Physiological and morphological responses to increase the mobilization and uptake of nutrients from the soil are subject to regulation by ethylene.
Abstract
To cope with nutrient deficiencies, plants develop both morphological and physiological responses. The regulation of these responses is not totally understood, but some hormones and signaling substances have been implicated. It was suggested several years ago that ethylene participates in the regulation of responses to iron and phosphorous deficiency. More recently, its role has been extended to other deficiencies, such as potassium, sulfur, and others. The role of ethylene in so many deficiencies suggests that, to confer specificity to the different responses, it should act through different transduction pathways and/or in conjunction with other signals. In this update, the data supporting a role for ethylene in the regulation of responses to different nutrient deficiencies will be reviewed. In addition, the results suggesting the action of ethylene through different transduction pathways and its interaction with other hormones and signaling substances will be discussed.
When plants suffer from a mineral nutrient deficiency, they develop morphological and physiological responses (mainly in their roots) aimed to facilitate the uptake and mobilization of the limiting nutrient. After the nutrient has been acquired in enough quantity, these responses need to be switched off to avoid toxicity and conserve energy. In recent years, different plant hormones (e.g. ethylene, auxin, cytokinins, jasmonic acid, abscisic acid, brassinosteroids, GAs, and strigolactones) have been implicated in the regulation of these responses (Romera et al., 2007, 2011, 2015; Liu et al., 2009; Rubio et al., 2009; Kapulnik et al., 2011; Kiba et al., 2011; Iqbal et al., 2013; Zhang et al., 2014).
Before the 1990s, there were several publications relating ethylene and nutrient deficiencies (cited in Lynch and Brown [1997] and Romera et al. [1999]) without establishing a direct implication of ethylene in the regulation of nutrient deficiency responses. In 1994, Romera and Alcántara (1994) published an article in Plant Physiology suggesting a role for ethylene in the regulation of Fe deficiency responses. In 1999, Borch et al. (1999) showed the participation of ethylene in the regulation of P deficiency responses. Since then, evidence has been accumulating in support of a role for ethylene in the regulation of both Fe (Romera et al., 1999, 2015; Waters and Blevins, 2000; Lucena et al., 2006; Waters et al., 2007; García et al., 2010, 2011, 2013, 2014; Yang et al., 2014) and P deficiency responses (Kim et al., 2008; Lei et al., 2011; Li et al., 2011; Nagarajan and Smith, 2012; Wang et al., 2012, 2014c). Both Fe and P may be poorly available in most soils, and plants develop similar responses under their deficiencies (Romera and Alcántara, 2004; Zhang et al., 2014). More recently, a role for ethylene has been extended to other deficiencies, such as K (Shin and Schachtman, 2004; Jung et al., 2009; Kim et al., 2012), S (Maruyama-Nakashita et al., 2006; Wawrzyńska et al., 2010; Moniuszko et al., 2013), and B (Martín-Rejano et al., 2011). Ethylene has also been implicated in both N deficiency and excess (Tian et al., 2009; Mohd-Radzman et al., 2013; Zheng et al., 2013), and its participation in Mg deficiency has been suggested (Hermans et al., 2010).
In this update, we will review the information supporting a role for ethylene in the regulation of different nutrient deficiency responses. For information relating ethylene to other aspects of plant mineral nutrition, such as N2 fixation and responses to excess of nitrate or essential heavy metals, the reader is referred to other reviews (for review, see Maksymiec, 2007; Mohd-Radzman et al., 2013; Steffens, 2014).
ETHYLENE SYNTHESIS AND SIGNALING UNDER NUTRIENT DEFICIENCIES
Nutrient deficiencies can influence both ethylene synthesis and signaling. In general, ethylene production increases under different nutrient deficiencies. Additionally, ethylene production can increase upon excess of some nutrients, like nitrate (Tian et al., 2009; Mohd-Radzman et al., 2013) or essential heavy metals (Maksymiec, 2007). In 1999, Romera et al. (1999) showed that Fe-deficient roots of several dicots produced more ethylene than the Fe-sufficient ones, even before the plants showed any other symptom of deficiency (which could lead to tissue necrosis and thereby, stimulation of wound ethylene; Lynch and Brown, 1997). At the same time, Borch et al. (1999) and Gilbert et al. (2000) showed that P-deficient roots produced more ethylene than the P-sufficient ones. After these reports, the higher ethylene production by Fe-deficient roots has been confirmed by other authors (cited in García et al. [2010] and Romera et al. [2015]). In relation to P, there has been research confirming higher ethylene production by P-deficient roots (Li et al., 2009) and showing higher ethylene production by P-deficient shoots (Kim et al., 2008).
In the last 10 years, increased ethylene production by roots and/or shoots has been described for other nutrient deficiencies, such as K (Shin and Schachtman, 2004; Benlloch-González et al., 2010), S (Zuchi et al., 2009; Moniuszko et al., 2013), N (Zheng et al., 2013), and Mg (Hermans et al., 2010).
