The plant hormone abscisic acid functions as a general negative regulator of branching and may contribute to the effects of other hormones and proteins that control the process.
Abstract
Branching is an important process controlled by intrinsic programs and by environmental signals transduced by a variety of plant hormones. Abscisic acid (ABA) was previously shown to mediate Arabidopsis (Arabidopsis thaliana) branching responses to the ratio of red light (R) to far-red light (FR; an indicator of competition) by suppressing bud outgrowth from lower rosette positions under low R:FR. However, the role of ABA in regulating branching more generally was not investigated. This study shows that ABA restricts lower bud outgrowth and promotes correlative inhibition under both high and low R:FR. ABA was elevated in buds exhibiting delayed outgrowth resulting from bud position and low R:FR and decreased in elongating buds. ABA was reduced in lower buds of hyperbranching mutants deficient in auxin signaling (AUXIN RESISTANT1), MORE AXILLARY BRANCHING (MAX) signaling (MAX2), and BRANCHED1 (BRC1) function, and partial suppression of branch elongation in these mutants by exogenous ABA suggested that ABA may act downstream of these components. Bud BRC1 expression was not altered by exogenous ABA, consistent with a downstream function for ABA. However, the expression of genes encoding the indole-3-acetic acid (IAA) biosynthesis enzyme TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1, the auxin transporter PIN-FORMED1, and the cell cycle genes CYCLIN A2;1 and PROLIFERATING CELL NUCLEAR ANTIGEN1 in buds was suppressed by ABA, suggesting that it may inhibit bud growth in part by suppressing elements of the cell cycle machinery and bud-autonomous IAA biosynthesis and transport. ABA was found to suppress bud IAA accumulation, thus confirming this aspect of its action.
Plant shoot architecture is determined to a large extent by the number, position, orientation and size of shoot branches. Branching exerts a profound influence on the architecture of the shoot and gives rise to much of the variation in shoot form observed between species, between accessions of a species and within accessions grown in disparate environments. Branching is a plastic trait that allows plant form to be tailored to ecological niche and dynamically changing environmental conditions (Juenger and Bergelson, 2000; Lortie and Aarssen, 2000; Bonser and Aarssen, 2003; Weinig et al., 2003). In the agricultural context, branching is an important trait that contributes to yield and the suitability of a given crop for particular environments and end uses (Zarrough et al., 1983; Peng et al., 1994; García del Moral and García del Moral, 1995; Zhao et al., 2006; Boe and Beck, 2008).
Shoot branches arise from buds that develop from meristems in the leaf axils. Once a bud is formed, it may remain dormant or quasidormant, or it may grow to form a branch immediately or after a variable interval of time (Bennett and Leyser, 2006). In common Arabidopsis (Arabidopsis thaliana) ecotypes grown under long days, axillary buds are initiated and form branches beginning at the top of the rosette, and this pattern progresses downward (Hempel and Feldman, 1994). Arabidopsis buds do not exhibit characteristics of true dormancy, because even buds at lower rosette positions that normally do not form elongated branches display continual, though slow, growth and development until the plant senesces. The existing evidence suggests that Arabidopsis axillary bud development comprises a continuum of bud formation and outgrowth, with distinctions made between bud and branch being essentially arbitrary (Finlayson et al., 2010; Su et al., 2011; Reddy et al., 2013). Although not entirely dormant, the fate of lower buds is plastic, and their growth and development can be retarded or accelerated by a number of factors.
Several hormones play prominent roles in regulating bud outgrowth. A variety of studies using diverse approaches have shown that cytokinins can promote the outgrowth of buds that would otherwise remain inhibited and that cytokinin levels in or near the bud correlated with bud fate (Sachs and Thimann, 1967; Medford et al., 1989; Emery et al., 1998; Tanaka et al., 2006). Branching is inhibited by a strigolactone pathway that has been demonstrated to function in many species (Waldie et al., 2010; Janssen et al., 2014). In Arabidopsis, this pathway is defined by the MORE AXILLARY BRANCHING (MAX) proteins involved in the synthesis and perception of the strigolactone or derivative, which may be carlactonoic acid methyl ester (Abe et al., 2014). Additionally, auxin in the main shoot polar auxin transport stream (PATS) is well established as a major inhibitor of branch development, acting to suppress the initiation of bud growth (Domagalska and Leyser, 2011) and to inhibit bud elongation (Chatfield et al., 2000; Stirnberg et al., 2012; Reddy and Finlayson, 2014). However, the observation that auxin acts indirectly without entering the bud has provoked contrasting, though not mutually exclusive, hypotheses concerning the mechanisms involved in transducing the auxin signal. One hypothesis postulates that the inhibitory effects of auxin are communicated by second messengers that act more directly on the bud, potentially including cytokinins (depletion) and/or the MAX-associated strigolactone (Brewer et al., 2009; Dun et al., 2012, 2013). The other hypothesis contends that competition between the main shoot and the bud for auxin transport capacity limits bud outgrowth, and thus auxin in the main shoot PATS suppresses bud outgrowth by preventing the bud itself from establishing auxin export into the main shoot (Bennett et al., 2006; Prusinkiewicz et al., 2009; Shinohara et al., 2013).
Bud outgrowth has also been proposed to respond to various limiting nutrients and resources, with sugars drawing considerable scrutiny (Ballard and Wildman, 1964; Girault et al., 2010; Henry et al., 2011; Kebrom et al., 2012; Rabot et al., 2012; Kebrom and Mullet, 2015). A recent report provided evidence that in pea (Pisum sativum), rapid bud outgrowth in response to decapitation results from signals communicated by the redirection of Suc to the region containing the buds (Mason et al., 2014).
The general branching form of a plant is directed to a large extent by intrinsic developmental programs that generate a branching habit characteristic of a particular species or accession. However, environmental signals, including the ratio of red light (R) to far-red light (FR), may modify the intrinsic branching program and add a level of developmental plasticity. The R:FR may decrease due to the selective absorption of R and reflection or transmission of FR in incident light by neighboring vegetation. The R:FR is perceived by phytochromes, including the major R:FR sensor phytochrome B (phyB). A decrease in the R:FR can occur long before actual shading begins and thus informs plants of potential future competitors. In response to the threat of competition, plants may evoke the so-called shade avoidance syndrome, including rapid shoot elongation, early flowering and reduced branching (and other responses), which are believed to confer a fitness advantage in natural environments (Smith, 1995; Ballaré, 1999; Franklin and Whitelam, 2005; Casal, 2012). The response is highly dependent on the timing of exposure to low R:FR, with exposure late in development actually promoting some aspects of branching (Reddy et al., 2014). The inhibition of branching in Arabidopsis due to low R:FR has been shown to be dependent on phyB function, auxin and MAX signaling and the function of the TEOSINTE BRANCHED1 (TB1), CYCLOIDEA (CYC), and PROLIFERATING CELL FACTORs (PCFs; TCP) domain protein BRANCHED1 (BRC1; Finlayson et al., 2010; Su et al., 2011; González-Grandío et al., 2013; Reddy and Finlayson, 2014; Reddy et al., 2014).
The effects of the hormones and other signals described are transduced by BRC1, and its homolog in grasses, TB1. BRC1 is a negative regulator of branching that is expected to function as a transcription factor and is expressed predominantly in unelongated buds (Aguilar-Martínez et al., 2007; Finlayson, 2007). BRC1 expression is responsive to developmental and environmental cues, and BRC1 function is necessary for proper regulation of bud outgrowth in response to a wide variety of stimuli, leading to its designation as a branching integrator (Aguilar-Martínez et al., 2007).
Abscisic acid (ABA) was hypothesized to play a role in branching for many years. Studies using a wide variety of species showed that ABA abundance in buds negatively correlated with bud activity (Tucker and Mansfield, 1971; Tucker, 1977; Tamas et al., 1979; Knox and Wareing, 1984; Gocal et al., 1991; Mader et al., 2003). Exogenous ABA inhibited bud growth in pea (Arney and Mitchell, 1969), Arabidopsis (Chatfield et al., 2000), Ipomoea nil (Cline and Oh, 2006), and tomato (Solanum lycopersicum; Cline and Oh, 2006), while the carotenoid (and ABA) biosynthesis inhibitor fluridone promoted bud growth in Rosa hybrida (Le Bris et al., 1999). Isolated poplar (Populus × canescens) explants with transgenically reduced ABA sensitivity exhibited increased lateral bud growth (Arend et al., 2009), and transcriptome studies of branch development in Arabidopsis (González-Grandío et al., 2013) and sugarcane (Saccharum officinarum; Ortiz-Morea et al., 2013) provided additional support for an association of ABA with the process. Definitive proof for the function of ABA in regulating Arabidopsis axillary bud outgrowth responses to the R:FR was demonstrated using a combination of transcriptomic, biochemical, and genetic approaches (Reddy et al., 2013). Bud ABA levels and the expression of ABA-related genes decreased when buds suppressed by growth under low R:FR were exposed to high R:FR, and branching responses to low R:FR were defective in the ABA-deficient aba deficient2-1 (aba2-1) and 9-cis-epoxycarotenoid dioxygenase3-2 (nced3-2) biosynthetic mutants.
While there is now considerable evidence that ABA inhibits bud outgrowth under low R:FR, the function of ABA in favorable high R:FR environments is less clear. Additionally, how ABA integrates with other established regulators of branching such as auxin, strigolactones, and BRC1 is unknown. In this study, the hypothesis that ABA acts as a general regulator of branching under high and low R:FR was tested, as was the hypothesis that ABA acts downstream or independently of auxin and strigolactone signaling and BRC1 function. Potential targets of ABA action were also explored.
