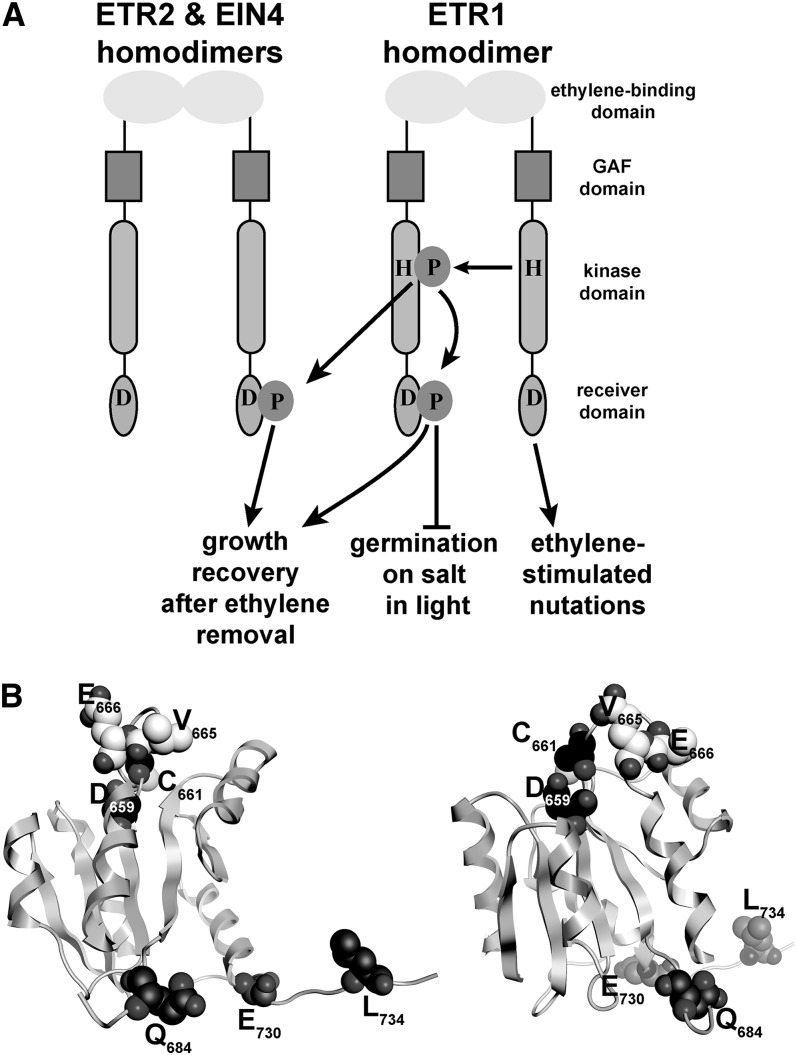
The receiver domain of an ethylene receptor has regions that control specific traits, leading to receptor subfunctionalization.
Abstract
Ethylene influences the growth and development of Arabidopsis (Arabidopsis thaliana) via five receptor isoforms. However, the ETHYLENE RESPONSE1 (ETR1) ethylene receptor has unique, and sometimes contrasting, roles from the other receptor isoforms. Prior research indicates that the receiver domain of ETR1 is important for some of these noncanonical roles. We determined that the ETR1 receiver domain is not needed for ETR1’s predominant role in mediating responses to the ethylene antagonist, silver. To understand the structure-function relationship underlying the unique roles of the ETR1 receiver domain in the control of specific traits, we performed alanine-scanning mutagenesis. We chose amino acids that are poorly conserved and are in regions predicted to have altered tertiary structure compared with the receiver domains of the other two receptors that contain a receiver domain, ETR2 and ETHYLENE INSENSITIVE4. The effects of these mutants on various phenotypes were examined in transgenic, receptor-deficient Arabidopsis plants. Some traits, such as growth in air and growth recovery after the removal of ethylene, were unaffected by these mutations. By contrast, three mutations on one surface of the receiver domain rendered the transgene unable to rescue ethylene-stimulated nutations. Additionally, several mutations on another surface altered germination on salt. Some of these mutations conferred hyperfunctionality to ETR1 in the context of seed germination on salt, but not for other traits, that correlated with increased responsiveness to abscisic acid. Thus, the ETR1 receiver domain has multiple functions where different surfaces are involved in the control of different traits. Models are discussed for these observations.
Ethylene is a phytohormone that affects the growth and development of plants and mediates plant stress responses (Mattoo and Suttle, 1991; Abeles et al., 1992). Mutational, molecular, and biochemical analyses have identified components in the ethylene signal transduction pathway. The current model speculates that ethylene binding to the receptors reduces the activity of the receptors, leading to reduced activity of the CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) protein kinase (Kieber et al., 1993). Lower CTR1 activity results in reduced phosphorylation of ETHYLENE INSENSITIVE2 (EIN2) protein (Chen et al., 2011; Ju et al., 2012; Qiao et al., 2012). The reduction in EIN2 phosphorylation leads to a decrease in the ubiquitination of EIN2, resulting in a rise in EIN2 protein levels and proteolytic release of the C-terminal portion of the protein (Qiao et al., 2009, 2012; Ju et al., 2012; Wen et al., 2012). The C-terminal portion of EIN2, through mechanisms not completely understood, modulates levels of the EIN3 and EIN3-LIKE1 transcription factors and leads to most ethylene responses (Chao et al., 1997; Solano et al., 1998; Alonso et al., 1999; Guo and Ecker, 2003; Yanagisawa et al., 2003; Binder et al., 2004a; Gagne et al., 2004; Qiao et al., 2012). As predicted by this model, loss of multiple ethylene receptors results in a constitutive ethylene response (Hua and Meyerowitz, 1998; Hall and Bleecker, 2003; Wang et al., 2003; Qu et al., 2007). Even though this signaling pathway was mostly worked out in the model plant Arabidopsis (Arabidopsis thaliana), similar genes have been described in other plants such as rice (Oryza sativa), strawberry (Fragaria vesca), and tomato (Solanum lycopersicum), as well as in plants from ancient divergent lineages such as Physcomitrella patens and Selaginella moellendorffii and the charaphyte Spirogyra pratensis (Rensing et al., 2008; Rzewuski and Sauter, 2008; Ma et al., 2010; Banks et al., 2011; Klee and Giovannoni, 2011; Shulaev et al., 2011; Ju et al., 2015). This suggests that a similar signaling pathway is found in all land plants and likely evolved prior to colonization of land.
Responses to ethylene are mediated by a receptor family that is predominantly located in the membrane of the endoplasmic reticulum (Chen et al., 2002; Ma et al., 2006; Grefen et al., 2008; Bisson et al., 2009). In Arabidopsis, there are five receptor isoforms called ETHYLENE RESPONSE1 (ETR1), ETR2, ETHYLENE RESPONSE SENSOR1 (ERS1), ERS2, and EIN4 (Chang et al., 1993; Hua and Meyerowitz, 1998; Hua et al., 1998; Sakai et al., 1998; Gao et al., 2003). The plant receptors form homodimers, with each monomer predicted to have three membrane-spanning α helices at the amino terminus containing the ethylene-binding site (Schaller and Bleecker, 1995; Schaller et al., 1995; Rodríguez et al., 1999). This is followed by the cytosolic portion of the receptor consisting of a GAF (for cGMP-specific phosphodiesterases, adenylyl cyclases, and FhlA) domain, kinase domain, and in a subset of the receptors (ETR1, ETR2, and EIN4), a receiver domain (Fig. 1A). We know that all five ethylene receptor isoforms in Arabidopsis are involved with ethylene signaling because all five members bind ethylene with high affinity (Schaller and Bleecker, 1995; Hall et al., 2000; O’Malley et al., 2005; McDaniel and Binder, 2012) and specific missense mutations in the ethylene-binding domain of any single isoform leads to dominant ethylene insensitivity that affects responses throughout the plant (Bleecker et al., 1988; Hua et al., 1995; Hua and Meyerowitz, 1998; Wang et al., 2006). The nature of signal transmission through the ethylene receptors is unknown. They have homology to bacterial two-component receptors (Chang et al., 1993; Hua and Meyerowitz, 1998; Hua et al., 1998; Sakai et al., 1998), which transduce signal via the autophosphorylation of a His residue in the kinase domain, followed by the transfer of phosphate to a conserved Asp residue in the receiver domain of a response regulator protein (West and Stock, 2001). Biochemical studies show that some of the receptor isoforms possess functional His kinases (Gamble et al., 1998; Moussatche and Klee, 2004) and ethylene binding might modulate His-kinase activity of ETR1 (Voet-van-Vormizeele and Groth, 2008). However, genetic studies suggest that His-kinase activity is not required for responses to ethylene (Wang et al., 2003; Binder et al., 2004b; Qu and Schaller, 2004; Xie et al., 2006; Cho and Yoo, 2007; Hall et al., 2012). Rather, this activity modulates growth in air, sensitivity to ethylene, and growth recovery after ethylene removal (Gamble et al., 1998; Binder et al., 2004b; Cho and Yoo, 2007; Hall et al., 2012). Thus, the plant ethylene receptors have diverged in functional output from other two-component receptors.
Figure 1.
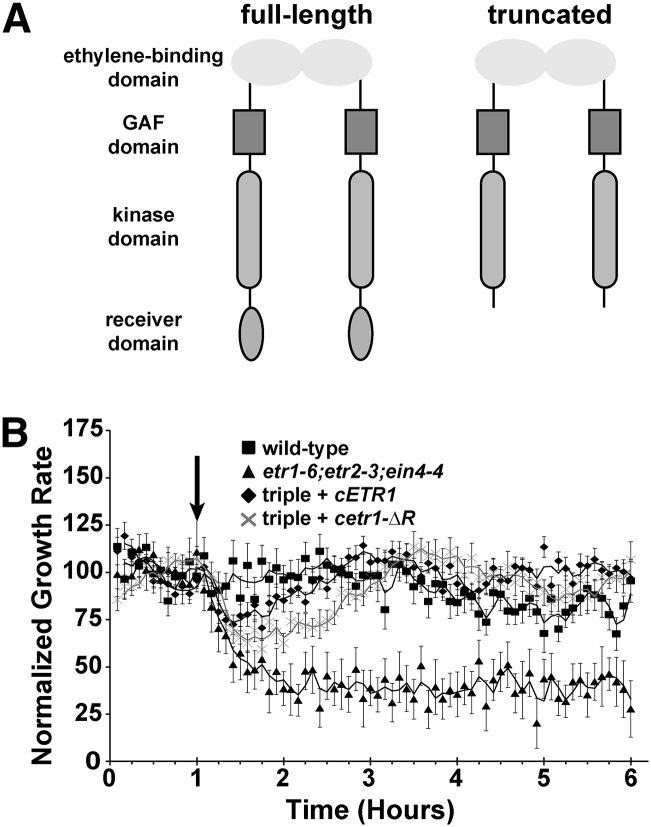
The ETR1 receiver domain is not required for response to silver ions. A, Domain structure of full-length and truncated ETR1 receptor lacking the receiver domain used in this study. B, Growth kinetic profiles in the presence of 100 µm AgNO3 are shown for etr1-6;etr2-3;ein4-4 triple mutants transformed with cDNA encoding for either a full-length ETR1 (cETR1) or a truncated transgene lacking the receiver domain (cetr1-ΔR). For comparison, responses of wild-type and triple mutant seedlings are shown. Seedlings were allowed to grow for 1 h in air, followed by the addition of 1 µL L–1 ethylene (down arrow). Growth rate was normalized to the growth rate in the air pretreatment and represents the average ± sem. Lines are a moving average calculated with a period of 2 using Microsoft Excel.
