The balance between the ethylene produced by the fruit and cell-specific expression of ethylene receptor genes in the seed affects the determinism of apple fruitlet abscission.
Abstract
Apple (Malus × domestica) is increasingly being considered an interesting model species for studying early fruit development, during which an extremely relevant phenomenon, fruitlet abscission, may occur as a response to both endogenous and/or exogenous cues. Several studies were carried out shedding light on the main physiological and molecular events leading to the selective release of lateral fruitlets within a corymb, either occurring naturally or as a result of a thinning treatment. Several studies pointed out a clear association between a rise of ethylene biosynthetic levels in the fruitlet and its tendency to abscise. A direct mechanistic link, however, has not yet been established between this gaseous hormone and the generation of the abscission signal within the fruit. In this work, the role of ethylene during the very early stages of abscission induction was investigated in fruitlet populations with different abscission potentials due either to the natural correlative inhibitions determining the so-called physiological fruit drop or to a well-tested thinning treatment performed with the cytokinin benzyladenine. A crucial role was ascribed to the ratio between the ethylene produced by the cortex and the expression of ethylene receptor genes in the seed. This ratio would determine the final probability to abscise. A working model has been proposed consistent with the differential distribution of four receptor transcripts within the seed, which resembles a spatially progressive cell-specific immune-like mechanism evolved by apple to protect the embryo from harmful ethylene.
Vegetative and reproductive organs that are no longer needed or that are infected, damaged, or senescent may shed from the main plant body following a specific sequence of highly regulated events, known as abscission (González-Carranza et al., 1998; Taylor and Whitelaw, 2001; Estornell et al., 2013). This process relies upon a complex regulatory network, activated by the abscising organ, that leads to the activation of the abscission zones (AZs; Addicott, 1982; Zanchin et al., 1995; del Campillo and Bennett, 1996; Taylor and Whitelaw, 2001; González-Carranza et al., 2002, 2007; Lashbrook and Cai, 2008).
Fruit trees have set up a developmental strategy aimed at controlling fruit load according to nutrient availability, thus making efficient use of resources. This strategy is achieved through the so-called physiological drop or June drop, involving the abscission of young developing fruits mainly due to a correlative dominance effect of adjacent fruit and/or nearby shoots (Bangerth, 2000). This process differs from senescence-driven abscission, which consists of a developmentally programmed process occurring at or after ripening. The correlative effect is mainly transduced in nutritional terms (i.e. sugar starvation), thus generating intraorgan metabolic rearrangements and signals leading to AZ activation. The currently accepted model for correlatively driven abscission implies that auxin, produced by the subtending organ and transported through the AZ, can reduce its sensitivity to ethylene and delay its activation. Once the auxin flow through the AZ decreases or its transport is depolarized, the AZ becomes sensitive to ethylene and is activated (Dhanalakshmi et al., 2003; Blanusa et al., 2005; Meir et al., 2006, 2010). This downstream model, however, describes only the events occurring when abscission is induced, whereas it does not tell anything about how and why the auxin flow changes (i.e. the origin of the abscission signal).
Within this context, apple (Malus × domestica) was revealed to be a good model system to study the generation of the abscission signal in young developing fruits (Botton et al., 2011; Eccher et al., 2013, 2014), as it develops corymbs with a clear gradient of correlative dominance related to the position and size of the fruit. This dominance is naturally responsible for the physiological fruit drop, which, however, is not able to guarantee high fruit quality and, on the other hand, a suitable return to flowering in the following season. Fortunately, this dominance can be magnified by means of chemical treatments, thus inducing a significantly higher rate of fruitlet abscission (Greene et al., 1992; Bangerth, 2000). Benzyladenine (BA) is a widely known chemical thinner (Bangerth, 2000; Buban, 2000) that can induce abscission in a controlled, inducible, and selective way through the enhancement of correlative inhibitions (Dal Cin et al., 2005, 2007, 2009a, 2009b; Botton et al., 2011). As a result, a model experiment with BA can provide different populations of fruitlets with clearly predictable abscission potentials: (1) small lateral fruitlets that abscise spontaneously even upon the thinning treatment (L1); (2) big lateral fruitlets that would naturally persist (L3); (3) big lateral fruitlets that abscise upon the BA treatment (LB3); and (4) big central fruitlets that would persist (C3) also upon the thinning treatment (CB3). This experimental system allowed light to be shed on the signaling pathways mediating the induction of apple fruitlet abscission, which can be summarized in a hypothetical model describing the cortex as the primary sensor of the nutritional stress occurring within the tree. In this tissue, the molecular mechanisms linking nutrient starvation to hormone signaling are consequently activated, mainly involving abscisic acid (Eccher et al., 2013) and ethylene, whose levels peak at 2 and 4 to 5 d after abscission induction, respectively. The seeds seem to perceive the situation at a later stage and with a less pronounced transcriptional and metabolic reprogramming, leading to their abortion (Botton et al., 2011). Failure of embryo development is the critical irreversible step of abscission induction (Goldschmidt and Koch, 1996; Yuan and Greene, 2000), which is somehow triggered by the primary reaction of the cortex. Since the seed is the primary source of auxin within the fruit, its abortion decreases the supply of this hormone to the AZ, leading to its activation according to the same model proposed for the leaf by Sakamoto et al. (2008).
Despite its robustness, confirmed by physiological, metabolic, and transcriptional data, this model still lacks a critical point: how is the reaction of the seed triggered? In other words, how does the cortex communicate the critical situation to the seed? Although the involvement of the gaseous hormone ethylene in apple fruitlet abscission has already been pointed out and discussed in several studies (Dal Cin et al., 2005, 2007; Botton et al., 2011), a direct mechanistic link with the physiology of this process is still missing. In this study, the relationship between ethylene and fruitlet shedding is revisited under a new perspective, pointing out a role for ethylene perception in the seed tissues as a possible main factor involved in transducing the signal generated in the cortex. Experimental data also give some important indications about the molecular and cellular mechanisms that most likely determine the final destiny of the fruit, discriminating fruitlets destined to persist from those designated to abscise.
RESULTS
The Physiological Link between Ethylene and Apple Fruitlet Abscission
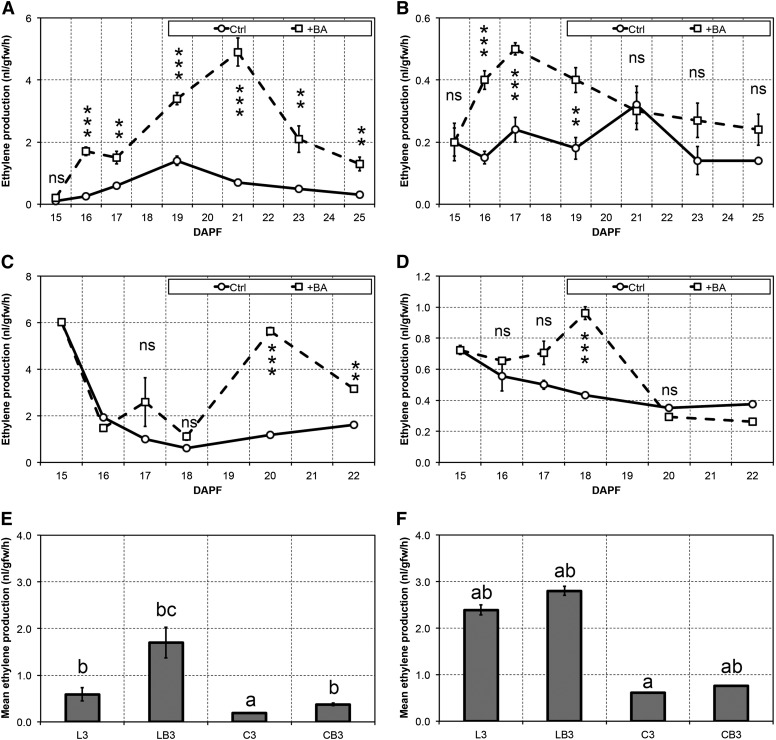
Ethylene biosynthesis was measured in the cortex of two fruitlet classes: (1) lateral fruitlets (control and BA treated) that abscise only upon a successful thinning treatment; and (2) central fruitlets (control and BA treated) that persist even upon the treatment (Fig. 1). These measurements were carried out during both a successful experiment (i.e. the level of fruit drop in treated trees was significantly higher than in controls; year 2008) and an unsuccessful one (year 2009). The former data have already been partially reported and discussed by Botton et al. (2011). However, additional information is given herein and discussed under a new perspective. Concerning the latter experiment, despite the fact that it was carried out according to the usual standards in terms of time of BA application (mean cross diameter of central fruitlets equal to 13 mm; Botton et al., 2011), the fruit drop of treated trees did not differ significantly from the controls (Supplemental Fig. S1). When the treatment succeeded, both lateral and central fruitlets that were treated with BA (LB3 and CB3) displayed, already at 16 days after petal fall (DAPF), an enhanced ethylene biosynthesis, significantly higher than that found in control samples. LB3 fruitlets, however, produced approximately 5-fold the amount of ethylene synthesized by the centrals, and, while in the centrals, ethylene biosynthesis continued to increase up to 17 DAPF and then decreased to the same levels of the control, in the laterals, it remained constant up to 17 DAPF and started to increase only thereafter, reaching its maximum peak at 21 DAPF. During the whole experimental period, all the control fruitlets showed almost stable ethylene emissions.
