Two transcriptional regulators of ethylene signaling play differential roles in ethylene and salt stress responses, distinct from their orthologs in Arabidopsis.
Abstract
Ethylene plays important roles in plant growth, development, and stress responses. The ethylene signaling pathway has been studied extensively, mainly in Arabidopsis (Arabidopsis thaliana). However, the molecular mechanism of ethylene signaling is largely unknown in rice (Oryza sativa). Previously, we have isolated a set of rice ethylene-response mutants. Here, we characterized the mutant maohuzi6 (mhz6). Through map-based cloning, we found that MHZ6 encodes ETHYLENE INSENSITIVE3-LIKE1 (OsEIL1), a rice homolog of ETHYLENE INSENSITIVE3 (EIN3), which is the master transcriptional regulator of ethylene signaling in Arabidopsis. Disruption of MHZ6/OsEIL1 caused ethylene insensitivity mainly in roots, whereas silencing of the closely related OsEIL2 led to ethylene insensitivity mainly in coleoptiles of etiolated seedlings. This organ-specific functional divergence is different from the functional features of EIN3 and EIL1, both of which mediate the incomplete ethylene responses of Arabidopsis etiolated seedlings. In Arabidopsis, EIN3 and EIL1 play positive roles in plant salt tolerance. In rice, however, lack of MHZ6/OsEIL1 or OsEIL2 functions improves salt tolerance, whereas the overexpressing lines exhibit salt hypersensitivity at the seedling stage, indicating that MHZ6/OsEIL1 and OsEIL2 negatively regulate salt tolerance in rice. Furthermore, this negative regulation by MHZ6/OsEIL1 and OsEIL2 in salt tolerance is likely attributable in part to the direct regulation of HIGH-AFFINITY K+ TRANSPORTER2;1 expression and Na+ uptake in roots. Additionally, MHZ6/OsEIL1 overexpression promotes grain size and thousand-grain weight. Together, our study provides insights for the functional diversification of MHZ6/OsEIL1 and OsEIL2 in ethylene response and finds a novel mode of ethylene-regulated salt stress response that could be helpful for engineering salt-tolerant crops.
The simple hydrocarbon phytohormone ethylene regulates a variety of basic plant processes throughout whole-plant growth and development (Abeles et al., 1992). In Arabidopsis (Arabidopsis thaliana), genetic studies of ethylene-responsive mutants have uncovered a linear ethylene signaling framework from hormone perception in the endoplasmic reticulum to transcriptional regulation in the nucleus. Ethylene is sensed by five endoplasmic reticulum-localized receptors that are negative regulators of the signaling pathway (Chang et al., 1993; Hua et al., 1995, 1998; Hua and Meyerowitz, 1998; Sakai et al., 1998). Without ethylene, the receptors may recruit and activate CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), which further phosphorylates the C-terminal domain of ETHYLENE INSENSITIVE2 (EIN2) to repress the downstream ethylene response (Kieber et al., 1993; Alonso et al., 1999; Ju et al., 2012). When ethylene is present, the receptors and CTR1 are inactive. The C terminus of EIN2 is cleaved and translocated to the nucleus to participate in the stabilization and accumulation of EIN3/ETHYLENE INSENSITIVE3-LIKE1 (EIL1) and, consequently, induces ethylene-responsive genes (Chao et al., 1997; Ju et al., 2012; Qiao et al., 2012; Wen et al., 2012). EIN2 and EIN3/EIL1 are regulated by proteasomal degradation through ETHYLENE INSENSITIVE2 TARGETING PROTEIN1 (ETP1) and ETP2 and ETHYLENE INSENSITIVE3-BINDING F-BOX PROTEIN1 (EBF1) and EBF2, respectively (Guo and Ecker, 2003; Potuschak et al., 2003; Gagne et al., 2004; Qiao et al., 2009).
Semiaquatic rice (Oryza sativa) is one of the most important crops in the world. Ethylene plays essential roles in the adaptive responses to the hypoxia conditions of rice (Fukao and Bailey-Serres, 2008; Rzewuski and Sauter, 2008; Ma et al., 2010; Yang et al., 2015). Different from the triple response observed in etiolated Arabidopsis seedlings, ethylene inhibits root growth but promotes coleoptile growth of dark-grown rice seedlings, which is called the double response (Ma et al., 2010, 2013, 2014; Yang et al., 2015). Several homologous genes of Arabidopsis ethylene signaling components have been identified and characterized in rice, including the ethylene receptor genes ETHYLENE RESPONSE SENSOR1 (OsERS1) and ETHYLENE RESPONSE2 (OsETR2), REVERSION TO ETHYLENE SENSITIVITY1 (RTE1) homolog (RTH1), OsCTRs, OsEIN2, and OsEIL1 (Jun et al., 2004; Mao et al., 2006; Wuriyanghan et al., 2009; Zhang et al., 2012; Ma et al., 2013, 2014; Wang et al., 2013). Overexpression of OsETR2 reduces ethylene sensitivity and delays the floral transition in transgenic rice. Conversely, OsETR2 RNA interference (RNAi) plants and knockdown mutants show enhanced ethylene sensitivity, early flowering, and increased thousand-seed weight (Wuriyanghan et al., 2009). Overexpression of OsRTH1, the Arabidopsis RTH1 in rice, rescues the ethylene-insensitive phenotype of the RTE1 loss-of-function mutant rte1-2 (Zhang et al., 2012). Ectopic expression of OsCTR2 largely complements Arabidopsis ctr1-1, while OsCTR2 loss-of-function plants show several aspects of the constitutive ethylene-response phenotypes (Wang et al., 2013). Based on the double response of rice, our laboratory has isolated a set of rice ethylene-response mutants, and MAOHUZI7 (MHZ7)/OsEIN2 was identified. The mhz7 mutant shows complete insensitivity to ethylene in both root and coleoptile growth, whereas MHZ7-OX rice seedlings show constitutive or enhanced ethylene response without or with ethylene treatment, respectively (Ma et al., 2013). Recently, the study of MHZ4 revealed a novel mode of interplay between ethylene and abscisic acid (ABA) in the control of rice growth and development (Ma et al., 2014). MHZ4 encoded a chloroplast-localized membrane protein homologous to Arabidopsis ABA4. MHZ4 mutation leads to reduced ethylene response in roots but enhanced ethylene response in coleoptiles of etiolated seedlings. And overexpression of MHZ4 resulted in enhanced and reduced ethylene response in roots and coleoptiles, respectively (Ma et al., 2014). More recently, through analysis of the mhz5 mutant, we found that the MHZ5/carotenoid isomerase-mediated ABA pathway acts downstream of ethylene signaling, and ethylene induces the expression of MHZ5 and MHZ4 and drives the metabolic flux of carotenogenesis into ABA biosynthesis to regulate root growth in rice (Yin et al., 2015).
Ethylene is known to play important roles in regulating biotic and abiotic stress responses (Morgan and Drew, 1997; van Loon et al., 2006). Over the years, the functions of components of ethylene biosynthesis and signaling in response to salt stress were investigated in dicotyledonous plants. Our previous studies have demonstrated that subfamily II ethylene receptor genes from tobacco (Nicotiana tabacum), tobacco HISTIDINE KINASE1 (NTHK1) and NTHK2, are induced by different stresses (Zhang et al., 2001a, 2001b). NTHK1 overexpression plants show a salt-sensitive phenotype; however, the presence of 1-aminocyclopropane-1-carboxylic acid suppresses the salt sensitivity (Cao et al., 2006, 2007). The kinase domain and kinase activity of NTHK1 are functionally differential in the regulation of the salt stress response and growth response (Zhou et al., 2006; Chen et al., 2009). Recently, two NTHK1-interacting proteins, including the ankyrin-domain protein NTHK1 ETHYLENE RECEPTOR-INTERACTING PROTEIN2 (NEIP2) and the translationally controlled tumor protein TRANSLATIONALLY CONTROLLED TUMOR PROTEIN (TCTP), have been identified. NEIP2 can associate with NTHK1 to promote growth and stress tolerance (Cao et al., 2015). TCTP can stabilize NTHK1 to reduce the ethylene response but enhance plant growth through the promotion of cell proliferation (Tao et al., 2015). The Arabidopsis ein2-1 and ein2-5 mutants also show a salt-sensitive response to salt stress, whereas overexpression of the C-terminal end of EIN2 in ein2-5 exhibits a salt-tolerant phenotype (Cao et al., 2007; Lei et al., 2011). The ctr1-1 and ebf1-1ebf2-1 mutants that cause EIN3 accumulation exhibit increased salt tolerance, whereas the ein3-1 mutant exhibits reduced salt tolerance (Achard et al., 2006; Lei et al., 2011). Recently, Peng et al. (2014) reported that plants pretreated with ethylene show increased tolerance to salt stress, and EIN3/EIL1 are both necessary and sufficient for salt tolerance. The mutation of ETHYLENE OVERPRODUCER1 (ETO1) function causing enhanced ethylene production promotes soil salinity tolerance by reducing root-to-shoot Na+ delivery (Jiang et al., 2013). These studies support that enhanced ethylene production or signaling improves salt tolerance in dicotyledonous plants. However, the roles of ethylene and its signaling components in salt stress responses remain unclear in rice.
Recently, we isolated a set of rice ethylene-response mutants and identified MHZ7/OsEIN2, MHZ4/ABA4, and MHZ5 through map-based cloning (Ma et al., 2013, 2014; Yin et al., 2015). In this study, we characterize mhz6, a mutant exhibiting ethylene insensitivity in root growth. Through map-based cloning, we found that mhz6 is a loss-of-function mutation of the previously described OsEIL1 locus, whose encoded protein is homologous to EIN3, the key transcription factor of the ethylene signaling pathway in Arabidopsis (Chao et al., 1997; Mao et al., 2006). We further demonstrate that, different from the incomplete ethylene insensitivity of ein3 and eil1 in Arabidopsis, MHZ6/OsEIL1 and OsEIL2 differentially regulate ethylene responses of the roots and coleoptiles of etiolated seedlings. We next show that MHZ6/OsEIL1 and OsEIL2 negatively regulate salt tolerance in rice, which is different from the positive salt-tolerant regulation of EIN3/EIL1 in Arabidopsis. Furthermore, this negative regulation by MHZ6/OsEIL1 and OsEIL2 in salt tolerance is likely in part attributable to the direct regulation of HIGH-AFFINITY K+ TRANSPORTER2;1 (OsHKT2;1) expression and Na+ uptake in roots. Our results suggest that MHZ6/OsEIL1 and OsEIL2, in contrast to Arabidopsis EIN3/EIL1, may play distinct roles not only in ethylene signal transduction but also in regulating salt-stress responses.
