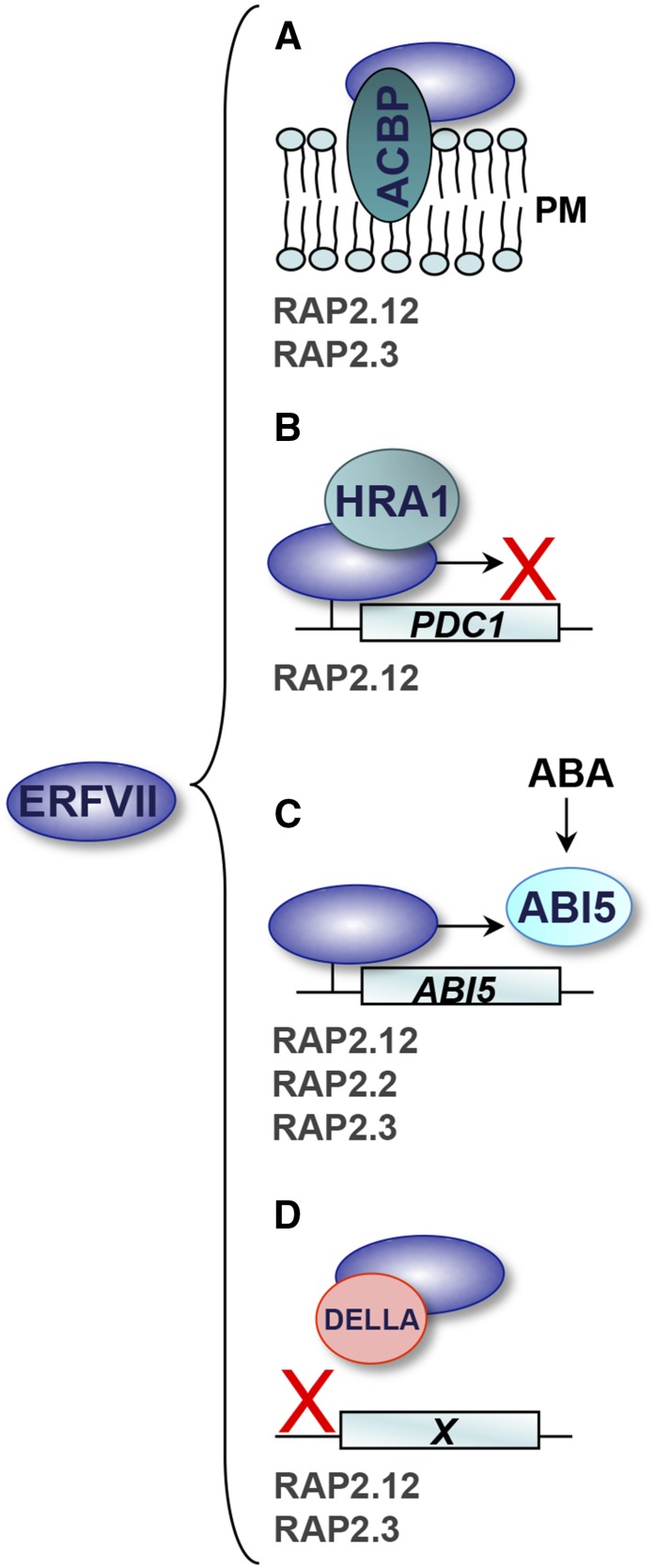
Licausi et al. (2011) discovered that, under normoxic conditions, RAP2.12 is localized to the plasma membrane (PM) via interaction with acyl-CoA binding protein1 (ACBP1) and ACBP2, two PM-associated members of the six-member ACBP family (Fig. 3A; Xiao and Chye, 2009; Licausi et al., 2011). RAP2.3 had also previously been identified as a direct interaction partner of ACBP2 (Li and Chye, 2004). In normoxia, this protein association seems to protect a pool of ERFVIIs from degradation, whereas hypoxia induces a localization shift from the PM to the nucleus (Licausi et al., 2011). This relocalization is triggered once oxygen levels decrease to approximately one-half that of normal air, and accumulation in the nucleus at this oxygen tension coincides with the first induction of hypoxia-responsive gene expression (Kosmacz et al., 2015). In addition, reduction in oxygen availability would also permit stabilization of de novo synthesized ERFVIIs; therefore, the pool of active ERFVIIs in the nucleus likely comprises factors of these two different origins. This is supported by the finding that RAP2.12 transcript levels in polysomal complexes are not dramatically altered by hypoxia (Mustroph et al., 2009). Once normal oxygen levels return, RAP2.12 degradation occurs within 3 h, coinciding with down-regulation of hypoxia-adaptive gene expression (Kosmacz et al., 2015). The PM localization of RAP2.12 is speculated to be a key component of the hypoxia-sensing mechanism, and it will be interesting to see whether other members of the ERFVII family behave similarly, whether interactions with soluble ACBP proteins also occur, and whether this mechanism is conserved across species. A key focus should be placed on understanding how RAP2.12 relocalization occurs in response to low oxygen, and whether changes in NO availability play a role. Furthermore, exactly how a small pool of RAP2.12 evades degradation to make it to the PM remains unanswered.
Figure 3.