Figure 1.
A to D, Ethylene production in big lateral (left) and big central (right) fruitlets measured in untreated control (Ctrl; circles, continuous lines) and BA-treated (+BA; squares, dashed lines) samples in a successful (A and B) and in an unsuccessful (C and D) experiment. Statistically significant differences are indicated: ***, P < 0.001; and **, P < 0.01. Error bars represent se (n = 4). ns, Nonsignificant. E and F, Mean ethylene production as measured during abscission induction in a successful (E) and in an unsuccessful (F) trial. Error bars represent se (n = 16 in E), whereas letters indicate significant differences as identified by the Waller-Duncan test (P < 0.05).
The situation was substantially different when the thinning treatment did not succeed. Within the first 3 d after the BA treatment, both LB3 and CB3 did not show any increased ethylene production with respect to the controls. Ethylene biosynthesis was significantly enhanced in the treated laterals only at 20 DAPF, while in the centrals the treatment was able to stimulate the emissions of this hormone transiently and only at 18 DAPF. It is noteworthy that the untreated laterals at 15 DAPF displayed very high ethylene emissions (comparable to those achieved by the treated laterals at 21 DAPF in the successful experiment), with a decreasing trend throughout the experiment.
Considering the average amount of ethylene emitted by the different samples during the abscission induction period (i.e. within 3–4 d after the treatment), it is worthy to note that, when the thinning treatment was successful (Fig. 1E), an approximately 3-fold increase of mean ethylene emission (P ≤ 0.05) was observed in the BA-treated laterals that will abscise. Similarly, BA-treated centrals of the same experiment displayed a highly significant (P ≤ 0.01) 2-fold increase, although maintaining lower levels of ethylene production than the persisting laterals (i.e. the controls), consistent with their destiny. These differences, both in terms of entity and statistical significance, were not observed in the failed experiment (Fig. 1F). In particular, L3 and LB3 fruitlets showed similar mean ethylene emissions during the induction period, while the CB3 fruitlets displayed a significant increase of the biosynthetic rate of this hormone (approximately +25%), although not comparable to that observed in the previous experiment (approximately +95%).
Variations of the Ethylene-Related Transcriptome during Abscission Induction
Ninety-four genes encoding different elements of ethylene biosynthesis, perception, and signal transduction were identified using a BLASTP-validated Hidden Markov Models approach carried out on the apple genome version 1.0 (see Supplemental Tables S1 and S2). Detailed descriptions of both the rationale on which gene selection was based and the identification pipeline are given as Supplemental Text S1 and Supplemental Figures S2 to S11.
The amount of gene transcripts was then measured by quantitative PCR (qPCR) in the cortex and seeds excised from lateral and central fruitlets sampled during the abscission induction (15–19 DAPF) in the successful experiment (year 2008). Genes that were not expressed and those with either unreliable expression levels among the replicates or too close to the detection limit were discarded from the following analyses, giving a final list of 64 targets (Supplemental Table S3). Principal component analysis (PCA) was performed separately for cortex and seed on a selection of the most variable ones (52 genes), chosen according to their coefficient of variation among the samples, in order to avoid the background noise.
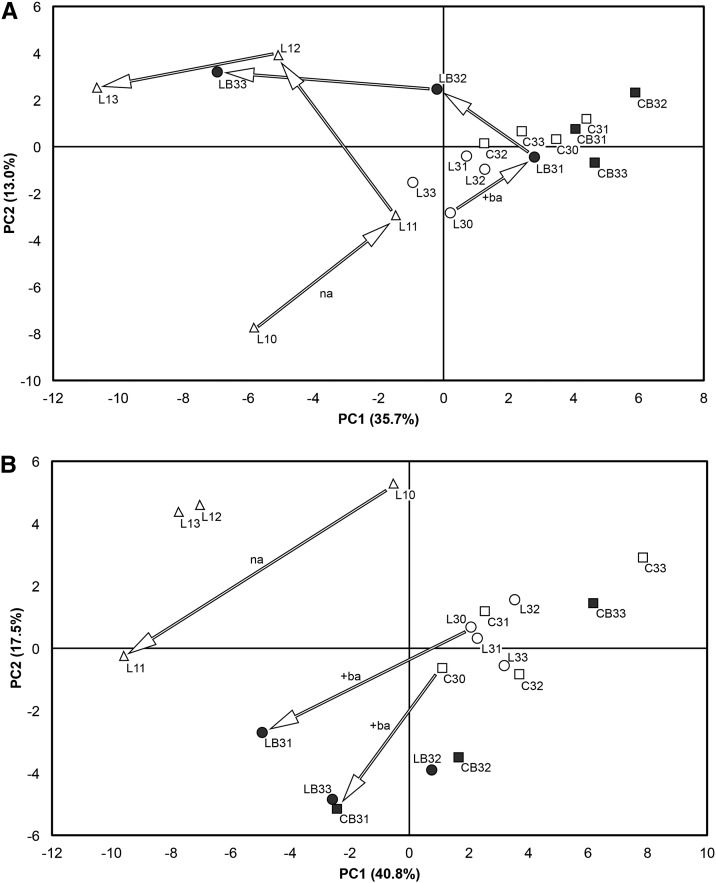
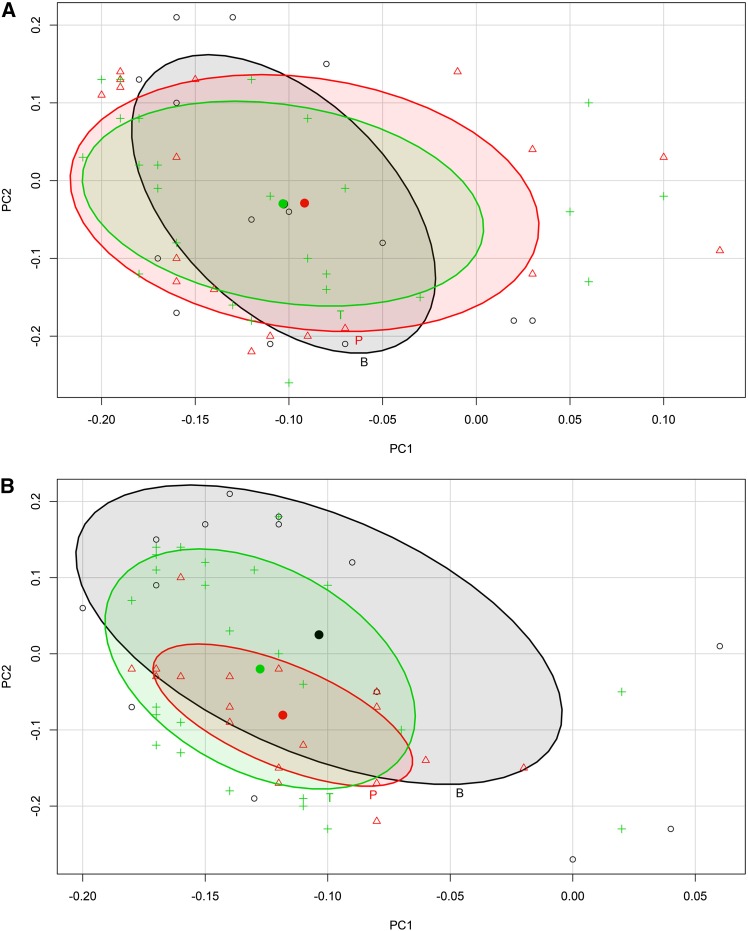
The PCA scores of the cortex based upon the first two principal components (Fig. 2) revealed that, upon abscission induction, the ethylene-related transcriptome of big lateral fruitlets (LB3) followed a pathway that substantially overlapped that of the small laterals undergoing natural abscission (L1). Abscising fruitlets showed an increase of both principal components from 15 to 16 DAPF, followed by a decrease of PC1 concurrent with a further increase of PC2 up to 17 DAPF. Then, from 17 to 19 DAPF, PC1 continued to decrease, whereas PC2 stayed almost stable. All the persisting fruitlets, including the BA-treated centrals, were closely grouped and did not show any significant and/or continuous and/or treatment-dependent variation of their ethylene-related transcripts. The first two principal components accounted for 48.7% of the total variance, with 35.7% explained by PC1 and 13% by PC2. An increase up to 60% of total variance could be achieved by including PC3, at the expense of the overall readability of the chart and without giving any additional information. Examination of the loadings (Fig. 3) indicated that the differences explained by the PCA were due to equal contributions of genes belonging to any of the three categories (i.e. biosynthesis, perception, and signal transduction). Moreover, the tendency to abscise was largely accompanied by a general increase of all the ethylene-related transcripts, as pointed out by the colocalization given by the loadings with respect to the samples as shown by the PCA scores.