RESULTS
The mhz6 Mutants Display Insensitivity to Ethylene in Roots
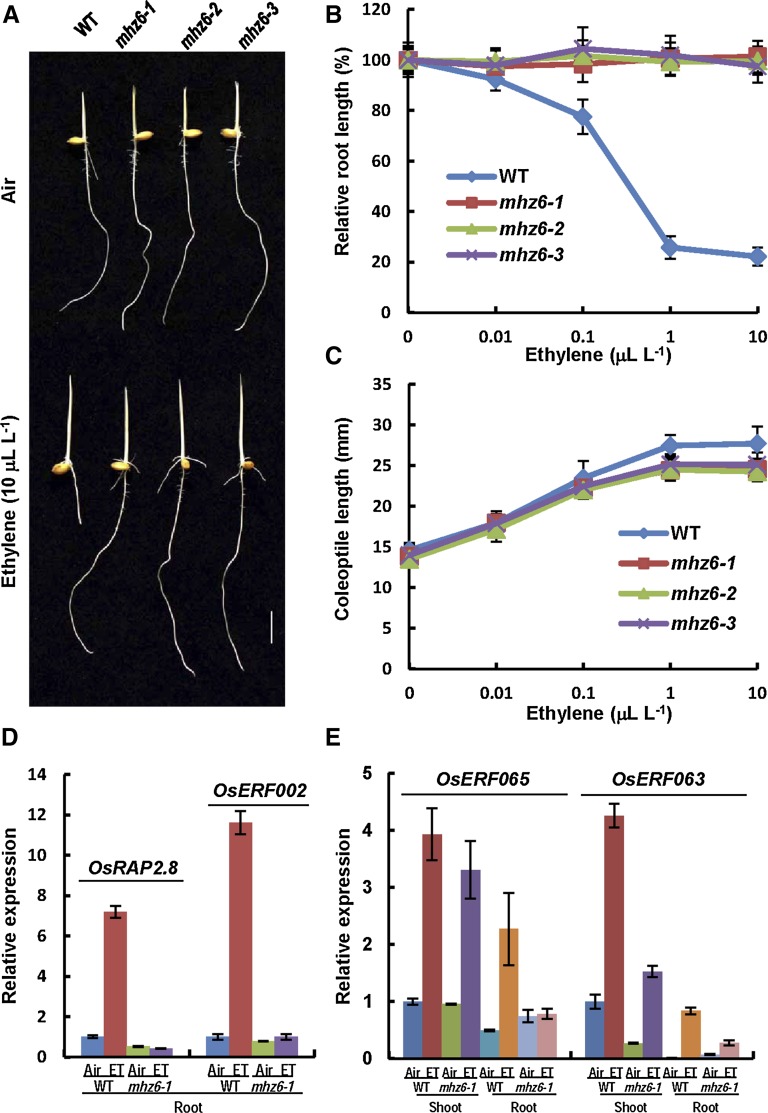
In an effort to clarify the ethylene signal pathway in a model monocot plant, a set of rice ethylene-response mutants were isolated in our laboratory (Ma et al., 2013). One group of the mutants, named mhz6, was analyzed. Compared with the wild type, etiolated seedlings of three allelic mutants, mhz6-1, mhz6-2, and mhz6-3, had similar phenotypes in air but showed longer roots in 10 µL L−1 ethylene (Fig. 1A). The ethylene dose-response curve revealed that wild-type root length was drastically reduced under ethylene treatments from 0.1 to 10 µL L−1, whereas the mutant root growth was not inhibited by ethylene, indicating a completely ethylene-insensitive phenotype (Fig. 1B). Coleoptile growth of wild-type and mhz6 mutant seedlings was promoted similarly after ethylene treatment, although the mutants seemed to have slightly shorter coleoptiles (Fig. 1C). These results indicate that mhz6 is completely insensitive to ethylene in root growth and slightly insensitive in coleoptile promotion.
Figure 1.
Characterization of ethylene responses of mhz6 seedlings. A, Ethylene-insensitive phenotypes of the wild type (WT) and different mhz6 alleles. Bar = 10 mm. B, Ethylene dose-response curves for relative root length of the wild type and mhz6 mutants. Values are means ± sd (n ≥ 30). C, Ethylene dose-response curves of coleoptile length of the wild type and mhz6 mutants. Values are means ± sd (n ≥ 30). D, Real-time PCR analysis of the expression of ethylene-inducible genes in roots of the wild type and mhz6-1. Two-day-old etiolated seedlings were treated with or without 10 µL L−1 ethylene (ET) for 8 h, and the RNA was isolated for analysis. Values are means ± sd (n = 3). E, Real-time PCR analysis of the expression of ethylene-inducible genes in both shoots and roots of the wild type and mhz6-1. Details are as in D.
To further examine the ethylene response of the mhz6 mutants, we determined the expression of ethylene-responsive genes (Ma et al., 2013). OsRAP2.8 and rice ETHYLENE RESPONSE FACTOR002 (OsERF002) were found to be induced to higher levels by ethylene only in roots of wild-type seedlings but not in roots of mhz6-1 seedlings (Fig. 1D). OsERF063 and OsERF065 were found to be induced by ethylene in both roots and shoots of wild-type seedlings. In mhz6-1, the expression of the two genes in roots remained at a very low level after ethylene treatment, while in shoots the ethylene induction of the two genes was similar or mildly reduced when compared with those in the wild type (Fig. 1E). These results indicate that the mhz6-1 mutant is ethylene insensitive in roots for some ethylene-responsive gene expression.
Molecular Cloning and Functional Verification
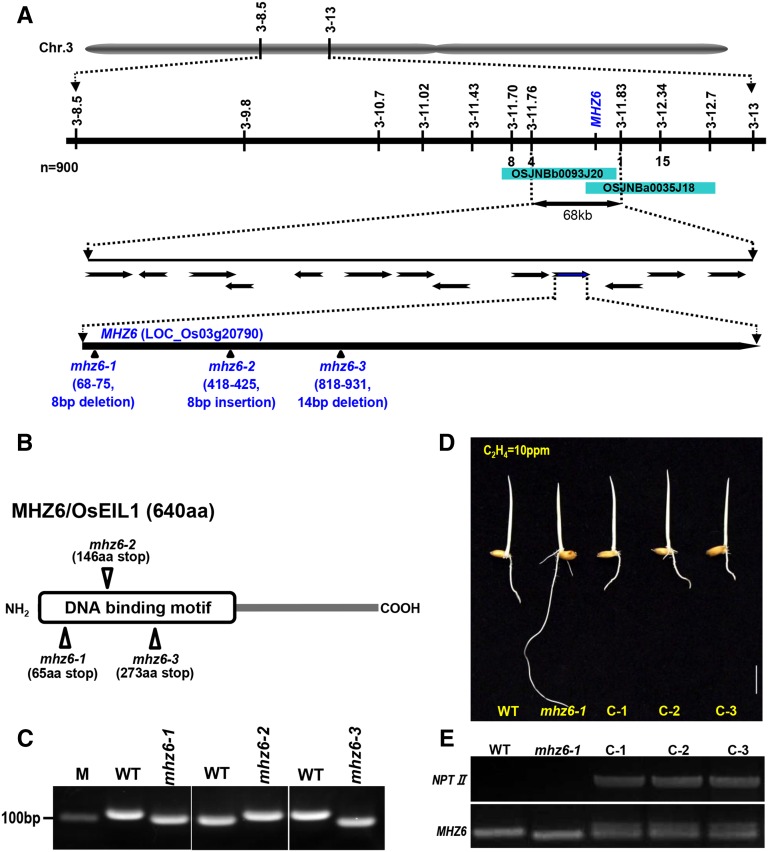
Segregation analysis revealed that the mhz6-1 ethylene-insensitive phenotype was recessive (Ma et al., 2010). The mhz6-1 mutant was crossed with four indica cultivars, and 900 segregated mutant individuals from the F2 population were used for fine mapping. Finally, we restricted MHZ6 to a 68-kb interval between insertion/deletion markers 3-11.76 and 3-11.83 on chromosome 3 (Fig. 2A). Sequencing of all the genes in this region revealed that 8-bp deletions were identified in the exon of Os03g20790 (Fig. 2A). The Os03g20790 locus encoded a homologous protein of EIN3, which is an important transcription factor of ethylene signaling in Arabidopsis (Chao et al., 1997; Fig. 2B). In rice, this gene has been named as OsEIL1 and analyzed through an ectopic overexpression approach (Mao et al., 2006); however, the ethylene response controlled by OsEIL1 in plants is still unclear. The mhz6-2 mutant harbored 8-bp insertions at position 418; mhz6-3 harbored 14-bp deletions at position 818. All the mutations produced stop codons (Fig. 2, A and B). The mutation was also confirmed by nested PCR through examination of DNA fragment length polymorphism (Fig. 2C).
Figure 2.
Molecular characterization of MHZ6. A, Map-based cloning of MHZ6. The MHZ6 locus was mapped in a 68-kb interval on chromosome 3 between markers 3-11.76 and 3-11.83. The number under the marker indicates the number of recombinant individuals. Mutation sites of three allelic mutants are indicated in the schematic diagram of MHZ6. B, Mutation sites of the three allelic mutants shown on the schematic structure of the MHZ6 protein predicted using the SMART software (http://smart.embl-heidelberg.de). C, Confirmation of mutation sites in mhz6-1, mhz6-2, and mhz6-3 by nested PCR analysis. D, Functional complementation of the mhz6-1 mutant. The wild type (WT), mhz6-1, and three complementary lines were treated with 10 µL L−1 ethylene. Bar = 10 mm. E, PCR-based analyses of NPTII and MHZ6 genes in the wild type, mhz6-1, and the three complementary lines.
MHZ6/OsEIL1 complementation transgenic lines were generated by introducing the pMHZ6C construct into mhz6-1 plants. The wild-type phenotype was segregated from T1 lines after 10 µL L−1 ethylene treatment (Fig. 2D), indicating that MHZ6 complemented the mhz6-1 phenotype. PCR-based analyses of NEOMYCIN PHOSPHOTRANSFERASE II (NPTII) and MHZ6 with genomic DNA confirmed the transgenic lines (Fig. 2E). All these results indicate that MHZ6 corresponds to the locus Os03g20790.
Overexpression of MHZ6/OsEIL1 Leads to a Constitutive Ethylene Response
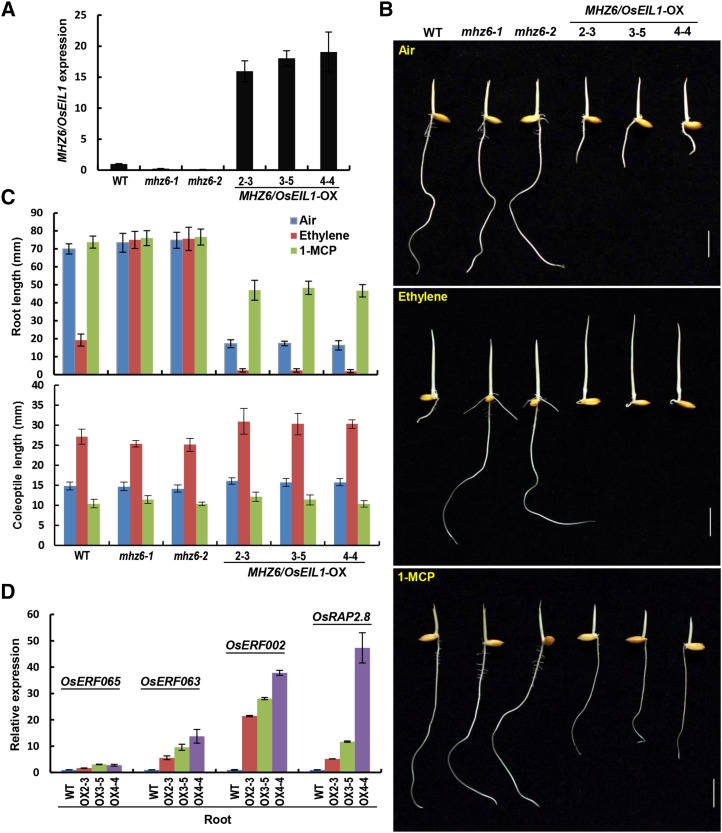
To further investigate the function of MHZ6/OsEIL1, we introduced the plasmid p35S:MHZ6 containing the MHZ6/OsEIL1 gene into wild-type plants. MHZ6/OsEIL1 expression levels of all plant materials were examined by real-time PCR, and three overexpressing (OX) lines (OX-2-3, OX-3-5, and OX-4-4) were chosen for further analysis (Fig. 3A). In air, etiolated seedlings of the three lines all showed shorter roots and slightly longer coleoptiles compared with the wild type, suggesting the presence of a constitutive ethylene response mainly in roots (Fig. 3, B and C). After ethylene treatment, the overexpression lines showed much shorter and extremely twisted roots, along with slightly longer coleoptiles, in comparison with the wild type and mhz6 mutants, indicating enhanced ethylene-response phenotypes mainly in roots (Fig. 3, B and C). We further used 1-methylcyclopropene (1-MCP; Serek et al., 1995), a chemical that can tightly bind to the ethylene receptors in plants to block ethylene perception, and examined its effect on the ethylene response of the overexpression lines. The constitutive ethylene response of etiolated seedlings of MHZ6/OsEIL1-OX lines is largely eliminated in air-grown roots and completely blocked in coleoptiles by the application of 1-MCP (Fig. 3, B and C).