The higher ethylene production described for nutrient deficiencies has been further supported by results showing up-regulation of genes implicated in ethylene synthesis. Ethylene is synthesized from Met through a pathway that requires the enzymes S-adenosyl methionine synthetases (SAMS), 1-aminocyclopropane-1-carboxylic acid synthase (ACS), and 1-aminocyclopropane-1-carboxylic acid oxidade (ACO; Sauter et al., 2013). SAMS, ACS, and ACO genes (Table I shows gene names and functions) were up-regulated under Fe deficiency (for review, see Romera et al., 2015) and also, P deficiency (Hernández et al., 2007; Lei et al., 2011; O’Rourke et al., 2013; Wang et al., 2014b). Shin and Schachtman (2004) have found up-regulation of two Arabidopsis (Arabidopsis thaliana) ACOs under K deficiency, Nikiforova et al. (2003) have found up-regulation of AtSAMS under S deficiency, Zhao et al. (2015) have found up-regulation of an AtACO under N deficiency, and Hermans et al. (2010) have found up-regulation of several AtACS under Mg deficiency.
Table I. Genes related to nutrient deficiency responses used in this update.
ER, endoplasmic reticulum; TF, transcription factor.
| Name | Function |
|---|---|
| Ethylene synthesis genes | |
| SAMS | SAMS |
| ACS | ACS |
| ACO | ACO |
| Ethylene signaling genes | |
| ETRs and ERSs | Ethylene receptors |
| CTR1a | Kinase |
| EIN2b | Protein acting downstream of CTR1 (localized to ER membrane) |
| EIN3c | TF acting downstream of EIN2 |
| EILsc | TFs acting downstream of EIN2 |
| SLIM1 (EIL3) | SLIM1 is an allele of EIL3 |
| ERFs | TFs acting downstream of EIN3 |
| RAP2.11 | Is an ERF |
| Fe-related genes | |
| FIT (FER homolog) | TF (master regulator of most Fe acquisition genes) |
| MED16 | Mediator (interacts with EIN3/EIL1 for FIT transcription) |
| P-related genes | |
| PT1, PT2, and PT5 | Phosphate transporters |
| ACP5 and PAP1 | Acid phosphatases |
| PHO2 | E2 conjugase (negative regulator of P deficiency responses) |
| S-related genes | |
| SULTRs | Sulfate transporters |
| APR | Adenosine 5′-phosphosulfate reductase |
| APS4 | ATP sulfurylase (negative regulator of S deficiency responses) |
| K-related genes | |
| HAK5 | K+ transporter |
| N-related genes | |
| NRTs | Nitrate transporters |
Its mutation leads to a constitutive triple-response phenotype, similar to the one of wild-type plants treated with ethylene.
Its mutation leads to an ethylene-insensitive phenotype.
Their mutations lead to ethylene-insensitive phenotypes.
Other than ethylene synthesis, nutrient deficiencies can also affect ethylene responsiveness. He et al. (1992) showed increased sensitivity to ethylene in N- and P-deficient roots, which has been further supported in more recent publications (Ma et al., 2003; Kim et al., 2008). Although ethylene’s mode of action is not fully understood, a linear signaling pathway has been proposed in Arabidopsis (Shakeel et al., 2013; Wang et al., 2013):
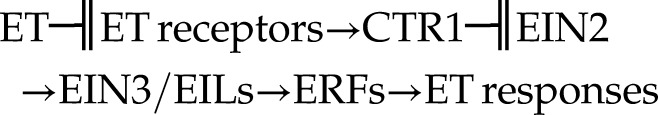 |
where ET indicates ethylene, ─╢ indicates negative effect, → indicates positive effect, CTR1 indicates Constitutive Triple Response1, EIN2 indicates Ethylene Insensitive2, EIL indicates Ethylene Insensitive-Like, and ERF indicates Ethylene Response Factor. In the absence of ethylene, the kinase CTR1 phosphorylates EIN2 (which is localized to the endoplasmic reticulum membrane), preventing the cleavage and translocation of the EIN2 C-terminal fragment into the nucleus. In the presence of ethylene, this is bound to its receptors, and CTR1 is inactivated, resulting in dephosphorylation of EIN2 and its cleavage. The EIN2 C-terminal fragment is then translocated into the nucleus, where it participates in stabilization of the transcription factor EIN3 and downstream gene activation (Shakeel et al., 2013; Wang et al., 2013). EIN3 belongs to a small family of transcription factors that also includes various EIL proteins: EIL1, EIL2, and EIL3 (Wang et al., 2013). Mutants of CTR1 present constitutive activation of ethylene signaling, whereas mutants of EIN2 and EIN3/EILs display reduced sensitivity to ethylene (Shakeel et al., 2013; Wang et al., 2013). The ERF transcription factors act downstream of EIN3 to activate or repress ethylene-responsive genes, although some ERFs can be activated by ethylene-independent transcription factors not related to EIN3 (Wang et al., 2013; Thirugnanasambantham et al., 2015).