RESULTS
ABA Suppresses Branching under Both High and Low R:FR
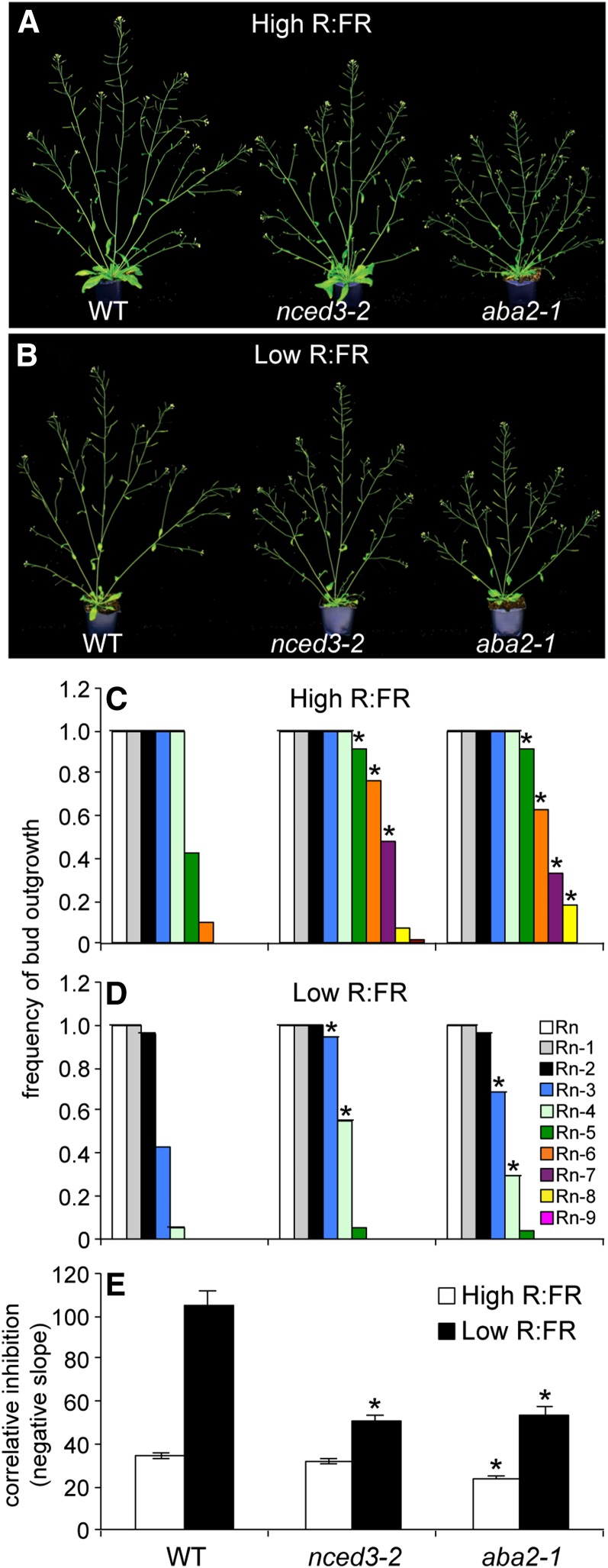
ABA was previously shown to restrict the outgrowth of lower buds of Arabidopsis grown under low R:FR from 1 d after sowing, and ABA levels in buds were shown to be modulated by the R:FR (Reddy et al., 2013). To determine if ABA more generally regulated branching, the wild type and the ABA-deficient lines aba2-1 and nced3-2 were grown under both low and high R:FR, and architectural analyses were conducted at 10 DPA. Although healthy, both nced3-2 and aba2-1 showed reduced shoot growth compared with the wild type under both high and low R:FR (Fig. 1). Plants grown under low R:FR appeared less branched than their counterparts grown under high R:FR (Fig. 1). The frequency of outgrowth of lower buds was elevated in aba2-1 and nced3-2 compared with the wild type under both high and low R:FR (Fig. 1). This effect was apparent beginning with bud n-5 (bud n is the topmost bud; n-5 is the sixth bud down in the rosette) under high R:FR and with bud n-3 under low R:FR. The lengths of the top three rosette branches were used to calculate a correlative inhibition index (by regressing branch lengths versus position in the rosette), as described previously (Finlayson et al., 2010; Su et al., 2011; Reddy and Finlayson, 2014). This index integrates the timing of the initiation of bud outgrowth and the elongation rate of branches from these upper positions, providing a quantitative estimate of branch growth inequality, used as an index of branching vigor. Under high R:FR, the correlative inhibition index of aba2-1 was reduced compared with the wild type, indicating increased branching strength in the mutant (Fig. 1). Correlative inhibition was reduced in both aba2-1 and nced3-2 under low R:FR, indicating that normal ABA levels are necessary for typical correlative inhibition responses to the R:FR. ABA status had modest effects on total leaf numbers. Total leaf numbers differed significantly from the wild type only in aba2-1 grown under low R:FR, but the effect was small (9.7 in aba2-1 versus 8.9 in the wild type). Thus, the ABA effects could be attributed to specific alteration of the branching program rather than to collateral effects on development.
Figure 1.
Visual phenotypes in high (A) and low R:FR (B), frequency of bud outgrowth by bud position in high (C) and low R:FR (D), and correlative inhibition index in high and low R:FR (E) of the wild type (WT), nced3-2, and aba2-1. Rn indicates rosette bud n, the topmost bud. Sequentially lower buds are numbered in order. Data are means ± se with n = 54. Asterisks indicate a significant difference between the wild type and mutants at α = 0.05.
While attenuation was evident, both aba2-1 and nced3-2 responded to low R:FR with reduced frequencies of bud outgrowth and increased correlative inhibition. Because neither mutant is totally ABA deficient, these responses could be due to modulation of the residual ABA. It is perhaps more likely that the R:FR affects auxin signaling in these lines to alter these branching parameters, as has been demonstrated with phyB deficiency (Reddy and Finlayson, 2014).
ABA Abundance Is Elevated in Inferior Buds and under Low R:FR and Decreases in Elongated Buds
ABA levels were assessed in buds displaying sequentially delayed outgrowth patterns (bud n, bud n-1, and bud n-2), just prior to the predicted onset of outgrowth of bud n. A graded increase in ABA was observed from bud n to bud n-2 (Fig. 2), which correlated inversely with the timing of outgrowth and elongation rates of buds from these positions (Finlayson et al., 2010; Su et al., 2011). The ABA abundance in bud n-2 was 2.4 (low R:FR) to 3.4 (high R:FR) times higher than that of bud n. ABA was also elevated in buds of plants grown under low R:FR compared with high R:FR (Fig. 2), consistent with weaker branching from buds n-1 and n-2 observed under low R:FR (Fig. 1; Finlayson et al., 2010). ABA in bud n was also elevated under low R:FR, though growth from this position was previously shown to be enhanced compared with high R:FR (Finlayson et al., 2010).
Figure 2.
ABA abundance in the top three buds of the wild type grown in high and low R:FR. Data are means ± se with n = 4. Bars with different letters are significantly different at α < 0.05, within light treatments. Asterisks indicate a significant difference between high and low R:FR at α = 0.05. FW, Fresh weight.
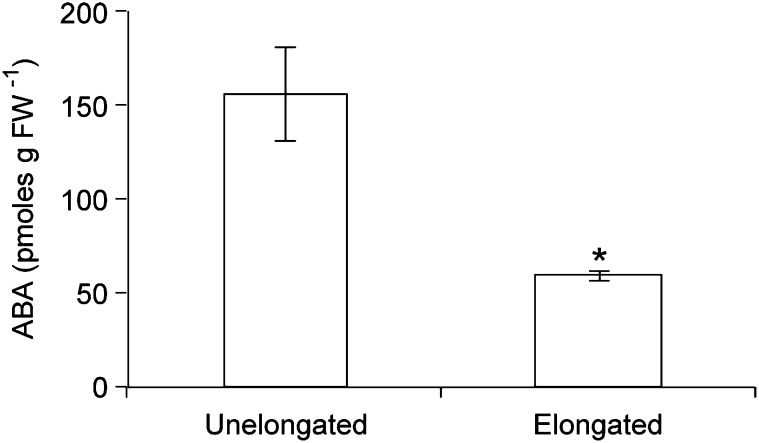
The correlation between ABA abundance and bud status was investigated in bud n-2, which was previously shown to exhibit delayed outgrowth and a reduced maximum elongation rate compared with superior buds (Finlayson et al., 2010; Su et al., 2011). ABA abundance was high in unelongated buds (less than 2 mm) and decreased to about 38% of the initial level in buds that had begun to elongate (3–4 mm; Fig. 3). Thus, ABA abundance was negatively correlated with the developmental potential of the buds (i.e. by bud position, except for bud n under low R:FR) and also with bud activity.
Figure 3.
ABA abundance in unelongated and elongated wild-type bud n-2 grown under high R:FR. Data are means ± se with n = 7. Asterisk indicates a significant difference between unelongated and elongated buds at α = 0.05. FW, Fresh weight.
Auxin, the MAX Pathway, and BRC1 Promote ABA Accumulation in Lower, But Not Upper, Buds
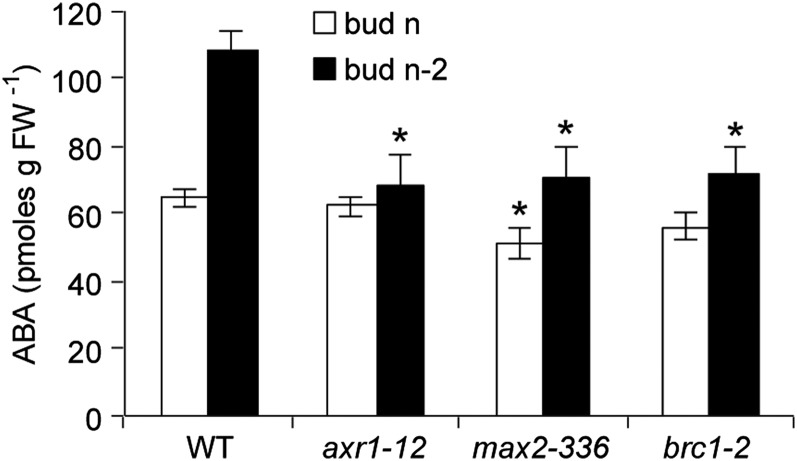
To discover how ABA regulation of bud growth integrates with other established branching pathways, ABA abundances were assessed in buds n and n-2 of the wild type and the hyperbranching mutants auxin resistant1-12 (axr1-12; deficient in auxin signaling), max2-336 (deficient in strigolactone signaling), and brc1-2 (deficient in a bud-suppressing TCP domain protein) just prior to the predicted onset of outgrowth of bud n. Buds from equivalent positions in all genotypes were similar in size. ABA levels were comparable in bud n of all genotypes, although max2-336 showed significantly reduced accumulation compared with the wild type (Fig. 4). ABA abundance in the lower bud n-2 was significantly reduced in all the mutant genotypes compared with the wild type (Fig. 4), demonstrating the necessity for auxin signaling, signaling through the MAX pathway, and BRC1 function for the maintenance of elevated ABA in lower buds.
Figure 4.
ABA abundance in buds n and n-2 of the wild type (WT), axr1-12, max2-336, and brc1-2. Data are means ± se with n = 4. Asterisks indicate a significant difference between wild-type and mutant buds at α = 0.05. FW, Fresh weight.
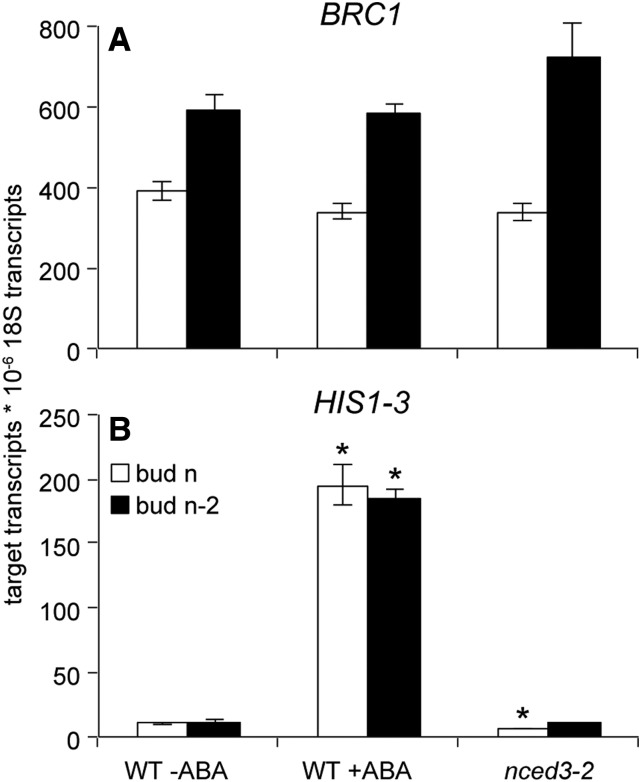
ABA Does Not Affect BRC1 Expression
BRC1 expression has previously been shown to be responsive to a variety of pathways that influence branching, including auxin signaling (Finlayson, 2007) and signaling through the MAX pathway (Aguilar-Martínez et al., 2007; Finlayson, 2007). The effect of ABA on BRC1 expression was examined by applying ABA to unelongated buds and also by using the nced3-2 mutant that has reduced ABA abundance in buds (Reddy et al., 2013). As previously shown, BRC1 expression was elevated in the lower buds compared with upper buds (Finlayson, 2007), but expression was not altered by the ABA treatment and was also not impacted by NCED3 function (Fig. 5). To determine if the ABA treatment was effective in inducing ABA-responsive genes, the expression of the HISTONE H1-3 (HIS1-3) gene was measured. HIS1-3 expression is known to be induced by ABA and is directly regulated by the ABA-signaling transcription factor ABSCISIC ACID-RESPONSIVE ELEMENTS-BINDING FACTOR1 (Fujita et al., 2005). HIS1-3 was robustly induced by the ABA treatment, and its expression was significantly reduced in bud n of nced3-2 compared with the wild type (Fig. 5). Thus, the ABA treatment was effective, but ABA did not influence the expression of BRC1.