Figure 2.
PCA of ethylene-related genes. Gene expression was measured by qPCR in the following samples: L10 to L13 (small lateral fruitlets at 15–17 and 19 DAPF); L30 to L33 (big lateral fruitlets at 15–17 and 19 DAPF); LB31 to LB33 (BA-treated big lateral fruitlets at 16, 17, and 19 DAPF); C30 to C33 (big central fruitlets at 15–17 and 19 DAPF); and CB31 to CB33 (BA-treated big central fruitlets at 16, 17, and 19 DAPF). A, PCA scores plot of the first two principal components (PC1 and PC2) explaining 48.7% of the total variance in the cortex. Arrows indicate the path followed by naturally abscising fruitlets (na) and those abscising because of the BA treatment (+ba). B, PCA scores plot of the first two principal components explaining 58.3% of the total variance in the seed. Arrows indicate the early reaction of the ethylene-related transcriptome in the naturally abscising fruitlets and in those treated with BA.
Figure 3.
PCA loadings plot. The loadings of cortex (A) and seed (B) are indexed according to three categories: ethylene biosynthesis (B; black circles), perception (P; red triangles), and signal transduction (T; green crosses). Data ellipses and their centers are also shown with a confidence interval of 50%. The colors are the same as for the loading symbols described above.
A different behavior was displayed by the seeds, as shown by PCA scores of the first two principal components (Fig. 2). First, the overall variations in terms of gene expression differed between the samples undergoing natural abscission and those induced to shed by the BA treatment. In this case, moreover, the ethylene-related transcripts behaved similarly in the BA-treated laterals and centrals, although only during the early abscission induction. A clear differentiation was displayed thereafter, in that the CB3 at 19 DAPF returned to be consistently grouped with the nonabscising fruitlets, while the LB3 at the same time point remained distant from the persisting samples, although not colocalizing with the naturally abscising ones (L1). Also in this case, the persisting fruitlets, including the BA-treated centrals at 19 DAPF, were closely grouped and did not show any significant continuous or treatment-dependent variation of their ethylene-related transcripts. The first two principal components accounted for 58.3% of the total variance, with 40.8% explained by PC1 and 17.5% by PC2. Also in this case, including PC3 would not significantly affect the final result but only increase the overall explained variance up to 66% of the total. The loadings plot (Fig. 3) indicated that the different behaviors shown by the seeds of the different fruitlet classes could be ascribed to diverse components of the ethylene-related transcriptome. On the one hand, the variations observed in the seeds of the naturally abscising fruitlets were largely determined by genes encoding biosynthetic elements, while on the other hand, LB3 and CB3 fruitlets displayed variations of the expression of genes coding for ethylene perception components.
Expression of Ethylene Perception Genes
The overall analysis of the ethylene-related transcriptome in the seed pointed out that (1) BA-induced abscission may determine a different transcriptional reprogramming with respect to natural fruit shedding; (2) the BA treatment induced similar transcriptomic reactions, at least as far as the ethylene-related genes, in lateral and central fruitlets during early abscission induction; and (3) the common reactions in the two fruitlet classes, mainly due to ethylene perception elements, determined a different physiological outcome (i.e. the LB3 abscised, while the CB3 persisted).
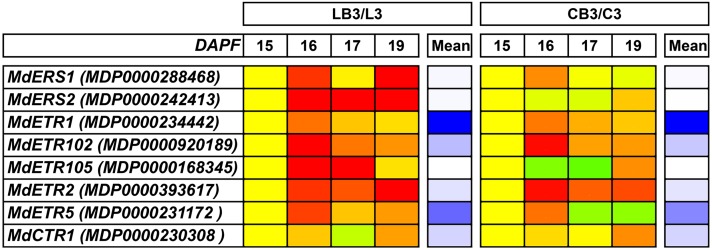
Based upon these basic observations, attention was focused on seven genes encoding ethylene receptors: Apple ETHYLENE RESPONSE SENSOR1 (MdERS1; MDP0000288468), MdERS2 (MDP0000242413), Apple ETHYLENE RESISTANT1 (MdETR1; MDP0000234442), MdETR102 (MDP0000920189), MdETR105 (MDP0000168345), MdETR2 (MDP0000393617), and MdETR5 (MDP0000231172), as well as on Apple CONSTITUTIVE TRIPLE RESPONSE1 (MdCTR1; MDP0000230308). Among the receptor-coding genes, MdETR1, MdETR5, MdETR102, and MdETR2, in this order, showed the highest expression levels in the seeds (Fig. 4). Their expression patterns well represent the situation highlighted by the PCA. In particular, all four of these genes were overexpressed with respect to the control in the seeds of BA-treated fruitlets at 16 DAPF (i.e. 24 h after the thinning treatment). In the following days, they remained substantially up-regulated, although to lower extents, with the only exception of MdETR5 in the centrals. It is noteworthy that this gene even reversed its expression, showing higher transcript levels in the control seeds than in the BA-treated ones. It is also worthy to note that, in LB3, the genes with lower expression levels also were in general up-regulated, including two ERS genes. Concerning MdCTR1, only a slight, but not significant, up-regulation was observed at 16 DAPF, higher in LB3 than in CB3; no difference was pointed out thereafter between the two fruitlet classes.
Figure 4.
Heat map showing the expression (as a log ratio) of eight selected genes during abscission induction (15–17 and 19 DAPF) in the seeds of BA-treated fruitlets with respect to their control ones (LB3/L3, BA-treated versus control big laterals; CB3/C3, BA-treated versus control big centrals). Mean expression levels are also shown with a white-blue color scale separately for each fruitlet class. The gene name and identifier are reported.