Even though the receptors have largely overlapping roles for many phenotypes, there are traits where the receptors are not redundant (Binder et al., 2004b, 2006; Plett et al., 2009a, 2009b; Liu et al., 2010; Kim et al., 2011; McDaniel and Binder, 2012; Wilson et al., 2014a, 2014b). For instance, ETR1, ETR2, and EIN4 have a role in normal growth recovery after ethylene removal, whereas ERS1 and ERS2 do not (Binder et al., 2004b). Another example of nonredundancy is that ETR1 has the predominant role in mediating the inhibitory effects of silver ions (McDaniel and Binder, 2012). Silver ions inhibit ethylene perception in plants (Beyer, 1976), but etr1 loss-of-function mutants have little response to silver ions (McDaniel and Binder, 2012). Surprisingly, there are also some traits where ethylene receptor isoforms have contrasting roles. For example, ethylene stimulates nutational bending of the hypocotyls (Binder et al., 2006). Mutant seedlings lacking ETR1 fail to nutate when treated with ethylene, whereas mutants lacking the other four isoforms have constitutive nutations in air (Binder et al., 2006; Kim et al., 2011). Additionally, we recently reported that ETR1 and ETR2 have contrasting roles in the control of Arabidopsis seed germination in darkness or during salt stress in the light where ETR1 inhibits germination and ETR2 stimulates germination (Wilson et al., 2014a, 2014b). Correlating with these seed germination results are the observations that etr1 loss-of-function mutants have reduced sensitivity to abscisic acid (ABA), whereas etr2 loss-of-function mutants have increased sensitivity to ABA (Wilson et al., 2014b). These observations are not explained by current models of ethylene signaling and point to unique roles for the ETR1 receptor. It has been suggested that the receptors may have ethylene signaling-independent roles (Gamble et al., 1998; Beaudoin et al., 2000; Desikan et al., 2005; Binder et al., 2006; Wilson et al., 2014a, 2014b), which may underlie these observations. Determining the basis for noncanonical receptor signaling is of wide interest because noncanonical signal transduction has been observed in other signal transduction pathways, including Hedgehog and Wingless/int-1) signaling in animal cells (Jenkins, 2009; Miller and McCrea, 2010) and quorum sensing in bacteria (Federle and Bassler, 2003).
The receiver domain of ETR1 is required for both ethylene-stimulated nutations and the inhibitory role of ETR1 on seed germination during salt stress (Kim et al., 2011; Wilson et al., 2014). Interestingly, this domain is not required for the inhibitory role of ETR1 on seed germination in the dark (Wilson et al., 2014a). Additionally, a common feature of the three receptors that have a role in growth recovery is that they contain a receiver domain, and genetic evidence indicates that ETR1 His autophosphorylation followed by phosphotransfer through these receiver domains is important for normal growth recovery (Binder et al., 2004b; Kim et al., 2011). To understand the structure-function relationship leading to the unique role of the ETR1 receiver domain in the control of various traits, we performed Ala-scanning mutagenesis. For this, we chose amino acids that are poorly conserved and are in regions predicted to have altered tertiary structure when compared to the receiver domains of ETR2 and EIN4. The effects of these mutants on various phenotypes were examined in transgenic, receptor-deficient Arabidopsis plants. From these experiments, we identified regions of the receiver domain important for the control of specific phenotypes.
RESULTS
Responses to Silver Ions Do Not Require the ETR1 Receiver Domain
ETR1 has been shown to have the major role in mediating the effects of the ethylene antagonist AgNO3, and etr1-6;etr2-3;ein4-4 triple mutants have no response to silver ions (McDaniel and Binder, 2012). We previously showed silver responses were rescued when the triple mutants were transformed with a full-length genomic ETR1 transgene, a transgene deficient in His-kinase activity, or a transgene lacking the conserved Asp-659 required for phosphorelay (McDaniel and Binder, 2012). However, it is unknown whether the ETR1 receiver domain is required for this trait. To address this, we examined the growth response kinetics to the application of 1 µL L–1 ethylene in the presence of 100 µm AgNO3. Our prior studies determined that there are two phases of ethylene-induced growth inhibition that are genetically distinct (Binder et al., 2004a, 2004b). The first phase starts approximately 10 min after the addition of ethylene and reaches a plateau approximately 10 min later. This plateau lasts approximately 30 min and is followed by a second phase of growth inhibition that lasts for as long as ethylene is present (Binder et al., 2004a, 2004b). The application of 100 µm AgNO3 severely attenuates or eliminates the first phase and entirely blocks the second phase of growth inhibition (McDaniel and Binder, 2012). Consistent with our prior study (McDaniel and Binder, 2012), in the presence of AgNO3, etr1-6;etr2-3;ein4-4 triple mutants showed two phases of growth inhibition when ethylene was added and wild-type seedlings showed no response to ethylene (Fig. 1B). Transformation of the etr1-6;etr2-3;ein4-4 triple mutants with a complementary DNA (cDNA) encoding for either a full-length ETR1 transgene (cETR1) or a truncated transgene lacking the receiver domain (cetr1-ΔR) resulted in seedlings that had a small, transient growth inhibition response when ethylene was applied (Fig. 1B). Neither transformant had a long-term response to ethylene in the presence of AgNO3, indicating that the receiver domain of ETR1 has little or no role in mediating ETR1 output important for responses to silver ions.
Identification of Amino Acid Residues in the ETR1 Receiver Domain for Mutagenesis
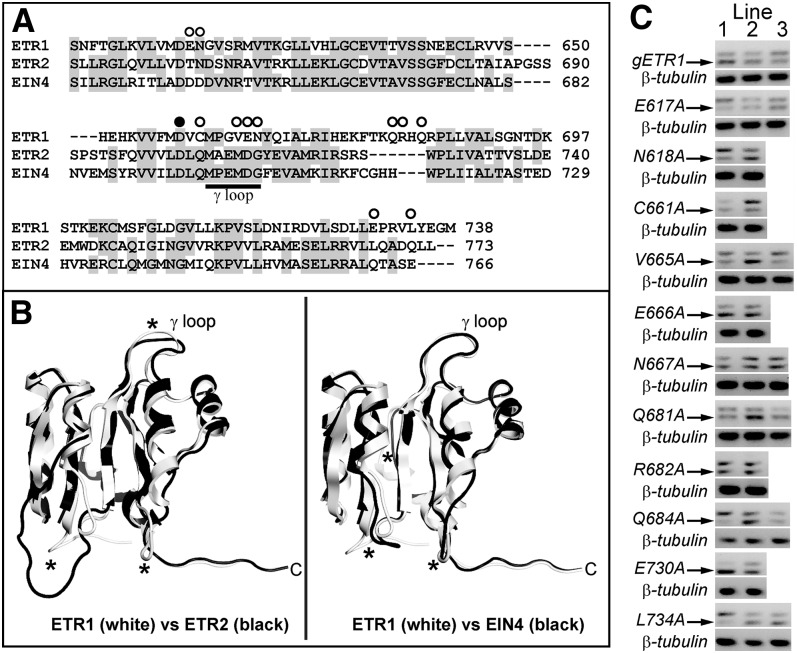
To better define unique regions within the ETR1 receiver domain that may be important for ETR1 subfunctionalization for other traits, we carried out site-directed mutagenesis on the receiver domain of full-length ETR1 genomic DNA. To define which amino acids residues to target, we first looked for amino acids not conserved in the ETR1 receiver domain compared with the receiver domains of ETR2 and EIN4 as well as regions where the structure of the ETR1 receiver domain is predicted to diverge from the other two receptors. As shown in Figure 2, a comparison of the predicted amino acid sequences of the receiver domains shows that there are over 60 residues not conserved between ETR1 and the other two receptors that could potentially underlie the unique functions of ETR1 (Fig. 2A). One region that shows high divergence in amino acid sequence is the γ loop, which consists of six amino acids just beyond the conserved Asp required for phosphorelay. The γ loop of ETR1 is in a different orientation from other structurally characterized γ loops and may underlie functional differences (Müller-Dieckmann et al., 1999). We therefore mutagenized several amino acids in this loop. We also targeted amino acids in the C-terminal tail, which has been identified as a region that might be important in ETR1-protein interactions (Müller-Dieckmann et al., 1999) and is another region where there is high amino acid divergence. Finally, autodephosphorylation activity has been observed in receiver domains, and amino acids corresponding to Asn-618, Cys-661, and Asn-694 in ETR1 have been implicated as critical for this activity in other receiver domains (Pazy et al., 2009). We also homology modeled the receiver domains of ETR2 and EIN4 and compared these models to the crystal structure of the ETR1 receiver domain (Fig. 2B). This modeling revealed that there are several loops where the tertiary structures may be diverged (marked with asterisks in Fig. 2B). Several amino acids in these regions were targeted for mutagenesis. Based on these criteria, 11 amino acids were changed to Ala (marked with white circles in Fig. 2A). These mutant transgenes and wild-type genomic ETR1 (gETR1) were transformed into etr1-6;etr2-3;ein4-4 triple loss-of-function mutants. Two to three transgenic lines were created for each mutant, and transcript levels were measured with reverse transcription (RT)-PCR (Fig. 2C). For each transgene construct, we chose the line with the highest expression level for physiological analyses.
Figure 2.