Ethylene responsiveness could be related to changes in the expression of genes implicated in ethylene signaling. Several of these genes were up-regulated under Fe deficiency, like Ethylene Triple Responses (ETRs; coding for ethylene receptors), Ethylene Response Sensors (ERSs; coding for ethylene receptors), EIN2, EIN3, EILs, and ERFs (O’Rourke et al., 2007; García et al., 2010, 2014; Wang et al., 2014a). Similarly, Shin and Schachtman (2004) found increased expression of AtETR2 (encoding an ethylene receptor) and Kim et al. (2012) found increased expression of Arabidopsis Rhoptry-Associated Protein2.11 (AtRAP2.11; encoding an ERF) under K deficiency. In relation to N, Zheng et al. (2013) have shown increased expression of AtEIN3 and AtEIL1, and Zhao et al. (2015) have shown increased expression of several AtERFs under this deficiency. Very recently, Ramaiah et al. (2014) have described the up-regulation of AtERF070 (encoding an ERF) in P-deprived roots and shoots.
Whether the expression of these genes enhances or decreases the sensitivity to ethylene deserves a deeper investigation. Because ethylene receptors act as negative regulators of ethylene signaling, their increase would decrease sensitivity to ethylene (Wang et al., 2013). Possibly, the induction of ethylene receptor genes may function as a dampening mechanism, slowing down an ethylene response after it has been initiated.
ETHYLENE PARTICIPATION IN NUTRIENT DEFICIENCY RESPONSES
In addition to the higher ethylene production of nutrient deficient plants (see above), other results also support a role for ethylene in the regulation of nutrient deficiency responses. These other results are mainly based on the use of ethylene inhibitors, like cobalt or silver thiosulfate (STS), the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), the ethylene-releasing substance ethephon, ethylene itself, ethylene mutants (ethylene insensitive, ethylene constitutive, or ethylene overproducers), and molecular biology techniques, such as transgenic lines, transcriptomics, fluorescence imaging, luciferase imaging, GUS assay, and yeast (Saccharomyces cerevisiae) two-hybrid assay (Romera and Alcántara, 2004; Maruyama-Nakashita et al., 2006; Jung et al., 2009; García et al., 2010; Lei et al., 2011; Kim et al., 2012; Yang et al., 2014). In most cases, ethylene, with production that increases under the nutrient deficiency, acts as activator of the responses. Consequently, ethylene inhibitors block the responses (Fig. 1), whereas ethylene itself or ethylene precursors (ACC and ethephon) promote them (Romera and Alcántara, 1994, 2004; Jung et al., 2009; Tian et al., 2009; Lei et al., 2011; Li et al., 2011; Wang et al., 2012).
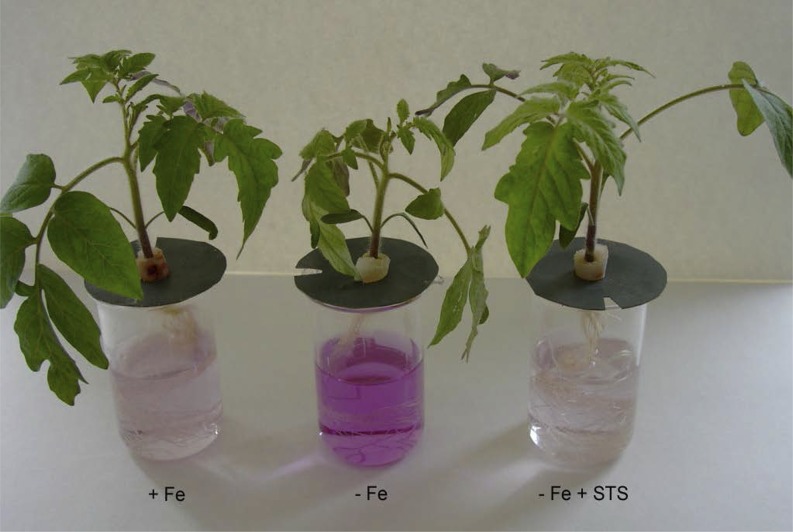
Figure 1.
Ethylene generally acts as an activator of responses to nutrient deficiencies. Consequently, ethylene inhibitors block these responses. As an example, ferric reductase activity is enhanced under Fe deficiency (denoted by the purple color of the assay solution) but blocked upon application of the ethylene inhibitor STS. Tomato plants grown in complete nutrient solution (+Fe) were transferred for the last 3 d to nutrient solution either without Fe (−Fe) or without Fe plus 400 μm STS (−Fe + STS). Ferric reductase activity was determined as described by Romera and Alcántara (1994).
Ethylene has been implicated in the regulation of both morphological and physiological responses to nutrient deficiencies (Romera and Alcántara, 1994, 2004; Jung et al., 2009; Lei et al., 2011; Wang et al., 2012, 2014c). Morphological responses include responses like changes in root system architecture (RSA), development of root hairs, development of cluster or proteoid roots (clusters of closely spaced short lateral rootlets formed in some plant species adapted to poor soils; Wang et al., 2014b), and development of root transfer cells (cells with increased surface area because of invaginations of the plasma membrane; Kramer et al., 1980). Most of these root modifications enhance nutrient uptake by increasing the surface of contact of roots with soil and chemically modifying the soil environment (Wang et al., 2014b). Physiological responses are changes in processes aimed to facilitate the mobilization and uptake of nutrients. Some physiological responses are the acidification of the rhizosphere, the release of chelating agents into the medium, the increased amount of specific transporters in root epidermal cells, the increase of internal transporters and chelating agents (to improve mobilization of nutrients inside the plant), the enhancement of root ferric reductase activity, and the enhancement of root acid phosphatase activity.