Figure 5.
Abundance of BRC1 (A) and HIS1-3 (B) mRNAs in buds n and n-2 of the wild type (WT) with and without 50 pmol of (+) ABA and of nced3-2, just prior to the predicted outgrowth of bud n. Buds were harvested 3 h after the start of the treatments. Data are means ± se with n = 3 (bud n) and 4 (bud n-2). Asterisks indicate a significant difference between the wild type without ABA and the wild type with ABA or nced3-2 at α = 0.05.
ABA Inhibits Bud Growth Downstream or Independently of AXR1, MAX2, and BRC1 Function
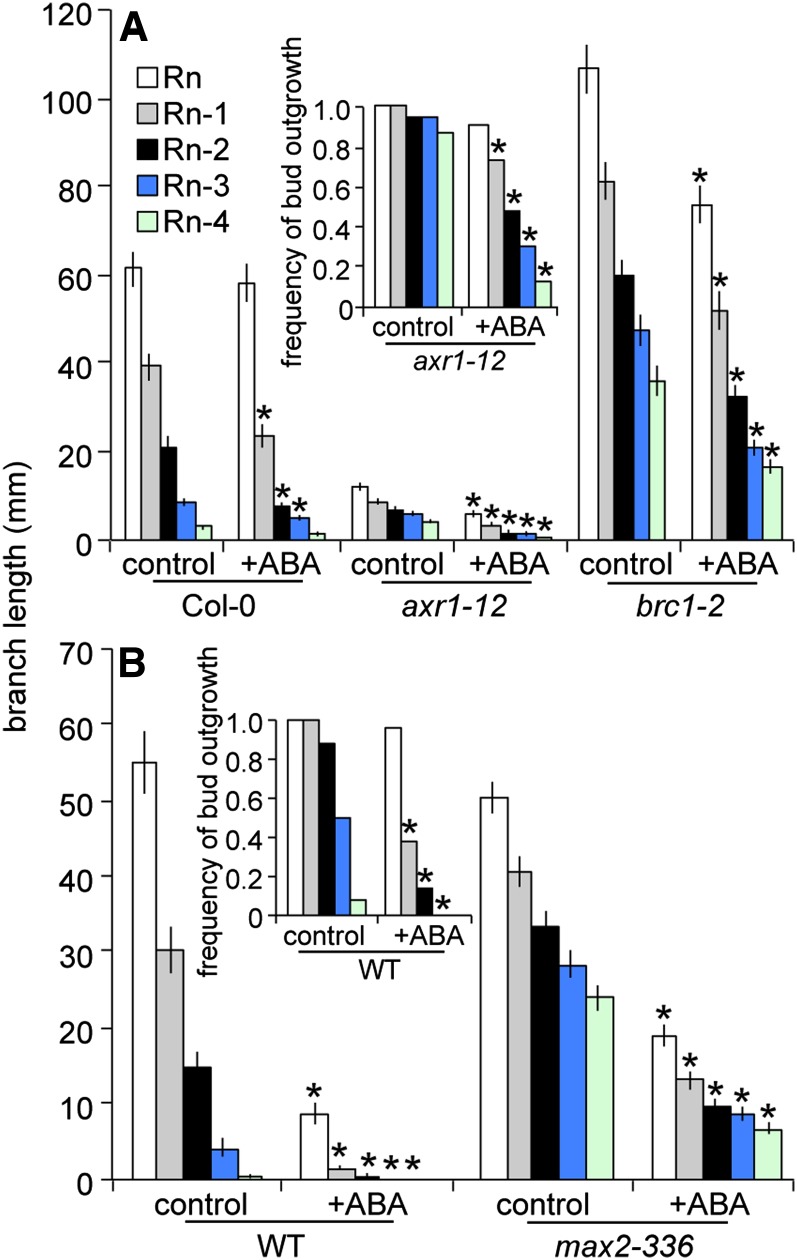
The effect of daily ABA application to unelongated buds of axr1-12, max2-336, and brc1-2 was investigated to directly test how ABA interacts with signaling mediated by the corresponding genes. ABA was able to partially inhibit the elongation of treated buds of all the genotypes tested (Fig. 6), indicating that it can act downstream or independently of these components. Lower buds of the wild type were completely arrested at the higher ABA level (100 pmol), whereas the upper buds still showed some elongation. The lower level of ABA also reduced the frequency of outgrowth of axr1-12 buds n-1 through n-4 but did not impact the frequency of wild-type or brc1-2 bud outgrowth. At the lower level of ABA (50 pmol), the elongation of wild-type buds from most positions was modestly attenuated. Buds of brc1-2 and max2-336 treated with ABA achieved greater lengths than those of the wild type provided with the same level of the hormone, whereas those of axr1-12 were always shorter. The ABA treatment did not result in any obvious detrimental effects associated with the hormone, such as chlorosis.
Figure 6.
Elongation of wild-type (WT), axr1-12, and brc1-2 branches with and without 50 pmol of (+) ABA (A) and wild-type and max2-336 branches with and without 100 pmol of (+) ABA (B) applied to each of the top five buds daily. Rn indicates rosette bud n, the topmost bud. Sequentially lower buds are numbered in order. Insets provide the frequency of bud outgrowth for genotypes where significant differences were observed with ABA treatment. Treatments began at anthesis, and branch lengths were determined after 5 d. Data are means ± se with n = 22 to 27. Asterisks indicate a significant difference between control and ABA treatments at α = 0.05.
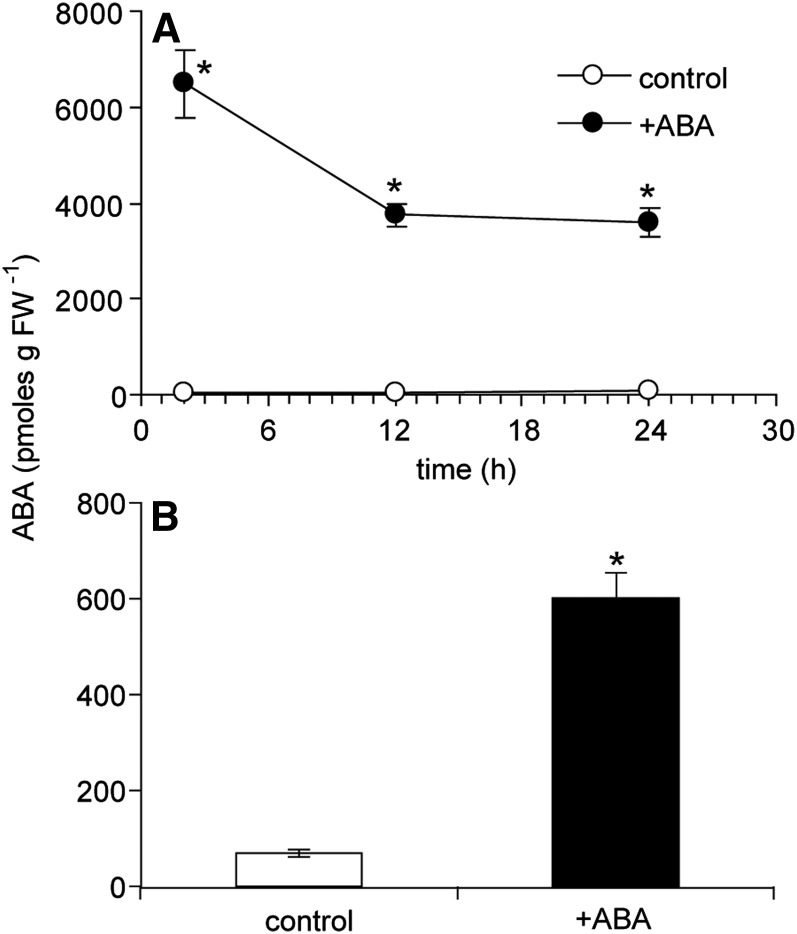
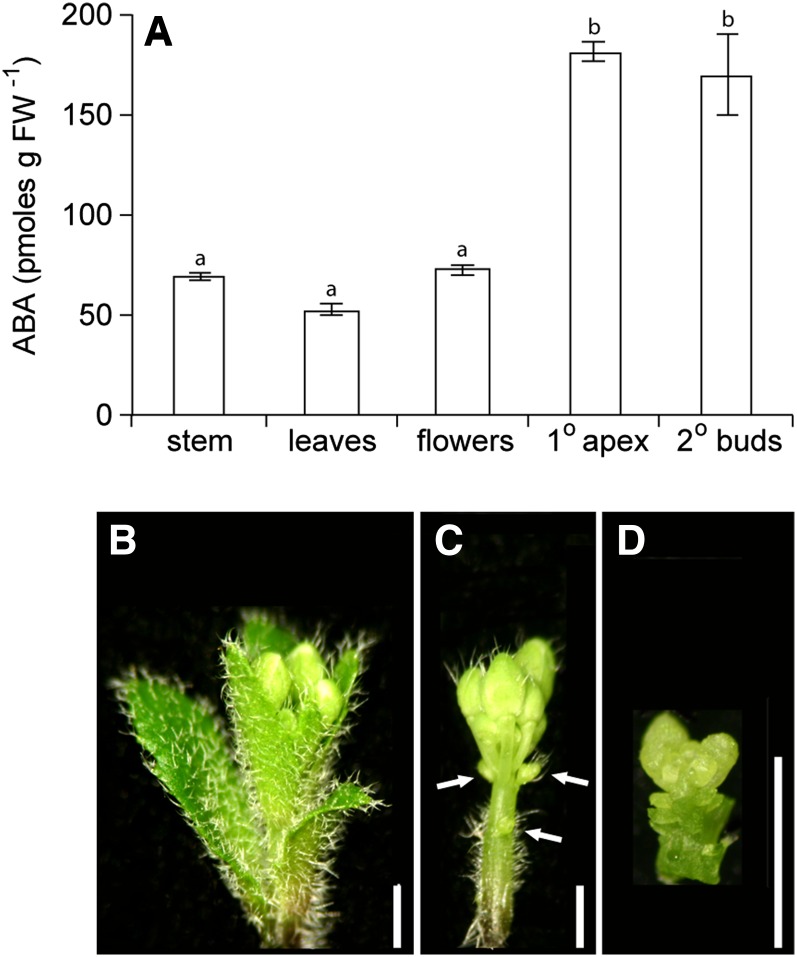
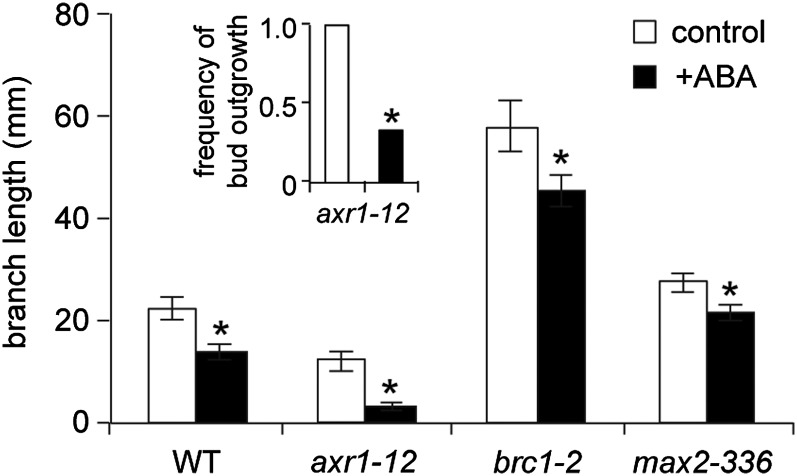
ABA levels were assessed in wild-type buds with and without the application of 50 pmol of ABA to determine how the treatment altered hormone abundance. ABA levels were about 150 times higher in treated buds compared with controls at 2 h and declined to about 50 times higher at 24 h (Fig. 7). While these levels are very high, it is important to note that the ABA solution was applied to the end of the bud and thus was absorbed to a great extent by the young leaves and flowers that might not play a major role in transducing ABA effects on bud growth processes. Additionally, it is possible that some portion of the applied ABA did not enter the cells but remained in the apoplast. To determine where ABA naturally accumulated, its distribution within the bud was examined by finely dissecting wild-type bud n-2 into stem, young leaves, young flowers, primary shoot apex, and secondary bud tissues (Fig. 8). ABA accumulation was relatively low in stem, leaves, and flowers but was higher in portions enriched in meristematic tissue. Because the elevated ABA abundance in the shoot apex could indicate a special function for ABA at this site, an additional experiment investigating the effect of ABA on bud elongation was conducted employing a smaller amount of ABA (5 pmol) applied to the shoot apical meristem of bud n-2 of the wild type, axr1-12, max2-336, and brc1-2. This treatment resulted in a more modest increase in ABA of wild-type bud apices of about 8.8 times at 24 h (Fig. 7) but still significantly inhibited the elongation of the buds of all the genotypes (Fig. 9). The repressive effects of ABA were most apparent in axr1-12, which also exhibited a reduced frequency of bud outgrowth not observed in the other genotypes.