Receiver domain amino acids targeted for mutagenesis. A, An amino acid sequence alignment of the receiver domains from ETR1, ETR2, and EIN4 was performed using ClustalW. Conserved residues are shaded gray. Amino acids targeted for mutagenesis to Ala in this study are marked with white circles. Amino acid residues for each protein are numbered at the right. The black circle marks the conserved Asp (D659 in ETR1) involved in phosphotransfer that has previously been mutated (Binder et al., 2004b). B, Homology models of the ETR2 and EIN4 receiver domains (black) were generated as described in “Materials and Methods” and compared to the crystal structure of ETR1 (white). Regions where the backbone carbons of the models diverge from the ETR1 structure are marked with asterisks. C, Transcript levels of receptor transgenes. The location of each point mutation is labeled. All constructs were genomic DNA constructs and were transformed into the etr1-6;etr2-3;ein4-4 triple mutant background as described in “Materials and Methods.” RNA expression level for each transgene was analyzed using RT-PCR. The transgene transcripts (marked with arrow) ran as a smaller product than the etr1-6 product as we have previously described (Kim et al., 2011). Transcript levels for β-tubulin in each plant line are shown as a control.
Effect of ETR1 Receiver Domain Point Mutations on ETR1 Signaling
To delineate amino acids in the ETR1 receiver domain important for subfunctionalization, we studied the ability of these 11 point mutants to rescue several traits when transformed into the etr1-6;etr2-3;ein4-4 triple mutants. This triple mutant was chosen because it has reduced growth in air (Hua and Meyerowitz, 1998; Binder et al., 2004b; Qu and Schaller, 2004; Liu et al., 2010), has very slow growth recovery after ethylene removal (Binder et al., 2004b), fails to nutate when ethylene is applied (Binder et al., 2006), and has an accelerated germination time course under salt stress (Wilson et al., 2014b). Thus, we could compare the rescue of these various traits by the mutant transgenes to determine residues important for one or more functions.
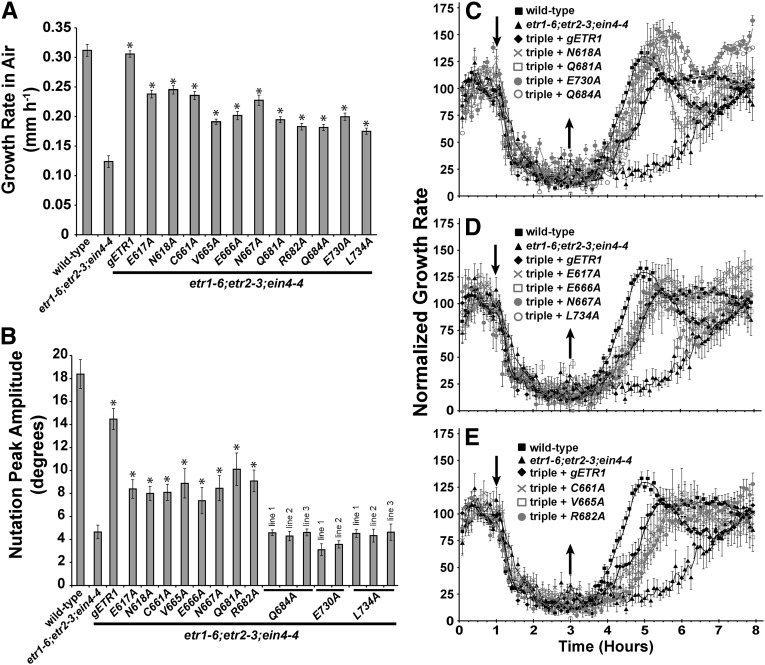
Using high-resolution, time-lapse imaging of growing seedlings in the dark, we analyzed growth rate in air, ethylene-stimulated nutations, and growth response kinetics when ethylene was applied and removed (Fig. 3). Transformation of the etr1-6;etr2-3;ein4-4 triple null mutant with a wild-type gETR1 transgene reversed the effects of the triple mutant, resulting in faster growth in air (Fig. 3A), larger ethylene-stimulated nutational amplitude (Fig. 3B), and accelerated growth recovery after removal of ethylene (Fig. 3C). Different patterns of rescue were obtained with the point mutants, but all were functional for at least one trait (summarized in Table I). All were at least partially functional in the rescue of growth in air and growth recovery after ethylene removal. For this later trait, several transgenes rescued slightly better than wild-type gETR1 (Fig. 3C), some equally well (Fig. 3D), and some slightly worse than gETR1 (Fig. 3E). In addition, all of the transformants had growth inhibition kinetics similar to wild-type seedlings. In general, the mutant transgenes were less effective at rescuing nutations than gETR1 (Fig. 3B; Table I). This is consistent with our previous observations that indicate this trait is easily disrupted (Kim et al., 2011). Nonetheless, eight mutant transgenes partially rescued ethylene-stimulated nutations. Three of the mutant transgenes failed to rescue ethylene-stimulated nutations; this was confirmed by examining multiple transgenic lines for each mutant (Fig. 3B). One of the mutations (Q684A) is in a loop region, and two (E730A and L734A) are in the C-terminal tail of ETR1.
Figure 3.
Growth, nutations, and growth recovery are differentially rescued by etr1 point mutant transgenes. The etr1-6;etr2-3;ein4-4 triple mutants were transformed with genomic DNA encoding a mutant etr1 transgene containing the indicated point mutation. For comparison, data from the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with a wild-type genomic ETR1 (gETR1) transgene are included. A, The growth rates in air of triple mutants transformed with the indicated receptor are plotted. Growth rate in air was determined from the first hour of growth kinetic measurements prior to the introduction of ethylene. Data represents the average growth rate ± sem. B, Nutations in response to 10 µL L–1 ethylene were measured in etr1-6;etr2-3;ein4-4 triple mutants transformed with the indicated receptor. The average peak nutation amplitude ± sem is plotted. C to E, Growth kinetic profiles for triple mutants transformed with receptors are shown. Seedlings were allowed to grow for 1 h in air, followed by the addition of 10 µL L–1 ethylene (down arrow). Ethylene was removed 2 h later (up arrow), and seedlings were grown in air for an additional 5 h. Growth rate was normalized to growth rate in the air pretreatment and represents the average ± sem. Lines are a moving average calculated with a period of 2 using Microsoft Excel. In A and B, data were analyzed with Student’s t tests. *, Increases caused by the transgene over the etr1-6;etr2-3;ein4-4 triple mutants were considered statistically significant for P < 0.05.
Table I. Summary of rescue of traits by ETR1 transgenes.
| etr1-6;etr2-3;ein4-4 Transformed with: | Phenotype Rescueda |
|||
|---|---|---|---|---|
| Growth in Airb | Growth Recovery after Ethylene Removalc | Ethylene-Stimulated Nutationsb | Germination Rate during Salt Stressd | |
| gETR1 | +++ | +++ | +++ | +++ |
| E617A | +++ | +++ | + | +++ |
| N618A | +++ | ++++ | + | +++ |
| D659A | +++e | +e | +++f | + |
| C661A | +++ | ++ | + | + |
| V665A | ++ | ++ | + | +++++ |
| E666A | ++ | +++ | + | +++++ |
| N667A | +++ | +++ | + | ++ |
| Q681A | ++ | ++++ | + | ++++ |
| R682A | ++ | ++ | + | +++ |
| Q684A | ++ | ++++ | – | ++ |
| E730A | ++ | ++++ | – | ++ |
| L734A | ++ | +++ | – | ++ |
Rescue for each mutant transgene was scored relative to the rescue obtained with the gETR1 transgene.
Results of growth in air and nutations (Fig. 3) were scored as follows: more than 70% rescue, +++; 50% to 70% rescue, ++; 30% to 50% rescue, +; and no rescue, –.
Results for growth recovery (Fig. 3) were scored as follows: recovery time was faster, ++++; recovery time was comparable, +++; recovery time was slightly slower, ++; and recovery time was very slow and only slightly faster than the etr1-6;etr2-3;ein4-4 mutants, +.
Results for the time for 50% germination on salt (Supplemental Fig. S1) were scored as follows: more than 65% slower, +++++; 20% to 65% slower, ++++; comparable, +++; 15% to 35% faster, ++; and more than 35% faster, +.
We also examined the effect of the point mutants on seed germination in the presence of 150 mm NaCl. Previously, we generated and characterized an Asp-659Ala mutant (D659A) transformed into the etr1-6;etr2-3;ein4-4 triple mutant background to assess the role of phosphotransfer in the control of growth in air, growth recovery after ethylene removal, and ethylene-stimulated nutations (Binder et al., 2004b; Kim et al., 2011). In addition to the 11 point mutants described above, we used this mutant in these seed germination experiments to determine the role of phosphotransfer in this phenotype. In the absence of NaCl, most seed lines germinated with a time course indistinguishable from wild-type seeds (Fig. 4). However, several transformants (C661A, V665A, E666A, and Q681A) germinated slightly slower than wild-type seeds, with the E666A mutant receptor giving the slowest germination time course with a time for 50% seed germination of approximately 1.8 d compared with 1.3 d for the wild-type seeds (Supplemental Fig. S1).
Figure 4.
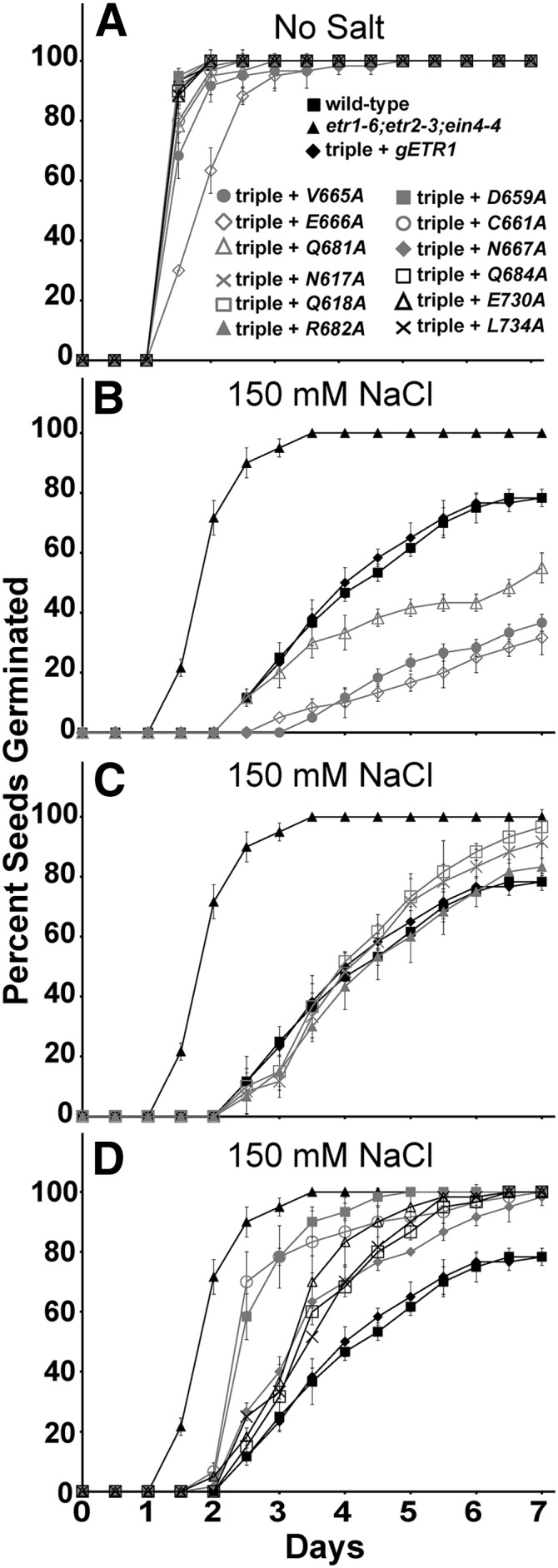
The rapid germination on salt of etr1;etr2;ein4 triple mutants is differentially reversed by etr1 point mutant transgenes. Germination time courses of etr1-6;etr2-3;ein4-4 triple mutants transformed with genomic DNA encoding a mutant etr1 transgene containing the indicated point mutation were determined in the absence (A) or presence (B–D) of 150 mm NaCl. For comparison, data from the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with a wild-type genomic ETR1 (gETR1) transgene are included in each section. The percentage of seeds that germinated was determined every 12 h. All experiments were performed in triplicate. The average percentage of seed germination ± sd at each time is plotted for each seed line.