In Situ Localization of ETR1, ETR2, ETR5, and ETR102 Transcripts in the Seed
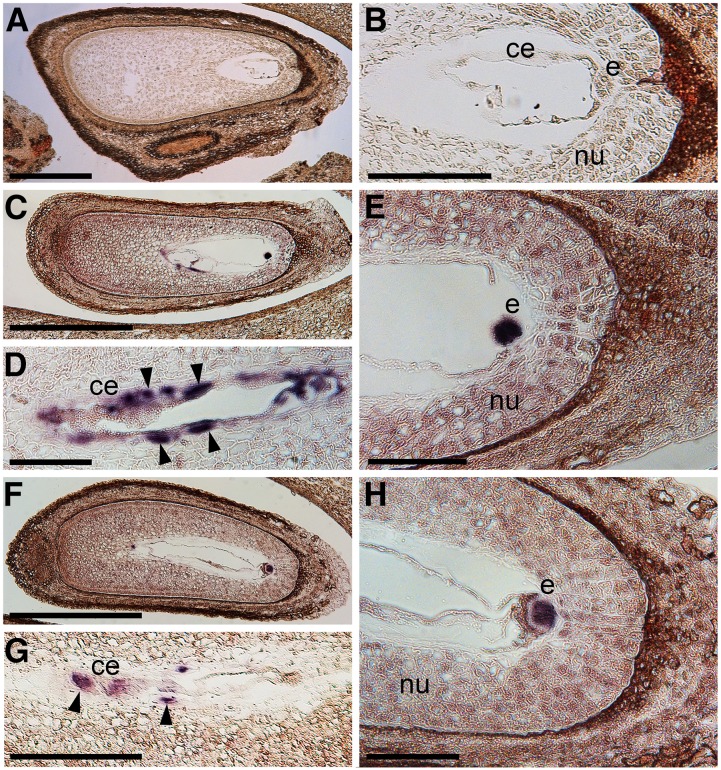
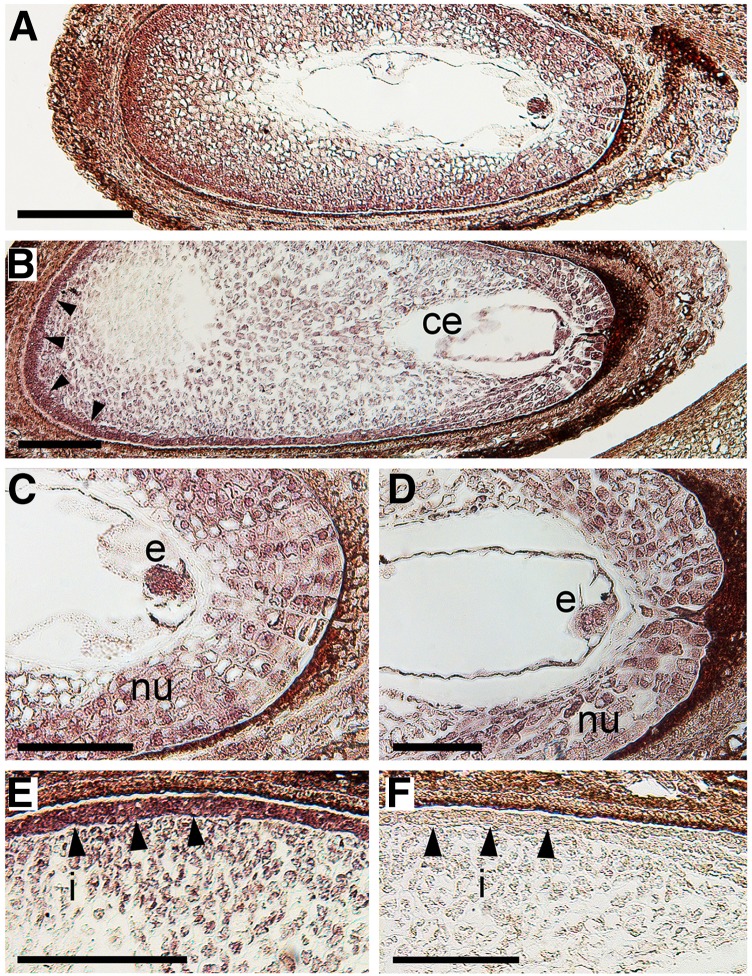
The seed encloses a series of complex structures, made up of different cell types of either maternal or filial origin. Therefore, a deep insight into the distribution within the different seed tissues and structures of the ethylene receptor transcripts may help in ascribing a functional meaning to the up-regulation of these genes during abscission induction. For this reason, in situ hybridizations were carried out with gene-specific probes (Supplemental Fig. S12) to detect the transcripts of MdETR1, MdETR2, MdETR5, and MdETR102.
Two main expression domains were pointed out by these analyses: (1) filial tissues (i.e. embryo and endosperm), and (2) maternal tissues (i.e. nucellus and inner integument). At the time when the experiments were carried out, the embryo was at the globular stage, while the endosperm was showing different degrees of cellularization, starting from the chalazal side toward the embryo. MdETR1 transcripts were mainly localized at the level of the first domain with a very strong and unequivocal signal in the embryo and in most endosperm cells (Fig. 5). Moreover, a weak but distinguishable hybridization was detectable also in the nucellus cells. MdETR2 showed a similar expression pattern (i.e. embryo and endosperm), although with weaker hybridization signal than MdETR1, consistent with its lower expression levels as detected by qPCR. In the different replicates, however, the extension of the signal of MdETR2 hybridization in the endosperm seemed more restricted to the cells at the chalazal side (data not shown), which are those resulting from earlier events of cellularization. Taken as a whole, MdETR1 and MdETR2 transcripts mainly showed filial tissue localizations. On the other hand, MdETR102 and MdETR5 transcripts were mainly localized in maternal tissues (Fig. 6), although also in this case with a specific pattern. MdETR102 signal was weakly detectable in the small and compact cells of the embryo and undetectable in the endosperm, whereas its transcripts were clearly visible in the nucellus cells, with a positive gradient toward the outer layers and a visible signal in the cells of the inner integument. Finally, MdETR5 was mainly localized in the inner integument, with only weak signals in the nucellus and in filial tissues. Its localization in the inner integument was marked especially at the chalazal side, although it was visible also around the micropyle, where cells are less compact and thus may bias the signal detection.
Figure 5.
In situ hybridization analyses of MdETR1 (C–E) and MdETR2 (F–H) expression in apple seeds collected from persisting fruitlets during the abscission induction period (17 DAPF). Longitudinal sections of the seeds were hybridized with gene-specific antisense digoxigenin-labeled probes. A hybridization without probe was used as a negative control (A and B) in order to discern the specificity of the signal in terms of color and intensity, especially at the level of the embryo and endosperm. In the latter, arrowheads indicate the strongest signals. ce, Cellularized endosperm; e, embryo; nu, nucellus. Bars = 500 μm (A, C, and F), 200 μm (B and G), and 100 μm (D, E, and H).
Figure 6.
In situ hybridization analyses of MdETR102 (A and C) and MdETR5 (B, D, and E) expression in apple seeds collected from persisting fruitlets during the abscission induction period (17 DAPF). Longitudinal sections of the seeds were hybridized with gene-specific antisense digoxigenin-labeled probes. A hybridization without probe is shown as a negative control (F) in order to discern the specificity of the signal in terms of color and intensity. Arrowheads indicate the inner integument. ce, Cellularized endosperm; e, embryo; i, inner integument; nu, nucellus. Bars = 200 μm (A and B) and 100 μm (C–F).
DISCUSSION
The role of ethylene in different abscission processes has long been investigated in diverse plant model and crop species, although most of these studies were focused on the involvement of this hormone at the level of the AZ, where only the last steps of organ shedding take place (Patterson and Bleecker, 2004; Butenko et al., 2006; Meir et al., 2010; Bar-Dror et al., 2011; Sawicki et al., 2015). Taken together, the available information indicates two anatomical layers of ethylene functional involvement: (1) within the AZ, and (2) within the abscising organ. An organ may undergo shedding because of different cues, either developmentally programmed or as a response to particular physiological contexts, such as ongoing abiotic and/or biotic stress. However, although the context in which abscission occurs may vary, it can be hypothesized that one or more signals/signal cascades always start from the abscising organ to finally communicate to the AZ that the process is induced.
As far as apple fruitlet abscission is concerned, recent studies were able to shed light, at least in part, on the molecular cascades occurring in the cortex and seed during the early inductive phases of the process upon a chemical thinning induction (Botton et al., 2011; Eccher et al., 2013). The current model describes the early reaction of the cortex of abscising fruitlets to abscission induction mainly in terms of (1) enhanced ethylene biosynthesis, (2) increased abscisic acid production, and (3) increased reactive oxygen species production. Then, later on, the seed would react in terms of (1) ethylene signaling, (2) reactive oxygen species signaling, (3) block of embryogenesis, and (4) a likely block of auxin biosynthesis. Consequently, the reduced supply of auxin to the AZ concurrent with a likely depolarization of its transport would enhance its sensitivity to ethylene and the consequent activation of cell wall-degrading enzymes (Sexton and Roberts, 1982; Taylor and Whitelaw, 2001).
But how does the seed perceive the situation? Does the cortex communicate with the seed? If yes, how? Again, what determines the different responses observed in lateral versus central fruitlets?