Morphological Responses
Ethylene has been implicated in the development of subapical root hairs, root transfer cells, and cluster roots induced under Fe or P deficiency (Kramer et al., 1980; Romera and Alcántara, 1994, 2004; Schmidt et al., 2000a; Waters and Blevins, 2000; Schmidt and Schikora, 2001; Schikora and Schmidt, 2002; Zaid et al., 2003; Zhang et al., 2003, 2014; Wang et al., 2014b). Other than these deficiencies, ethylene has also been implicated in the development of root hairs caused by K (Jung et al., 2009) or B deficiency (Martín-Rejano et al., 2011).
Nutrient deficiencies can also change RSA by altering the number, length, and diameter of roots (Gruber et al., 2013). The RSA modifications depend on each specific nutrient and the extent of the deficiency (mild, moderate, or severe). Generally, nutrient-deficient plants exhibit a shallower architecture that results from inhibition of primary root elongation (Kramer et al., 1980; López-Bucio et al., 2003; Ma et al., 2003; Jung et al., 2009; Martín-Rejano et al., 2011; Nagarajan and Smith, 2012, Wang et al., 2012; Gruber et al., 2013; Zhang et al., 2014). Some exceptions are S deficiency, with relatively little influence on the morphology of roots, and N deficiency, which stimulates primary root elongation and particularly, lateral root elongation (Gruber et al., 2013). Several deficiencies, like B, Fe, or P deficiency, can cause an increase in lateral root density (Kramer et al., 1980; López-Bucio et al., 2003; Miura et al., 2011; Gruber et al., 2013; Zhang et al., 2014). Ethylene has been implicated in most of these RSA changes (Borch et al., 1999; Gilbert et al., 2000; Ma et al., 2003; Zhang et al., 2003, 2014; Jung et al., 2009; Martín-Rejano et al., 2011; Miura et al., 2011; Chérel et al., 2014) along with auxin and other hormones and signaling substances (“Ethylene Interacts with Other Signals for the Regulation of Nutrient Deficiency Responses”). Very recently, Ramaiah et al. (2014) have found that AtERF070 (encoding an ERF) was greatly induced under P deprivation and can affect RSA. This indicates that ethylene, through an ERF transcription factor that participates in ethylene signaling (see above), can modify the architecture of roots.
The participation of ethylene in the regulation of the morphological responses described above is supported by results showing that ethylene inhibitors negatively affect these changes in nutrient-deficient plants, whereas ethylene precursors promote them in nutrient-sufficient plants (Romera and Alcántara, 1994; Borch et al., 1999; Schmidt et al., 2000a; Ma et al., 2003; Zaid et al., 2003; Zhang et al., 2003, 2014; Romera et al., 2007; Wang et al., 2012, 2014b). As an example, ethylene inhibitors almost totally block the development of subapical root hairs in Fe-, K-, or P-deficient plants (Zhang et al., 2003; Romera and Alcántara, 2004; Jung et al., 2009). Additionally, results obtained with ethylene mutants support this participation (Jung et al., 2009; Wang et al., 2012; see below).
Physiological Responses
In addition to a role for ethylene in the regulation of morphological responses, many data support its role in the regulation of physiological responses. In relation to Fe nutrition, ethylene participates in the up-regulation of many genes implicated in Fe acquisition and homeostasis (Lucena et al., 2006; Waters et al., 2007; García et al., 2010; Romera et al., 2015). Some of the genes up-regulated by ethylene encode transcription factors that are key regulators of most of the responses to Fe deficiency, such as Arabidopsis Fer-like Fe Deficiency Transcription Factor (AtFIT; Colangelo and Guerinot, 2004) or its tomato (Solanum lycopersicum) homolog tomato Fe Efficiency Response (SlFER; Brumbarova and Bauer, 2005). Very recently, it has been shown that AtEIN3 and AtEIL1, both related to ethylene signaling (see above), interact with Arabidopsis Mediator16 (AtMED16) to form a complex implicated in the transcription of AtFIT (Yang et al., 2014).