Consistent with our previous results (Wilson et al., 2014b), 150 mm NaCl delayed germination of all seed lines (Fig. 4; Supplemental Fig. S1). The triple mutant seeds were the least affected by 150 mm NaCl, having a slight increase in the time for 50% seed germination from approximately 1.3 d in the absence of salt to approximately 1.8 d in the presence of salt (Supplemental Fig. S1). The wild type and triple mutants transformed with gETR1 reached approximately 78% germination after 7 d, whereas the triple mutants reached 100% germination by 3.5 d. There were three patterns of rescue for germination on 150 mm NaCl (Fig. 4; Table I; Supplemental Fig. S1). Some transgenes (V665A, E666A, and Q681A) caused germination to be slower than germination of triple mutants transformed with gETR1 and resulted in a lower percentage of seeds that germinated (Fig. 4B). These results were confirmed in multiple transgenic lines for each mutant (data not shown). This indicates that these mutant receptors are more functional than the wild-type receptor for this phenotype. Interestingly, these mutants are not more functional for the other traits examined (Table I). The two mutations causing the slowest germination time course (V665A and E666A) are both in the γ loop, and these seed lines failed to reach 50% germination within the time span of these experiments (Supplemental Fig. S1). Three mutant transgenes (N617A, Q618A, and R682A) rescued germination on salt to the same extent as gETR1 (Fig. 4C), and six mutant transgenes were less functional, resulting in a faster germination time course and better overall germination compared with gETR1 (Fig. 4D). Four of these less-functional transgenes (N667A, Q684A, E730A, and L734A) were only slightly less functional than gETR1 (Fig. 4D; Table I). By contrast, two mutant transgenes (D659A and C661A) resulted in a faster germination time course on salt, with a time for 50% seed germination over 30% faster than the gETR1 transformants (Table I; Supplemental Fig. S1). The D659A transgene affects the Asp that is believed to be involved in phosphotransfer based on homology to other receiver domains, and C661A affects a Cys that hydrogen bonds with Asp-659 (Müller-Dieckmann et al., 1999). Both amino acid residues are located just before the γ loop. Thus, the γ loop and phosphotransfer through the receiver domain have important roles in mediating germination on salt.
Effect of the EIN4 Receiver Domain on Seed Germination on Salt
We have previously generated cDNAs encoding chimeric ETR1-EIN4 receptors to examine the role of each domain in the regulation of various traits (Kim et al., 2011). These chimeric receptor transgenes were transformed into etr1-6;etr2-3;ein4-4 triple mutants, and the rescue of various traits was compared to rescue obtained with a cDNA encoding a full-length ETR1 (cETR1). From this, we found that transformation with a chimeric ETR1-EIN4 receptor transgene containing the receiver domain of EIN4 (1114) can rescue growth in air and growth recovery after ethylene removal but not ethylene-stimulated nutations (Kim et al., 2011). This suggests that the ETR1 receiver domain is unique and needed for the control of some, but not all, phenotypes. Therefore, we were curious to know whether the EIN4 receiver domain can substitute for the ETR1 receiver domain in the control of seed germination on salt. To determine this, we examined the germination time course of triple mutants transformed with the 1114 transgene. The germination time course of this transformant was compared to the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with either cETR1 or cetr1-ΔR. All seed lines had similar germination time courses in the absence of salt (Fig. 5; Supplemental Fig. S2). Consistent with our prior results (Wilson et al., 2014b), on salt, the etr1-6;etr2-3;ein4-4 triple mutants transformed with cETR1 had slow germination comparable to wild-type seeds, whereas the cetr1-ΔR transgene was nonfunctional for this trait, resulting in fast germination similar to the germination time course of the triple mutant seeds (Fig. 5; Supplemental Fig. S2). Similarly, the 1114 transgene did not have an effect on seed germination on salt, indicating that the EIN4 receiver domain cannot substitute for the ETR1 receiver domain for this trait. Both cetr1-ΔR and 1114 are expressed and functional for other traits (Kim et al., 2011), providing evidence that the ETR1 receiver domain is specifically required to mediate germination on salt.
Figure 5.
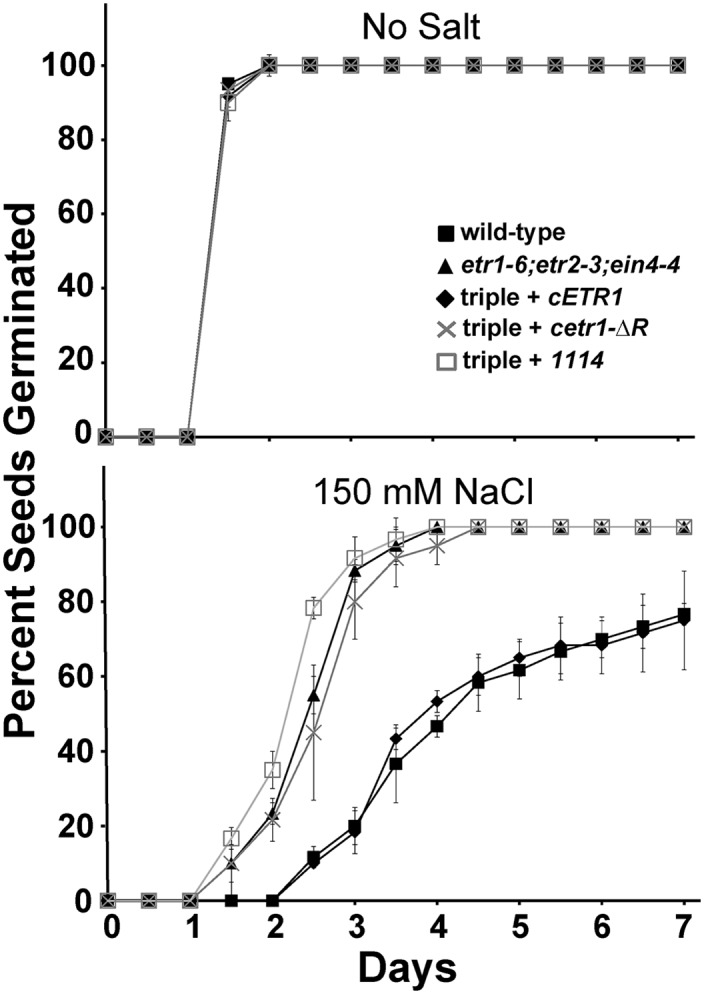
The rapid germination on salt of etr1;etr2;ein4 triple mutants is not reversed by an ETR1-EIN4 chimeric receptor transgene containing the EIN4 receiver domain. Germination time courses of etr1-6;etr2-3;ein4-4 triple mutants transformed with cDNA for a chimeric ETR1-EIN4 receptor containing the receiver domain of EIN4 (1114) were determined in the presence and absence of 150 mm NaCl. Germination time courses of the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with cDNA for full-length ETR1 (cETR1) or a truncated ETR1 lacking the receiver domain (cetr1-ΔR) are included for comparison. All experiments were performed in triplicate. The average percentage of seed germination ± sd at each time is plotted for each seed line.
Responses to ABA Are Altered to Affect Seed Germination
The hormone ABA is an inhibitor of germination that accumulates in plants in response to salt stress (Garciarrubio et al., 1997; Jakab et al., 2005). In a previous study, we demonstrated that the faster germination of etr1-6 loss-of-function mutants on salt correlated with a decrease in responsiveness to ABA, rather than changes in levels or responses to ethylene, GA3, or cytokinin (Wilson et al., 2014b). We therefore wished to determine whether ABA was involved in the alterations we observe in seed germination on salt in this study. We first compared the germination time courses of the wild type to etr1-6;etr2-3;ein4-4 triple mutant seeds (Fig. 6). In the absence of ABA, both seed lines had similar germination time courses. As we previously reported (Wilson et al., 2014), 1 µm ABA inhibited the germination of wild-type seeds (Fig. 6B), causing the time for 50% germination to increase by approximately 3 d (Supplemental Fig. S3). ABA had a smaller effect on the etr1-6;etr2-3;ein4-4 triple mutants, showing that they are less responsive to this hormone. We also wished to determine if the altered germination of certain point mutants on salt involved ABA. For this, we chose the D659A transformant, which germinates better than gETR1 on salt, and the V665A and E666A transformants, which germinate slower than gETR1 on salt. In solvent control conditions (Fig. 6), germination time courses were similar to what was observed in the absence of solvent (Fig. 4), with the E666A transgene causing a slight increase in the germination time course (Fig. 6; Supplemental Fig. S3). Transformation of the triple mutant with gETR1 caused the seeds to have a larger response to ABA and have slower germination that was comparable to wild-type seeds. Correlating with our germination results on salt, the D659A transformants germinated faster on ABA than gETR1 and had a germination time course that was very similar to the triple mutants (Fig. 6; Supplemental Fig. S3). By contrast, the V665A and E666A transgenes caused slower seed germination in response to ABA than what was observed with gETR1 seeds (Fig. 6). These seed lines never reached 50% germination within the time frame of these experiments (Supplemental Fig. S3). These data demonstrate that application of ABA phenocopies the effects of NaCl on the seed lines tested, suggesting that the difference in germination caused by these mutant transgenes is due to differences in responsiveness to ABA.