Figure 3.
MHZ6/OsEIL1 overexpression conferred constitutive and enhanced ethylene responses strongly in roots but slightly in coleoptiles in the absence and presence of ethylene, respectively. A, MHZ6/OsEIL1 expression in etiolated seedlings of the wild type (WT), mhz6 alleles, and MHZ6/OsEIL1-OX transgenic lines by real-time PCR. Values are means ± sd (n = 3). B, Ethylene-response phenotypes of the wild type, mhz6 mutants, and MHZ6/OsEIL1-OX lines. The etiolated seedlings were grown in air, 10 µL L−1 ethylene, or 1 µL L−1 1-MCP for 3 d. Bars = 10 mm. C, Length of roots and coleoptiles in the wild type, mhz6 mutants, and MHZ6/OsEIL1-OX lines in response to ethylene and 1-MCP. Values are means ± sd (n ≥ 30). D, Expression of ethylene-inducible genes in roots of the wild type and three MHZ6/OsEIL1-OX lines. Values are means ± sd (n = 3).
We further identified MHZ6/OsEIL1-regulated ethylene-responsive genes in roots of MHZ6/OsEIL1-OX lines. The expression levels of OsRAP2.8, OsERF002, OsERF063, and OsERF065 were substantially enhanced in comparison with the wild type in air-grown etiolated seedlings, which proved the constitutive ethylene response in roots of MHZ6-OX lines (Fig. 3D). All these data indicate that MHZ6 overexpression leads to a constitutive ethylene response and confers an enhanced response to ethylene treatment mainly in roots.
Transactivation Analysis and Subcellular Localization of OsEILs
There are six EIL homologs, designated as OsEIL1 to OsEIL6, in rice. Phylogenetic analysis indicates that MHZ6/OsEIL1 and OsEIL2 showed the highest similarity to the Arabidopsis EIN3 (Supplemental Fig. S1A). The transcriptional activation abilities of OsEILs were investigated using a dual-luciferase reporter assay system in the Arabidopsis protoplast. OsEIL genes were fused to the GAL4 (yeast [Saccharomyces cerevisiae] transcription activator Gal4) DNA-binding domain (DBD) coding sequence and constructed into pRT107 to generate effector plasmid pRTBD-OsEILs. Compared with the GAL4-DBD negative control, MHZ6/OsEIL1, OsEIL2, OsEIL3, and OsEIL4 strongly activated the reporter gene activity, while OsEIL5 and OsEIL6 showed less activity than the negative control (Supplemental Fig. S1B). These results indicate that OsEIL1 to OsEIL4 can activate transcription, whereas OsEIL5 and OsEIL6 appear to lack this activity. We next determined the subcellular localization of OsEIL1 to OsEIL4. Each gene was fused to the GFP gene in a transient expression vector, and the fusion genes driven by the 35S promoter were transfected into Arabidopsis protoplasts. All the OsEILs were located in the nucleus, and the GFP control was mainly located in the cytoplasm (Supplemental Fig. S1C). These results indicate that the OsEIL1 to OsEIL4 proteins are nuclear proteins.
OsEIL2 Is Required for Ethylene-Promoted Coleoptile Elongation
The ethylene-response phenotypes of mhz6 mutants indicated that MHZ6/OsEIL1 is fully required for ethylene-inhibited root growth but only slightly required the ethylene-promoted coleoptile elongation. We then examined whether other OsEILs have any roles in the regulation of the ethylene response in coleoptiles. To elucidate the function of other OsEILs, we generated RNAi constructs of OsEIL2 and OsEIL3 and identified an OsEIL4 knockout mutant (oseil4) with Tos17 insertion from the stock seeds in Rice Genome Resource Center (http://tos.nias.affrc.go.jp/∼miyao/pub/tos17/index.html.en). We also generated overexpression constructs of OsEIL2, OsEIL3, and OsEIL4. All the plasmids were introduced into wild-type rice for further analysis.
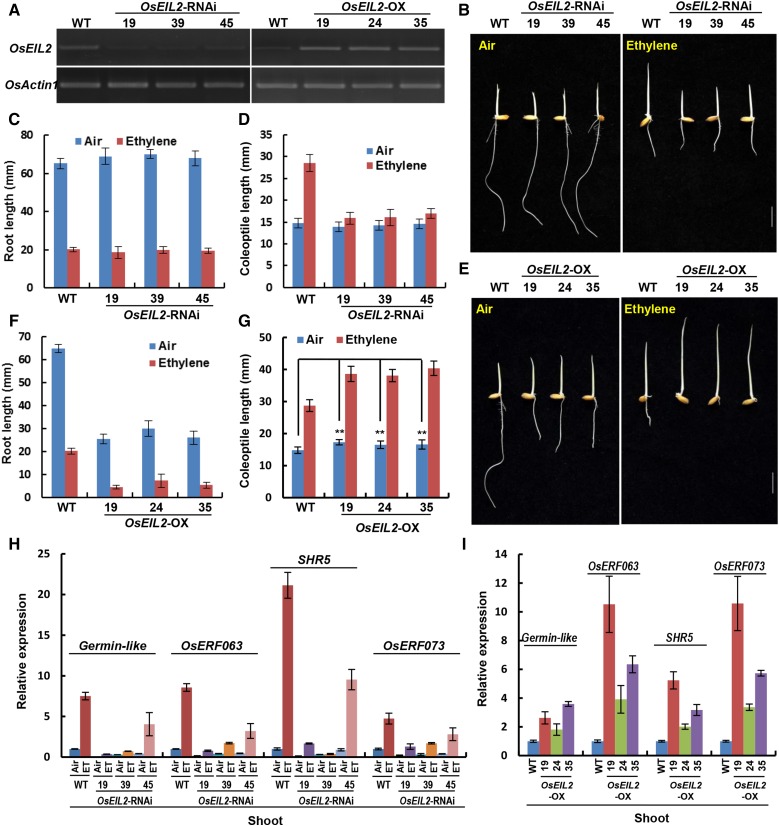
For OsEIL2 transgenic plants, OsEIL2 expression levels of all plant materials were examined by semiquantitative PCR, and three knockdown lines (19, 39, and 45) and three OX lines (19, 24, and 35) were chosen for further analysis (Fig. 4A). Compared with the wild type, the etiolated seedlings of three OsEIL2-RNAi lines had almost normal ethylene-response phenotypes in roots but exhibited shorter coleoptiles under 10 µL L−1 ethylene (Fig. 4, B–D). These results indicated that knockdown of OsEIL2 conferred strong insensitivity in ethylene-promoted coleoptile elongation.
Figure 4.
Knockdown of OsEIL2 expression by RNAi confers strong ethylene insensitivity in coleoptiles, and overexpression of OsEIL2 confers constitutive and enhanced ethylene responses in the absence and presence of ethylene, respectively. A, Expression levels of OsEIL2 in OsEIL2-RNAi and OsEIL2-OX transgenic lines compared with the wild type (WT) by semiquantitative PCR. OsACTIN1 was amplified as a loading control. B, Ethylene-response phenotypes of the wild type and OsEIL2-RNAi lines. The etiolated seedlings were grown in air or 10 µL L−1 ethylene for 3 d. Bar = 10 mm. C, Length of roots in the wild type and OsEIL2-RNAi lines in response to ethylene. Values are means ± sd (n ≥ 30). D, Length of coleoptiles in the wild type and OsEIL2-RNAi lines in response to ethylene. Values are means ± sd (n ≥ 30). E, Ethylene-response phenotypes of the wild type and OsEIL2-OX lines. Details are as in B. F, Length of roots in the wild type and OsEIL2-OX lines in response to ethylene. Values are means ± sd (n ≥ 30). G, Length of coleoptiles in the wild type and OsEIL2-OX lines in response to ethylene. Values are means ± sd (n ≥ 30). Asterisks indicate significant differences from the corresponding controls at P < 0.01. H, Expression of ethylene-inducible genes in shoots of 2-d-old dark-grown wild-type and OsEIL2-RNAi plants with or without ethylene (10 µL L−1) for 8 h. Values are means ± sd (n = 3). I, Expression of ethylene-inducible genes in shoots of the wild type and three OsEIL2-OX lines. Values are means ± sd (n = 3).
We further analyzed the ethylene response of OsEIL2-OX lines. In air, the three lines all showed shorter roots and slightly but significantly longer coleoptiles compared with the wild type. After ethylene treatment, the lines exhibited much shorter and twisted roots, elongated mesocotyls, and long coleoptiles in comparison with the wild type (Fig. 4, E–G). These results indicated that OsEIL2 overexpression conferred constitutive ethylene responses in air especially for roots but enhanced ethylene responses in ethylene for both roots and coleoptiles.
The expression of ethylene-inducible genes in shoots was examined in OsEIL2 transgenic lines. GERMIN-LIKE GENE, OsERF063, SHR5, and OsERF073 were found to be induced by ethylene in shoots of wild-type etiolated seedlings, but this induction was greatly blocked in OsEIL2-RNAi lines (Fig. 4H). Both OsERF063 and OsERF073 were found to be induced by ethylene in roots of wild-type seedlings. In roots of OsEIL2-RNAi lines, the ethylene induction of the two genes was moderately reduced when compared with that in the wild type (Supplemental Fig. S2). Furthermore, the expression levels of these genes were substantially enhanced in comparison with the wild type in shoots of air-grown etiolated overexpression seedlings (Fig. 4I). All these data indicate that OsEIL2 is required for ethylene-induced expression of a subset of genes, especially in shoots.
We examined the expression of MHZ6/OsEIL1 and OsEIL2 by real-time PCR and promoter-GUS analysis. The results showed that MHZ6/OsEIL1 and OsEIL2 expression was detected in both roots and coleoptiles of etiolated seedlings (Supplemental Fig. S3, A and B). Their expression was also detected in organs from vegetative to reproductive stages and found to be more abundant in young leaves in plants (Supplemental Fig. S3, A–D).
For OsEIL3 transgenic plants, three knockdown lines (1, 2, and 19) and three OX lines (17, 25, and 30) were selected, and they all exhibited almost similar phenotypes in both roots and coleoptiles compared with the wild type in the absence and presence of ethylene (Supplemental Fig. S4, A–C). Similar results were obtained in oseil4 and three OsEIL4-OX lines (34, 47, and 57; Supplemental Fig. S5, A–C). These data suggest that OsEIL3 and OsEIL4 have no significant effects on the ethylene response in plants. Together with the results obtained with the OsEILs, we conclude that MHZ6/OsEIL1 and OsEIL2 mainly mediated ethylene signaling in roots and coleoptiles of rice etiolated seedlings, respectively, whereas the other related proteins play no roles or very limited roles, if any, in ethylene signal transduction.