In the last 10 years, the role of ethylene in the regulation of physiological responses has been extended to other nutrient deficiencies. In K nutrition, ethylene has been shown to be implicated in the up-regulation of the K+ transporter Arabidopsis High Affinity K+ Transporter5 (AtHAK5; Jung et al., 2009), possibly through RAP2.11 (an ERF; Kim et al., 2012). In S nutrition, the up-regulation of several sulfate transporter genes (Arabidopsis Sulfate Transporter1;1 [AtSULTR1;1], AtSULTR1;2, AtSULTR3;4, and AtSULTR4;2) as well as other S-responsive genes is greatly diminished in sulfur limitation1 (slim1; eil3) mutants (Maruyama-Nakashita et al., 2006). The similarity of AtEIL3 with AtEIN3 suggests that it could be a positive regulator of ethylene signaling (see above). The participation of ethylene in S deficiency responses has also been supported by other experimental results (Koprivova et al., 2008; Wawrzyńska et al., 2010; Iqbal et al., 2012; Moniuszko et al., 2013). Koprivova et al. (2008) found up-regulation of several Arabidopsis Adenosine 5′-Phosphosulfate Reductases (AtAPRs; encoding adenosine 5′-phosphosulfate reductase, a key enzyme of sulfate assimilation) upon ACC treatment, and Iqbal et al. (2012) found increased ATP-sulfurylase activity (also implicated in sulfate assimilation) upon ethephon application. However, Wawrzyńska et al. (2010) showed that tobacco (Nicotiana tabacum) Upregulated by Sulfur Deficit 9C, a tobacco gene strongly induced by S deficiency, is activated by the NtEIL2 transcription factor related to ethylene signaling (see above). In P nutrition, Lei et al. (2011) in Arabidopsis and Li et al. (2011) in Medicago falcata have implicated ethylene in up-regulation of phosphate transporter genes (Arabidopsis Phosphate Transporter1 [AtPT1], AtPT2, MfPT1, and MfPT5) and enhanced phosphatase activity (through higher expression of Arabidopsis Acid Phosphatase5 (AtACP5) and M. falcata Purple Acid Phosphatase1 (MfPAP1) encoding phosphatases) of P-deficient roots. In N nutrition, ethylene has been shown to up-regulate Arabidopsis Nitrate Transporter1.1 (AtNRT1.1), whereas it down-regulates AtNRT2.1 (both encoding nitrate transporters; Tian et al., 2009; Zheng et al., 2013). The negative effect of ethylene on the regulation of some nutrient deficiency responses has also been shown in P starvation-induced anthocyanin (Lei et al., 2011; Wang et al., 2012) and N starvation-induced anthocyanin (Wang et al., 2015), both of them inhibited by ethylene.
ARE THE DIFFERENT NUTRIENT DEFICIENCY RESPONSES REGULATED SIMILARLY BY ETHYLENE?
Because ethylene has been implicated in the regulation of many nutrient deficiency responses, the question arises as to whether ethylene regulates all of them through the same transduction pathway. The answer to this question is clearly that different responses can be regulated by ethylene through different transduction pathways based on results with ethylene mutants. However, many results suggest that ethylene does not act alone but acts in conjunction with other hormones and signaling substances to regulate the responses.
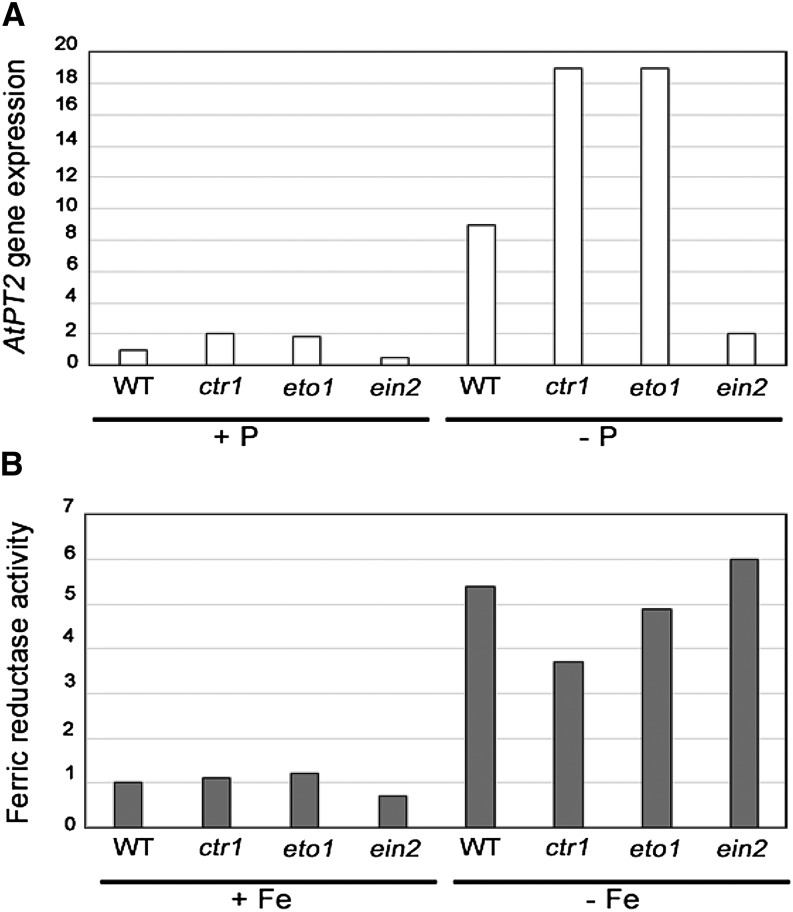
Results from ethylene-insensitive mutants suggest that, even within a deficiency, different responses can be regulated through different transduction pathways. The tomato ethylene-insensitive mutant Never ripe does not increase adventitious root formation under P deficiency but is normal in other morphological responses (Kim et al., 2008). Similarly, the development of subapical root hairs is impaired in the Arabidopsis ethylene-insensitive mutant ein2 under Fe deficiency (Schmidt and Schikora, 2001), whereas the enhanced ferric reductase activity (Fig. 2) and the expression of Fe acquisition genes are not impaired (García et al., 2010).