Figure 6.
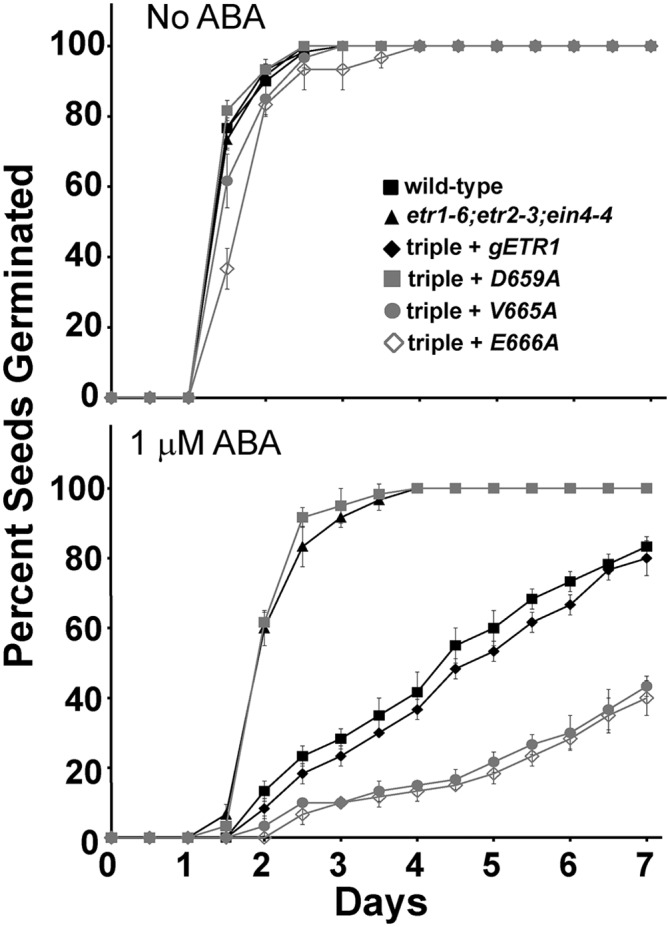
ABA phenocopies the effects of NaCl. Germination time courses of etr1-6;etr2-3;ein4-4 triple mutants transformed with genomic DNA encoding a mutant etr1 transgene containing the indicated point mutation were determined. For comparison, data from the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with a wild-type genomic ETR1 (gETR1) transgene are included. Seeds were germinated in the presence or absence of 1 µm ABA. All experiments were performed in triplicate. The average percentage of seed germination ± sd at each time is plotted for each seed line.
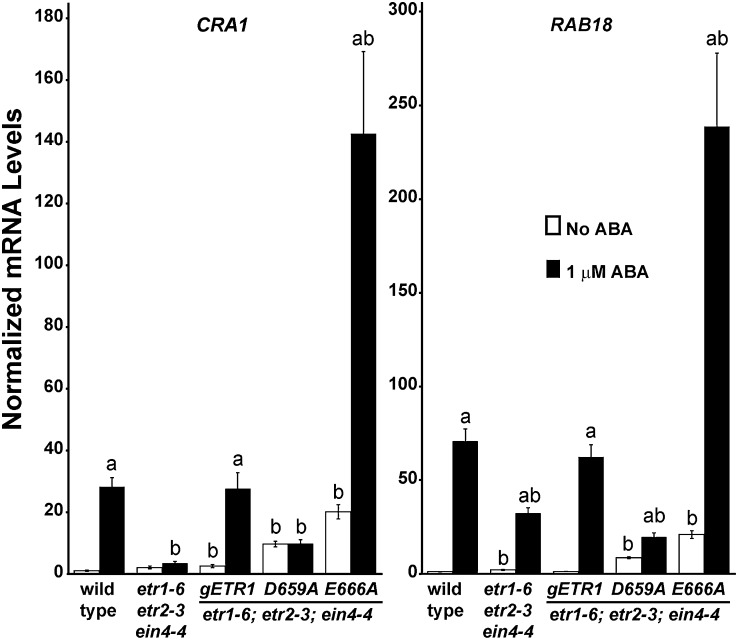
To further explore the link between ABA and germination, we used quantitative real-time reverse transcriptase (qRT)-PCR to examine the transcript abundance of two ABA-responsive genes in response to ABA treatment. For this, we chose CRUCIFERIN1 (CRA1), which encodes for a major seed storage protein, and RESPONSIVE TO ABA18 (RAB18), which encodes for a dehydrin (Lång and Palva, 1992; Gliwicka et al., 2012). As shown in Figure 7, treatment with 1 µm ABA for 2 d increased the transcript abundance of both genes in wild-type seeds (P < 0.05). Interestingly, the transcript levels of both genes were lower in the triple mutant than the wild-type seeds after treatment with ABA (P < 0.05). ABA had no effect on CRA1 transcript levels in the etr1-6;etr2-3;ein4-4 triple mutants and had a smaller effect on RAB18 transcript levels than seen with wild-type seeds. These data support the idea that the triple mutant seeds are less responsive to ABA. Similar changes in CRA1 and RAB18 transcript abundance are seen in response to 150 mm NaCl (Supplemental Fig. S4A). We also examined the changes in CRA1 and RAB18 transcript levels in triple mutants transformed with gETR1, D659A, or E666A. The patterns of changes in these transcripts correlated with germination, with the gETR1 transformants having responses similar to wild-type seeds, the D659A transformants having little or no response to ABA, and the E666A transformants having larger responses to ABA (Fig. 7).
Figure 7.
ABA changes the transcript abundance of RAB18 and CRA1. The levels of transcript for CRA1 and RAB18 were measured using qRT-PCR. For this, seeds were germinated for 2 d in the presence or absence of 1 µm ABA and mRNA extracted from the wild type and etr1-6;etr2-3;ein4-4 triple mutants as well as triple mutants transformed with the indicated mutant transgene. Data were normalized to the levels of At3g12210 in each seed line to determine the relative transcript level for each gene. These were then normalized to levels of the transcript in untreated wild-type seeds. The average ± sem for two biological replicates with three technical replicates each is shown. The letter a indicates that ABA caused a statistically significant increase in transcript levels (P < 0.05), and the letter b indicates a statistical difference from wild-type seeds in the same condition (P < 0.05). All P values were calculated by Student’s t test.
To further examine the involvement of ABA, we treated seedlings with the ABA biosynthesis inhibitor norflurazon in the presence of 150 mm NaCl. Solvent control seedlings had germination time courses in response to 150 mm NaCl (Fig. 8) that were similar to what we observe in the absence of solvent (Fig. 4). Application of 10 µm norflurazon caused germination time courses to be faster (Fig. 8) and significantly (P < 0.05) reduced the time for 50% seed germination for all lines tested (Supplemental Fig. S5). Application of 100 µm norflurazon almost entirely eliminated the germination differences between the seed lines tested (Fig. 8; Supplemental Fig. S5). Application of 100 µm norflurazon also almost entirely reversed the effects of 150 mm NaCl on these germinating seeds, suggesting that the effects of the mutant transgenes on seed germination during salt stress are largely mediated by ABA. Correlating with these changes, norflurazon reversed the effects that salt treatment had on the transcript levels of CRA1 and RAB18 (Supplemental Fig. S4B).
Figure 8.
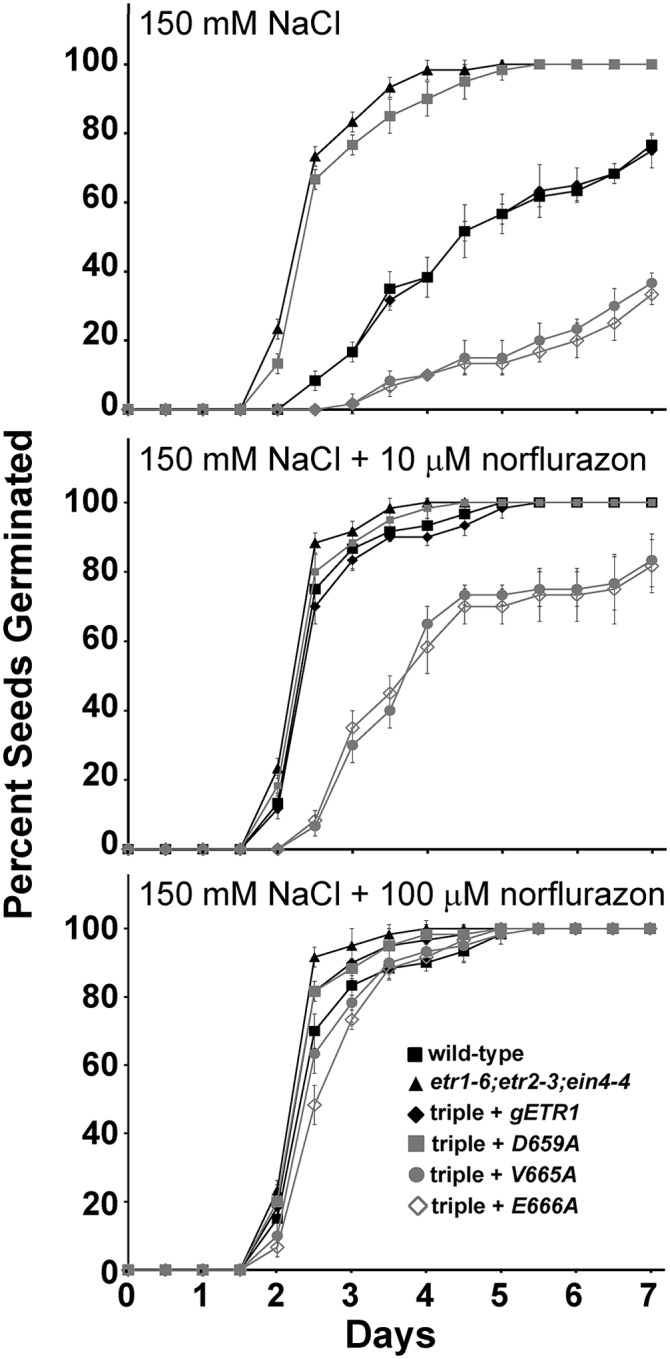
Norflurazon reduces differences in germination between select point mutants. Germination time courses of etr1-6;etr2-3;ein4-4 triple mutants transformed with genomic DNA encoding a mutant etr1 transgene containing the indicated point mutation were determined. For comparison, data from the wild type, etr1-6;etr2-3;ein4-4 triple mutants, and triple mutants transformed with a wild-type genomic ETR1 (gETR1) transgene are included. Seeds were germinated on 150 mm NaCl in the absence or presence of the indicated concentrations of the ABA biosynthesis inhibitor norflurazon. All experiments were performed in triplicate. The average percentage of seed germination ± sd at each time is plotted for each seed line.