Figure 7.
ABA abundance in unelongated wild-type bud n-2 grown under high R:FR. A, Time course following application of 50 pmol of (+) ABA to distal bud tissues. B, ABA abundance in shoot apices of wild-type bud n-2 24 h after application of 5 pmol of (+) ABA to the bud shoot apical meristem. Data are means ± se with n = 4. Asterisks indicate a significant difference between treated and control tissues at α = 0.05. FW, Fresh weight.
Figure 8.
A, ABA distribution in dissected portions of wild-type bud n-2. Bars with different letters are significantly different at α < 0.05. B to D, Dissection of wild-type bud n-2 for the determination of ABA distribution. Intact bud (B), bud with leaves removed (C), and bud primary shoot apex (D). Arrows in C indicate secondary buds. FW, Fresh weight. Bars = 1 mm.
Figure 9.
Elongation of the wild type (WT), axr1-12, brc1-2, and max2-336 branch n-2 with and without 5 pmol of (+) ABA applied to the bud shoot apical meristem daily. Treatments began at anthesis, and branch lengths were determined after 5 d for the wild type, brc1-2, and max2-336 or after 7 d for axr1-12. Inset provides the frequency of bud n-2 outgrowth of axr1-12, the only genotype showing a significant difference for this parameter with ABA treatment. Data are means ± se with n = 9. Asterisks indicate a significant difference between control and ABA-treated branch lengths at α = 0.05.
ABA Alters the Bud-Autonomous Expression of Genes That Function in Cytokinin, Auxin, and Cell Cycle Pathways
Although the data presented above establish that ABA acts downstream or independently of other major branch-regulating pathways, how ABA influences bud development is obscure. Several potential pathways were therefore investigated using analyses of gene expression in buds 3 h following the application of exogenous ABA to wild-type buds and in buds of nced3-2. A recent report showed that bud outgrowth of pea was rapidly regulated by a redistribution of sugar to buds of decapitated plants (Mason et al., 2014). The expression responses of three sugar signaling-responsive genes were surveyed using complementary DNA (cDNA) pooled from the replicate samples. The genes included PEROXIDASE4 and β-AMYLASE3, previously shown to be induced and repressed by Suc (Bläsing et al., 2005), respectively. The set also included VACUOLAR INVERTASE2 (VI2), an Arabidopsis homolog of R. hybrida RHVI1, which was shown to be induced by Suc and positively associated with bud burst (Rabot et al., 2012). None of the genes showed substantial differences in abundance in buds with or without ABA treatment, suggesting that ABA by itself may not have a strong impact on sugar distribution or signaling in the buds (Table I).
Table I. ABA has minimal effects on the expression of sugar signaling-related and specific cyclin genes in unelongated buds.
Data are from surveys employing pooled sample replicates and thus are not represented by biological replication. Results are the average threshold cycle of selected target mRNAs normalized to the average 18S RNA threshold cycle and represent three technical replicates.
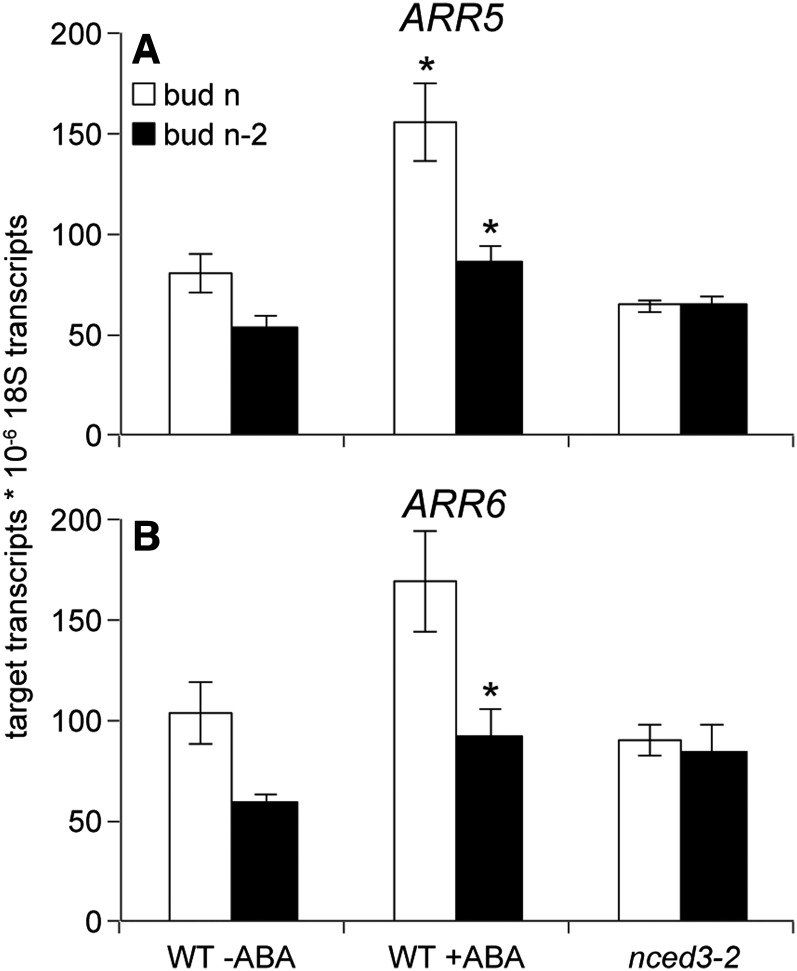
Because many studies have implicated cytokinins in the regulation of bud outgrowth, the expression of the cytokinin-responsive type A response regulators RESPONSE REGULATOR5 (ARR5) and ARR6 was examined. ARR5 expression was elevated in both bud n and n-2 of the wild type treated with ABA, and ABA induced ARR6 accumulation in bud n-2 (Fig. 10). Although the expression of these genes is induced by cytokinins, they act as negative regulators of cytokinin signaling (To et al., 2004). These data may indicate that ABA represses cytokinin signaling by inducing the expression of type A response regulators independent of cytokinin action. However, because nced3-2 did not show differential expression of these genes, it is unlikely that they play a major role in transducing sustained endogenous ABA signaling.
Figure 10.
Abundance of cytokinin type A response regulators ARR5 (A) and ARR6 (B) in buds n and n-2 of the wild type (WT) with and without 50 pmol of (+) ABA and of nced3-2, just prior to the predicted onset of bud n outgrowth. Buds were harvested 3 h after the start of the treatments. Data are means ± se with n = 3 (bud n) and 4 (bud n-2). Asterisks indicate a significant difference between the wild type without ABA and the wild type with ABA at α = 0.05.
The transition of a bud from a state of dormancy or quasidormancy to more active growth has been associated with increased expression of genes that regulate or are involved in the cell cycle (Devitt and Stafstrom, 1995; Shimizu and Mori, 1998; Kebrom et al., 2010). The effects of ABA on the expression of a variety of cell cycle-related genes were therefore tested. A scouting experiment using cDNA pooled from the experimental replicates showed that neither CYCLIN D2;1 (CYCD2;1) nor CYCD3;1 varied substantially in abundance in response to ABA treatment (Table I). Conversely, PROLIFERATING CELL NUCLEAR ANTIGEN1 (PCNA1) expression was repressed by ABA in both bud n and bud n-2 and was elevated in both buds of nced3-2 (Fig. 11). CYCA2;1 abundance was similarly lower in bud n-2 of the wild type treated with ABA but was not significantly altered in nced3-2 (Fig. 11). Therefore, ABA may exert some of its effects on bud growth by altering the expression of specific components of the cell cycle machinery, including PCNA1.
Figure 11.
Abundance of cell cycle-related mRNAs PCNA1 (A) and CYCA2;1 (B) in buds n and n-2 of the wild type (WT) with and without 50 pmol of (+) ABA and of nced3-2, just prior to the predicted onset of bud n outgrowth. Buds were harvested 3 h after the start of the treatments. Data are means ± se with n = 3 (bud n) and 4 (bud n-2). Asterisks indicate a significant difference between the wild type without ABA and the wild type with ABA or nced3-2 at α = 0.05.