Figure 2.
Effect of P deficiency on AtPT2 gene expression (A) and Fe deficiency on ferric reductase activity (B) in Arabidopsis wild-type (WT) Columbia-0 plants and ethylene mutants (ctr1, ethylene-constitutive mutant; ein2, ethylene-insensitive mutant; eto1, ethylene overproducer mutant). Fold changes were normalized to transcript levels of the wild type on P sufficiency (A) and ferric reductase activity of the wild type on Fe sufficiency (B). Data for P treatments were redrawn from Lei et al. (2011) with permission, and data for Fe were from García et al. (2010, 2014) and M.J. García, F.J. Romera, C. Lucena, E. Alcántara, and R. Pérez-Vicente (unpublished data).
The idea of multiple transduction pathways for regulating nutrient deficiency responses by ethylene is further reinforced by comparing different deficiencies and looking at the results from ethylene overproducer and ethylene-constitutive mutants. The up-regulation of the AtPT2 gene (induced under P deficiency; Fig. 2) and the AtHAK5 gene (induced under K deficiency) is impaired in the Arabidopsis ein2 mutant (Jung et al., 2009; Lei et al., 2011), whereas increased ferric reductase activity under Fe deficiency is not impaired (Fig. 2). Both the Arabidopsis ethylene-constitutive mutant ctr1 and the Arabidopsis ethylene overproducer mutant eto have constitutive subapical root hairs (a nutrient deficiency symptom) in complete nutrient solution; however, neither of these mutants has full constitutive activation of P, Fe, or K physiological responses (Fig. 2; Schmidt et al., 2000b; Romera and Alcántara, 2004; García et al., 2007, 2014; Jung et al., 2009; Lei et al., 2011; Wang et al., 2012). In the same way, root hairs, transfer cells, and cluster roots are almost fully induced by ACC or ethephon in plants grown with high levels of P, Fe, or K, whereas physiological responses are activated to a lesser degree than when applied to plants grown with low levels or in absence of these nutrients (Romera and Alcántara, 1994; Schmidt et al., 2000a; Zaid et al., 2003; Zhang et al., 2003; Jung et al., 2009; Lucena et al., 2006; Lei et al., 2011; Li et al., 2011; García et al., 2013).
From all of the results above, several conclusions can be drawn. First, different nutrient deficiency responses can be regulated by ethylene through distinct transduction pathways. Second, for some responses, like Fe physiological responses, ethylene could act through a pathway where EIN2 and possibly, CTR1 are not strictly required (Fig. 3). Third, morphological and physiological responses can be differently regulated by ethylene. Fourth, for the regulation of physiological responses, ethylene could act in conjunction with nutrient-related repressive signals. The existence of an alternate route for ethylene signaling, other than the conventional one including CTR1 and EIN2 (Fig. 3; Shakeel et al., 2013), is further supported by results showing that the Arabidopsis ctr1 and ein2 mutants respond to both ACC (García et al., 2010, 2014) and ethylene inhibitors (García et al., 2007; Jung et al., 2009) for some physiological responses. In relation to the last two conclusions, we can speculate that, because physiological responses are not fully activated in the ctr1 and eto mutants (Fig. 2), whereas morphological responses (at least in root hairs) are, some nutrient-related signals act negatively to block physiological responses. These signals probably act downstream of CTR1 in the ethylene signaling pathway. This does not preclude that these signals can also affect ethylene synthesis.
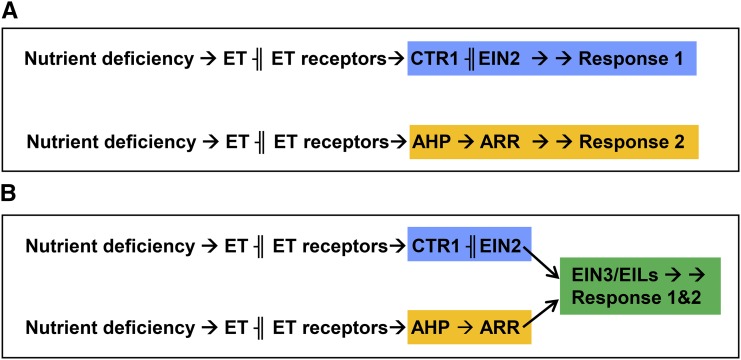
Figure 3.
Ethylene (ET) could regulate different nutrient deficiency responses through two distinct signal transduction pathways. One pathway is CTR1-EIN2 dependent, and the other is CTR1-EIN2 independent (instead, using Arabidopsis His Phosphotransfer [AHP] proteins and Arabidopsis Response Regulators [ARRs]; Shakeel et al., 2013). This model is supported by data showing that ctr1 and ein2 mutants respond to both ACC and ET inhibitors for some physiological responses (García et al., 2007, 2010, 2014; Jung et al., 2009). It is possible that both pathways can act independently (A) or that both can interact and converge downstream through EIN3/EILs (B) depending on the responses (Table I). ─╢, Inhibition; →, promotion.