Functional ERFVII protein-protein and protein-gene interactions in Arabidopsis. A, ERFVIIs interact with PM-associated ACBP1 and ACBP2 under normoxic conditions. When oxygen levels decline, this association is reduced and ERFVIIs accumulate in the nucleus (Licausi et al., 2011; Kosmacz et al., 2015). B, The ERFVII RAP2.12 induces expression of HYPOXIA RESPONSE ATTENUATOR1 (HRA1), which physically associates with RAP2.12 and attenuates anaerobic gene expression (for example, PYRUVATE DECARBOXYLASE1 [PDC1]) as part of a negative feedback module (Giuntoli et al., 2014). C, Stabilized ERFVIIs in the mature seed endosperm promote ABI5 expression, enhancing ABA responsiveness and maintaining seed dormancy. NO-mediated abolishment of ERFVIIs down-regulates ABI5, reducing ABA sensitivity and initiating germination (Gibbs et al., 2014b). D, DELLA proteins interact with ERFVIIs via the AP2 domain, which inhibits the ability of ERFVIIs to bind to target genes (X). This suggests that when GA levels are low, accumulated DELLA proteins inhibit ERFVII-mediated gene expression (Marín-de la Rosa et al., 2014). For each interaction, the validated Arabidopsis ERFVII family members are listed.
Once ERFVIIs accumulate in the nucleus, a number of essential anaerobic response genes are switched on, including many of the “core 49” hypoxia response mRNAs previously identified (Mustroph et al., 2009). Sustained expression of many of these genes can be detrimental, and so counterbalancing mechanisms must be in place. In addition to the PCO enzymes discussed earlier, the trihelix transcription factor HRA1 plays such a role (Giuntoli et al., 2014; Fig. 3B). HRA1 is both induced by RAP2.12 and counteracts anaerobic gene induction by physically interacting with RAP2.12. HRA1 was shown to be particularly important for attenuating hypoxia responses in young tissues and meristematic regions, and may play a role in dampening low-oxygen responses under aerobic conditions in regions of the plant that are experiencing physiological hypoxia. In addition to antagonizing RAP2.12-mediated gene expression, HRA1 also negatively regulates its own transcription, providing a second feedback loop. Interestingly, HRA1 did not interact with any of the other four Arabidopsis ERFVIIs, further indicating that each family member may have specific interaction partners (Giuntoli et al., 2014).