The relationship between ethylene and apple fruitlet abscission has long been discussed (Dennis, 2002), and the role of this hormone is still controversial. The data presented here, however, strengthen the direct relationship existing between ethylene and apple fruitlet abscission, although under a different perspective with respect to previous studies. First, an early (i.e. within 24 h from the thinning treatment) stimulation of ethylene biosynthesis (Fig. 1) seems a conditio sine qua non for abscission to occur. Actually, the importance of the first 24 h was already inferred (Botton et al., 2010), although indirectly and based upon limited evidence. Moreover, the peak of ethylene at 5 d after the thinning treatment in fruitlets from BA-treated trees was observed also in 2009 (i.e. the unsuccessful experiment), but in that case, it was neither preceded by an early increase of its biosynthesis nor followed by an enhanced abscission rate. In addition, the fact that the BA-treated central fruitlets that also will persist in the successful experiment do show an enhanced production of the hormone indicates that, although indispensable, this early increase itself is not sufficient for abscission to occur, as stated previously (Dennis, 1987), and that it is rather the degree of amplification of ethylene biosynthesis with respect to the basal levels at the time of the thinning treatment (Fig. 1, E and F) that most likely determines a successful abscission induction, as inferred previously (Botton et al., 2010). Summarizing, two strictly related conditions are required for a successful thinning with BA: (1) stable basal ethylene biosynthesis at the time of the treatment, and (2) its early stimulation upon the BA treatment. The strict relationship between these two conditions may depend upon an immediate availability of ethylene precursors and, thus, on the level of ethylene biosynthesis at the time of the treatment. In fact, when the thinning experiment did not succeed, both central and lateral fruitlets were already showing high ethylene emissions at 15 DAPF, leading us to hypothesize that an early increase of its production could not be achieved due to the limited availability of its precursors. Later on, a stimulation of the upstream biosynthetic steps may have allowed the increased ethylene production observed at 20 DAPF in lateral fruitlets.
Ethylene biosynthesis was shown previously to depend on temperature, especially at the level of 1-aminocyclopropane-1-carboxylate synthase activity, often with an optimum within the range of 20°C to 25°C (Burg and Thimann, 1959; Field, 1985; Biggs et al., 1988; Atta-Aly, 1992). Nevertheless, it is known to decline just after fruit set in several species (Vriezen et al., 2008; Wang et al., 2009; Martínez et al., 2013), most likely along with the availability of its immediate precursor 1-aminocyclopropane-1-carboxylate (A. Botton, unpublished data). According to the data provided by the meteorological station located in the experimental orchards, during the 5 d preceding the thinning treatment (10–14 DAPF), mean and maximum temperatures in 2009 were 3.3°C (11.2°C versus 14.5°C) and 6.2°C (15°C versus 21.2°C) lower than in 2008, respectively. Additionally, total rainfalls during the same period were 101.4 mm in 2009 versus 7.3 mm in 2008. Such conditions may have affected photosynthesis, thus causing an enhanced limitation of assimilates and a partial block/slowdown of fruit development. Since BA efficacy relies mainly upon an active competition for assimilates between central and lateral fruitlets and between fruitlets and shoots, the thinning treatment performed in nonoptimal conditions of fruit growth was ineffective. A possible physiological explanation for the high production of ethylene observed at 15 DAPF and the following progressive drop of its biosynthesis observed thereafter, when mean temperatures recovered to normal values (23.2°C), in untreated fruit of 2009 could be related to two main reasons: (1) the rise in mean and maximum temperatures that occurred from 14 to 15 to 18 DAPF allowed a rapid recovery of ethylene biosynthetic rates at 15 DAPF, due to a suddenly wide availability of precursors that were not consumed before, and (2) a greater receptor degradation rate, as observed recently by Shakeel et al. (2015) at elevated temperatures, which may have caused a higher ethylene sensitivity of the whole system at 15 DAPF (both in laterals and centrals), in turn causing a homeostatic reaction and a decreased ethylene biosynthesis. Within this context, the BA treatment, besides being performed under nonoptimal fruitlet growth conditions, could not induce an immediate rise of ethylene biosynthesis because of the shortage of precursors, whereas thereafter, from 18 to 20 DAPF, a transient burst of ethylene biosynthesis was allowed by their increased availability.
Expression analyses carried out on ethylene-related genes in the cortex indicate that a successful chemically induced abscission (i.e. in LB3) determined a series of transcriptional events closely similar to those occurring during natural abscission (i.e. in L1), whose synchronism was due to the perfect timing chosen for the thinning treatment (see “Materials and Methods”; Botton et al., 2011). The early burst of ethylene observed in LB3 was associated with an increase of both the principal components (Fig. 2A), which in turn was negatively correlated to most of the genes analyzed (Supplemental Table S4). This would mean that this burst, achieved within the first 24 h also in persisting central fruitlets, was not accompanied by a transcriptional activation of ethylene-related genes but rather was due to an enhanced activity of rate-limiting ethylene biosynthetic enzymes and a concurrent sufficient availability of precursors. A general down-regulation was observed for all three gene categories (biosynthesis, perception, and signal transduction) at 16 DAPF. Among the down-regulated genes, those encoding elements of ethylene perception (i.e. the receptors) may play a relevant role in determining the following events and responses. Fewer receptors means more sensitivity to the hormone (Chang et al., 1993; Cancel and Larsen, 2002; Kevany and Klee, 2007). Therefore, during this early inductive phase, ethylene produced by the cortex most likely saturated the receptors (i.e. was perceived) and determined the response that was observed thereafter, including the transcriptional activation of ethylene-responsive genes and the strong increase of biosynthesis, with a peak at 21 DAPF, observed in the laterals.
In spite of the initial increase of ethylene production, the cortex of BA-treated central fruitlets did not show any significant transcriptional rearrangement of the considered genes, behaving similarly to untreated fruitlets (L3 and C3). This may be explained in part by observations carried out on the seeds, whose early response was exactly the opposite of that shown by the cortex. Indeed, as a general trend, the ethylene-related transcriptome of the seeds was up-regulated within the first 24 h, with BA-treated fruitlets, both laterals and centrals, showing closely similar variations. Despite these similarities, the final outcome is known to be completely divergent, as confirmed by the different PCA scores at 21 DAPF of the BA-treated laterals (LB33) and the BA-treated centrals (CB33), the latter being positioned among the persisting fruitlets. The loading plots (Fig. 3) as well as the correlation coefficients (Supplemental Table S5) indicate that, while the cortex of abscising fruitlets reacted by coordinating the whole ethylene-related transcriptome (i.e. the ethylene was likely synthesized, perceived, and its response triggered), the seeds of treated fruits, especially the centrals, mainly reacted in terms of ethylene perception genes, without activating the large transcriptional response observed in the cortex. This would indicate that the seeds may have perceived the ethylene produced by the cortex, at higher levels in LB3 than in CB3, within the first 24 h and then responded by selectively up-regulating the receptor genes, more strongly in CB3 than in LB3, as shown by PCA and loading plots taken together. This would resemble a homeostatic reaction aimed at restoring the normal situation. The up-regulation of ethylene receptor genes (Fig. 4), in particular MdETR1, MdETR5, MdETR102, and MdETR2, was stronger and longer in seeds of laterals, while MdETR5 was even down-regulated in the centrals already at 17 DAPF. Taken as a whole, these results indicate a different response of CB3 seeds with respect to LB3. Considering the final outcome, in the centrals the attempted homeostatic recovery was successful, while in the laterals it was not. This may be due to the lower sensitivity to ethylene achieved in seeds of central fruitlets by up-regulating the expression of its receptor genes. A similar mechanism was found to be involved in female cucumber (Cucumis sativus) flower development (Wang et al., 2010). In that case, the down-regulation of ETR1 and the concurrent enhancement of ethylene sensitivity were shown to promote DNA damage specifically in primordial anthers, resulting in female flowers whose development was paralleled by a reduced sensitivity to the hormone caused by higher levels of its receptors. Nucleic acid degradation was also observed in the seeds of naturally abscising fruitlets, as indirectly shown by the progressively lower yields of RNA that could be purified from those samples already at 16 DAPF (data not shown).