The role of auxin in the main shoot PATS as an inhibitor of bud outgrowth is well established. However, auxin is also expected to play a positive role within the bud to promote elongation, as is the case in the main shoot apex (Gray et al., 1998; Lincoln et al., 1990; Jouve et al., 1999). The expression of several genes involved in auxin biosynthesis (TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 [TAA1]), transport (PIN-FORMED1 [PIN1]), conjugation (GH3.5), and signaling (INDOLE-3-ACETIC ACID INDUCIBLE2 [IAA2] and IAA3) was tested to discover if ABA could influence bud-autonomous aspects of auxin physiology. ABA suppressed the expression of TAA1 in bud n-2, and TAA1 expression was elevated in bud n-2 of the nced3-2 ABA-deficient mutant (Fig. 12). Similar expression patterns were observed for PIN1, although the numerical increase in nced3-2 was not significantly different from the wild type (Fig. 12). These results support a role for ABA in repressing auxin biosynthesis in, and auxin transport out of, axillary buds, especially in bud n-2, which has delayed and weaker outgrowth compared with bud n (Finlayson et al., 2010; Su et al., 2011). Although the abundances of GH3.5, IAA2, and IAA3 increased with ABA treatment, their role in bud repression by endogenous ABA is suspect given that corresponding changes in expression in nced3-2 buds were not observed (Fig. 12).
Figure 12.
Abundance of auxin-related mRNAs including TAA1 (A), PIN1 (B), GH3.5 (C), IAA2 (D), and IAA3 (E) in buds n and n-2 of the wild type (WT) with and without 50 pmol of (+) ABA and of nced3-2, just prior to the predicted onset of bud n outgrowth. Buds were harvested 3 h after the start of the treatments. Data are means ± se with n = 3 (bud n) and 4 (bud n-2). Asterisks indicate a significant difference between the wild type without ABA and the wild type with ABA or nced3-2 at α = 0.05.
IAA Accumulation Is Repressed in ABA-Treated Buds
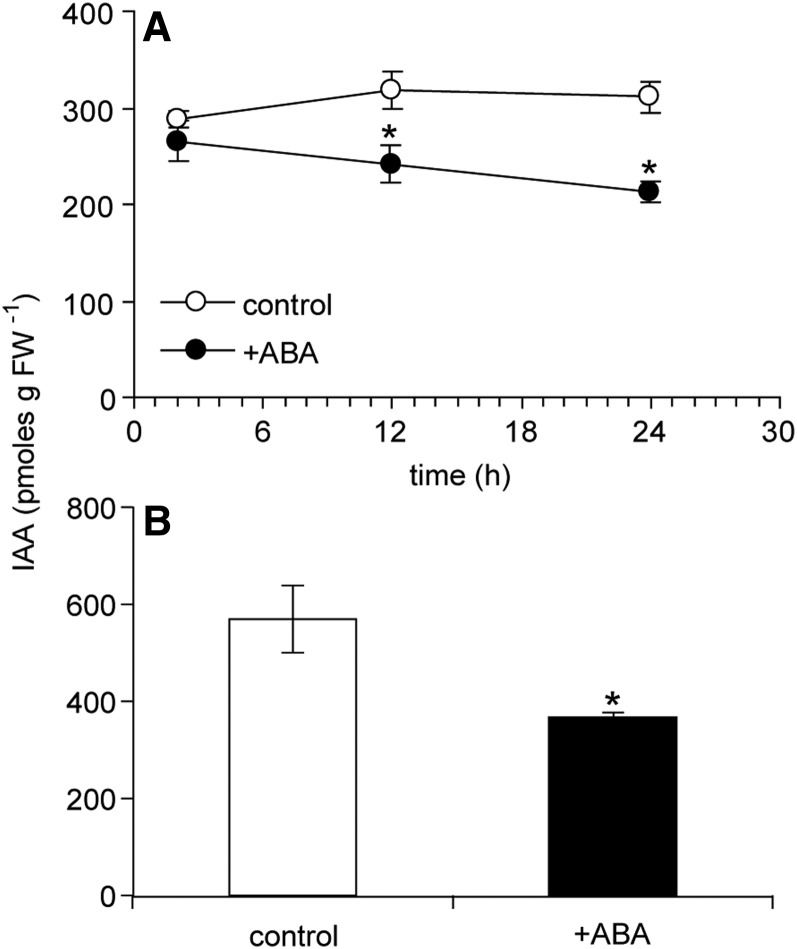
Because the gene expression analyses indicated that auxin homeostasis might be altered by ABA, the effect of exogenous ABA on the accumulation of indole-3-acetic acid (IAA) in wild-type bud n-2 was investigated. A high level of ABA (50 pmol) applied to the distal bud tissues resulted in a decline in bud IAA abundance at 12 and 24 h after treatment (Fig. 13). A lower level of ABA (5 pmol) applied to the bud shoot apical meristem also resulted in a significant reduction in IAA accumulation in the apex by 24 h after treatment (Fig. 13). The growth-inhibiting effect of ABA was correlated with its inhibitory effect on bud IAA abundance.
Figure 13.
IAA abundance in unelongated wild-type bud n-2 grown under high R:FR. A, Time course following application of 50 pmol of (+) ABA to distal bud tissues. B, IAA abundance in shoot apices of wild-type bud n-2 24 h after application of 5 pmol of (+) ABA to the bud shoot apical meristem. Data are means ± se with n = 4. Asterisks indicate a significant difference between treated and control tissues at α = 0.05. FW, Fresh weight.
DISCUSSION
ABA Is a General Inhibitor of Axillary Bud Growth
A previous study investigating the mechanisms associated with the regulation of branching by competition signals demonstrated a dynamic role for ABA in restricting bud outgrowth under low R:FR (Reddy et al., 2013). While this work provided clear evidence that ABA was involved in suppressing branching under low R:FR, the role of ABA in regulating branching more generally was not tested. This study demonstrated a role for ABA in suppressing the frequency of outgrowth and elongation of lower rosette buds both under high and low R:FR using the aba2-1 and nced3-2 ABA biosynthesis mutants. As noted previously, ABA contributed to correlative inhibition under low R:FR. However, this effect was also apparent in aba2-1 grown under high R:FR. Thus, while ABA is integral to inhibiting branching under low R:FR, it also suppresses lower bud outgrowth and elongation and is a factor contributing to the coordination of growth between branches (correlative inhibition), even when low R:FR competition signals are not prevalent.
Both aba2-1 and nced3-2 showed increased branching phenotypes; however, promotion of branching was not observed in a sextuple ABA receptor mutant (González-Grandío et al., 2013). The sextuple ABA receptor mutant shows a substantial dwarfing phenotype (Gonzalez-Guzman et al., 2012) that may counter the promotive effects of reduced ABA signaling on branching. For instance, the repression of shoot growth may limit the energy available to support branch growth. While the aba2-1 and nced3-2 mutants also show reduced shoot growth, their phenotypes (especially nced3-2) more closely resemble the wild type than does the sextuple ABA receptor mutant. It should also be noted that the increased branching observed in the ABA biosynthetic mutants is not as obvious as that in strong hyperbranching lines such as the max mutants or brc1. It is possible that a more detailed analysis of the sextuple ABA receptor mutant could reveal weaker branching effects. Alternatively, the branching program may be affected by an ABA-signaling pathway that does not include the PYRABACTIN RESISTANCE1/PYRABACTIN RESISTANCE1-LIKE proteins.
The abundance of ABA correlated with the retarded development of lower buds (Finlayson et al., 2010; Su et al., 2011), suggesting that growth is partially dependent on endogenous control of bud ABA homeostasis and/or transport. ABA abundance was elevated in lower buds, and ABA accumulation was promoted by low R:FR, indicating that modulation occurs both through intrinsic developmental programming as well as in response to environmental signals. Low R:FR also promoted ABA accumulation in the uppermost bud (bud n), which has previously been shown to initiate outgrowth earlier and to grow at a higher rate than the corresponding bud of plants grown under high R:FR (Finlayson et al., 2010). Bud ABA is therefore not likely to be the sole factor contributing to bud fate, and the inhibitory effects of high ABA may be overridden by the promotive effects of other factors. The extensive changes in the transcriptional programming of buds in response to the R:FR suggests that many pathways in addition to ABA are likely involved in regulating bud fate (González-Grandío et al., 2013; Reddy et al., 2013).
The developmental stage of the bud also correlated with ABA levels, which declined in parallel with bud elongation. The data suggest that ABA inhibits growth of lower buds and that growth accelerates once ABA levels decline. This hypothesis was supported by the architectural analysis of the ABA-deficient mutants aba2-1 and nced3-2, discussed above, and by the effect of exogenous ABA on branch development, discussed below. An alternative hypothesis is that growth of the bud simply dilutes an existing static pool of ABA, accounting for the lower abundance in the larger upper buds and buds beginning to elongate. ABA accumulation has been shown to be rapidly modulated by the R:FR (Reddy et al., 2013) and low R:FR promoted accumulation in buds of equivalent sizes (Fig. 2). Thus, plants have the capacity to specifically alter bud ABA abundance in response to environmental cues and potentially also in response to developmental signals controlling bud growth.
Exogenous ABA applied directly to the buds partially inhibited elongation, and the strength of the effect was dependent on the amount of ABA used and the genotype. The upper buds were generally less responsive to ABA than those from lower positions. It is possible that the upper buds were intrinsically less responsive to ABA or that they had achieved a more advanced stage of development that rendered them less sensitive. Similarly, buds of brc1-2 and max2-336 appeared less sensitive to ABA than those of the corresponding wild-type controls. This reduced sensitivity could reflect an overall advancement in the developmental programming of the mutant buds, could result from a mutation-dependent reduction in ABA sensitivity, or may simply have stemmed from their lower levels of endogenous ABA. Given that the buds of brc1-2 and max2-336 show very rapid growth, it seems possible that their precocious developmental programming may be the source of the reduced sensitivity to ABA.
ABA Acts Downstream or Independently of BRC1
The TCP domain protein BRC1 (and its homolog in grasses, TB1) has been termed a branching integrator because it acts downstream of many other pathways that influence branching, including auxin (Finlayson, 2007; Chen et al., 2013), the strigolactone pathway (Aguilar-Martínez et al., 2007; Finlayson, 2007; Braun et al., 2012; Dun et al., 2012), cytokinins (Braun et al., 2012; Dun et al., 2012), decapitation (Aguilar-Martínez et al., 2007; Martin-Trillo et al., 2011; Chen et al., 2013), the R:FR (Kebrom et al., 2006, 2010; Finlayson et al., 2010; Su et al., 2011; González-Grandío et al., 2013), planting density (Takeda et al., 2003; Kebrom et al., 2006; Aguilar-Martínez et al., 2007; Chen et al., 2013), and sugar signals (Mason et al., 2014). Integration of these signaling pathways occurs at least in part by altering the expression of the BRC1/TB1 gene. For instance, the auxin-signaling-deficient axr1-12 mutant had reduced BRC1 expression in lower buds (Finlayson, 2007), auxin supplied to excised Dendranthema grandiflorum nodes increased the expression of the BRC1 homolog DgBRC1 in buds (Chen et al., 2013), and loss of BRC1 function conferred hyperbranching to hypobranching auxin-overproducing YUCCA lines (Aguilar-Martínez et al., 2007; Finlayson, 2007). The inhibitory effects of ABA, however, do not appear to be integrated by BRC1 because ABA inhibited bud elongation of the BRC1-deficient mutant, and BRC1 mRNA abundance was not altered in buds of the ABA-deficient nced3-2 mutant, nor in wild-type buds supplemented with ABA. Although it is possible that ABA affects branching independently of BRC1, BRC1 promoted the accumulation of ABA in lower buds, and thus ABA more likely acts downstream of BRC1 to transduce some of the effects of BRC1 function. The BRC1-dependent expression of ABA-related genes reported previously (González-Grandío et al., 2013) may have resulted from BRC1 promotion of ABA accumulation and subsequent signaling, though it is possible that BRC1 could also influence ABA signaling independently of its effects on ABA accumulation.