DISCUSSION
The ethylene receptors have become subfunctionalized and have both overlapping and nonoverlapping roles (for review, see Shakeel et al., 2013). Prior studies showed that ETR1 has a prominent role in the control of several phenotypes, including ethylene-stimulated nutations, growth recovery after removal of ethylene, inhibition of ethylene responses by silver ions, seed germination during salt stress in the light, and seed germination in darkness (Binder et al., 2004b, 2006; Kim et al., 2011; McDaniel and Binder, 2012; Wilson et al., 2014a, 2014b). Interestingly, the ETR1 receiver domain was previously shown to be important for normal growth recovery, ethylene-stimulated nutations, and germination during salt stress in the light (Binder et al., 2004b, 2006; Kim et al., 2011; Wilson et al., 2014b) but not for the control of seed germination in darkness (Wilson et al., 2014a). In this study, we demonstrated that the ETR1 receiver domain also has little or no role in mediating the inhibitory effects of silver ions. These results in combination with other studies demonstrate that the control of various traits have different ETR1 domain requirements. Three traits (growth recovery after ethylene removal, ethylene-stimulated nutations, and germination during salt stress in the light) require the full-length ETR1 receptor (Binder et al., 2004b, 2006; Kim et al., 2011; Wilson et al., 2014b). By contrast, growth in air, growth inhibition by ethylene, germination in darkness, and response to silver ions do not require the receiver domain (Gamble et al., 2002; Binder et al., 2004b; Hall et al., 2012; Wilson et al., 2014a). There is also evidence that ETR1 signaling occurs from the ethylene-binding and GAF domains (Gamble et al., 2002; Qiu et al., 2012). This signaling appears to occur via REVERSION TO ETHYLENE1, which modulates ETR1 function by interactions with the ethylene-binding and GAF domains, and may signal via a CTR1-independent pathway (Resnick et al., 2006; Dong et al., 2008, 2010; Qiu et al., 2012).
Within those traits that require the receiver domain, there are differences. Genetic studies indicate that phosphotransfer through the receiver domain is important for both normal growth recovery after the removal of ethylene (Binder et al., 2004b) and germination under salt stress. However, using ETR1-EIN4 chimeric receptors containing the receiver domain of EIN4 (1114), we show that the receiver domain of EIN4 can substitute for the receiver domain of ETR1 in the control of growth recovery (Kim et al., 2011) but not germination on salt. This suggests a model where there are two patterns of phosphotransfer from ETR1 His kinase, where phosphotransfer to ETR1, ETR2, and EIN4 controls growth recovery and phosphotransfer solely to ETR1 controls seed germination during salt stress (Fig. 9A). The phosphotransfer solely to ETR1 appears to inhibit germination under salt stress, but we cannot rule out that subtle changes in tertiary structure have occurred in the chimeric receptor, leading to these results. Because ETR2 has the opposite effect on seed germination from ETR1, it will be interesting to determine whether a chimeric ETR1-ETR2 receptor containing the receiver domain of ETR2 has the opposite effect on seed germination from the ETR1 receptor. Interestingly, the changes in seed germination on salt involve ABA because there are differences in ABA responsiveness. However, the mechanism for this is unknown. In contrast to these two traits, genetic studies show that ETR1 His-kinase activity and phosphotransfer are not required for ethylene-stimulated nutations, even though the ETR1 receiver domain is specifically needed for this trait (Kim et al., 2011). Thus, the ETR1 receiver domain has several functions that are not entirely redundant in the control of these three traits.
Figure 9.
Models for ETR1 receiver domain output. A, The ETR1, ETR2, and EIN4 receptors form homodimers with each monomer containing an ethylene-binding, GAF, kinase, and receiver domain as labeled. The conserved His (H) and Asp (D) amino acid residues involved in phosphotransfer are shown. The receiver domain is predicted to have several roles in the control of several phenotypes. In this model, two outputs require ETR1 His-kinase activity where transphosphorylation from one monomer to a conserved His in the other monomer occurs. This phosphate then transfers to conserved Asp residues in the receiver domains of ETR1, ETR2, and EIN4. In this model, growth recovery after the removal of ethylene is stimulated by phosphotransfer to all three receptors. By contrast, germination on salt is only controlled by phosphotransfer to the receiver domain of ETR1, which acts to inhibit germination. Ethylene-stimulated nutations require the ETR1 receiver domain but are stimulated via a phosphotransfer-independent mechanism. B, Two views are shown of the tertiary structure of the backbone carbons of the ETR1 receiver domain based on the published crystal structure (Müller-Dieckmann et al., 1999). The positions of the amino acid residues determined to have the largest effects on ethylene-stimulated nutations (Q684, E730, and L734) and germination on salt (D659, C661, V665, and E666) are shown as space-filled representations.
In this study, we found that there are regions in the receiver domain of ETR1 that are important for the control of ethylene-stimulated nutations and germination under salt stress. Using site-specific mutagenesis of amino acids in the receiver domain, we found that Q684, E730, and L734 are required for a functional ETR1 protein in the control of ethylene-stimulated nutations but not for the other traits studied. Q684 is in a loop region, whereas E730 and L734 are in the C-terminal tail of the protein. When placed in the crystal model, all three amino acid residues fall along a single face of the receiver domain (Fig. 9B). This surface of the receiver domain is modeled to face the kinase domain (Mayerhofer et al., 2015). It is also noteworthy that ethylene-stimulated nutations are reduced by most point mutations studied, suggesting that the entire domain may be involved in the regulation of this phenotype. We also identified two regions at or near the γ loop of the receiver domain that are important in the control of germination on salt. One region is defined by D659 and C661, which are located just before the γ loop and are needed to inhibit seed germination on salt. The other region is in the γ loop and is defined by V665 and E666, which are located at the other end of the γ loop from D659 and C661 (Fig. 9B). Mutations in V665 and E666 result in a receptor that inhibits germination more than the wild-type receptor. These mutations do not result in a hyperfunctional receptor for the other traits studied. It is interesting to note that the D659A transgene affects growth recovery after ethylene removal (Binder et al., 2004b), but C661A, V665A, and E666A do not.
The mechanism by which these regions of the receiver domain affect receptor output is not known. One possibility is that these regions are important for receptor-protein interactions. Bacterial two-component receptors are modeled to form higher order clusters through direct interactions with adjacent receptors, where the signaling state of one receptor affects the signaling state of surrounding receptors (Bray et al., 1998; Duke and Bray, 1999; Shimizu et al., 2003). Similar models have been invoked for ethylene receptors (Binder and Bleecker, 2003; Binder et al., 2004a, 2004b; Gao et al., 2008; Grefen et al., 2008; Gao and Schaller, 2009; Liu and Wen, 2012). The crystal structure of the ETR1 receiver domain has been determined, revealing a homodimer that is stabilized by the C terminus, which forms an extended β sheet (Müller-Dieckmann et al., 1999). However, in solution, dimerization may not occur (Hung et al., 2015), and a more recent structural model of the cytosolic domains of ETR1 indicates that the receiver domain of each monomer in a receptor may not come close enough to dimerize (Mayerhofer et al., 2015). Nonetheless, the modeling shows that the C-terminal tail could have a role in interactions with other proteins, including the receiver domains of adjacent receptor dimers (Mayerhofer et al., 2015). It is thus possible that the Q684A, E730A, and L734A mutations interfere with these interactions. Interestingly, the γ loop of ETR1 is in a different orientation from other structurally characterized γ loops and has also been proposed to be involved in receptor-protein interactions (Müller-Dieckmann et al., 1999). Thus, amino acids in the γ loop or C-terminal tail or both may mediate specific interactions, leading to the regulation of specific traits. Because these regions are on opposite sides of the receiver domain (Fig. 9B), it is likely each region modulates interactions with different proteins. It is currently unclear what receptor-protein interactions are mediated by these two regions of the receiver domain, but based on prior receptor-protein interaction studies, some possibilities include other ethylene receptors, CTR1, EIN2, His-containing phosphotransfer proteins, and response regulator proteins (Clark et al., 1998; Urao et al., 2000; Cancel and Larsen, 2002; Gao et al., 2003, 2008; Hass et al., 2004; Grefen et al., 2008; Scharein et al., 2008; Zhong et al., 2008; Bisson et al., 2009; Bisson and Groth, 2010).
Our data also support a model where phosphotransfer through the receiver domain is important in the control of certain traits. Support for this is that the D659A mutant affects both growth recovery after ethylene removal (Binder et al., 2004b) and germination on salt. The downstream target for phosphotransfer from the ethylene receptors is unknown. Prior research has documented interactions between ETR1 and both His-containing phosphotransfer proteins and response regulator proteins (Urao et al., 2000; Hass et al., 2004; Scharein et al., 2008; Scharein and Groth, 2011), leading to a model where phosphotransfer occurs from ETR1 to these proteins (for review, see Mason and Schaller, 2005; Bishopp et al., 2006; Shakeel et al., 2013). A direct demonstration of these proteins being downstream targets of ETR1 is still needed. However, it is noteworthy that some response regulator proteins affect seed germination on salt (Mason et al., 2010), which supports the possibility that these proteins are downstream targets for phosphorelay from ETR1. Because phosphorylation can affect bacterial receiver domain structure, leading to changes in dimerization and interactions with other proteins (for review, see Gao and Stock, 2009; Bourret, 2010; Galperin, 2010; Capra and Laub, 2012), it is possible that both receptor-protein interactions and phosphotransfer-induced changes in some of these interactions are involved in ETR1 subfunctionalization. Based on our data, such a phosphorylation-induced conformational change affects the face of the receiver domain containing the γ loop but not the face that includes the C-terminal tail.
The exact output of the ethylene receptors is not yet known. The results presented above show that ETR1 has multiple outputs via the receiver domain that modulate various traits. Future work will better delineate ETR1 functions in the control of plant growth and development and identify the underlying mechanisms for these functions.
MATERIALS AND METHODS
Plant Materials and Chemicals
All mutants are in the Columbia background of Arabidopsis (Arabidopsis thaliana), which was used as a wild-type control. The etr1-6;etr2-3;ein4-4 triple mutants and the triple mutants transformed with gETR1, cETR1, D659A, cetr1-ΔR, and 1114 have previously been described (Hall and Bleecker, 2003; Wang et al., 2003; Binder et al., 2004b, 2006; Kim et al., 2011). The D659A mutant was previously referred to as getr1-[D] (Binder et al., 2004b; Kim et al., 2011). ABA was from ACROS Organics, and norflurazon was from Fluka.