According to this interpretation, ethylene produced by the cortex functions as an alarm signal, which is perceived by the seeds of both laterals and centrals but may lead to different outcomes according to the ratio between receptors and ethylene levels, which is higher in centrals than in laterals. The lower the amount of receptors, the higher the ethylene sensitivity (i.e. receptors are saturated; LB3) and, consequently, its downstream effects (i.e. seed abortion). On the contrary, the higher the amount of receptors, the lower the sensitivity to the hormone (CB3), and since the receptors are not fully saturated by the lower levels of ethylene produced by central fruitlets, the ethylene signaling remains blocked, the seed survives, and abscission does not occur (Fig. 7). Actually, from a mechanistic point of view, the ethylene perception is governed by a very complex interplay among ethylene, its receptors, and CTR1, given by differential kinetics and rates of transcription, protein turnover, interactions, and subcellular localization of the players involved. The article recently published by Shakeel et al. (2015) sheds light on most of these aspects and proposes a model for Arabidopsis (Arabidopsis thaliana) that partially overlaps with the findings presented here. Although the internal concentration of ethylene in the fruitlets was not assessed, the two physiological contexts of lateral and central fruitlets may be fairly positioned within the model of Shakeel et al. (2015) as follows. (1) The seeds of central fruitlets were perceiving, already before the thinning treatment, the low amount of basal ethylene produced by the cortex; such low levels were most likely counterbalanced in terms of sensitivity by suitable transcription rates of ethylene receptor genes and CTR1 and a relatively low receptor protein turnover. Upon the BA treatment, these seeds immediately perceived the increased ethylene production, which transiently up-regulated the receptor genes, thus lowering ethylene sensitivity; once the ethylene wave decreased, the system recovered to the normal sensitivity. (2) The seeds of the laterals were closer to the one-way threshold, as they produced more ethylene and more receptors than the centrals, with a likely higher rate of receptor degradation; however, ethylene sensitivity was kept low enough to prevent a harmful response. The BA treatment leads to a strong up-regulation of receptors due to the high amounts of ethylene produced but also a likely high turnover of the respective proteins; consequently, ethylene sensitivity is enhanced and a response is achieved, leading to seed abortion.
Figure 7.
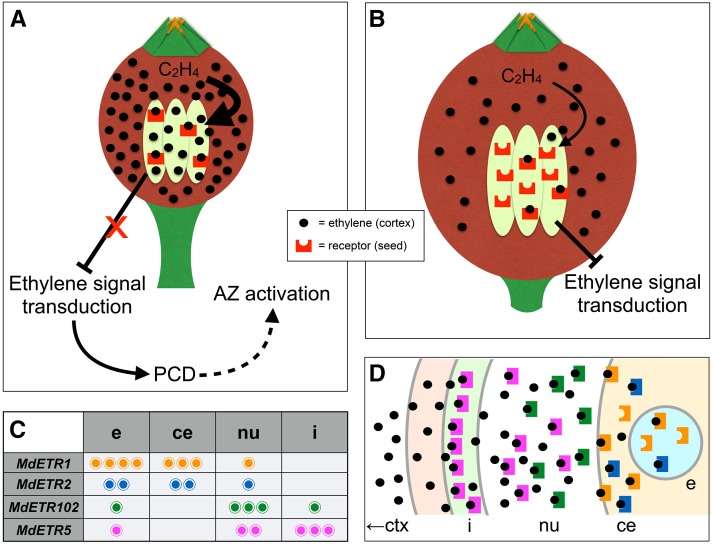
Hypothetical model for the ethylene receptor-based defense mechanism adopted by the seeds of central fruitlets to prevent them from abscising. In the lateral fruitlets that were stimulated to shed by BA (A), the high amount of ethylene produced by the cortex can saturate the receptors and thus trigger the following signal transduction pathway leading to programmed cell death (PCD), seed abortion, and AZ activation. In the central fruitlets (B), instead, the proportion between ethylene biosynthesis and the expression of ethylene receptor genes is more in favor of the latter. Therefore, since the low levels of ethylene production stimulated by the thinning treatment cannot saturate the receptors, the signaling of this hormone remains blocked and abscission does not occur. Within the seed, four ethylene receptor genes are mainly expressed: MdETR1, MdETR2, MdETR102, and MdETR5. Their transcripts are differentially distributed within the seed’s tissues, as shown in the table (C). ce, Cellularized endosperm; e, embryo; i, inner integument; nu, nucellus. These different expression domains (D) may lead one to think about a spatially progressive interception of ethylene by the different receptors at different cell layers. The ethylene produced by the cortex (ctx) also diffuses through the inner tissues toward the seeds, but once it enters the seed, it is first intercepted by the receptors being expressed at the level of the inner integument, then by those expressed in the nucellus cells, followed by those present in the cellularized endosperm, and, finally, by those expressed by the embryo. In this way, the amount of ethylene is progressively depleted, so that the receptors cannot be fully saturated, especially at the level of the embryo, and not leading to seed abortion such as in the lateral fruitlets.
The different expression domains of the four up-regulated ethylene receptor genes (Figs. 5 and 6) give some additional hints about the importance of this mechanism and explain, at least in part, the early down-regulation observed for MdETR5 in seeds of CB3. First, it is worthy to note that not only the maternal tissues are involved in this reaction. The specific localization of MdETR1 and MdETR2 transcripts at the level of the embryo and endosperm, both filial tissues, further strengthens the indispensability of this defense mechanism, whose final aim is to protect the embryo from the potential damage caused by ethylene, at least at this stage of seed development. Embryos with altered expression levels of these genes may undergo abortion as a selective strategy. A higher level of protection is achieved by expressing specific ethylene receptors also in the outermost cell layers, especially MdETR5, localized in cells of the inner integument. Also, the gradient-like localization of MdETR102 seems to resemble a progressive barrier toward ethylene, aimed at intercepting as much hormone as possible and as soon as possible, to prevent it from reaching the embryo (Fig. 7D). The success of this strategy is proved by the fact that, once the ethylene wave passed, MdETR5 immediately returned to its expression levels. More importantly, the central fruitlets persist.
CONCLUSION
The chemical nature of ethylene and its state allow it to work as the perfect mobile signal. Once again, this hormone was shown to take advantage of these features, functioning as an efficient way to exchange physiological information between different organs/tissues/cells. In this study, ethylene was shown to link the destiny of the whole fruit to that of the seed, by putting in communication the cortex with the seed, the maternal tissues with filial tissues (i.e. embryo and endosperm), thus determining a relevant developmental outcome, whether persistence or abscission. The destiny of the fruit may thus depend on the balance between ethylene biosynthesis in the cortex and the number of receptors in the seed, which in turn depends on the developmental phase resulting from the position of the fruitlet within the cluster. This occurs within a short temporal window, during which the nutritional stress, either natural or induced, triggers the whole mechanism. At this point, it would be important to shed light on the regulatory background on which a certain developmental stage relies, at least within the thinning window, in order to integrate the proposed model into the overall model of fruit development. For example, the role of auxin and its synergism with ethylene would deserve more attention.
For the first time, to our knowledge, the differential localization of four ethylene receptor gene transcripts was reported in seed tissues within the context of a well-defined physiological process (i.e. apple fruitlet abscission). A working model has been proposed consistently with their differential distribution within the seed, which resembles a spatially progressive cell-specific immune-like mechanism evolved by apple to protect the embryo from harmful ethylene action. A validation of this model in other apple cultivars (with different self-thinning behaviors) and species is necessary in order to determine if it represents an apple-specific mechanism or a conserved strategy aimed at selecting the fruits having the highest probability to survive until the end of development. Further confirmations can also be obtained by quantifying the number of receptor proteins, although it requires a high number of starting samples (i.e. seeds), which are difficult to obtain especially from abscising fruitlets.
Once this model is fully validated and/or integrated with additional evidence, it may allow the setting up of species-specific and, hopefully, cultivar-specific thinning strategies able to finely tune fruit load at the desired level.
MATERIALS AND METHODS
Plant Material and Measurements
Experiments were conducted in 2008 and 2009 on 8-year-old apple (Malus × domestica ‘Golden Delicious’/‘M9’) trees trained with standard horticultural practices at the experimental farm Maso Part (Mezzolombardo) of the Istituto Agrario San Michele all’Adige, Edmund Mach Foundation.
A randomized block design was adopted in all experiments, with four blocks, each including five trees, for each experimental thesis. Each block represented a biological replicate. Chemical thinning was performed by spraying BA at 200 μL L−1 (Brancher Dirado; Agrimport) when fruits had an average size of 13 mm (approximately 15 DAPF). Fruit drop was monitored daily up to the conclusion of June drop (i.e. the physiological fruitlet drop) to establish the effect and success of the treatment. A more detailed description of the experimental strategy can be found in Botton et al. (2011). Cortex and seeds of each biological replicate were collected from a suitable number of fruitlets (varying from 10 to 15, according to their size) of the following classes: L1, small lateral fruitlets undergoing naturally induced abscission; L3, big lateral fruitlets that would naturally persist; LB3, big lateral fruitlets from trees treated with BA that abscise; C3, big central fruitlets that would naturally persist; CB3, big central fruitlets from trees treated with BA that persist. All samples were immediately frozen in liquid nitrogen and stored at −80°C for later molecular analyses.