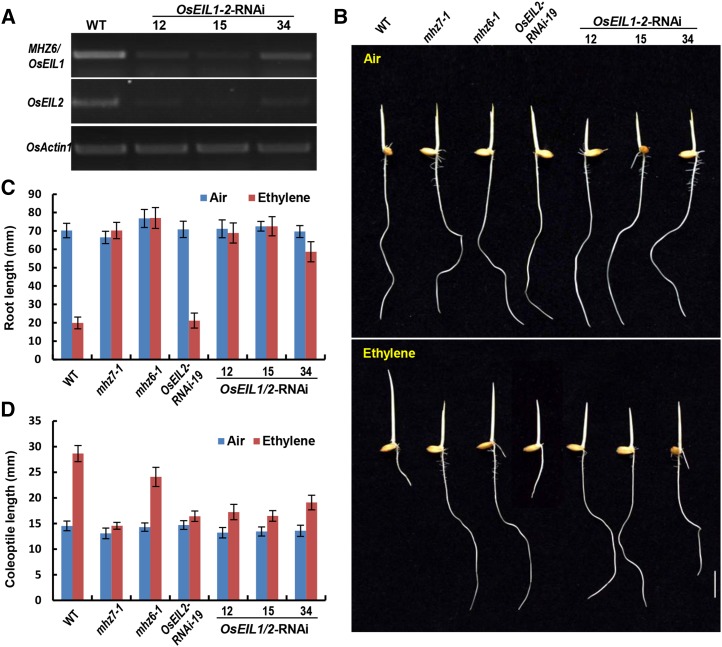
Simultaneous Knockdown of MHZ6/OsEIL1 and OsEIL2 Expression in Plants Confers Ethylene Insensitivity in Both Roots and Coleoptiles
To further confirm this conclusion, we generated an RNAi construct that harbors a 407-bp fragment of the MHZ6/OsEIL1 coding sequence in the reverse and forward directions and introduced it into rice plants. The sequence shares 50% identity with that of OsEIL2. Three OsEIL1-2-RNAi lines (12, 15, and 34), with both MHZ6/OsEIL1 and OsEIL2 knocked down, were obtained by semiquantitative PCR analysis (Fig. 5A). The three OsEIL1-2-RNAi lines showed largely insensitive phenotypes in both roots and coleoptiles, including longer roots and shorter coleoptiles, compared with the wild type in 10 µL L−1 ethylene (Fig. 5, B–D). These results indicate that MHZ6/OsEIL1 and OsEIL2 may control the ethylene response collectively downstream of OsEIN2 in rice.
Figure 5.
Knockdown of both MHZ6/OsEIL1 and OsEIL2 expression by RNAi (OsEIL1-2-RNAi) confers ethylene insensitivity in roots and coleoptiles. A, Expression levels of MHZ6/OsEIL1 and OsEIL2 in OsEIL1-2-RNAi transgenic lines compared with the wild type (WT) by semiquantitative PCR. OsACTIN1 was amplified as a loading control. B, Ethylene-response phenotypes of the wild type, osein2/mhz7-1, mhz6-1, OsEIL2-RNAi-19, and three OsEIL1-2-RNAi lines. The etiolated seedlings were grown in air or 10 µL L−1 ethylene for 3 d. Bar = 10 mm. C, Length of roots in the wild type, osein2/mhz7-1, mhz6-1, OsEIL2-RNAi-19, and three OsEIL1-2-RNAi lines in response to ethylene. Values are means ± sd (n ≥ 30). D, Length of coleoptiles in the wild type, osein2/mhz7-1, mhz6-1, OsEIL2-RNAi-19, and three OsEIL1-2-RNAi lines in response to ethylene. Values are means ± sd (n ≥ 30).
Mutation of MHZ6/OsEIL1 and OsEIN2/MHZ7 and Knockdown of OsEIL2 Improve Salt Tolerance in Rice Plants
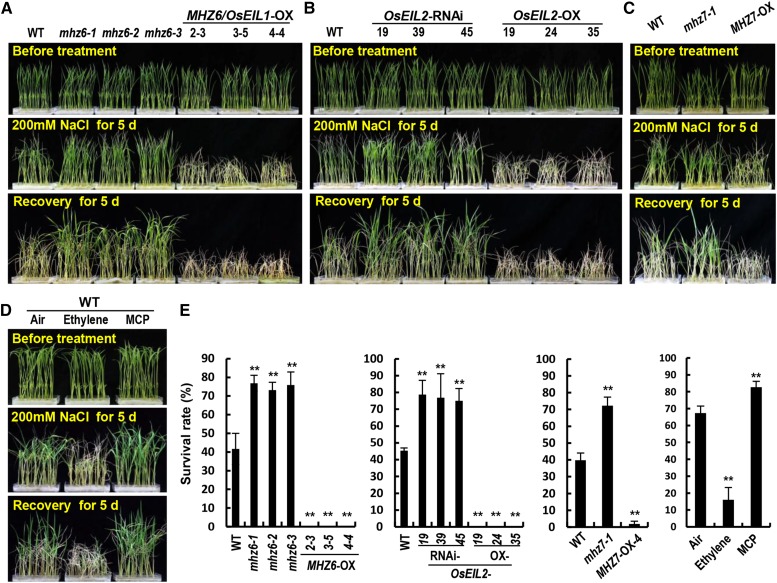
Previous studies in dicots have demonstrated that the components of the ethylene signaling pathway play important roles in plant salinity stress adaptation (Achard et al., 2006; Cao et al., 2006, 2007; Lei et al., 2011; Jiang et al., 2013; Li et al., 2014; Peng et al., 2014). However, their roles in rice remain largely unknown. Thus, we examined whether MHZ6/OsEIL1 and OsEIL2 contribute to salt tolerance. Eight-day-old seedlings of the wild type, the three mhz6 allelic mutants, and 9-d-old MHZ6/OsEIL1-OX lines were treated with 200 mm NaCl for 5 d and subsequently grown by regular hydroponic culture for 5 d. The mutant plants showed significantly higher survival rates compared with the wild type, whereas MHZ6/OsEIL1-OX seedlings were all wilted to death (Fig. 6, A and E). A similar performance was observed in OsEIL2 knockdown and overepression lines (Fig. 6, B and E). We also examined the salt tolerance of mhz7/osein2-1 and OsEIN2/MHZ7-OX-4 plants obtained in our previous study (Ma et al., 2013). Compared with the wild type, more mhz7-1 plants but fewer OsEIN2/MHZ7-OX-4 seedlings survived (Fig. 6, C and E). These results indicated that MHZ6/OsEIL1, OsEIL2, and OsEIN2/MHZ7 play negative roles in salt stress tolerance in rice, in contrast to our and other previous studies in Arabidopsis in which mutants deficient for ethylene responses display salt hypersensitivity (Achard et al., 2006; Cao et al., 2007; Lei et al., 2011; Peng et al., 2014).
Figure 6.
Performance of various plants under salt stress. A, Comparison of the wild type (WT), mhz6 alleles, and MHZ6/OsEIL1-OX lines under salt stress. Eight- and 9-d-old seedlings were used as described in “Materials and Methods.” B, Comparison of the wild type, OsEIL2-RNAi, and OsEIL2-OX lines under salt stress. C, Comparison of the wild type, mhz7-1, and MHZ7/OsEIN2-OX-4 lines under salt stress. D, Comparison of the wild type in air, 10 µL L−1 ethylene, or 5 µL L−1 1-MCP under salt stress. E, Survival rates after salt stress in A to D. Values are means ± sd (n = 3). Asterisks indicate significant differences from the corresponding controls at P < 0.01.
To corroborate the above results, we further investigated the effect of ethylene on salt stress. The wild-type seedlings of under 200 mm NaCl treatment were grown in air, 10 µL L−1 ethylene, or 5 µL L−1 1-MCP. Upon the treatment with 1-MCP, which blocks ethylene perception, the wild type displayed enhanced tolerance to salt compared with air-grown controls, as revealed from the higher survival rate. By comparison, the wild type under ethylene treatment showed a lower survival rate. These results indicate that ethylene treatment leads to salt sensitivity, whereas suppressed ethylene signaling improves salt tolerance in rice.
MHZ6/OsEIL1 and OsEIL2 Mutant/RNAi Plants Accumulate Less Na+, Whereas the OX Lines Accumulate More Na+, Than the Wild Type in Both Shoots and Roots under Salt Stress
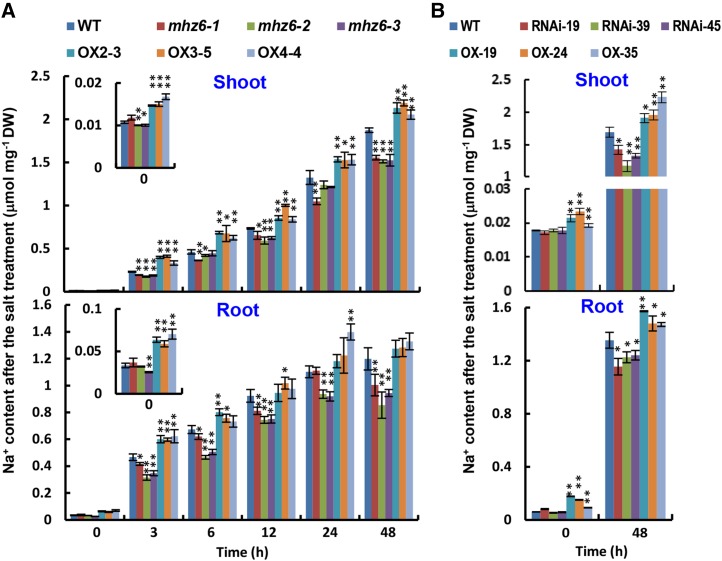
Salt tolerance in plants is the ultimate manifestation of several physiological processes. An important aspect is the avoidance of Na+ accumulation. To investigate whether the performance of mhz6 mutants and MHZ6/OsEIL1-OX lines under salt stress was related to Na+ accumulation, 8-d-old hydroponically grown wild-type plants, three allelic mutants, and 9-d-old MHZ6/OsEIL1-OX lines were subjected to treatment with 200 mm NaCl in Hoagland solution. After the stress, roots and shoots were harvested separately for analysis. The shoots of all the mhz6 seedlings had substantially less Na+ than the wild type during the salt stress, whereas the shoots of all MHZ6/OsEIL1-OX lines accumulated significantly more Na+ than the wild type at all time points (Fig. 7A). Similarly, mhz6 roots had significantly less Na+ than the wild type, whereas roots of all OX lines accumulated more Na+ than the wild type, especially at early hours of the salt stress (Fig. 7A). In contrast to Na+, levels of K+ declined gradually in both shoots and roots of all plant lines tested during salt stress (Supplemental Fig. S6). It should be noted that the OX lines accumulated slightly more Na+ and K+ in shoots before treatment (Fig. 7A; Supplemental Fig. S6A). These results suggest that MHZ6/OsEIL1 promotes Na+ accumulation in rice seedlings under NaCl stress.
Figure 7.
Na+ content in shoot and root of various plants under salt stress. A, Comparison of Na+ content in shoots and roots of the wild type (WT), mhz6 alleles, and MHZ6/OsEIL1-OX lines under salt stress. Eight-day-old seedlings were subjected to Hoagland solution with 200 mm NaCl. The roots and shoots were harvested separately at the indicated times. Values are means ± sd (n = 3). Asterisks indicate significant differences from the corresponding controls at **, P < 0.01 and *, P < 0.05. DW, Dry weight. B, Comparison of Na+ content in shoots and roots of the wild type, OsEIL2-RNAi, and OsEIL2-OX seedlings under salt stress. Details are as in A.
We also examined the Na+ accumulation level in wild-type, OsEIL2-RNAi, and OsEIL2-OX lines. OsEIL2 and MHZ6/OsEIL1 had similar roles in Na+ accumulation. Compared with the wild type, all OsEIL2-RNAi lines accumulated significantly less Na+, whereas all OX lines accumulated more Na+ in both shoots and roots of seedlings treated with NaCl for 48 h (Fig. 7B). These results imply that the Na+ level regulated by OsEILs may be one of the contributing mechanisms for salt-stress responses in rice.
OsHKT Genes Are Regulated by Ethylene and MHZ6/OsEIL1 and OsEIL2
The results obtained above indicate that MHZ6/OsEIL1 and OsEIL2 positively regulate the Na+ accumulation in rice seedlings under NaCl stress. In rice and Arabidopsis, there is evidence indicating that members of the HKT family function as Na+ and Na+/K+ transporters in controlling Na+ accumulation (Garciadeblás et al., 2003; Platten et al., 2006; Horie et al., 2009; Almeida et al., 2013). In Arabidopsis, there is only one HKT gene (Uozumi et al., 2000). In rice, the HKT gene family splits into two subfamilies, class I (OsHKT1;1, OsHKT1;3, OsHKT1;4, and OsHKT1;5) and class II (OsHKT2;1, OsHKT2;3, and OsHKT2;4; Platten et al., 2006). Therefore, we determined if ethylene signaling regulates the expression levels of HKT transporter genes.