Homology Modeling
Three-dimensional structural models of the ETR2 and EIN4 receiver domains were generated with Molecular Operating Environment (MOE) version 2012.10 using the ETR1 receiver domain as a template structure (Protein Data Bank 1DCF). This template was used because, of the possible templates identified by MOE, it had the highest Z value. The ETR1 receiver domain protein sequence and each of the other two receiver domain sequences were aligned, with structural alignment enabled. For the alignment between the ETR1 and ETR2 receiver domains, a blosum35 substitution matrix was used, and for the alignment between the ETR1 and EIN4 receiver domains, a blosum45 substitution matrix was used. The structures were prepared and errors were fixed both automatically with MOE and manually. The Protonate 3D function of MOE was used to assign protonation states, and homology models were then obtained using the CHARMM27 force field (Foloppe and MacKerell, 2000). An ensemble of 10 possible structures for each receiver domain was generated and the models were ranked using MOE to calculate the root-mean-square deviation values of atomic positions. These values ranged from 0.31 to 0.68 Å for EIN4 models and 0.36 to 0.90 Å for ETR2 models. The model for each receiver domain with the lowest value was then superposed on the crystal structure of the ETR1 receiver domain, and alterations in the positions of backbone carbons were evaluated.
Plasmid Construction, Generation of Transgenic Lines, and RT-PCR
The cloning of gETR1 into pBluescript and pPZP211 has been previously described (Wang et al., 2003). Several silent mutations were incorporated into pBluescript II SK– gETR1 and subsequently cloned into pPZP211-gETR1 with MfeI-HF and AflII (now called pPZP211-gETR1 silent), leading to a unique avrII restriction site in gETR1. These mutations, along with the following ones, were made using LaTaq polymerase (TaKaRa Bio) according to the manufacturer’s instructions. The following point mutations were made in pBluescript II SK– gETR1 and then cloned into pPZP211-gETR1 silent using AflII and KpnI: E617A, N618A, C661A, V665A, E666A, N667A, Q681A, R682A, Q684A, E730A, and L734A. All plasmid constructs and point mutations were confirmed by sequencing. Primers for the generation of these mutants are listed in Supplemental Table S1.
All of the transgene constructs created above were transformed into Agrobacterium tumefaciens strain GV3101 pMP90 and then transformed into etr1-6;etr2-3;ein4-4 Arabidopsis plants using the floral dip method (Clough and Bent, 1998). Two to three homozygous lines were identified for each transgenic line. RNA was extracted from 10 or more seedlings using the RNA Plant Extraction Kit (Qiagen), DNA was cleaned using the Turbo DNase Kit (Ambion), and PCR amplification was carried out using the One-Step RT-PCR Kit (Qiagen) and primers described previously (Kim et al., 2011). The resultant amplification products were run on a 1.5% (w/v) agarose gel and detected with UV illumination. Transcript levels of β-tubulin were analyzed as a control using primers described previously (Gao et al., 2008).
High-Resolution Time-Lapse Imaging and Analysis of Growth Rate and Nutation Angles
Arabidopsis seeds were surface sterilized with 70% (v/v) alcohol for 30 s, placed on sterile filter paper to dry, and then placed on agar plates containing 0.8% (w/v) agar and one-half-strength Murashige and Skoog basal salt mixture (Murashige and Skoog, 1962), pH 5.7, fortified with vitamins and with no added sugar. Seeds were treated for 2 to 8 d at 4°C, treated with light for 4 to 8 h under continuous fluorescent lights, and then allowed to grow in the dark for 2 d on vertically orientated plates for time-lapse imaging experiments. Time-lapse imaging of dark-grown Arabidopsis hypocotyls was carried out using methods previously described with Marlin CCD cameras (Allied Vision Technology) and infrared lighting (Binder et al., 2004a, 2004b, 2006; Kim et al., 2011). For growth kinetics measurements, images were taken every 5 min. To measure growth response kinetics, seedlings were grown in air for 1 h followed by treatment for 2 h with 10 µL L–1 ethylene to examine growth inhibition kinetics. This was followed by a 5-h treatment with air to examine growth recovery kinetics. For treatment with silver, 100 µm AgNO3 was included in the agar. In these experiments, seedlings were grown for 1 h in air followed by treatment with 1 µL L–1 ethylene to examine growth inhibition kinetics as described by McDaniel and Binder (2012). The growth rate of each seedling was analyzed using custom software (Parks and Spalding, 1999; Folta and Spalding, 2001) and normalized to the growth rate in air prior to application of ethylene. Growth rate in air was quantified from the first hour of measurements before ethylene was added. To measure nutational bending, seedlings were treated with 10 µL L–1 ethylene for 24 h and images were acquired every 15 min. The angles of each hypocotyl were measured manually and nutation amplitude was determined as previously described (Binder et al., 2006). All experiments under all conditions were repeated in at least three separate experiments.
Seed Germination Experiments
Seed germination experiments were carried out according to the methods of Wilson et al. (2014a, 2014b). Briefly, to reduce biological variation, seeds were collected from plants grown together under uniform conditions (Hensel et al., 1993). Seeds were collected on the same day, stored in a desiccator for at least 3 weeks, and mechanically sorted using the methods of Elwell et al. (2011), and seeds between 250 and 300 µm in size were used. Seeds were surface sterilized with 70% (v/v) ethanol for 30 s, dried, and then placed on agar plates as described above that contained either no salt or 150 mm NaCl. In some experiments, ABA or the ABA biosynthesis inhibitor norflurazon was included at the indicated concentration. These were prepared as 1,000× stocks in ethanol and added after autoclaving. Solvent control plates contained 0.1% (v/v) ethanol. The plates were sealed with porous surgical tape (3M) to prevent accumulation of ethylene (Buer et al., 2003). Twenty seeds of a genotype were placed on a plate, and three plates were used per condition. Plates were kept vertically under a long-day (16-h-light/8-h-dark) photoperiod at 20°C to 21°C under 12 to 13 µmol m–2 s–1 white light. The number of seeds that germinated on each plate was determined every 12 h for 7 d. Germination was scored as the rupture of the testa (seed coat).
RNA Isolation and qRT-PCR
The transcript abundance of several Arabidopsis genes was examined using qRT-PCR. This included gene transcripts for CRA1 and RAB18, which are ABA responsive (Lång and Palva, 1992; Gliwicka et al., 2012). For this, total RNA was isolated from 25 mg (dry weight) of seeds that were sown in the presence or absence of 1 µm ABA or 150 mm NaCl. RNA was isolated using the methods of Meng and Feldman (2010) as modified by Wilson et al. (2014a). Transcript data were normalized to At3g12210 (Dekkers et al., 2012) using the method of Livak and Schmittgen (2001) for each seed line for each condition to obtain the relative amounts of target gene transcripts between plant backgrounds for each treatment. These levels were then normalized to the levels observed in untreated wild-type seeds. The primers used for the analysis of these transcripts have been previously described (Fujii et al., 2007; Gliwicka et al., 2012).
Statistics
Data were analyzed using Student’s t tests and considered statistically different at P < 0.05.
Arabidopsis Genome Initiative accession numbers for genes studied in this article are ETR1, At1g66340; EIN4, At3g04580; ETR2, At3g23150; CRA1, At5g44120; and RAB18.
Supplemental Material
The following supplemental materials are available.
Supplemental Figure S1. Time for 50% of point mutant seeds to germinate in response to NaCl.
Supplemental Figure S2. Time for 50% of chimeric receptor seeds to germinate in response to NaCl.
Supplemental Figure S3. Time for 50% of select point mutant seeds to germinate in response to ABA.
Supplemental Figure S4. Change in transcript abundance of RAB18 and CRA1 in response to NaCl and norflurazon.
Supplemental Figure S5. Time for 50% of select point mutant seeds to germinate on NaCl in response to norflurazon.
Supplemental Table S1. Primers used for site-directed mutagenesis.
Supplementary Material
Acknowledgments
We thank Jerome Baudry and Karan Kapoor for useful discussions on homology modeling and Rachel Barker, Gabriel Dagotto, Katrina Deponte, Rebecca Murdaugh, Katherine Odom, David Pease, Amanda Wehner, and Zack Zenn for technical help.
Glossary
- ABA
abscisic acid
- cDNA
complementary DNA
- RT
reverse transcription
- qRT
quantitative real-time reverse transcriptase
- MOE
Molecular Operating Environment
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–0918430 to B.M.B. and fellowship to R.L.W.).
Articles can be viewed without a subscription.