ABA Acts Downstream or Independently of Signaling by the MAX Pathway and the Main Shoot PATS But May Influence Bud Growth by Bud-Autonomous Effects on Auxin Biosynthesis and Transport
The data suggested that ABA may act downstream of BRC1, and it is therefore logical that it should also function downstream of main shoot PATS and MAX signaling, because these pathways are integrated by BRC1 (Aguilar-Martínez et al., 2007; Finlayson, 2007). The experimental evidence supported this contention, as ABA levels were suppressed in lower buds of axr1-12 and max2-336, and exogenous ABA inhibited the elongation of the branches of these mutants. Although it is possible that ABA acts independently of these pathways, the fact that ABA levels are misregulated in lower buds of axr1-12 and max2-336 suggests it may be more likely to function downstream.
Interactions between ABA and strigolactones and/or strigolactone-signaling components have previously been noted. Studies on tomato ABA biosynthesis mutants and seedlings provided ABA biosynthesis inhibitors suggested that ABA may promote the accumulation of several strigolactones in roots (Lopez-Raez et al., 2010), and application of exogenous ABA to soybean (Glycine max) elevated the expression of several putative strigolactone biosynthetic genes (Wang et al., 2013). While exogenous strigolactone application was reported to inhibit bud outgrowth in Oryza sativa (Minakuchi et al., 2010) and Arabidopsis (Crawford et al., 2010), the majority of such studies show that exogenous (and thus elevated) strigolactones generally do not affect branching in intact wild-type Arabidopsis (Gomez-Roldan et al., 2008; Nelson et al., 2011), O. sativa (Umehara et al., 2008), petunia (Petunia hybrida; Hamiaux et al., 2012; Kretzschmar et al., 2012), pea (Braun et al., 2012), tomato (Koltai et al., 2010), and maize (Zea mays; Guan et al., 2012). It is possible that ABA effects on branching could result from elevation of strigolactone levels, but current evidence suggests that supplemental strigolactone has little effect on the process, making this seem unlikely.
The application of the synthetic strigolactone GR24 to seeds of the parasitic plant Phelipanche ramosa resulted in elevated expression of a gene encoding an ABA catabolic enzyme and decreased accumulation of ABA (Lechat et al., 2012). Similar results were obtained in thermoinhibited Arabidopsis seeds and in Striga hermonthica (Toh et al., 2012). In this study, analyses of ABA in buds demonstrated equivalent levels in upper buds of the wild type and max2 but reduced levels in lower buds of max2. It appears that MAX2 status does not exert a global effect on ABA accumulation but rather modulates accumulation in buds that exhibit a more plastic developmental fate.
The potential role of strigolactones or MAX2 signaling in modulating ABA responsiveness appears complex. Loss of MAX2 (Shen et al., 2012; Bu et al., 2014) or MAX2, MAX3, or MAX4 (Ha et al., 2014) function was reported to either increase (Shen et al., 2012; Bu et al., 2014) or decrease (Ha et al., 2014) the inhibitory effects of ABA on seed germination and early growth/development. Additionally, Bu et al. (2014) concluded that strigolactones per se were not involved in altering ABA responsiveness because loss of the biosynthetic function of MAX1, MAX3, and MAX4 had no effect. In this study, the branching inhibition of max2 was similar to that of the wild type at low ABA levels but was possibly reduced at higher levels. Because seed germination and branching are rather disparate processes, direct comparisons may not be warranted.
The inhibitory effects of ABA were most apparent in the axr1-12 mutant, which could indicate an interaction between the ABA and auxin pathways. The hyperbranching phenotype of axr1-12 has been attributed to defects in AXR1-dependent auxin signaling arising from the PATS that normally inhibits bud growth indirectly (Booker et al., 2003). While hyperbranching eventually becomes obvious in this mutant (Lincoln et al., 1990; Finlayson et al., 2010), it is also obvious that the plant exhibits a general dwarfing phenotype, and bud growth is initially quite slow. The rather sluggish bud growth may be a secondary manifestation of impaired auxin signaling within the bud/branch, because sufficient auxin signaling is necessary for normal shoot growth. The deficiency in bud-autonomous auxin signaling may counter some of the promotive effects of decreased ABA accumulation in lower buds of these plants. ABA was found to influence the bud-autonomous auxin pathway by decreasing the expression of the IAA biosynthesis gene TAA1 and the auxin efflux transporter gene PIN1. Additionally, bud IAA accumulation was significantly suppressed by exogenous ABA. The relatively strong repression of axr1-12 branching by ABA may therefore result from suppression of bud IAA accumulation (and potentially transport) compounded with its impaired auxin responsiveness.
A broader role for ABA regulation of auxin homeostasis may be indicated because ABA signaling mediated by ABSCISIC ACID INSENSITIVE4 (ABI4) has also been shown to suppress PIN1 protein accumulation and auxin transport in Arabidopsis roots, thus inhibiting lateral root formation (Shkolnik-Inbar and Bar-Zvi, 2010). Similarly, ABI5 was shown to repress PIN1 accumulation and auxin activity in Arabidopsis root tips in response to Glc (Yuan et al., 2014). Because ABI5 is also a component of the ABA-signaling pathway, it is possible that ABA effects are partially mediated by ABI5. These results are in general agreement with a previous study that proposed a role for ABA in inhibiting auxin transport in Arabidopsis stem segments (Chatfield et al., 2000). Conversely, ABA promoted AXR1 and PIN2 expression in Arabidopsis root tips, which was associated with the maintenance of primary root elongation under low water potential (Xu et al., 2013). The effect of ABA on auxin transport may therefore be complex, with differential effects on specific tissues and transport components. Bud-autonomous PIN1 protein function appears to regulate bud outgrowth in pea (Balla et al., 2002), and TAA1 expression has previously been associated with the process in Arabidopsis (Finlayson et al., 2010; Su et al., 2011). Although direct evidence for a role of ABA in regulating bud IAA transport remains to be demonstrated, this study clearly shows that ABA suppresses bud IAA accumulation, supporting the hypothesis that ABA represses branching, in part, by its effects on auxin homeostasis.
ABA Was Not Found to Strongly Influence Sugar or Cytokinin Pathways within Axillary Buds
Additional pathways were tested to assess the potential mechanisms of ABA action on branching. In decapitated pea, the remobilization of sugars has been shown to modulate rapid bud outgrowth responses to decapitation (Mason et al., 2014). While it has been established that sugar (Glc) can inhibit seed germination and seedling growth by promoting ABA biosynthesis and signaling (León and Sheen, 2003), these data contrast with the expected antagonistic effects of sugar and ABA on bud development. Additionally, because preliminary gene expression analyses did not reveal any changes in the expression of several sugar-responsive genes in buds with exogenous ABA application, this pathway may not be involved in modulating Arabidopsis bud responses to ABA. This scenario is consistent with the apparent function of BRC1 downstream of sugar signals (Mason et al., 2014), because ABA itself may function downstream of BRC1. A more thorough investigation is needed to definitively resolve the relationship between ABA and sugar signals in the regulation of branching.
Cytokinins are recognized as important regulators of bud function, and thus cytokinin-regulated pathways could potentially be targets of ABA action on bud growth. However, the expression patterns of cytokinin-responsive genes did not provide strong support for ABA modulation of cytokinin response. Previous studies have shown that the relationship between ABA and cytokinins is complex. ABA suppressed cytokinin accumulation in roots and shoots of wheat (Triticum aestivum) and stimulated cytokinin oxidase activity, which presumably contributed to the decline in cytokinins (Vysotskaya et al., 2009). On the other hand, there is additional evidence that cytokinins may regulate ABA levels and response. In Arabidopsis, endogenous cytokinins promoted ABA accumulation but suppressed ABA response (Nishiyama et al., 2011), and the cytokinin receptors HIS KINASE2, HIS KINASE3, and CYTOKININ RESPONSE1 were shown to negatively regulate ABA response in Arabidopsis germination assays (Tran et al., 2007). Another study demonstrated that application of the cytokinin 6-benzylaminopurine transiently increased Arabidopsis shoot ABA levels (Žd’árská et al., 2013), while overexpression of the cytokinin biosynthesis enzyme isopentenyl transferase resulted in reduced ABA accumulation in petunia flowers (Chang et al., 2003). Overall, the existing evidence may suggest that it is more probable that some of the effects of cytokinins are transduced by ABA rather than vice versa.
ABA Suppresses the Expression of the Cell Cycle Genes PCNA1 and CYCA2;1
The expression of CYCA2;1 and PCNA1 was suppressed by ABA, and PCNA1 expression was elevated in nced3-2. Previous studies showed that PCNA expression was up-regulated in buds of pea within 4 h following a decapitation treatment that promoted bud outgrowth (Shimizu and Mori, 1998) and was down-regulated in sorghum (Sorghum bicolor) axillary buds suppressed by low R:FR or defoliation (Kebrom et al., 2010). ABA had little effect on PCNA protein accumulation in maize seeds (Herrera et al., 2000), suggesting that its effects on expression may depend on the type or developmental stage of the tissue. PCNA1 is a multifunctional protein that is involved in DNA repair and also acts as a processivity factor during DNA replication (Strzalka and Ziemienowicz, 2011). Human PCNA1 can interact with CYCLIN-DEPENDENT KINASE2-cyclin A and recruit the associated phosphorylation activity to DNA replication proteins (Koundrioukoff et al., 2000) and thus may represent a regulatory mechanism promoting the cell cycle, beyond its role as a necessary component in DNA replication required for mitosis. Repression of PCNA1 and CYCA2;1 expression represents a potential mechanism by which ABA may act to suppress the bud’s cell cycle to inhibit outgrowth.
CONCLUSION
The data support a general role for ABA in restricting Arabidopsis branching. Given the quantity of reports that have previously implicated ABA as a branching repressor in other species, it is possible that future studies will demonstrate that it functions similarly in many, if not all, higher plants. ABA appears to act downstream of the main shoot PATS, the MAX pathway, and the branching integrator BRC1 and thus is likely to contribute to the repressive effects of these other components. In fact, ABA increased correlative inhibition, a process associated with auxin that coordinates growth among branches, indicating that the effects of auxin are partly dependent on ABA function. If ABA also transduces some of the functions of BRC1, as the evidence suggests, then it would occupy a far-downstream position in both the second messenger and PATS competition models described earlier, because both models incorporate BRC1 as a bud-autonomous integrator of bud development.
The full extent of the influence of ABA on bud development remains unknown. The ABA biosynthetic mutants employed in this study are not totally ABA deficient, and therefore the residual bud ABA is likely to provide some level of growth repression. Furthermore, ABA-deficient plants show generally weaker growth compared with the wild type, and anecdotal observations suggest that weakened overall growth is associated with a substantial decrease in branching. It may be necessary to use advanced techniques that abrogate ABA effects specifically and more completely in the axillary bud to develop a clearer picture of the role of ABA. The plants used in this study were grown under long days, and therefore the buds transitioned very early to the floral program. Whether ABA influences branching under short days, where the buds remain vegetative for a much longer duration, is unknown but should be tested. Finally, while this study indicated that ABA affects auxin homeostasis/transport and the cell cycle machinery in the bud, a complete understanding of the pathways involved awaits discovery.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The ecotype Columbia (Col-0) of Arabidopsis (Arabidopsis thaliana) was used throughout. Wild-type Col-0 (CS60000), aba2-1, and axr1-12 seed was obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Other mutants, including brc1-2 (Aguilar-Martínez et al., 2007), max2-336 (Finlayson, 2007), and nced3-2 (Urano et al., 2009), have previously been described.