ETHYLENE INTERACTS WITH OTHER SIGNALS FOR THE REGULATION OF NUTRIENT DEFICIENCY RESPONSES
Morphological and physiological responses work together to effectively increase nutrient uptake (Lucena et al., 2006; Jung et al., 2009; Wang et al., 2014b). Consequently, their regulation is coordinated through the participation of similar signals for both kind of responses, like hormones (e.g. auxin, ethylene, cytokinins, jasmonic acid, brassinosteroids, gibberellins, abscisic acid, and strigolactones) and other signaling substances, such as nitric oxide (NO), reactive oxygen species (ROS), and sugars (Romera et al., 2007, 2011, 2015; Rubio et al., 2009; Hammond and White, 2011; Kapulnik et al., 2011; Lei and Liu, 2011; Iqbal et al., 2013; Zhang et al., 2014). Other than these common signals, there are other more nutrient-specific signals, such as mineral ions, microRNAs, reduced glutathione (GSH), and peptides (Lappartient et al., 1999; Liu et al., 2009; Buhtz et al., 2010; García et al., 2013; Zeng et al., 2014; Zhang et al., 2014), that could confer specificity (at least a certain degree of specificity) to the different nutrient deficiency responses. Despite this, cross talk in the activation of physiological responses under different nutrient deficiencies (e.g. a K physiological response can be activated under P deficiency) has been described (Shin et al., 2005; Waters et al., 2012; Wang et al., 2014b), probably because of the common implication of ethylene and other signals in their regulation.
Different results suggest that some nutrient-related repressive signals (e.g. mineral ions, peptides, and GSH) can move from shoots to roots through the phloem (Dong et al., 1998; Lappartient et al., 1999; García et al., 2013; Zhang et al., 2014). Additionally, other signals, like auxin, sugars, and microRNAs, can also move through the phloem (Romera et al., 2007, 2011; Buhtz et al., 2010; Lei and Liu, 2011). This would provide a way for shoots to inform roots of their nutrient status and could serve to integrate the role of both shoots and roots in the regulation of nutrient deficiency responses.
Auxin, sugars, NO, and ROS generally accumulate in roots under nutrient deficiencies (Shin et al., 2005; Tewari et al., 2006; Graziano and Lamattina, 2007; Romera et al., 2007; Jung et al., 2009; Wang et al., 2010, 2014b; Kiba et al., 2011; Iqbal et al., 2013) and can positively interact with ethylene to regulate nutrient deficiency responses. Auxin can stimulate ethylene production, and ethylene can affect auxin transport and accumulation (Romera et al., 2007, 2011; Muday et al., 2012). The exact relationship of sugars and ethylene is not totally known, but hexokinases are a critical node in mediating plant Glc and ethylene responses (Karve et al., 2012). Ethylene can stimulate both ROS and NO accumulation (Shin et al., 2005; Jung et al., 2009; García et al., 2011; Steffens, 2014), whereas both ROS and NO can stimulate ethylene production (Ahlfors et al., 2009; García et al., 2011; Iqbal et al., 2013). Furthermore, ROS can stimulate ethylene production through NO accumulation (Ahlfors et al., 2009). Probably, ROS and NO influence the production of ethylene in a positive feedback loop, leading to enhancement of the nutrient deficiency signal as described for ethylene and NO (Fig. 4; García et al., 2011).
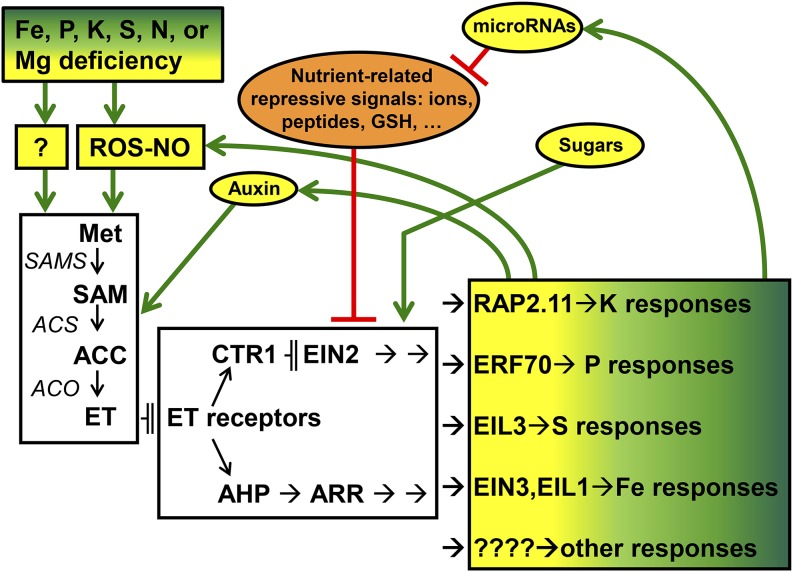
Figure 4.