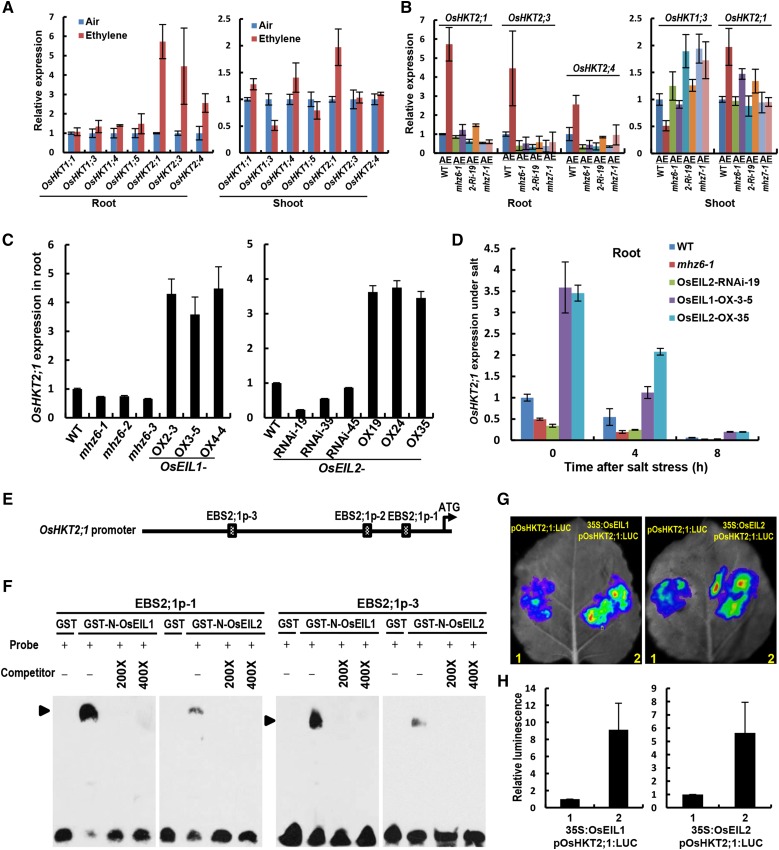
In roots of wild-type seedlings, class II HKT genes, including OsHKT2;1, OsHKT2;3, and OsHKT2;4, were induced to a higher level by ethylene (Fig. 8A, left), while class I HKT gene expression showed little change under ethylene treatment. In shoots, OsHKT2;1 was induced to a level approximately 2-fold higher than the control, whereas OsHKT1;3 expression was reduced by half in response to ethylene (Fig. 8A, right). The expression of other genes was only slightly changed (Fig. 8A, right). Further experiments showed that the ethylene induction of OsHKT2;1 in roots and shoots, the induction of OsHKT2;3 and OsHKT2;4 in roots, and the suppression of OsHKT1;3 in shoots were completely or largely blocked in seedlings of mhz6-1, OsEIL2-RNAi-19, and mhz7-1 (Fig. 8B). These results indicate that ethylene regulates the expression of these HKT genes in an MHZ7/OsEIN2- and OsEIL1/2-dependent manner.
Figure 8.
MHZ6/OsEIL1 and OsEIL2 directly activate the expression of OsHKT2;1. A, Expression of OsHKT family genes in both shoot and root of the wild type. Light-grown seedlings were treated with or without 10 µL L−1 ethylene for 8 h. Values are means ± sd (n = 3). B, Expression of various OsHKTs in both shoot and root of wild-type (WT), mhz6-1, OsEIL2-RNAi-19 (2-Ri-19), and mhz7/osein2-1 plants. Details are as in A. C, Expression of OsHKT2;1 in roots of MHZ6/OsEIL1 and OsEIL2 mutation/RNAi and OX lines. Details are as in A. D, Expression of OsHKT2;1 in root of wild-type, mhz6-1, OsEIL2-RNAi-19, OsEIL1-OX-3-5, and OsEIL2-OX-35 plants under salt stress. Details are as in A. E, Schematic diagrams of putative EBS in the promoter of OsHKT2;1. F, MHZ6/OsEIL1 and OsEIL2 proteins bind to the EBS-containing region of OsHKT2;1. Glutathione S-transferase (GST)-tagged MHZ6/OsEIL1 and OsEIL2 N-terminal fusion proteins were incubated with biotin-labeled DNA fragments (Probe). Competition for the biotin-labeled promoter region was done by adding an excess of unlabeled wild-type probe (Competitor). Two biological replicates and two technical replicates were performed with similar results. G, MHZ6/OsEIL1 and OsEIL2 activated the promoter activity of OsHKT2;1 by transient expression in tobacco leaves. Five biological replicates were performed with similar results. H, Quantitative analysis of luminescence intensity for each comparison in G.
MHZ6/OsEIL1 and OsEIL2 Activate the Expression of OsHKT2;1 through Direct Binding to Its Promoter
Previous studies have identified that OsHKT2;1 and its homologous wheat (Triticum aestivum) protein TaHKT2;1 had similar roles in Na+ uptake from the external medium (Laurie et al., 2002; Horie et al., 2007). Because ethylene induces OsHKT2;1 expression via OsEIL1/2 functions, we further examined the expression of OsHKT2;1 in roots of the mutants/RNAi lines and OX lines of MHZ6/OsEIL1 and OsEIL2. In comparison with the wild type, OsHKT2;1 was slightly down-regulated in the three mhz6 allelic mutants, whereas the expression was dramatically increased in the MHZ6/OsEIL1-OX lines (Fig. 8C, left). Similarly, OsHKT2;1 expression was weakly repressed in OsEIL2-RNAi plants but apparently induced in the OsEIL2-OX lines (Fig. 8C, right). We next examined the expression of OsHKT2;1 in roots of various plants under 200 mm NaCl. OsHKT2;1 was repressed in response to salt treatment in all the plants. However, compared with the wild type, the expression level of OsHKT2;1 was still much higher in MHZ6/OsEIL1-OX-3-5 and OsEIL2-OX-35 plants but lower in mhz6-1 and OsEIL2-RNAi-19 plants under salt treatment (Fig. 8D). These data indicate that MHZ6/OsEIL1 and OsEIL2 promote the expression of OsHKT2;1 in roots and that salt stress reduces this gene expression.
We next analyzed the promoter region of OsHKT2;1 and revealed three putative EIN3-binding sites (EBS-2;1-1, EBS-2;1-2, and EBS-2;1-3; Fig. 8E). To test the possibility that MHZ6/OsEIL1 and OsEIL2 directly bind to the promoter of OsHKT2;1, we carried out an electrophoresis mobility shift assay (EMSA) using the recombinant MHZ6/OsEIL1 and OsEIL2-DBD. Two fragments of OsHKT2;1 promoter sequence harboring EBS-2;1-1 or EBS-2;1-3 were specifically bound by MHZ6/OsEIL1 or OsEIL2-DBD, respectively (Fig. 8F). These results indicate that both MHZ6/OsEIL1 and OsEIL2 directly bind to the OsHKT2;1 promoter in vitro.
Using a tobacco transient expression assay system, we then tested whether MHZ6/OsEIL1 and OsEIL2 could activate the expression of OsHKT2;1 in tobacco leaves. The 2,500-bp promoter sequence upstream from the ATG codon of OsHKT2;1 was fused to the LUCIFERASE (LUC) reporter gene and cotransfected with each of the effector plasmids harboring 35S:OsEIL1 or 35S:OsEIL2 into the tobacco leaves. In contrast to the reporter vector control, the cotransfected effectors significantly increased the OsHKT2;1 promoter-driven LUC activities (Fig. 8, G and H). These results demonstrate that MHZ6/OsEIL1 and OsEIL2 could activate the expression of OsHKT2;1. Together, these data indicate that MHZ6/OsEIL1 and OsEIL2 regulate the Na+ uptake of plants through direct binding to the promoter and the activation of OsHKT2;1 expression.
Effects of MHZ6/OsEIL1 and OsEIL2 on Plant Growth and Yield-Related Traits
Alterations of the components in ethylene signaling and ethylene biosynthesis could affect grain- and/or yield-related traits (Wuriyanghan et al., 2009; Ma et al., 2013, 2014; Wang et al., 2013). Seedling growth from various plants in regular hydroponic culture for 7 d was examined under light. Compared with the wild type, the mhz6 mutants had relatively straight and longer roots, whereas MHZ6/OsEIL1-OX plants had twisted and shorter roots and shorter shoots (Supplemental Fig. S7, A and B). Similar results were obtained from the OsEIL2-RNAi and OsEIL2-OX plants (Supplemental Fig. S7, C and D). These results suggest that light-grown MHZ6/OsEIL1 and OsEIL2 mutants/RNAi seedlings may have insensitive responses to the internal ethylene, whereas the OX plants have a constitutive ethylene response, especially in roots during adaptive growth in hydroponic culture. The plant height of mhz6 and OsEIL1-OX plants at maturity stage exhibited no significant difference in comparison with that of the wild type (data not shown). OsEIL2-RNAi plants also had similar height to wild-type plants (Supplemental Fig. S7, E and G). However, the OsEIL2-OX plants were obviously shorter than the wild-type plants (Supplemental Fig. S7, F and G). These results indicate that alteration of the OsEIL1 and OsEIL2 expression could affect the growth of seedlings and/or mature plants.
The grain size was examined in various plants. The mhz6 mutants had a slight reduction in grain length, and all three mutants showed significant decreases in both grain width and thickness compared with wild-type grains (Supplemental Fig. S7, H and I). In MHZ6/OsEIL1-OX plants, grain length and width were increased significantly in all three lines compared with that in the wild type (Supplemental Fig. S7, H and I). These results indicate that MHZ6/OsEIL1 mutation reduces grain size, whereas MHZ6 overexpression increases grain size. The thousand-grain weight of various plants was further measured. The results showed that mhz6 mutants had significant reductions, whereas the three OX plants had significant increases in this parameter (Supplemental Fig. S7I). The grain size and thousand-grain weight of two OsEIL2-OX plants (19 and 24) were smaller than those of the wild type, whereas the two parameters of the OsEIL2-RNAi plants showed some fluctuation compared with those of the wild type (Supplemental Fig. S7, J and K). These results indicate that the effects of MHZ6/OsEIL1 and OsEIL2 on grain size and weight are differential and that MHZ6/OsEIL1 increases the grain size and thousand-grain weight in rice.
DISCUSSION
In this study, we characterized a rice ethylene response-deficient mutant, mhz6, which showed ethylene insensitivity in roots. MHZ6 encoded a homologous protein to EIN3, which is the master transcription factor in the ethylene signaling pathway of Arabidopsis, suggesting that ethylene signaling in rice may be conserved. However, our results reveal distinct roles for MHZ6/OsEIL1 and its related gene OsEIL2, in contrast to Arabidopsis EIN3/EIL1, in both ethylene response and salt stress response. In rice, MHZ6/OsEIL1 and OsEIL2 regulate the ethylene responses of roots and coleoptiles, respectively, and both cause salt-sensitive responses. In Arabidopsis, EIN3 and EIL1 regulate similar ethylene responses in seedlings but confer salt tolerance.