The laser-based photoacoustic detection of ethylene was carried out on four biological replicates using a previously described experimental device (Harren et al., 1990a, 1990b; Harren and Reuss, 1997) as reported by Dal Cin et al. (2005, 2007). A suitable number of fruitlets, varying from 10 to 15 (according to their size), were assessed for each biological replicate.
RNA Isolation and qPCR
For qPCR analyses, total RNA was extracted in 1 or 10 mL of extraction buffer from 0.02 g of seeds or 0.6 g of cortex following the method of Ruperti et al. (2001), with a few adaptations as described by Botton et al. (2009, 2011). Total RNA was quantified with the NanoDrop 2000c spectrophotometer (Thermo Scientific), and its integrity was checked by running 1 µg on a 1% (w/v) agarose gel stained with SYBR Safe (Life Technologies).
Complementary DNA was synthesized with the SuperScript VILO cDNA Synthesis Kit (Life Technologies) from 1 µg of DNA-free total RNA in a final volume of 40 µL, according to the instructions provided by the manufacturer. The reaction was performed in a Gene Amp PCR System 9700 thermocycler (Applied Biosystems).
Real-time PCR relative quantification was performed in triplicate on two biological replicates, as described by Botton et al. (2011). The nucleotide sequences of the primers for both the target and reference genes are reported in Supplemental Table S3. Data were acquired, elaborated, and exported with the StepOne Software version 2.1 (Applied Biosystems), whereas all the final calculations were made with the automated Excel spreadsheet Q-Gene designed by Simon (2003), using the modifications of the delta cycle threshold method suggested by Pfaffl (2001). Gene expression values in the seed were normalized to Md18S (Dal Cin et al., 2005) as described by Botton et al. (2011), while those of the cortex were normalized to three housekeeping genes (MDP0000375455, putatively encoding for a Leu-rich repeat protein kinase; MDP0000767855, encoding for a Δ1-pyrroline-5-carboxylate dehydrogenase, GenBank accession no. ACL13550; and MdUBIQUITIN, GenBank accession no. DQ438989, the same used by Dal Cin et al. [2005]) as described by Eccher et al. (2013). Expression levels were then reported as arbitrary units of mean normalized expression, calculated using Equation 2 of the Q-Gene spreadsheet.
Plant Fixation, Embedding, and in Situ Hybridization
Seeds were excised from persisting lateral fruitlets (sampled at 16 DAPF) along with the surrounding ovary tissues, fixed, and embedded as described by Canãs et al. (1994). Longitudinal sections of 7 μm were hybridized with sense MdETR1, MdETR2, MdETR5, and MdETR102 RNA probes labeled with digoxigenin-11-UTP using T3 polymerase following the protocol of the manufacturer (Roche). Gene-specific probes were obtained by PCR carried out on fruitlet complementary DNA using the primers listed in Supplemental Table S6, designed on nonconserved coding regions of each gene (Supplemental Fig. S1). All in situ hybridization steps, with the exception of staining, were carried out using the Gene Paint suite accessories (Freedom EVO100; Tecan) as described by Begheldo et al. (2013). The signal was developed with detection buffer containing nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche) following the manufacturer’s instructions. Sections were mounted in 50% (v/v) glycerol and observed with an Olympus BX50 microscope (Olympus) equipped with differential interference contrast optics. Images were captured with an MRc5 Axiocam color camera (Carl Zeiss) and processed with Photoshop (Adobe) for brightness/contrast corrections and normalization.
Statistical Analysis
Basic statistical analyses were performed with the StatPlus:mac LE.2009 version 5.8.3.7 package (AnalystSoft) for Microsoft Excel.
PCA and all multiple comparison statistics were calculated with R software version 3.1.1 (http://www.r-project.org/). Specifically, normality was verified with the Shapiro-Wilk test, homoscedasticity with both Bartlett and/or nonparametric Levene tests, differences among samples with either ANOVA (normality and homogenous variances) or Welch one-way ANOVA (normality and nonhomogenous variances) followed by posthoc lsd or Waller-Duncan test, respectively, and with Kruskal-Wallis (nonnormality and homogenous variances) or Friedman test (nonnormality and nonhomogenous variances).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Fruit drop dynamics in control and BA-treated trees in 2009 trials.
Supplemental Figure S2. Phylogenetic tree constructed with ACS proteins of apple and other species.
Supplemental Figure S3. Phylogenetic tree constructed with ACO proteins of apple and other species.
Supplemental Figure S4. Phylogenetic tree constructed with mitogen-activated protein kinase kinase and mitogen-activated protein kinase proteins of apple and other species.
Supplemental Figure S5. Phylogenetic tree constructed with REVERSION TO ETHYLENE SENSITIVITY1 proteins of apple and other species.
Supplemental Figure S6. Phylogenetic tree constructed with CTR proteins of apple and other species.
Supplemental Figure S7. Phylogenetic tree constructed with ERS/ETR proteins of apple and other species.
Supplemental Figure S8. Phylogenetic tree constructed with ETHYLENE INSENSITIVE2 (EIN2) proteins of apple and other species.
Supplemental Figure S9. Phylogenetic tree constructed with EIN3/EIN3-like proteins of apple and other species.
Supplemental Figure S10. Phylogenetic trees constructed with EIN3 BINDING F-box and EIN5/EXORIBONUCLEASE4 proteins of apple and other species.
Supplemental Figure S11. Chromosomal localization of the genes encoding elements of ethylene biosynthesis, perception, and signaling.
Supplemental Figure S12. Positions of the in situ hybridization probes along the coding sequences of MdETR1, MdETR2, MdETR5, and MdETR102.
Supplemental Table S1. Proteins used for generating Hidden Markov Model patterns.
Supplemental Table S2. Apple ethylene-related genes.
Supplemental Table S3. Oligonucleotides used for qPCR amplification.
Supplemental Table S4. Correlations between gene expression in the cortex and the first two principal components.
Supplemental Table S5. Correlations between gene expression in the seed and the first two principal components.
Supplemental Table S6. Oligonucleotides used for in situ hybridization probe synthesis.
Supplemental Text S1. Pipeline adopted for the identification of apple ethylene-related genes.
Acknowledgments
We thank Alberto Dorigoni and Paolo Lezzer for setting up field trials and treatments.
Glossary
- AZ
abscission zone
- BA
benzyladenine
- DAPF
days after petal fall
- qPCR
quantitative PCR
- PCA
principal component analysis
Footnotes
This work was supported by the Progetto Agroalimentare e Ricerca (grant no. 2010–2119) and by the Provincia Autonoma di Trento (Grandi Progetti 2012, Transcrapple project).
Articles can be viewed without a subscription.