References
- Abeles F, Morgan P, Saltveit MJ (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, dePamphilis C, Albert VA, Aono N, Aoyama T, Ambrose BA, et al. (2011) The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332: 960–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EM. (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58: 268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Bleecker AB (2003) A model for ethylene receptor function and 1-methylcyclopropene action. Acta Hortic 628: 177–187 [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB (2004a) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004b) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’Malley RC, Wang W, Zutz TC, Bleecker AB (2006) Ethylene stimulates nutations that are dependent on the ETR1 receptor. Plant Physiol 142: 1690–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Mähönen AP, Helariutta Y (2006) Signs of change: hormone receptors that regulate plant development. Development 133: 1857–1869 [DOI] [PubMed] [Google Scholar]
- Bisson MMA, Bleckmann A, Allekotte S, Groth G (2009) EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem J 424: 1–6 [DOI] [PubMed] [Google Scholar]
- Bisson MMA, Groth G (2010) New insight in ethylene signaling: Autokinase activity of ETR1 modulates the interaction of receptors and EIN2. Mol Plant 3: 882–889 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bourret RB. (2010) Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol 13: 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D, Levin MD, Morton-Firth CJ (1998) Receptor clustering as a cellular mechanism to control sensitivity. Nature 393: 85–88 [DOI] [PubMed] [Google Scholar]
- Buer CS, Wasteneys GO, Masle J (2003) Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol 132: 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancel JD, Larsen PB (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra EJ, Laub MT (2012) Evolution of two-component signal transduction systems. Annu Rev Microbiol 66: 325–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen R, Binder BM, Garrett WM, Tucker ML, Chang C, Cooper B (2011) Proteomic responses in Arabidopsis thaliana seedlings treated with ethylene. Mol Biosyst 7: 2637–2650 [DOI] [PubMed] [Google Scholar]
- Chen YF, Randlett MD, Findell JL, Schaller GE (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277: 19861–19866 [DOI] [PubMed] [Google Scholar]
- Cho YH, Yoo SD (2007) ETHYLENE RESPONSE1 histidine kinase activity of Arabidopsis promotes plant growth. Plant Physiol 143: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95: 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dekkers BJW, Willems L, Bassel GW, van Bolderen-Veldkamp RP, Ligterink W, Hilhorst HWM, Bentsink L (2012) Identification of reference genes for RT-qPCR expression analysis in Arabidopsis and tomato seeds. Plant Cell Physiol 53: 28–37 [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol 137: 831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Jang M, Scharein B, Malach A, Rivarola M, Liesch J, Groth G, Hwang I, Chang C (2010) Molecular association of the Arabidopsis ETR1 ethylene receptor and a regulator of ethylene signaling, RTE1. J Biol Chem 285: 40706–40713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Rivarola M, Resnick JS, Maggin BD, Chang C (2008) Subcellular co-localization of Arabidopsis RTE1 and ETR1 supports a regulatory role for RTE1 in ETR1 ethylene signaling. Plant J 53: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke TAJ, Bray D (1999) Heightened sensitivity of a lattice of membrane receptors. Proc Natl Acad Sci USA 96: 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell AL, Gronwall DS, Miller ND, Spalding EP, Brooks TLD (2011) Separating parental environment from seed size effects on next generation growth and development in Arabidopsis. Plant Cell Environ 34: 291–301 [DOI] [PubMed] [Google Scholar]
- Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112: 1291–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foloppe N, MacKerell AD Jr (2000) All-atom empirical force field for nucleic acids: (1) parameter optimization based on small molecule and condensed phase macromolecular target data. J Comput Chem 21: 86–104 [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu JK (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY. (2010) Diversity of structure and function of response regulator output domains. Curr Opin Microbiol 13: 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1: role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Stock AM (2009) Biological insights from structures of two-component proteins. Annu Rev Microbiol 63: 133–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Gao Z, Schaller GE (2009) The role of receptor interactions in regulating ethylene signal transduction. Plant Signal Behav 4: 1152–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wen CK, Binder BM, Chen YF, Chang J, Chiang YH, Kerris RJ III, Chang C, Schaller GE (2008) Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J Biol Chem 283: 23801–23810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203: 182–187 [DOI] [PubMed] [Google Scholar]
- Gliwicka M, Nowak K, Cieśla E, Gaj MD (2012) Expression of seed storage product genes (CRA1 and OLEO4) in embryonic cultures of somatic tissues of Arabidopsis. Plant Cell Tissue Organ Cult 109: 235–245 [Google Scholar]
- Grefen C, Städele K, Růzicka K, Obrdlik P, Harter K, Horák J (2008) Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant 1: 308–320 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Hall AE, Bleecker AB (2003) Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 15: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB (2000) Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol 123: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BP, Shakeel SN, Amir M, Ul Haq N, Qu X, Schaller GE (2012) Histidine kinase activity of the ethylene receptor ETR1 facilitates the ethylene response in Arabidopsis. Plant Physiol 159: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass C, Lohrmann J, Albrecht V, Sweere U, Hummel F, Yoo SD, Hwang I, Zhu T, Schäfer E, Kudla J, et al. (2004) The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis. EMBO J 23: 3290–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel LL, Grbić V, Baumgarten DA, Bleecker AB (1993) Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell 5: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YL, Lin YJ, Sue SC (2015) 13C, 15N and 1H resonance assignments of receiver domain of ethylene receptor ETR1. Biomol NMR Assign 9: 119–122 [DOI] [PubMed] [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Métraux JP, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139: 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. (2009) Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal 21: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Ju C, Van de Poel B, Cooper ED, Thierer JH, Gibbons TR, Delwiche CF, Chang C (2015) Conservation of ethylene as a plant hormone over 450 million years of evolution. Nature Plants 1: 1–7 [DOI] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kim H, Helmbrecht EE, Stalans MB, Schmitt C, Patel N, Wen CK, Wang W, Binder BM (2011) Ethylene receptor ETHYLENE RECEPTOR1 domain requirements for ethylene responses in Arabidopsis seedlings. Plant Physiol 156: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Lång V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Liu Q, Wen CK (2012) Arabidopsis ETR1 and ERS1 differentially repress the ethylene response in combination with other ethylene receptor genes. Plant Physiol 158: 1193–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xu C, Wen CK (2010) Genetic and transformation studies reveal negative regulation of ERS1 ethylene receptor signaling in Arabidopsis. BMC Plant Biol 10: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Ma B, Chen SY, Zhang JS (2010) Ethylene signaling in rice. Chin Sci Bull 55: 2204–2210 [Google Scholar]
- Ma B, Cui ML, Sun HJ, Takada K, Mori H, Kamada H, Ezura H (2006) Subcellular localization and membrane topology of the melon ethylene receptor CmERS1. Plant Physiol 141: 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Jha D, Salt DE, Tester M, Hill K, Kieber JJ, Schaller GE (2010) Type-B response regulators ARR1 and ARR12 regulate expression of AtHKT1;1 and accumulation of sodium in Arabidopsis shoots. Plant J 64: 753–763 [DOI] [PubMed] [Google Scholar]
- Mason MG, Schaller GE (2005) Histidine kinase activity and the regulation of ethylene signal transduction. Canadian Journal of Botany-Revue Canadienne de Botanique 83: 563–570 [Google Scholar]
- Mattoo AK, Suttle JC (1991) The Plant Hormone Ethylene. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- Mayerhofer H, Panneerselvam S, Kaljunen H, Tuukkanen A, Mertens HDT, Mueller-Dieckmann J (2015) Structural model of the cytosolic domain of the plant ethylene receptor 1 (ETR1). J Biol Chem 290: 2644–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel BK, Binder BM (2012) ETHYLENE RECEPTOR1 (ETR1) is sufficient and has the predominant role in mediating inhibition of ethylene responses by silver in Arabidopsis thaliana. J Biol Chem 287: 26094–26103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Feldman L (2010) A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnol J 5: 183–186 [DOI] [PubMed] [Google Scholar]
- Miller RK, McCrea PD (2010) Wnt to build a tube: contributions of Wnt signaling to epithelial tubulogenesis. Dev Dyn 239: 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche P, Klee HJ (2004) Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. J Biol Chem 279: 48734–48741 [DOI] [PubMed] [Google Scholar]
- Müller-Dieckmann HJ, Grantz AA, Kim SH (1999) The structure of the signal receiver domain of the Arabidopsis thaliana ethylene receptor ETR1. Structure 7: 1547–1556 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, Bleecker AB (2005) Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J 41: 651–659 [DOI] [PubMed] [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazy Y, Wollish AC, Thomas SA, Miller PJ, Collins EJ, Bourret RB, Silversmith RE (2009) Matching biochemical reaction kinetics to the timescales of life: structural determinants that influence the autodephosphorylation rate of response regulator proteins. J Mol Biol 392: 1205–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett JM, Cvetkovska M, Makenson P, Xing T, Regan S (2009a) Arabidopsis ethylene receptors have different roles in fumonisin B1-induced cell death. Physiol Mol Plant Pathol 74: 18–26 [Google Scholar]
- Plett JM, Mathur J, Regan S (2009b) Ethylene receptor ETR2 controls trichome branching by regulating microtubule assembly in Arabidopsis thaliana. J Exp Bot 60: 3923–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR (2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Xie F, Yu J, Wen CK (2012) Arabidopsis RTE1 is essential to ethylene receptor ETR1 amino-terminal signaling independent of CTR1. Plant Physiol 159: 1263–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Hall BP, Gao Z, Schaller GE (2007) A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BMC Plant Biol 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Schaller GE (2004) Requirement of the histidine kinase domain for signal transduction by the ethylene receptor ETR1. Plant Physiol 136: 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69 [DOI] [PubMed] [Google Scholar]
- Resnick JS, Wen CK, Shockey JA, Chang C (2006) REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci USA 103: 7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996–998 [DOI] [PubMed] [Google Scholar]
- Rzewuski G, Sauter M (2008) Ethylene biosynthesis and signaling in rice. Plant Sci 175: 32–42 [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Ladd AN, Lanahan MB, Spanbauer JM, Bleecker AB (1995) The ethylene response mediator ETR1 from Arabidopsis forms a disulfide-linked dimer. J Biol Chem 270: 12526–12530 [DOI] [PubMed] [Google Scholar]
- Scharein B, Groth G (2011) Phosphorylation alters the interaction of the Arabidopsis phosphotransfer protein AHP1 with its sensor kinase ETR1. PLoS One 6: e24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharein B, Voet-van-Vormizeele J, Harter K, Groth G (2008) Ethylene signaling: identification of a putative ETR1-AHP1 phosphorelay complex by fluorescence spectroscopy. Anal Biochem 377: 72–76 [DOI] [PubMed] [Google Scholar]
- Shakeel SN, Wang X, Binder BM, Schaller GE (2013) Mechanisms of signal transduction by ethylene: overlapping and non-overlapping signalling roles in a receptor family. AoB Plants 5: plt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu TS, Aksenov SV, Bray D (2003) A spatially extended stochastic model of the bacterial chemotaxis signalling pathway. J Mol Biol 329: 291–309 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al. (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Miyata S, Yamaguchi-Shinozaki K, Shinozaki K (2000) Possible His to Asp phosphorelay signaling in an Arabidopsis two-component system. FEBS Lett 478: 227–232 [DOI] [PubMed] [Google Scholar]
- Voet-van-Vormizeele J, Groth G (2008) Ethylene controls autophosphorylation of the histidine kinase domain in ethylene receptor ETR1. Mol Plant 1: 380–387 [DOI] [PubMed] [Google Scholar]
- Wang W, Esch JJ, Shiu SH, Agula H, Binder BM, Chang C, Patterson SE, Bleecker AB (2006) Identification of important regions for ethylene binding and signaling in the transmembrane domain of the ETR1 ethylene receptor of Arabidopsis. Plant Cell 18: 3429–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hall AE, O’Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22: 1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AH, Stock AM (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci 26: 369–376 [DOI] [PubMed] [Google Scholar]
- Wilson RL, Bakshi A, Binder BM (2014a) Loss of the ETR1 ethylene receptor reduces the inhibitory effect of far-red light and darkness on seed germination of Arabidopsis thaliana. Front Plant Sci 5: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RL, Kim H, Bakshi A, Binder BM (2014b) The ethylene receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 have contrasting roles in seed germination of Arabidopsis during salt stress. Plant Physiol 165: 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Liu Q, Wen CK (2006) Receptor signal output mediated by the ETR1 N terminus is primarily subfamily I receptor dependent. Plant Physiol 142: 492–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Zhong S, Lin Z, Grierson D (2008) Tomato ethylene receptor-CTR interactions: visualization of NEVER-RIPE interactions with multiple CTRs at the endoplasmic reticulum. J Exp Bot 59: 965–972 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.