MHZ6/OsEIL1 and OsEIL2 Regulate the Ethylene Response of Root and Coleoptile of Rice Etiolated Seedlings, Respectively
There are six members of the EIN3 family in Arabidopsis. Of these, EIN3 and EIL1 are the most related proteins in ethylene-controlled transcriptional regulation (Chao et al., 1997; Alonso et al., 2003). Both ein3 and eil1 mutants exhibit incomplete ethylene insensitivity in etiolated seedlings (Alonso et al., 2003). Different from this phenomenon, the roots of our rice mhz6/oseil1 seedlings displayed completely insensitive phenotypes when treated with ethylene (Fig. 1, A and B). By contrast, the coleoptile growth of mhz6 seedlings was only slightly different from that of the wild type after ethylene treatment (Fig. 1, A and C). In the rice OsEIL family, OsEIL2s are closely related to MHZ6/OsEIL1. Silencing of OsEIL2 leads to strong insensitivity in ethylene-promoted coleoptile elongation but almost normal ethylene sensitivity in root growth (Fig. 4, B–D). These results demonstrate that MHZ6/OsEIL1 and OsEIL2 mainly regulate the ethylene response in roots and coleoptiles, respectively, indicating their functional divergence in rice. This organ-specific functional divergence has not been observed in EIN3 and EIL1 of Arabidopsis, since both control similar ethylene responses in etiolated seedlings. The reason for this functional diversification of MHZ6/OsEIL2 and OsEIL2 is not clear. It is possible that this functional difference was acquired during rice domestication, since rice plants need a mechanism to adapt to the water environment. We have discovered that ethylene promotion of coleoptile elongation in etiolated seedlings is specific for rice among the monocotyledonous plants examined (Yang et al., 2015). Rice lives in water-saturated soil almost in its entire life cycle, and faster growing coleoptiles promoted by OsEIL2 are conducive for rice to emerge from shallow waters, representing the mechanism adopted for rice adaptation in the water environment.
How MHZ6/OsEIL1 and OsEIL2 of the rice plants achieve their corresponding functions requires further investigation. In etiolated seedlings, both genes showed relatively higher expression in coleoptiles than in roots (Supplemental Fig. S2, A and B). Promoter-GUS analysis also reveals similar expression patterns (Supplemental Fig. S2, C and D). Therefore, the organ-specific functions of the two genes may not be due to differences in spatial expression patterns and/or intensities. It is possible that MHZ6/OsEIL1 and OsEIL2 may have different protein levels in roots and shoots. Moreover, the two proteins may activate different ERF-related genes for root inhibition or coleoptile promotion. In Arabidopsis, EIN3 may regulate different ERF genes for stress and ethylene responses (Zhong et al., 2009; Zhang et al., 2011; Chang et al., 2013; Peng et al., 2014). Alternatively, the two proteins may also function through the direct regulation of downstream genes other than ERFs. Further identification of the target genes of MHZ6/OsEIL1 and OsEIL2 should shed light on the mechanisms involved.
It is interesting that OsEIL2 promoted the expression of MHZ6/OsEIL1 mildly in OsEIL2-OX transgenic plants (Supplemental Fig. S8A). Compared with the wild type, the expression of MHZ6/OsEIL1 was slightly inhibited in roots but apparently inhibited in shoots of OsEIL2-RNAi-19 (Supplemental Fig. S8A). Similarly, MHZ6/OsEIL1 promoted OsEIL2 expression especially in roots of MHZ6/OsEIL1-OX plants (Supplemental Fig. S8B). Consistently, the expression of OsEIL2 had similar inhibition in roots and shoots of the mhz6-1 mutant (Supplemental Fig. S8B). These results indicate that MHZ6/OsEIL1 and OsEIL2 may regulate each other at the transcript level. Considering that MHZ6/OsEIL1 and OsEIL2 mainly regulate ethylene responses in roots and coleoptiles/shoots, respectively, the mutual positive regulation of the two genes may be beneficial for the survival of the whole plants when only roots or shoots are exposed to different stresses.
We analyzed the ethylene-responsive genes in roots of mhz7-2/osein2 and mhz6-1 and in shoots of mhz7-2/osein2 and OsEIL2-RNAi-19 compared with the wild type by RNA sequencing (RNA-seq; SRP041468). There are 1,046 ethylene-responsive genes in roots of the wild type, and OsEIN2 and MHZ6/OsEIL1 regulated 757 and 464 genes, respectively. Eighty-seven percent (404 genes) of the MHZ6/OsEIL1-regulated genes are also regulated by OsEIN2 in roots (Supplemental Fig. S9A; Supplemental Table S1). In shoots, there are 5,112 ethylene-responsive genes. Among these, OsEIN2 and OsEIL2 regulated 4,815 and 4,515 genes, respectively, and the overlapping genes are 4,328, representing 95% of the OsEIL2-regulated genes (Supplemental Fig. S9B; Supplemental Table S2). These results further support that MHZ6/OsEIL1 and OsEIL2 mainly regulate the ethylene response of roots and coleoptiles, respectively, downstream of OsEIN2.
Knockdown of both MHZ6/OsEIL1 and OsEIL2 leads to insensitive phenotypes in both roots and coleoptiles of OsEIL1-2-RNAi plants (Fig. 5B). This phenomenon may be consistent with that of the Arabidopsis ein3eil1 double mutants, which show complete ethylene insensitivity in both the triple response and repression of the ctr1 mutation (Alonso et al., 2003). Therefore, similar to EIN3 and EIL1 from Arabidopsis, MHZ6/OsEIL1 and OsEIL2 could collectively mediate most aspects of seedling growth responses to ethylene in rice. Other members of the Arabidopsis EIN3 family (EIL2–EIL5) may not play roles in ethylene signaling transduction. In this study, we found that the more distantly related members OsEIL3 and OsEIL4 also had transcriptional activities and were nuclear proteins (Supplemental Fig. S1, B and C). However, analyses of the RNAi/mutation and OX plants of OsEIL3 and OsEIL4 suggest that OsEIL3 and OsEIL4 likely have less effect on the ethylene response in etiolated seedlings (Supplemental Figs. S4, A–C, and S5, A–C).
Together, our results provide direct evidence that MHZ6/OsEIL1 and OsEIL2 mediate ethylene signaling in rice seedlings by spatially regulating the ethylene response of root and coleoptile, respectively.
MHZ6/OsEIL1 and OsEIL2 Negatively Regulate the Salt Tolerance of Rice Seedlings via the Direct Transcriptional Regulation of OsHKT2;1 in Roots
Our previous studies in the ethylene receptor NTHK1 in tobacco and EIN2 and EIN3/EIL1 in Arabidopsis have demonstrated that ethylene plays a positive role in salt stress tolerance in dicotyledonous plants (Cao et al., 2006, 2007; Chen et al., 2009; Lei et al., 2011). An ankyrin domain protein, NEIP2, that interacted with NTHK1 is also required for salt-stress tolerance in tobacco plants (Cao et al., 2015). Recently, studies on EIN3/EIL1 function in salt stress have further supported the above view (Peng et al., 2014). Increased in vivo ethylene production via loss of ETO1 function also confers salt tolerance in Arabidopsis (Jiang et al., 2013). At present, we found that, in contrast to the positive roles of EIN3 and EIL1 in Arabidopsis, MHZ6/OsEIL1 and OsEIL2 play negative roles in the salt tolerance of rice seedlings. Rice seedlings with ethylene treatment displayed reduced salt tolerance, whereas blocking ethylene perception with 1-MCP leads to enhanced tolerance of rice seedlings (Fig. 6D). These results further support that increased ethylene or enhanced ethylene signaling decreases the salt tolerance of rice seedlings. Consistent with this, the rice receptor-like kinase SALT INTOLERANCE1, which promotes ethylene production in rice, causes salt sensitivity (Li et al., 2014). Similarly, down-regulating the ethylene biosynthetic pathway by knocking down 1-aminocyclopropane-1-carboxylic acid synthase3 can improve the performance and grain yield of maize (Zea mays) under abiotic stress (Habben et al., 2014).
When plants are grown in conditions of high salt, the avoidance of Na+ uptake is an important aspect of salt tolerance (Munns and Tester, 2008). In Arabidopsis, EIN3ox seedlings accumulated less Na+, whereas ein3-1eil1-1 seedlings had higher Na+ content compared with the wild type (Peng et al., 2014). The plants lacking ETO1 function accumulated much less shoot Na+ than the wild type (Jiang et al., 2013). On the contrary, our observations indicate that, under high-salt conditions, loss of MHZ6/OsEIL1 and OsEIL2 functions reduced Na+ accumulation, whereas overexpression of them increased Na+ accumulation in plants (Fig. 7), suggesting that MHZ6/OsEIL1 and OsEIL2 promote Na+ accumulation and hence cause salt-sensitive responses in rice.
It should be noted that the ion content differences between mutant/RNAi or OX plants and wild-type plants are not that large in this study (Fig. 7), very similar to the cases in rice and wheat of other studies (Laurie et al., 2002; Ren et al., 2005). However, in Arabidopsis, the Na+ contents are strikingly different in the plants compared (Jiang et al., 2013; Peng et al., 2014). It is possible that the Na+ accumulation is differential in different plant species. Alternatively, in rice, ethylene signaling may control only a limited portion of Na+ accumulation. Other possibilities could not be excluded.
We further elucidated the specific downstream targets of the ethylene signaling cascade controlling the Na+ content in rice and found that MHZ6/OsEIL1 and OsEIL2 positively regulate the expression of OsHKT2;1 in roots by directly binding to its promoter. Ethylene also induced OsHKT2;1 expression via MHZ7/OsEIN2 and MHZ6/OsEIL1 and OsEIL2 functions. OsHKT2;1 is classified as a member of HKT subfamily II, which appears to be specific to monocotyledonous plants (Platten et al., 2006; Almeida et al., 2013). OsHKT2;1 mediates Na+ import in the presence of Na+ in the millimolar range (Jabnoune et al., 2009). In comparison with the wild type, the knockout mutants of OsHKT2;1 have large reductions in Na+ uptake (Horie et al., 2007). In addition to OsHKT2;1, two homologous genes, TaHKT2;1 and HvHKT2;1, of HKT subfamily II have been characterized in wheat and barley (Hordeum vulgare; Gassmann et al., 1996; Wang et al., 1998; Rubio et al., 1999; Buschmann et al., 2000; Golldack et al., 2002; Laurie et al., 2002; Haro et al., 2005; Bañuelos et al., 2008; Mian et al., 2011). The antisense repression of TaHKT2;1 expression resulted in a decrease in Na+ uptake by the roots and increased salt tolerance in wheat (Laurie et al., 2002). HvHKT2;1 overexpression increases Na+ content in plants grown at high external Na+ concentrations in barley (Mian et al., 2011). Together, these results reveal that the members of class II HKT have a role in Na+ uptake from the external medium. Moreover, OsHKT2;1, TaHKT2;1, and HvHKT2;1 are expressed in root periphery cells, which agrees with their functions in ion uptake from the external medium (Schachtman and Schroeder, 1994; Golldack et al., 2002; Jabnoune et al., 2009). Therefore, the MHZ6/OsEIL1- and OsEIL2-mediated regulation of OsHKT2;1 in roots may contribute to the Na+ accumulation and salt sensitivity of rice seedlings under salt stress.
Interestingly, it seems likely that HKT proteins evolved rapidly. The Arabidopsis genome contains a single HKT gene, whereas rice contains up to nine HKT genes (depending on variety). The main role of AtHKT1;1 is to avoid the accumulation of excessive Na+ in the shoots of Arabidopsis (Berthomieu et al., 2003; Sunarpi et al., 2005; Møller et al., 2009). In rice, ethylene and MHZ6/OsEIL1- and OsEIL2-regulated OsHKT2;1 have distinct roles in Na+ uptake from the external medium. The ethylene signaling-driven Na+ uptake may be useful for rice to adapt to the environment of high water potential by maintaining the ion homeostasis of plant cells. This mechanism is essential for the survival of rice plants under normal water conditions. However, when exposed to high salinity, the high OsHKT2;1 expression induced by the enhanced ethylene level and MHZ6/OsEIL1 and OsEIL2 expression result in excessive Na+ accumulation and, hence, salt sensitivity of plants. It should be noted that salt treatment represses OsHKT2;1 expression in rice, suggesting that rice plants may also try to avoid excessive Na+ uptake through the inhibition of OsHKT2;1 expression under high-salinity conditions. It will be interesting to test the performance of the plants with altered ethylene signaling under long-term salt stress conditions in the field.