ERFVII Cross Talk with Hormone Signaling Pathways
In addition to the discovery of functionally relevant protein-protein interactions, ERFVII proteins are known to interact with diverse hormone signaling pathways at the transcriptional and protein level. Gibbs et al. (2014b) discovered that ERFVIIs mediate cross talk between NO availability and ABA signaling during the regulation of seed dormancy through their positive control of ABSICISIC ACID INSENSITIVE5 (ABI5) expression, a major negative regulator of germination (Fig. 3C; Holdsworth et al., 2008). Constitutively expressed RAP2.2, RAP2.12, and RAP2.3 all induced ABI5 expression in protoplasts, and a physical interaction between RAP2.3 and a GCC box DNA motif in the ABI5 promoter was confirmed (Gibbs et al., 2014b). This functional interaction retrospectively explained why both prt6 and ate1ate2 mutants, which constitutively accumulate ERFVIIs, have heightened levels of seed dormancy and ABA hypersensitivity relative to the wild type (Holman et al., 2009). It has long been known that NO acts as a potent disrupter of seed dormancy, able to trigger germination (Bethke et al., 2007), and that following seed imbibition, there is a well-documented burst of NO (Liu et al., 2009). Rapid increases in cellular NO as well as oxygenation of the seed during imbibition would stimulate ERFVII degradation, leading to a down-regulation of ABI5 expression in the endosperm, reducing sensitivity to ABA, and promoting germination (Gibbs et al., 2014b). Future analyses should focus on whether other key ABA-regulated responses are also regulated by ERFVIIs.
A recent large-scale yeast two-hybrid screen of Arabidopsis proteins identified RAP2.3 as an interaction partner of the DELLA protein GA INSENSITIVE (GAI; Marín-de la Rosa et al., 2014). Interactions between RAP2.3 and the DELLA protein REPRESSOR OF THE ga1-3 MUTANT, and between GAI and RAP2.12 were also identified. These DELLAs associate with the ERFVII DNA-binding domain, and disrupt their ability to interact with target genes (Fig. 3D; Marín-de la Rosa et al., 2014). This suggests that, in the absence of GA, accumulated DELLA proteins act as negative regulators of ERFVII-mediated gene expression, whereas GA-induced destruction of DELLA proteins would relieve this repression. This hypothesis was investigated within the context of seedling apical hook development. The apical hook is a hallmark feature of etiolated seedlings; its formation is promoted by both ethylene and GA, which have additive effects enhancing hook angle (Abbas et al., 2013). In the pentuple erfVII mutant, which lacks all five ERFVIIs, hooks were able to respond similarly to the wild type for ethylene, but the single and additive effects of exogenous GA were significantly reduced compared with the wild type, indicating a role for ERFVIIs in GA regulation of early seedling development. As discussed earlier, there is a well-documented link between ERFVIIs and GA signaling in rice: SNORKELs and SUB1A-1 promote and inhibit downstream GA responses, respectively (Fukao and Bailey-Serres, 2008; Hattori et al., 2009). Furthermore, Arabidopsis ERFVIIs regulate processes dependent on cell elongation (e.g. germination and hypocotyl elongation; Holman et al., 2009; Gibbs et al., 2014b), suggesting that ERFVII interactions with GA signaling pathways may be more significant than is currently understood.
CONCLUSION
Recent data indicate that the long-known distinctive Nt structure of the ERFVIIs, conferring oxygen- and NO-dependant stability, provides these proteins with a conditional activity that has diverse consequences during growth and development, and in response to environmental stress. Many aspects of ERFVII biology remain to be determined to enable an understanding of how changed stability is related to distinct responses to different conditions. Future studies aimed at defining the specificity of ERFVII protein-protein and protein-gene interactions, as well as the contribution of non-N-end rule-mediated ubiquitination to their stability, will shed light onto the complex nature of ERFVII signal transduction, and may help understand how this small family of transcriptional regulators controls such an array of plant responses. In addition, determining the overlapping and unique roles of individual members of the group will help in understanding adaptive molecular responses to evolutionary pressure related to specific stresses. Further study of these factors and mechanisms influencing the changes in their stability should also provide a deeper understanding of how plants integrate development and stress responses. The observation that ERFVIIs from diverse species positively influence tolerance to multiple stresses in similar ways indicates that they are good targets for breeding and biotechnological approaches to increase stability of plant growth and yield in response to environmental stress.