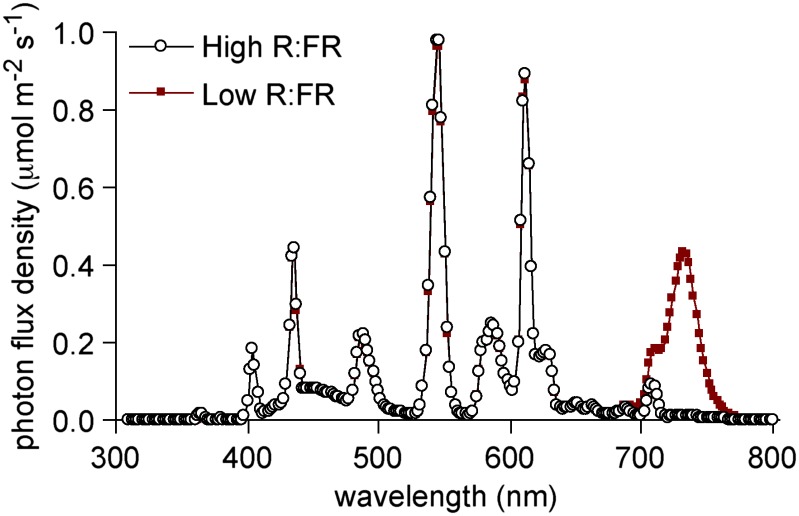
Seeds were stratified for 3 d at 4°C and sown in six-cell inserts filled with Sunshine LC1 potting mixture. Each cell was thinned to contain one plant and was fertilized weekly with 7.2 mL of 1× Hoagland’s solution. Plants were grown under 18-h-light/6-h-dark photoperiods with 24°C/18°C day/night temperatures in a growth chamber providing 180 μmol m–2 s–1 photosynthetic photon flux density with a R:FR of 2.84. Light was provided using T5 fluorescent lamps (6,400 K) and was supplemented with FR from an overhead array of 735-nm light-emitting diodes (L735-01AU, Epitex) mounted in a clear acrylic sheet producing a R:FR of 0.08 for some experiments. Light was measured with a Li-1800 spectroradiometer (Licor Biosciences). The R:FR was calculated as the quantum flux density from 654 to 666 nm divided by the quantum flux density from 724 to 736 nm. The spectra of the light sources are provided in Figure 14.
Figure 14.
Spectra of the light sources used for plant growth.
Branch Elongation Analyses
Architectural characteristics and branch elongation of wild-type Col-0 and ABA-deficient mutants were measured at 10 DPA as described in Finlayson et al. (2010), except that the correlative inhibition index was calculated for each record individually. The frequency of bud outgrowth was calculated as the frequency of buds growing to a size greater than 3 mm at a specific rosette position. Means for each treatment were calculated by pooling the results of three experiments with a total of 54 replicates.
Experiments assessing the effect of exogenous ABA application to distal bud tissues on bud outgrowth used 100 (wild type, axr1-12, and brc1-2) or 200 (wild type and max2-336) pmol of (±) ABA in 1% (v/v) ethanol with 0.03% (v/v) Silwet applied to each of the top five buds in a volume of 1 μL, giving 50 or 100 pmol of the active (+) isomer. ABA was applied beginning 1 d before predicted anthesis every 24 h for 5 d, at which time architectural analyses were made as described above. An identical solution without ABA was applied to an equal number of plants as controls. Twenty-three to 24 replicates (wild type, axr1-12, and brc1-2), or 27 replicates (wild type and max2-336), were used for each experiment.
To assess the effect of exogenous ABA application to the bud primary shoot apical meristem on bud n-2 outgrowth, 10 pmol of (±) ABA in 1% (v/v) ethanol with 0.03% (v/v) Silwet was applied to the bud primary shoot apical meristem in a volume of 100 nL, giving 5 pmol of the active (+) isomer. Flowers and leaves obscuring the shoot apical meristem were gently displaced (without damaging) using a probe under a dissecting microscope, and ABA was applied with a microliter syringe fitted with a fused silica needle, beginning at anthesis every 24 h for 5 d (for the wild type, brc1-2, and max2-336) or 7 d (for axr1-12 to allow for sufficient branch elongation in this retarded genotype), at which time branch lengths were determined. An identical solution without ABA was applied to an equal number of plants as controls. Nine replicates were employed for each genotype/treatment combination.
Analysis of ABA Abundance
ABA was extracted and quantified using isotope dilution selected ion monitoring gas chromatography-mass spectroscopy as described in Reddy et al. (2013), except that the pH of the solvent partitioning steps was adjusted to 8.0 and 6.0 and the mass spectrometer was operated in negative chemical ionization mode with methane as the reagent gas, monitoring mass-to-charge ratio 260.1, 266.1, 278.1, and 284.1 and using 278.1 and 284.1 for quantification. Buds were harvested just prior to the predicted onset of elongation of bud n and for the comparison of unelongated and elongated wild-type bud n-2, when bud n-2 had elongated no more than 4 mm. Four biological replicates (of 10–12 buds) were measured for each data point, except for the comparison of unelongated and elongated wild-type bud n-2, where the results of two experiments were pooled for a total of seven replicates (buds from approximately 70 plants in total).
Experiments assessing the effect of exogenous ABA application to distal bud tissues on bud ABA content used 100 pmol of (±) ABA in 1% (v/v) ethanol with 0.03% (v/v) Silwet applied to bud n-2 in a volume of 1 μL, giving 50 pmol of the active (+) isomer. ABA was applied at about 3 d after anthesis, and buds were harvested 2, 12, and 24 h later. An identical solution without ABA was applied to an equal number of plants as controls. Four biological replicates, each composed of 12 to 15 buds, were measured.
The distribution of ABA accumulation within various tissues of wild-type bud n-2 was determined at the time of the onset of elongation (approximately 2–3 d after anthesis). Buds were dissected into subterminal stem sections (stem) approximately 1.5 to 2 mm long, the youngest two to three leaves (leaves), young flowers, excluding the oldest three flowers and the smallest flowers and primordia (flowers), the shoot apex, including less than 1 mm of stem, the shoot apical meristem, and youngest flowers and flower primordia (1° apex), and intact secondary buds (2° buds). Four replicates of 21 dissected buds were used for each measurement.
Experiments assessing the effect of exogenous ABA application on bud primary shoot apex section ABA content used 10 pmol of (±) ABA in 1% (v/v) ethanol with 0.03% (v/v) Silwet. Flowers and leaves obscuring the shoot apical meristem were gently displaced with a probe under a dissecting microscope, and ABA was applied to bud n-2 primary shoot apical meristems in a volume of 100 nL, giving 5 pmol of the active (+) isomer. ABA was provided 4 d after anthesis, and buds were dissected and harvested 24 h later. An identical solution without ABA was applied to an equal number of plants as controls. Four biological replicates, each composed of 18 apex sections, were measured.
Analysis of IAA Abundance
IAA was extracted and quantified using isotope dilution selected ion monitoring gas chromatography-mass spectroscopy as described in Reddy et al. (2013), except that the pH of the solvent partitioning steps was adjusted to 8.0 and 6.0. Experiments assessing the effect of exogenous ABA application to distal bud tissues on bud IAA content are described above and employed four biological replicates, each composed of 12 to 15 buds. Experiments assessing the effect of exogenous ABA application on bud primary shoot apex section IAA content are described above and employed four biological replicates, each composed of 18 apex sections.
Analysis of Gene Expression
Buds of the wild type with and without exogenous ABA application and nced3-2 were used for the analysis of gene expression, just prior to the predicted onset of outgrowth of bud n. ABA treatment was applied as 100 pmol of (±) ABA in 1% (v/v) ethanol with 0.03% (v/v) Silwet to each of the top three buds of the wild type in a volume of 1 μL, giving 50 pmol of the active (+) isomer. Control plants received the same solution without the ABA. The treatment was started at 2 h after dawn, and buds were harvested 3 h later. The uppermost (n) and third from uppermost (n-2) buds were collected separately in liquid N2. Total RNA was extracted, and gene expression was measured by quantitative PCR (qPCR) using the methods of Su et al. (2011). Three (bud n) and four (bud n-2) biological replicates of 10 to 12 buds were measured for each genotype/treatment combination. Primers for BRC1 and GH3.5 were taken from Aguilar-Martínez et al. (2007) and Effendi et al. (2011), respectively. Primers for ARR5, CYCD2;1, CYCD3;1, PCNA1, and TAA1 are given in Finlayson et al. (2010). Primer sequences for IAA2 and IAA3 are given in Reddy and Finlayson (2014). Primer sequences for ARR6 and HIS1-3 are provided in Su et al. (2011). The sequences of other primers used are provided in Table II.
Table II. Sequence of primers used for qPCR.
| Target | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| 18S (At3g41768) | AAACGGCTACCACATCCAAG | ACTCGAAAGAGCCCGGTATT |
| BETA-AMYLASE3 (At5g18670) | TGATGGGAAGACTCCTATGGAGGT | GCATGTGTTTGTCGTAACACTGGA |
| CYCA2;1 (At5g25380) | TCGACCAATCTAACCATCCTTGG | TGTGTTCACACGTTCAGGAGATGT |
| PEROXIDASE4 (At1g14540) | AAGAGATTCCACCAACGCGTTT | CATTGAGCTTGCCCTAATGTGTGA |
| PIN1 (At1g73590) | TCATGCTCGTTGCTTCTTATGCC | GCGATCAACATCCCAAATATCACC |
| VI2 (At1g12240) | TAGCGTCGTACCGGTTCTAAAAGG | TGCTCCATAGATTGCAGTTGTTGG |
The expression patterns of sugar-responsive (β-AMYLASE3, PEROXIDASE4, and VACUOLAR INVERTASE2) and some cell cycle-regulating (CYCD2;1 and CYCD3;1) genes were surveyed with cDNA pooled from the experimental replicates described above. Equal volumes of each of the sample replicates were pooled and three technical replicates were measured by qPCR without biological replication.
Statistics
Comparisons between means were made using a two-tailed Student’s t test with α < 0.05 or ANOVA followed by Tukey’s honestly significant difference mean-separation test with α < 0.05. Comparisons between frequencies were made using a two-tailed Fisher Exact Probability test with α < 0.05.
Glossary
- ABA
abscisic acid
- R
red light
- FR
far-red light
- IAA
indole-3-acetic acid
- PATS
polar auxin transport stream
- phyB
phytochrome B
- cDNA
complementary DNA
- Col-0
ecotype Columbia
- qPCR
quantitative PCR
Footnotes
This work was supported by Texas A&M AgriLife Research (to S.A.F.).
Articles can be viewed without a subscription.