Besides OsHKT2;1, ethylene also affected the expression of some other members of the OsHKT family (Fig. 8, A and B). MHZ6/OsEIL1 and OsEIL2 positively regulated OsHKT2;3 and OsHKT2;4 in roots but negatively regulated OsHKT1;3 in shoots (Supplemental Fig. S10, A–C), most likely through direct binding to their promoters (Supplemental Fig. S10, D–G). OsHKT2;3 and OsHKT2;4, members of HKT subfamily II like OsHKT2;1, were marginally expressed in the roots (Lan et al., 2010; Horie et al., 2011). In Xenopus laevis oocytes, OsHKT1;3 mediated both inward and outward Na+ currents with weak inward rectification, although yeast cells expressing OsHKT1;3 did not mediate any type of transport (Garciadeblás et al., 2003; Jabnoune et al., 2009). The function of OsHKT1;3 in rice has not been studied yet. Another subfamily I member, OsHKT1;5, corresponding to Streptomyces lividans K+ Channel1, is an Na+-selective transporter and positively regulated K+/Na+ homeostasis under salt stress (Ren et al., 2005). It will be interesting to identify the functions of the above OsHKT transporters in plants to determine whether MHZ6/OsEIL1 and OsEIL2 regulation of their expression is involved in the salt stress responses of rice seedlings.
Taken together, different from the positive roles of ethylene and EIN3/EIL1 in Arabidopsis, our results prove that MHZ6/OsEIL1 and OsEIL2 negatively regulate the salt tolerance of rice seedlings by direct regulation of OsHKT2;1 expression and Na+ uptake. Understanding the molecular mechanism of the interaction between ethylene and salt stress in rice will provide insights for engineering salt tolerance in important crops.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rice (Oryza sativa ssp. japonica ‘Nipponbare’) was used as the wild type in this study. All plants were grown in the paddy field of the Experimental Station of the Institute of Genetics and Developmental Biology in Beijing from May to October of each year.
Ethylene Treatment
For the ethylene-response assay, seeds were soaked at 37°C for 2 d, and the germinated seeds were placed on stainless nets with a water level below the seeds. Ethylene gas was injected into the boxes using a syringe. The seedlings were grown at 28°C in the dark for 3 d (Ma et al., 2013). The length of roots and coleoptiles was measured.
For ethylene treatment of etiolated/green seedlings, germinated seeds were placed on stainless nets for 2/3 d in air and then treated with 10 µL L−1 ethylene for 8 h at 28°C in dark/constant light. The roots and coleoptiles were harvested for total RNA isolation.
Molecular Cloning of MHZ6
To map the MHZ6 locus, the mutant mhz6-1 was crossed with the indica cv 93-11 MH63, cv ZF802, and cv TN1. For rough mapping, we used a DNA pool generated from 15 mutant individuals selected from the F2 population derived from a cross between mhz7-1 and MH63. For fine mapping, a total of 900 mutant individuals selected from the four F2 populations were used. The MHZ6 locus was mapped to a 68-kb region (3-11.76, 5′-GCCAAGACGCCAACCAAA-3′ and 5′-GGTGGAGGGGAGTGGGAT-3′; 3-11.83, 5′-CCCTGATGTTTCCCCTTTTA-3′ and 5′-TATCAATTTTGATCCCCTCAT-3′), which contains 13 genes. The candidate gene was finally determined by DNA sequencing of all the genes in this region.
To confirm the mutation in mhz6, DNA fragment-length polymorphism between the wild type and the mutant was examined. Considering the duplication of the MHZ6 sequence, nested PCR was used. The primers are listed in Supplemental Table S1.
Gene Expression Analysis by Semiquantitative PCR or Real-Time PCR
Total RNA was extracted using TRIZOL reagent (Invitrogen). After eliminating DNA contamination by DNase I (TaKaRa), the complementary DNAs were synthesized using the cDNA Synthesis Kit (Moloney murine leukemia virus version; TaKaRa) and then subjected to semiquantitative PCR or real-time quantitative PCR. OsACTIN1 and/or OsACTIN2 were used as the internal controls. The primers used are listed in Supplemental Table S3.
Vector Construction and Plant Transformation
For complementation, the pCAMBIA2300 vector (pMHZ6C) contained the MHZ6 genomic sequence (1,923 bp), the upstream 2,588-bp sequence of the MHZ6 ATG (promoter), and the downstream 2,117-bp sequence of the MHZ6 TGA. This full sequence (6,628 bp) was cloned by adding two separate regions: the first region (1–3,303 bp) with EcoRI and KpnI sites, and the second region (3,304–6,628bp) with KpnI and Sse8387I.
For the OsEIL OX vectors, taking MHZ6-OX vector as an example, the full coding sequence (1,923 bp) and 3′ untranslated region (43 bp) were inserted into BamHI/XbaI-digested pCAMBIA2300-35S vector to generate the MHZ6-OX construct.
For the promoter:GUS constructs, 2,588- and 2,618-bp fragments upstream from MHZ6/OsEIL1 ATG and OsEIL2 ATG were amplified for MHZ6/OsEIL1:GUS and OsEIL2:GUS, respectively. The fragments were then inserted into pCAMBIA2300-GUS vector.
For the OsEIL RNAi vectors, taking OsEIL2-RNAi vector as an example, two copies of the 484-bp specific 3′ coding sequence were repeatedly inserted in opposing orientations into pUCCRNAi to form a hairpin, which was then cloned into pCAMBIA2300-pAct1. The primers used for the constructs are listed in Supplemental Table S3.
All the vectors were introduced into Agrobacterium tumefaciens strain EHA105 through electroporation, and the resulting strains were used to transform the japonica rice cv Nipponbare. Rice transformation was performed as described previously (Wuriyanghan et al., 2009).
Transactivation Analysis
The transactivation activity was examined in the Arabidopsis (Arabidopsis thaliana) protoplast system. The reporter was a plasmid harboring the firefly LUC gene, which was controlled by a modified 35S promoter with 5× the upstream activating sequence in it. OsEIL genes were fused to the GAL4 (yeast transcription activator Gal4) DBD-coding sequence and constructed into pRT107 to generate effector plasmid pRTBD-OsEILs. The fusion genes were under the control of the 35S promoter. pRT107 vector containing the binding domain sequence and the 375BD-VP16 fusion sequence were used as negative and positive controls, respectively. A pPTRL plasmid that contained a cauliflower mosaic virus 35S promoter and Renilla LUC was used as an internal control (Ohta et al., 2000). The primers used for the constructs are listed in Supplemental Table S3.
Subcellular Localization Assays
For the detection of subcellular localization of OsEILs, the full-length OsEIL complementary DNA sequence was amplified and cloned into the pBI221-GFP or pJIT163-hGFP vector in which the GFP-coding sequence was fused in frame to the 3′ end of the OsEIL gene sequence. Primers are listed in Supplemental Table S3. The constructs were transformed into Arabidopsis protoplasts by polyethylene glycol-mediated transformation (Yoo et al., 2007). After 20 h of incubation at 28°C, GFP fluorescence was observed with a confocal laser scanning microscope (Leica TCS SP5).
NaCl Treatment
All seeds were surface sterilized and germinated in water for 2 d, placed on the petri dishes (10 × 10 cm) with two layers of gauze, incubated in Hoagland solution (pH 6) for 8 or 9 d, and treated with 200 mm NaCl-containing Hoagland solution for another 5 d. After 5 d of recovery, the number of surviving seedlings was counted. All plants were grown under white fluorescent light (600 photons m−2 s−1, 14 h of light/10 h of dark) at 28°C/26°C and 75% relative humidity. These experiments were repeated at least three times with similar results.
Measurement of Na+ and K+ Concentrations
Ten-day-old seedlings grown in Hoagland solution were supplemented with 200 mm NaCl and grown for 2 d. Shoots and roots were harvested at the indicated times. Subsequently, shoots and roots were collected, washed six times with distilled water to eliminate the attached metal ions on the surface, and dried at 80°C for 72 h. Samples were then weighed and digested as follows: 15 mL of nitric acid, 160°C for 1 h; 2 mL of hydrogen peroxide, 20 min; 5 mL of nitric acid, 160°C for 30 min; and 1 mL of hydrogen peroxide, 10 min. Na+ and K+ contents were measured using an inductively coupled plasma optical emission spectrometer (Perkin Elmer) following a method described previously (Yuan et al., 2008). The means and sd were calculated from three biological replications.
EMSA
To construct plasmids for the expression of N-terminal OsEIL1 (amino acids 1–350) and OsEIL2 (amino acids 1–354) regions in Escherichia coli BL21, DNA fragments corresponding to the two regions were obtained and inserted into the pGEX-6p-1 vector (Amersham). Purification of the fusion protein was conducted according to the Glutathione Sepharose 4B (GE) handbook.
Single-stranded complementary oligonucleotide fragments corresponding to regions of OsHKT promoters (Supplemental Table S3) harboring the EBS elements were synthesized (Invitrogen) and biotinylated using the Biotin 3′-end DNA Labeling Kit (Thermo Fisher Scientific). Biotinylated and unlabeled complementary oligonucleotide pairs were annealed to make double-stranded biotin-labeled probes and competitors by mixing together equal amounts, boiling for 5 min, and cooling slowly overnight. EMSA reaction solutions were prepared according to the manufacturer’s protocol (LightShift Chemiluminescent EMSA Kit; Thermo Fisher Scientific). Reaction solutions were incubated for 20 min at room temperature. The protein-probe mixture was separated on a 6% polyacrylamide native gel and transferred to a nylon membrane (GE). After UV light cross-linking, the DNA on the membrane was detected using the Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific).
Transactivation of the Target Promoter in Tobacco Leaves
The 2.5-kb sequences upstream from the ATG codons of OsHKTs were inserted into pGWB435 to generate promoter:LUC reporter constructs using Gateway technology (Invitrogen). The reporter plasmid and the constructs containing 35S:OsEIL1 and 35S:OsEIL2 were transformed into A. tumefaciens strain EHA105. The strains were incubated in Luria-Bertani medium and finally resuspended in infiltration buffer (10 mm MES, 0.2 mm acetosyringone, and 10 mm MgCl2) to an ultimate concentration of optical density at 600 nm = 1. Equal amounts of different combined bacterial suspensions were infiltrated into the young leaves of 5-week-old tobacco (Nicotiana tabacum) plants using a needleless syringe. After infiltration, the plants were grown first in the dark for 12 h and then with 16 h of light/8 h of dark for 48 h at 24°C before CCD imaging. The leaves were sprayed with 100 μm luciferin (Promega) and placed in the dark for 5 min. LUC activity was observed with a low-light cooled CCD imaging apparatus (iXon; Andor Technology). Experiments were performed with three independent biological replicates.
Library Preparation and Bioinformatics Analysis
Two-day-old etiolated seedlings of the wild type, mhz7-2/osein2 (Ma et al., 2013), mhz6-1, and OsEIL2-RNAi-19 were treated with or without 10 µL L−1 ethylene for 8 h, and the RNA was isolated. The mRNA was purified from 2 μg of total RNA using the Dynabeads mRNA Purification Kit (Invitrogen) and then submitted to RNA-seq library construction for the transcriptome experiments using the Ultra RNA Library Prep Kit (New England Biolabs) according to the manufacturer’s protocol. Multiplex paired-end adapters (New England Biolabs) were used to multiplex libraries. The RNA-seq libraries were quantified using Bioanalyzer (Agilent) and then sequenced (paired end, 100 bp each) in the Illumina genome analyzer (HiSeq 2000). After removing adaptor and low-quality reads, clean reads were mapped to the rice genome MSU7.0 using TopHat and analyzed using Cufflinks according to Trapnell et al. (2012) with a little modification, which includes the Poisson dispersion model of fragments being used to conduct statistical analysis (false discovery rate < 0.05) and ethylene-responsive genes being identified by fragments per kilobase per million reads requiring more than 1.5-fold change between two samples.