Glossary
- Nt
N-terminal
- ROS
reactive oxygen species
- NO
nitric oxide
- H2O2
hydrogen peroxide
- MV
methyl viologen
- PM
plasma membrane
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (grant nos. BB/M002268/1 and BB/M007820/1 to M.J.H. and grant no. BB/K000144/1 to J.V.C. and M.J.H., including financial support from SABMiller), the University of Birmingham (Fellowship to D.J.G.), Government of India National Overseas Scholarship for higher studies (PhD studentship to G.P.), the University of Nottingham (PhD studentship to S.B.), and a Barry Axcell postdoctoral fellowship (to G.M.M.).
References
- Abbas M, Alabadí D, Blázquez MA (2013) Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci 4: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas M, Berckhan S, Rooney DJ, Gibbs DJ, Conde JV, Correia CS, Bassel GW, Marin-de la Rosa N, Leon J, Alabadi D. , et al (2015) Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Curr Biol 25: 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayano M, Kani T, Kojima M, Sakakibara H, Kitaoka T, Kuroha T, Angeles-Shim RB, Kitano H, Nagai K, Ashikari M (2014) Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ 37: 2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A, Finley D, Varshavsky A (1986) In vivo half-life of a protein is a function of its amino-terminal residue. Science 234: 179–186 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LA, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59: 21–39 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143: 1173–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui LT, Giuntoli B, Kosmacz M, Parlanti S, Licausi F (2015) Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci 236: 37–43 [DOI] [PubMed] [Google Scholar]
- Büttner M, Singh KB (1997) Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP), an ethylene-inducible, GCC box DNA-binding protein interacts with an ocs element binding protein. Proc Natl Acad Sci USA 94: 5961–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Song F, Goodman RM, Zheng Z (2006) Molecular characterization of four rice genes encoding ethylene-responsive transcriptional factors and their expressions in response to biotic and abiotic stress. J Plant Physiol 163: 1167–1178 [DOI] [PubMed] [Google Scholar]
- Chen N, Yang QL, Su MW, Pan LJ, Chi XY, Chen MN, He YA, Yang Z, Wang T, Wang MA. , et al. (2012) Cloning of six ERF family transcription factor genes from peanut and analysis of their expression during abiotic stress. Plant Mol Biol Rep 30: 1415–1425 [Google Scholar]
- Chen X, Pierik R, Peeters AJM, Poorter H, Visser EJW, Huber H, de Kroon H, Voesenek LACJ (2010) Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol 154: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, et al. (2003) BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 132: 1961–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Chang Y, Huang F, Xiong L (2014) GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice. J Integr Plant Biol [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA 105: 16814–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18: 2021–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23: 412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J (2012) The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol 160: 1795–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Bacardit J, Bachmair A, Holdsworth MJ (2014a) The eukaryotic N-end rule pathway: conserved mechanisms and diverse functions. Trends Cell Biol 24: 603–611 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J. , et al. (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Md Isa N, Movahedi M, Lozano-Juste J, Mendiondo GM, Berckhan S, Marín-de la Rosa N, Vicente Conde J, Sousa Correia C, Pearce SP, et al. (2014b) Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol Cell 53: 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Lee SC, Licausi F, Kosmacz M, Oosumi T, van Dongen JT, Bailey-Serres J, Perata P (2014) A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biol 12: e1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Hess N, Klode M, Anders M, Sauter M (2011) The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiol Plant 143: 41–49 [DOI] [PubMed] [Google Scholar]
- Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol 153: 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Kouketu E, Katoh H, Aya K, Ueguchi-Tanaka M, Matsuoka M (2012) The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity. Plant J 71: 443–453 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179: 33–54 [DOI] [PubMed] [Google Scholar]