References
- Abe S, Sado A, Tanaka K, Kisugi T, Asami K, Ota S, Kim HI, Yoneyama K, Xie X, Ohnishi T, et al. (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Natl Acad Sci USA 111: 18084–18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend M, Schnitzler JP, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J (2009) Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiol 151: 2110–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arney SE, Mitchell DL (1969) The effect of abscisic acid on stem elongation and correlative inhibition. New Phytol 68: 1001–1015 [Google Scholar]
- Balla J, Blažková J, Reinöhl V, Procházka S (2002) Involvement of auxin and cytokinins in initiation of growth of isolated pea buds. Plant Growth Regul 38: 149–156 [Google Scholar]
- Ballard LAT, Wildman SG (1964) Induction of mitosis by sucrose in excised and attached dormant buds of sunflower (Helianthus annuus L). Aust J Biol Sci 17: 36–43 [Google Scholar]
- Ballaré CL. (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Bennett T, Leyser O (2006) Something on the side: axillary meristems and plant development. Plant Mol Biol 60: 843–854 [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe A, Beck DL (2008) Yield components of biomass in switchgrass. Crop Sci 48: 1306–1311 [Google Scholar]
- Bonser SP, Aarssen LW (2003) Allometry and development in herbaceous plants: functional responses of meristem allocation to light and nutrient availability. Am J Bot 90: 404–412 [DOI] [PubMed] [Google Scholar]
- Booker J, Chatfield S, Leyser O (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Le Signor C, Bouteiller N, et al. (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q, Lv T, Shen H, Luong P, Wang J, Wang Z, Huang Z, Xiao L, Engineer C, Kim TH, et al. (2014) Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol 164: 424–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2012) Shade avoidance. The Arabidopsis Book 10: e0157, doi/10.1199/tab.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Jones ML, Banowetz GM, Clark DG (2003) Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol 132: 2174–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24: 159–169 [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou X, Xi L, Li J, Zhao R, Ma N, Zhao L (2013) Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema ×grandiflora cv. Jinba). PLoS One 8: e61717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG, Oh C (2006) A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot (Lond) 98: 891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29: 255–265 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2013) Dynamics of strigolactone function and shoot branching responses in Pisum sativum. Mol Plant 6: 128–140 [DOI] [PubMed] [Google Scholar]
- Effendi Y, Rietz S, Fischer U, Scherer GFE (2011) The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J 65: 282–294 [DOI] [PubMed] [Google Scholar]
- Emery RJN, Longnecker NE, Atkins CA (1998) Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J Exp Bot 49: 555–562 [Google Scholar]
- Finlayson SA. (2007) Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol 48: 667–677 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152: 1914–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96: 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García del Moral MB, García del Moral LF (1995) Tiller production and survival in relation to grain yield in winter and spring barley. Field Crops Res 44: 85–93 [Google Scholar]
- Girault T, Abidi F, Sigogne M, Pelleschi-Travier S, Boumaza R, Sakr S, Leduc N (2010) Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ 33: 1339–1350 [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol 95: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P (2013) BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al. (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95: 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JC, Koch KE, Suzuki M, Wu S, Latshaw S, Petruff T, Goulet C, Klee HJ, McCarty DR (2012) Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol 160: 1303–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CV, Leyva-Gonzalez MA, Osaka Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV, Yamaguchi-Shinozaki K, et al. (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111: 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC (2012) DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22: 2032–2036 [DOI] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ (1994) Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192: 276–286 [Google Scholar]
- Henry C, Rabot A, Laloi M, Mortreau E, Sigogne M, Leduc N, Lemoine R, Sakr S, Vian A, Pelleschi-Travier S (2011) Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ 34: 1776–1789 [DOI] [PubMed] [Google Scholar]
- Herrera I, de la Paz Sanchez M, Molina J, Plasencia J, Vazquez-Ramos JM (2000) Proliferating cell nuclear antigen expression in maize seed development and germination: regulation by phytohormones and its association with putative cell cycle proteins. Physiol Plant 110: 127–134 [Google Scholar]
- Janssen BJ, Drummond RSM, Snowden KC (2014) Regulation of axillary shoot development. Curr Opin Plant Biol 17: 28–35 [DOI] [PubMed] [Google Scholar]
- Jouve L, Gaspar T, Kevers C, Greppin H, Degli Agosti R (1999) Involvement of indole-3-acetic acid in the circadian growth of the first internode of Arabidopsis. Planta 209: 136–142 [DOI] [PubMed] [Google Scholar]
- Juenger T, Bergelson J (2000) The evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution 54: 764–777 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ 33: 48–58 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140: 1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W (2012) Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol 160: 308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Mullet JE (2015) Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ 38: 1471–1478 [DOI] [PubMed] [Google Scholar]
- Knox JP, Wareing PF (1984) Apical dominance in Phaseolus vulgaris L.: the possible roles of abscisic acid and indole-3-acetic acid. J Exp Bot 35: 239–244 [Google Scholar]
- Koltai H, LekKala SP, Bhattacharya C, Mayzlish-Gati E, Resnick N, Wininger S, Dor E, Yoneyama K, Yoneyama K, Hershenhorn J, et al. (2010) A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J Exp Bot 61: 1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundrioukoff S, Jónsson ZO, Hasan S, de Jong RN, van der Vliet PC, Hottiger MO, Hübscher U (2000) A direct interaction between proliferating cell nuclear antigen (PCNA) and Cdk2 targets PCNA-interacting proteins for phosphorylation. J Biol Chem 275: 22882–22887 [DOI] [PubMed] [Google Scholar]
- Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483: 341–344 [DOI] [PubMed] [Google Scholar]
- Le Bris M, Michaux-Ferriere N, Jacob Y, Poupet A, Barthe P, Guigonis JM, Le Page-Degivry MT (1999) Regulation of bud dormancy by manipulation of ABA in isolated buds of Rosa hybrida cultured in vitro. Aust J Plant Physiol 26: 273–281 [Google Scholar]
- Lechat MM, Pouvreau JB, Péron T, Gauthier M, Montiel G, Véronési C, Todoroki Y, Le Bizec B, Monteau F, Machere D, et al. (2012) PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J Exp Bot 63: 695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Raez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TDH, Thompson AJ, Ruyter-Spira C, et al. (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187: 343–354 [DOI] [PubMed] [Google Scholar]
- Lortie CJ, Aarssen LW (2000) Fitness consequences of branching in Verbascum thapsus (Scrophulariaceae). Am J Bot 87: 1793–1796 [PubMed] [Google Scholar]
- Mader JC, Emery RJN, Turnbull CGN (2003) Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol Plant 119: 295–308 [Google Scholar]
- Martin-Trillo M, Gonzalez Grandio E, Serra F, Marcel F, Rodriguez-Buey ML, Schmitz G, Theres K, Bendahmane A, Dopazo H, Cubas P (2011) Role of tomato BRANCHED1-like genes in the control of shoot branching. Plant J 67: 701–714 [DOI] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111: 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ (1989) Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell 1: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, Umehara M, Luo L, Kobayashi K, Hanada A, Ueno K, Asami T, Yamaguchi S, et al. (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol 51: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Scaffidi A, Dun EA, Waters MT, Flematti GR, Dixon KW, Beveridge CA, Ghisalberti EL, Smith SM (2011) F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 108: 8897–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, et al. (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Vicentini R, Silva GFF, Silva EM, Carrer H, Rodrigues AP, Nogueira FTS (2013) Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. J Exp Bot 64: 2307–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Khush GS, Cassman KG (1994) Evolution of the new plant ideotype for increased yield potential. In KG Cassman, ed, Breaking the Yield Barrier: Proceedings of a Workshop on Rice Yield Potential in Favorable Environments. International Rice Research Institute, Los Banos, Philippines, pp 5–20 [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot A, Henry C, Ben Baaziz K, Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S, et al. (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53: 1068–1082 [DOI] [PubMed] [Google Scholar]
- Reddy SK, Finlayson SA (2014) Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol 164: 1542–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA (2013) Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol 163: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA (2014) The timing of low R:FR exposure profoundly affects Arabidopsis branching responses. Plant Signal Behav 9: e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann K (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54: 136–144 [Google Scholar]
- Shen H, Zhu L, Bu Q-Y, Huq E (2012) MAX2 affects multiple hormones to promote photomorphogenesis. Mol Plant 5: 750–762 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Mori H (1998) Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant Cell Physiol 39: 255–262 [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22: 3560–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1995) Physiological and ecological function within the phytochrome family. Ann Rev Plant Physiol Mol Biol 46: 289–315 [Google Scholar]
- Stirnberg P, Liu JP, Ward S, Kendall SL, Leyser O (2012) Mutation of the cytosolic ribosomal protein-encoding RPS10B gene affects shoot meristematic function in Arabidopsis. BMC Plant Biol 12: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalka W, Ziemienowicz A (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot (Lond) 107: 1127–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Abernathy SD, White RH, Finlayson SA (2011) Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ 34: 1986–1998 [DOI] [PubMed] [Google Scholar]
- Takeda T, Suwa Y, Suzuki M, Kitano H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M, Ueguchi C (2003) The OsTB1 gene negatively regulates lateral branching in rice. Plant J 33: 513–520 [DOI] [PubMed] [Google Scholar]
- Tamas IA, Ozbun JL, Wallace DH, Powell LE, Engels CJ (1979) Effect of fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol 64: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- To JPC, Haberer G, Ferreira FJ, Deruère J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16: 658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tscuchiya Y (2012) Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol 53: 107–117 [DOI] [PubMed] [Google Scholar]
- Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104: 20623–20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DJ. (1977) Effects of far-red light on lateral bud outgrowth in decapitated tomato plants and associated changes in levels of auxin and abscisic acid. Plant Sci Lett 8: 339–344 [Google Scholar]
- Tucker DJ, Mansfield TA (1971) Effects of light quality on apical dominance in Xanthium strumarium and the associated changes in endogenous levels of abscisic acid and cytokinins. Planta 102: 140–151 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, et al. (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57: 1065–1078 [DOI] [PubMed] [Google Scholar]
- Vysotskaya LB, Korobova AV, Veselov SY, Dodd IC, Kudoyarova GR (2009) ABA mediation of shoot cytokinin oxidase activity: assessing its impacts on cytokinin status and biomass allocation of nutrient-deprived durum wheat. Funct Plant Biol 36: 66–72 [DOI] [PubMed] [Google Scholar]
- Waldie T, Hayward A, Beveridge CA (2010) Axillary bud outgrowth in herbaceous shoots: How do strigolactones fit into the picture? Plant Mol Biol 73: 27–36 [DOI] [PubMed] [Google Scholar]
- Wang RK, Wang CE, Fei YY, Gai JY, Zhao TJ (2013) Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol Biol Rep 40: 4737–4745 [DOI] [PubMed] [Google Scholar]
- Weinig C, Stinchcombe JR, Schmitt J (2003) Evolutionary genetics of resistance and tolerance to natural herbivory in Arabidopsis thaliana. Evolution 57: 1270–1280 [DOI] [PubMed] [Google Scholar]
- Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J (2013) Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol 197: 139–150 [DOI] [PubMed] [Google Scholar]
- Yuan TT, Xu HH, Zhang KX, Guo TT, Lu YT (2014) Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ 37: 1338–1350 [DOI] [PubMed] [Google Scholar]
- Zarrough KM, Nelson CJ, Coutts JH (1983) Relationship between tillering and forage yield of tall fescue. I. Yield. Crop Sci 23: 333–337 [Google Scholar]
- Žd’árská M, Zatloukalová P, Benítez M, Šedo O, Potěšil D, Novák O, Svačinová J, Pešek B, Malbeck J, Vašíčková J, et al. (2013) Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiol 161: 918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DL, Atlin GN, Bastiaans L, Spiertz JHJ (2006) Developing selection protocols for weed competitiveness in aerobic rice. Field Crops Res 97: 272–285 [Google Scholar]