Sequence data from this article can be found in the MSU7.0 database under the following accession numbers: MHZ6/OsEIL1, Os03g20790; OsEIL2, Os07g48630; OsEIL3, Os09g31400; OsEIL4, Os08g39830; OsEIL5, Os02g36510; OsEIL6, Os04g38400; SHR5, Os08g10310; GERMIN-LIKE, Os08g13440; OsERF063, Os09g11480; OsERF073, Os09g11460; OsERF002, Os06g08340; OsRAP2.8, Os11g05740; OsACTIN1, Os03g50885; OsACTIN2, Os10g36650; OsHKT1;1, Os04g51820; OsHKT1;3, Os02g07830; OsHKT1;4, Os04g51830; OsHKT1;5, Os01g20160; OsHKT2;1, Os06g48810; OsHKT2;3, Os01g34850; and OsHKT2;4, Os06g48800.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Transactivation analysis and subcellular localization of OsEILs.
Supplemental Figure S2. Expression of ethylene-inducible genes in roots of wild-type and OsEIL2-RNAi lines.
Supplemental Figure S3. Expression patterns of MHZ6/OsEIL1 and OsEIL2.
Supplemental Figure S4. Knockdown and overexpression of OsEIL3 in rice showed a similar response to ethylene compared with the wild type.
Supplemental Figure S5. Mutation and overexpression of OsEIL4 in rice showed a similar response to ethylene compared with the wild type.
Supplemental Figure S6. K+ content in shoots and roots of various plants under salt stress.
Supplemental Figure S7. Phenotype analysis of mutants and transgenic lines of MHZ6/OsEIL1 and OsEIL2.
Supplemental Figure S8. Expression of OsEIL1 and OsEIL2 in various plants.
Supplemental Figure S9. Venn diagrams showing the overlaps among the ethylene-responsive genes regulated by OsEIL1, OsEIL2 and OsEIN2.
Supplemental Figure S10. MHZ6/OsEIL1 and OsEIL2 affect the expression of OsHKTs by directly binding to their promoter.
Supplemental Table S1. Ethylene-responsive genes regulated by OsEIN2 and OsEIL1 analyzed by RNA-seq in roots of etiolated seedlings.
Supplemental Table S2. Ethylene-responsive genes regulated by OsEIN2 and OsEIL2 analyzed by RNA-seq in shoots of etiolated seedlings.
Supplemental Table S3. Primers and oligonucleotides used in this study.
Acknowledgments
We thank Dr. Tie-Gang Lu (Chinese Academy of Agricultural Sciences) for providing the original transfer DNA-tagged lines and Dr. Hong-Qing Ling (Chinese Academy of Sciences) for helping to determine the ion contents.
Glossary
- RNAi
RNA interference
- ABA
abscisic acid
- OX
overexpressing
- 1-MCP
1-methylcyclopropene
- DBD
DNA-binding domain
- EMSA
electrophoresis mobility shift assay
- RNA-seq
RNA sequencing
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 91317306), 973 Projects (grant nos. 2015CB755702, 2012CB114202, and 2013CB835205), the Chinese Academy of Sciences project (grant no. KSCX3–EW–N–07), and the State Key Laboratory of Plant Genomics.
C.Y., B.M., J.-S.Z., and S.Y.C. conceived and designed the experiments; C.Y. performed the experiments; B.M. isolated the mhz6 mutant; S.-J.H. carried out the rice transformation; Q.X., K.-X.D., C.-C.Y., H.C., and X.L. contributed to some material preparation; C.Y. and J.S.Z. wrote the article.
Articles can be viewed without a subscription.
References
- Abeles FB, Morgan PW, Saltveit JME (1992). Ethylene in Plant Biology. Academic Press, San Diego [Google Scholar]
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Almeida P, Katschnig D, de Boer AH (2013) HKT transporters: state of the art. Int J Mol Sci 14: 20359–20385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Bañuelos MA, Haro R, Fraile-Escanciano A, Rodríguez-Navarro A (2008) Effects of polylinker uATGs on the function of grass HKT1 transporters expressed in yeast cells. Plant Cell Physiol 49: 1128–1132 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al. (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI (2000) Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol 122: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Liu J, Zhou QY, Cao YR, Zheng SF, Du BX, Zhang JS, Chen SY (2006) Expression of tobacco ethylene receptor NTHK1 alters plant responses to salt stress. Plant Cell Environ 29: 1210–1219 [DOI] [PubMed] [Google Scholar]
- Cao YR, Chen HW, Li ZG, Tao JJ, Ma B, Zhang WK, Chen SY, Zhang JS (2015) Tobacco ankyrin protein NEIP2 interacts with ethylene receptor NTHK1 and regulates plant growth and stress responses. Plant Cell Physiol 56: 803–818 [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chang KN, Zhong S, Weirauch MT, Hon G, Pelizzola M, Li H, Huang SS, Schmitz RJ, Urich MA, Kuo D, et al. (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLife 2: e00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen T, Liu J, Lei G, Liu YF, Li ZG, Tao JJ, Hao YJ, Cao YR, Lin Q, Zhang WK, et al. (2009) Effects of tobacco ethylene receptor mutations on receptor kinase activity, plant growth and stress responses. Plant Cell Physiol 50: 1636–1650 [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J (2008) Ethylene: a key regulator of submergence responses in rice. Plant Sci 175: 43–51 [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI (1996) Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J 10: 869–882 [DOI] [PubMed] [Google Scholar]
- Golldack D, Su H, Quigley F, Kamasani UR, Muñoz-Garay C, Balderas E, Popova OV, Bennett J, Bohnert HJ, Pantoja O (2002) Characterization of a HKT-type transporter in rice as a general alkali cation transporter. Plant J 31: 529–542 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Habben JE, Bao X, Bate NJ, DeBruin JL, Dolan D, Hasegawa D, Helentjaris TG, Lafitte RH, Lovan N, Mo H, et al. (2014) Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol J 12: 685–693 [DOI] [PubMed] [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A (2005) HKT1 mediates sodium uniport in roots: pitfalls in the expression of HKT1 in yeast. Plant Physiol 139: 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Brodsky DE, Costa A, Kaneko T, Lo Schiavo F, Katsuhara M, Schroeder JI (2011) K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol 156: 1493–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26: 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14: 660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabnoune M, Espeout S, Mieulet D, Fizames C, Verdeil JL, Conéjéro G, Rodríguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C, et al. (2009) Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol 150: 1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Cao Y, Smith JA, Harberd NP (2013) An Arabidopsis soil-salinity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell 25: 3535–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun SH, Han MJ, Lee S, Seo YS, Kim WT, An G (2004) OsEIN2 is a positive component in ethylene signaling in rice. Plant Cell Physiol 45: 281–289 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Lan WZ, Wang W, Wang SM, Li LG, Buchanan BB, Lin HX, Gao JP, Luan S (2010) A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc Natl Acad Sci USA 107: 7089–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32: 139–149 [DOI] [PubMed] [Google Scholar]
- Lei G, Shen M, Li ZG, Zhang B, Duan KX, Wang N, Cao YR, Zhang WK, Ma B, Ling HQ, et al. (2011) EIN2 regulates salt stress response and interacts with a MA3 domain-containing protein ECIP1 in Arabidopsis. Plant Cell Environ 34: 1678–1692 [DOI] [PubMed] [Google Scholar]
- Li CH, Wang G, Zhao JL, Zhang LQ, Ai LF, Han YF, Sun DY, Zhang SW, Sun Y (2014) The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 26: 2538–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Chen SY, Zhang JS (2010) Ethylene signaling in rice. Chin Sci Bull 55: 2204–2210 [Google Scholar]
- Ma B, He SJ, Duan KX, Yin CC, Chen H, Yang C, Xiong Q, Song QX, Lu X, Chen HW, et al. (2013) Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant 6: 1830–1848 [DOI] [PubMed] [Google Scholar]
- Ma B, Yin CC, He SJ, Lu X, Zhang WK, Lu TG, Chen SY, Zhang JS (2014) Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet 10: e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Wang S, Jia Q, Wu P (2006) OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol Biol 61: 141–152 [DOI] [PubMed] [Google Scholar]
- Mian A, Oomen RJ, Isayenkov S, Sentenac H, Maathuis FJ, Véry AA (2011) Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J 68: 468–479 [DOI] [PubMed] [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type–specific alteration of Na+ transport in Arabidopsis. Plant Cell 21: 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, Drew MC (1997) Ethylene and plant responses to stress. Physiol Plant 100: 620–630 [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H (2000) Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J 22: 29–38 [DOI] [PubMed] [Google Scholar]
- Peng J, Li Z, Wen X, Li W, Shi H, Yang L, Zhu H, Guo H (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 10: e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn DJ, Horie T, Leigh RA, Lin HX, Luan S, et al. (2006) Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci 11: 372–374 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR (2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37: 1141–1146 [DOI] [PubMed] [Google Scholar]
- Rubio F, Schwarz M, Gassmann W, Schroeder JI (1999) Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J Biol Chem 274: 6839–6847 [DOI] [PubMed] [Google Scholar]
- Rzewuski G, Sauter M (2008) Ethylene biosynthesis and signaling in rice. Plant Sci 175: 32–42 [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370: 655–658 [DOI] [PubMed] [Google Scholar]
- Serek M, Tamari G, Sisler EC, Borochov A (1995) Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropene, a new inhibitor of ethylene action. Physiol Plant 94: 229–232 [Google Scholar]
- Sunarpi H, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, et al. (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J 44: 928–938 [DOI] [PubMed] [Google Scholar]
- Tao JJ, Cao YR, Chen HW, Wei W, Li QT, Ma B, Zhang WK, Chen SY, Zhang JS (2015) Tobacco translationally controlled tumor protein interacts with ethylene receptor tobacco Histidine Kinase1 and enhances plant growth through promotion of cell proliferation. Plant Physiol 169: 96–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11: 184–191 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang W, Yin Z, Wen CK (2013) Rice CONSTITUTIVE TRIPLE-RESPONSE2 is involved in the ethylene-receptor signalling and regulation of various aspects of rice growth and development. J Exp Bot 64: 4863–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TB, Gassmann W, Rubio F, Schroeder JI, Glass ADM (1998) Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol 118: 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22: 1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF, Wei W, Wu HJ, Chen LJ, Chen HW, et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21: 1473–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Lu X, Ma B, Chen SY, Zhang JS (2015) Ethylene signaling in rice and Arabidopsis: conserved and diverged aspects. Mol Plant 8: 495–505 [DOI] [PubMed] [Google Scholar]
- Yin CC, Ma B, Collinge DP, Pogson BJ, He SJ, Xiong Q, Duan KX, Chen H, Yang C, Lu X, et al. (2015) Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 27: 1061–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Wu H, Wang N, Li J, Zhao W, Du J, Wang D, Ling HQ (2008) FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res 18: 385–397 [DOI] [PubMed] [Google Scholar]
- Zhang JS, Xie C, Du BX, Wu XL, Chen SY (2001a) Tobacco two-component gene NTHK2. Chin Sci Bull 46: 574–577 [Google Scholar]
- Zhang JS, Xie C, Shen YG, Chen SY (2001b) A two component gene (NTHK1) encoding a putative ethylene receptor homolog is both developmentally and stress-regulated in tobacco. Theor Appl Genet 102: 815–824 [Google Scholar]
- Zhang L, Li Z, Quan R, Li G, Wang R, Huang R (2011) An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol 157: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhou X, Wen CK (2012) Modulation of ethylene responses by OsRTH1 overexpression reveals the biological significance of ethylene in rice seedling growth and development. J Exp Bot 63: 4151–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, Guo H (2009) EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci USA 106: 21431–21436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HL, Cao WH, Cao YR, Liu J, Hao YJ, Zhang JS, Chen SY (2006) Roles of ethylene receptor NTHK1 domains in plant growth, stress response and protein phosphorylation. FEBS Lett 580: 1239–1250 [DOI] [PubMed] [Google Scholar]