- Holman TJ, Jones PD, Russell L, Medhurst A, Ubeda Tomás S, Talloji P, Marquez J, Schmuths H, Tung SA, Taylor I, et al. (2009) The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc Natl Acad Sci USA 106: 4549–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu RG, Sheng J, Qi X, Xu Z, Takahashi TT, Varshavsky A (2005) The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 437: 981–986 [DOI] [PubMed] [Google Scholar]
- Jaba IM, Zhuang ZW, Li N, Jiang Y, Martin KA, Sinusas AJ, Papademetris X, Simons M, Sessa WC, Young LH. , et al. (2013) NO triggers RGS4 degradation to coordinate angiogenesis and cardiomyocyte growth. J Clin Invest 123: 1718–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Won SY, Suh SC, Kim H, Wing R, Jeong Y, Hwang I, Kim M (2007) The barley ERF-type transcription factor HvRAF confers enhanced pathogen resistance and salt tolerance in Arabidopsis. Planta 225: 575–588 [DOI] [PubMed] [Google Scholar]
- Jung KH, Seo YS, Walia H, Cao P, Fukao T, Canlas PE, Amonpant F, Bailey-Serres J, Ronald PC (2010) The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol 152: 1674–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402 [DOI] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmacz M, Parlanti S, Schwarzlander M, Kragler F, Licausi F, Van Dongen JT (2015) The stability and nuclear localization of the transcription factor RAP2.12 are dynamically regulated by oxygen concentration. Plant Cell Environ 38: 1094–1103 [DOI] [PubMed] [Google Scholar]
- Li HY, Chye ML (2004) Arabidopsis Acyl-CoA-binding protein ACBP2 interacts with an ethylene-responsive element-binding protein, AtEBP, via its ankyrin repeats. Plant Mol Biol 54: 233–243 [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199: 639–649 [DOI] [PubMed] [Google Scholar]
- Licausi F, van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P (2010) HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J 62: 302–315 [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi L, Ye N, Liu R, Jia W, Zhang J (2009) Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 183: 1030–1042 [DOI] [PubMed] [Google Scholar]
- Marín-de la Rosa N, Sotillo B, Miskolczi P, Gibbs DJ, Vicente J, Carbonero P, Oñate-Sánchez L, Holdsworth MJ, Bhalerao R, Alabadí D. , et al. (2014) Large-scale identification of gibberellin-related transcription factors defines group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiol 166: 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiondo GM, Gibbs DJ, Szurman-Zubrzycka M, Korn A, Marquez J, Szarejko I, Maluszynski M, King J, Axcell B, Smart K, et al. (2015) Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol J DOI: 10.1111/pbi.12334 [DOI] [PMC free article] [PubMed]
- Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Pan L, Kawai-Yamada M, Yu LH, Yamamura S, Koyama T, Kitajima S, Ohme-Takagi M, Sato F, Uchimiya H (2005) Functional analysis of Arabidopsis ethylene-responsive element binding protein conferring resistance to Bax and abiotic stress-induced plant cell death. Plant Physiol 138: 1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Li Y, Zhang H, Huang R, Liu W, Ming J, Liu S, Li X (2014) Expression of signalling and defence-related genes mediated by over-expression of JERF1, and increased resistance to sheath blight in rice. Plant Pathol 63: 109–116 [Google Scholar]
- Papdi C, Abrahám E, Joseph MP, Popescu C, Koncz C, Szabados L (2008) Functional identification of Arabidopsis stress regulatory genes using the controlled cDNA overexpression system. Plant Physiol 147: 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papdi C, Pérez-Salamó I, Joseph MP, Giuntoli B, Bögre L, Koncz C, Szabados L (2015) The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J doi: 10.1111/tpj.12848 [DOI] [PubMed]
- Park HY, Seok HY, Woo DH, Lee SY, Tarte VN, Lee EH, Lee CH, Moon YH (2011) AtERF71/HRE2 transcription factor mediates osmotic stress response as well as hypoxia response in Arabidopsis. Biochem Biophys Res Commun 414: 135–141 [DOI] [PubMed] [Google Scholar]