References
- Addicott FT. (1982) Abscission. University of California Press, Berkeley [Google Scholar]
- Atta-Aly MA. (1992) Effect of high temperature on ethylene biosynthesis by tomato fruit. Postharvest Biol Technol 2: 19–24 [Google Scholar]
- Bangerth F. (2000) Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regul 31: 43–59 [Google Scholar]
- Bar-Dror T, Dermastia M, Kladnik A, Znidaric MT, Novak MP, Meir S, Burd S, Philosoph-Hadas S, Ori N, Sonego L, et al. (2011) Programmed cell death occurs asymmetrically during abscission in tomato. Plant Cell 23: 4146–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begheldo M, Ditengou FA, Cimoli G, Trevisan S, Quaggiotti S, Nonis A, Palme K, Ruperti B (2013) Whole-mount in situ detection of microRNAs on Arabidopsis tissues using Zip Nucleic Acid probes. Anal Biochem 434: 60–66 [DOI] [PubMed] [Google Scholar]
- Biggs MS, Woodson WR, Anda AK (1988) Biochemical basis of high-temperature inhibition of ethylene biosynthesis in ripening tomato fruits. Physiol Plant 72: 572–578 [Google Scholar]
- Blanusa T, Else MA, Atkinson CJ, Davies WJ (2005) The regulation of sweet cherry fruit abscission by polar auxin transport. Plant Growth Regul 45: 189–198 [Google Scholar]
- Botton A, Andreotti C, Costa G, Ramina A (2009) Peach (Prunus persica L. Batsch) allergen-encoding genes are developmentally regulated and affected by fruit load and light radiation. J Agric Food Chem 57: 724–734 [DOI] [PubMed] [Google Scholar]
- Botton A, Eccher G, Forcato C, Ferrarini A, Begheldo M, Zermiani M, Moscatello S, Battistelli A, Velasco R, Ruperti B, et al. (2011) Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol 155: 185–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton A, Eccher G, Ruperti B, Ramina A (2010) A time-course model for BA action in apple fruitlet thinning. Acta Hortic 884: 407–412 [Google Scholar]
- Buban T. (2000) The use of benzyladenine in orchard fruit growing: a mini review. Plant Growth Regul 32: 381–390 [Google Scholar]
- Burg SP, Thimann KV (1959) The physiology of ethylene formation in apples. Proc Natl Acad Sci USA 45: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko MA, Stenvik GE, Alm V, Saether B, Patterson SE, Aalen RB (2006) Ethylene-dependent and -independent pathways controlling floral abscission are revealed to converge using promoter:reporter gene constructs in the ida abscission mutant. J Exp Bot 57: 3627–3637 [DOI] [PubMed] [Google Scholar]
- Canãs LA, Busscher M, Angenent GC, Beltran JP, van Tunen AJ (1994) Nuclear localization of the petunia MADS box protein FBP1. Plant J 6: 597–604 [Google Scholar]
- Cancel JD, Larsen PB (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Dal Cin V, Barbaro E, Danesin M, Murayama H, Velasco R, Ramina A (2009a) Fruitlet abscission: a cDNA-AFLP approach to study genes differentially expressed during shedding of immature fruits reveals the involvement of a putative auxin hydrogen symporter in apple (Malus domestica L. Borkh). Gene 442: 26–36 [DOI] [PubMed] [Google Scholar]
- Dal Cin V, Boschetti A, Dorigoni A, Ramina A (2007) Benzylaminopurine application on two different apple cultivars (Malus domestica) displays new and unexpected fruitlet abscission features. Ann Bot (Lond) 99: 1195–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cin V, Danesin M, Boschetti A, Dorigoni A, Ramina A (2005) Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). J Exp Bot 56: 2995–3005 [DOI] [PubMed] [Google Scholar]
- Dal Cin V, Velasco R, Ramina A (2009b) Dominance induction of fruitlet shedding in Malus × domestica (L. Borkh): molecular changes associated with polar auxin transport. BMC Plant Biol 9: 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campillo E, Bennett AB (1996) Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol 111: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EG. (1987) Apple. In Monselise SP, ed, Handbook of Fruit Set and Development. CRC Press, Boca Raton, FL, pp l–44 [Google Scholar]
- Dennis EG. (2002) Mechanisms of action of apple thinning chemicals. HortSci 37: 471–474 [Google Scholar]
- Dhanalakshmi R, Prasad TG, Udayakumar M (2003) Is auxin a diffusible signal mediating abscission of recessive sinks? Plant Sci 164: 689–696 [Google Scholar]
- Eccher G, Alessandro B, Mariano D, Andrea B, Benedetto R, Angelo R (2013) Early induction of apple fruitlet abscission is characterized by an increase of both isoprene emission and abscisic acid content. Plant Physiol 161: 1952–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccher G, Ferrero S, Populin F, Colombo L, Botton A (2014) Apple (Malus domestica L. Borkh) as an emerging model for fruit development. Plant Biosystems 148: 157–168 [Google Scholar]
- Estornell LH, Agustí J, Merelo P, Talón M, Tadeo FR (2013) Elucidating mechanisms underlying organ abscission. Plant Sci 199-200: 48–60 [DOI] [PubMed] [Google Scholar]
- Field RJ. (1985) The effect of temperature on ethylene production by plant tissues. In Roberts JA, Tucker GA, eds, Ethylene and Plant Development. Butterworths Publishing, Boston, pp 47–69 [Google Scholar]
- Goldschmidt EE, Koch KE (1996) Citrus. In Zaminski E, Schaffer AA, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relations. Marcel Dekker, New York, pp 797–823 [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA (2007) Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J Exp Bot 58: 3719–3730 [DOI] [PubMed] [Google Scholar]
- González-Carranza ZH, Lozoya-Gloria E, Roberts JA (1998) Recent developments in abscission: shedding light on the shedding process. Trends Plant Sci 3: 10–14 [Google Scholar]
- González-Carranza ZH, Whitelaw CA, Swarup R, Roberts JA (2002) Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in oilseed rape and Arabidopsis. Plant Physiol 128: 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DW, Autio WR, Erf JA, Mao ZY (1992) Mode of action of benzyladenine when used as a chemical thinner on apples. J Am Soc Hortic Sci 117: 775–779 [Google Scholar]
- Harren FJ, Bijnen FG, Reuss J, Voesenek LA, Blom CW (1990a) Sensitive intracavity photoacoustic measurements with a CO2 waveguide laser. Appl Phys B 50: 137–144 [Google Scholar]
- Harren FJ, Reuss J (1997) Spectroscopy, photoacoustic. In Trigg GL, ed, Encyclopedia of Applied Physics, Vol 19 Wiley, New York, pp 413–427 [Google Scholar]
- Harren FJ, Reuss J, Woltering EJ, Bicanic DD (1990b) Photo-acoustic measurements of agriculturally interesting gases and detection of C2H4 below the ppb level. Appl Spectrosc 44: 1360–1367 [Google Scholar]
- Kevany B, Klee H (2007) Changes in ethylene sensitivity by regulated expression of the tomato ethylene receptor family. In A Ramina, C Chang, J Giovannoni, H Klee, P Perata, E Woltering, eds, Advances in Plant Ethylene Research: Proceedings of the 7th International Symposium on the Plant Hormone Ethylene. Springer, Amsterdam, pp 123–128 [Google Scholar]
- Lashbrook CC, Cai S (2008) Cell wall remodeling in Arabidopsis stamen abscission zones: temporal aspects of control inferred from transcriptional profiling. Plant Signal Behav 3: 733–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Manzano S, Megías Z, Garrido D, Picó B, Jamilena M (2013) Involvement of ethylene biosynthesis and signalling in fruit set and early fruit development in zucchini squash (Cucurbita pepo L.). BMC Plant Biol 13: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir S, Hunter DA, Chen JC, Halaly V, Reid MS (2006) Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa. Plant Physiol 141: 1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KS, Burd S, Ophir R, Kochanek B, Reid MS, Jiang CZ, Lers A (2010) Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol 154: 1929–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SE, Bleecker AB (2004) Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol 134: 194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruperti B, Bonghi C, Rasori A, Ramina A, Tonutti P (2001) Characterization and expression of two members of the peach 1-aminocyclopropane-1-carboxylate oxidase gene family. Physiol Plant 111: 336–344 [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Munemura I, Tomita R, Kobayashi K (2008) Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. Plant J 56: 13–27 [DOI] [PubMed] [Google Scholar]
- Sawicki M, Aït Barka E, Clément C, Vaillant-Gaveau N, Jacquard C (2015) Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J Exp Bot 66: 1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton R, Roberts JA (1982) Cell biology of abscission. Annu Rev Plant Physiol 33: 133–162 [Google Scholar]
- Shakeel S, Gao Z, Amir M, Chen YF, Rai MI, Ul Haq N, Schaller GE (2015) Ethylene regulates levels of ethylene-receptor/CTR1 signaling complexes in Arabidopsis thaliana. J Biol Chem 290: 12415–12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P. (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19: 1439–1440 [DOI] [PubMed] [Google Scholar]
- Taylor JE, Whitelaw CA (2001) Signals in abscission. New Phytol 151: 323–339 [Google Scholar]
- Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177: 60–76 [DOI] [PubMed] [Google Scholar]
- Wang DH, Li F, Duan QH, Han T, Xu ZH, Bai SN (2010) Ethylene perception is involved in female cucumber flower development. Plant J 61: 862–872 [DOI] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech JC, Fernie AR, Bouzayen M (2009) Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21: 1428–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Greene DW (2000) ‘McIntosh’ apple fruit thinning by benzyladenine in relation to seed number and endogenous cytokinin levels in fruit and leaves. Sci Hortic (Amsterdam) 86: 127–134 [Google Scholar]
- Zanchin A, Marcato C, Trainotti L, Casadoro G, Rascio N (1995) Characterization of abscission zones in the flowers and fruits of peach, Prunus persica (L.) Batsch. New Phytol 129: 345–354 [DOI] [PubMed] [Google Scholar]