- Parlanti S, Kudahettige NP, Lombardi L, Mensuali-Sodi A, Alpi A, Perata P, Pucciariello C (2011) Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann Bot (Lond) 107: 1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Castro JM, van Zanten M, Lee SC, Patel MR, Voesenek LA, Fukao T, Bailey-Serres J (2011) Expression of rice SUB1A and SUB1C transcription factors in Arabidopsis uncovers flowering inhibition as a submergence tolerance mechanism. Plant J 67: 434–446 [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H (1984) Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber W, Müller JT, Visser EJ, Sasidharan R, Voesenek LA, Mustroph A (2015) The greening after extended darkness1 is an N-end rule pathway mutant with high tolerance to submergence and starvation. Plant Physiol 167: 1616–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Bhekasut P, Greenway H (2010) Desiccation of leaves after de-submergence is one cause for intolerance to complete submergence of the rice cultivar IR 42. Funct Plant Biol 37: 1096–1104 [Google Scholar]
- Shemorry A, Hwang CS, Varshavsky A (2013) Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol Cell 50: 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Charles TM, Newton RJ (2005) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol Biol 59: 603–617 [DOI] [PubMed] [Google Scholar]
- van Dongen JT, Licausi F (2015) Oxygen sensing and signaling. Annu Rev Plant Biol 66: 345–367 [DOI] [PubMed] [Google Scholar]
- van Veen H, Akman M, Jamar DC, Vreugdenhil D, Kooiker M, van Tienderen P, Voesenek LA, Schranz ME, Sasidharan R (2014) Group VII ethylene response factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ 37: 2421–2432 [DOI] [PubMed] [Google Scholar]
- van Veen H, Mustroph A, Barding GA, Vergeer-van Eijk M, Welschen-Evertman RA, Pedersen O, Visser EJ, Larive CK, Pierik R, Bailey-Serres J. , et al. (2013) Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 25: 4691–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. (2011) The N-end rule pathway and regulation by proteolysis. Protein Sci 20: 1298–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206: 57–73 [DOI] [PubMed] [Google Scholar]
- Wang D, Pan Y, Zhao X, Zhu L, Fu B, Li Z (2011) Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genomics 12: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Du Y, Hou YJ, Zhao Y, Hsu CC, Yuan F, Zhu X, Tao WA, Song CP, Zhu JK (2015) Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci USA 112: 613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, Hoefgen R, Perata P, van Dongen JT, Licausi F (2014) Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat Commun 5: 3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P (2007) Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol 145: 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Chye ML (2009) An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physiol Biochem 47: 479–484 [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L. , et al. (2007) Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 65: 719–732 [DOI] [PubMed] [Google Scholar]
- Yang CY, Hsu FC, Li JP, Wang NN, Shih MC (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol 156: 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yu C, Yan J, Wang X, Chen F, Zhao Y, Wei W (2014) Overexpression of the Jatropha curcas JcERF1 gene coding an AP2/ERF-type transcription factor increases tolerance to salt in transgenic tobacco. Biochemistry (Mosc) 79: 1226–1236 [DOI] [PubMed] [Google Scholar]
- Yi SY, Kim JH, Joung YH, Lee S, Kim WT, Yu SH, Choi D (2004) The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol 136: 2862–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Lamattina L, Spoel SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202: 1142–1156 [DOI] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH. , et al. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268 [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen M, Li L, Xu Z, Chen X, Guo J, Ma Y (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60: 3781–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Huang Z, Xie B, Chen Q, Tian X, Zhang X, Zhang H, Lu X, Huang D, Huang R (2004) The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 220: 262–270 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li F, Li D, Zhang H, Huang R (2010) Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 232: 765–774 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wei T, Yin KQ, Chen Z, Gu H, Qu LJ, Qin G (2012) Arabidopsis RAP2.2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses. New Phytol 195: 450–460 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Shi J, Xu W, Li H, He M, Xu Y, Xu T, Yang Y, Cao J, Wang Y (2013) Three ERF transcription factors from Chinese wild grapevine Vitis pseudoreticulata participate in different biotic and abiotic stress-responsive pathways. J Plant Physiol 170: 923–933 [DOI] [PubMed] [Google Scholar]