Working model to explain the role of ethylene (ET) on the regulation of responses to different nutrient deficiencies in plants. Nutrient deficiencies can enhance ET production by up-regulating SAMS, ACS, and ACO genes (Shin and Schachtman, 2004; García et al., 2010; Hermans et al., 2010), although the steps leading to this up-regulation are not yet clear. A possibility is that, at first, nutrient deficiencies cause oxidative stress and consequently, ROS accumulation and possibly, NO accumulation (Shin et al., 2005; Tewari et al., 2006; Graziano and Lamattina, 2007; Ahlfors et al., 2009; Jung et al., 2009; García et al., 2011; Iqbal et al., 2013; Steffens, 2014). This ROS-NO accumulation would stimulate ET production, which in turn, could increase ROS-NO production (Ahlfors et al., 2009; Jung et al., 2009; García et al., 2011; Iqbal et al., 2013) in a positive feedback loop, leading to enhancement of the nutrient deficiency signal (García et al., 2011). When ET is perceived by the receptors, it could act through a CTR1-EIN2-dependent pathway for the regulation of some responses or a CTR1-EIN2-independent pathway for the regulation of other responses (Fig. 3). At the end of these transduction pathways, different ET-related transcription factors, such as RAP2.11, ERF070, EIL3, EIN3/EIL1, or others, would activate different nutrient responses (Maruyama-Nakashita et al., 2006; Kim et al., 2012; Ramaiah et al., 2014; Yang et al., 2014). Under nutrient sufficiency, several signals that can move through the phloem (mineral ions, peptides, GSH, etc.; Lappartient et al., 1999; García et al., 2013; Zhang et al., 2014) could negatively interact with ET to inactivate nutrient responses. However, under nutrient deficiency, other signals that can move through the phloem (microRNAs, auxin, sugars, etc.; Kasajima et al., 2007; Lejay et al., 2008; Hammond and White, 2011; Lei and Liu, 2011; Hu et al., 2015) could positively interact with ET to activate the responses. Additionally, ET can influence auxin accumulation and distribution and the expression of some microRNAs (see text for details). Yellow background indicates signals that activate responses, orange background indicates signals that repress responses. AHP, Arabidopsis His Phosphotransfer; ARR, Arabidopsis Response Regulators; ─╢, inhibition; →, promotion.
Signals Interacting with Ethylene for the Regulation of Morphological Responses
One of the signals most closely related to ethylene for the regulation of morphological responses is auxin. In supporting this view, it should be noted that subapical root hairs (Schmidt and Schikora, 2001; Zhang et al., 2003; Romera et al., 2007; Martín-Rejano et al., 2011), transfer cells (Schmidt et al., 2000a; Schikora and Schmidt, 2002), and cluster roots (Zaid et al., 2003; Wang et al., 2014b) are similarly affected by either ethylene or auxin treatments. In the same way, lateral root formation and inhibition of root elongation under different deficiencies (“Ethylene Participation in Nutrient Deficiency Responses”) have been associated with auxin and ethylene (Zhang et al., 2003; López-Bucio et al., 2003; Miura et al., 2011; Muday et al., 2012; Chérel et al., 2014). Both hormones synergistically inhibit root elongation and play an antagonistic role on lateral root formation, where auxin stimulates while ethylene inhibits it (López-Bucio et al., 2003; Miura et al., 2011; Muday et al., 2012; Chérel et al., 2014; Wang et al., 2014b). Ethylene increases rootward auxin transport by up-regulating PIN-FORMED3 (PIN3) and PIN7 (auxin efflux carriers) in the central cylinder, which may deplete the lateral root-forming zone of auxin while increasing auxin accumulation in the root apex. This effect may be responsible for the negative regulation of lateral root formation by ethylene (Muday et al., 2012; Chérel et al., 2014). At the root tip, up-regulation of AUXIN RESISTANT1 (AUX1; auxin influx carrier) and PIN2 (auxin efflux carrier) enhances shootward auxin transport into the elongation zone, thereby reducing primary root elongation and promoting the development of subapical root hairs by up-regulating root hair-specific genes (Muday et al., 2012; Lee and Cho, 2013).
Strigolactones are other hormones that participate in the regulation of root hair elongation, and their role has been related to interactions with auxin and ethylene (Kapulnik et al., 2011).
NO has also been implicated in the development of subapical root hairs (Graziano and Lamattina, 2007) and cluster roots (Wang et al., 2010) under Fe or P deficiency. Similarly, ROS has also been implicated in the development of subapical root hairs under K, N, and P deficiency (Shin et al., 2005; Jung et al., 2009). As previously described, both NO and ROS can influence the production of ethylene and vice versa. Moreover, ACC and ethephon induce NO and ROS accumulation in the subapical region of the roots, where subapical root hairs develop (Jung et al., 2009; García et al., 2011).
Signals Interacting with Ethylene for the Regulation of Physiological Responses
Different signals could interact with ethylene to activate or suppress physiological responses depending on the nutrient status of the plants. Under nutrient sufficiency, several nutrient-related repressive signals, some of them moving through the phloem (e.g. mineral ions, peptides, and GSH), could negatively interact with ethylene to inactivate nutrient responses (Fig. 4; Lappartient et al., 1999; García et al., 2013; Zhang et al., 2014). It has been proposed that some Fe compound moves through the phloem (probably an Fe peptide) and could negatively interact with ethylene signaling to regulate Fe physiological responses (García et al., 2013; Romera et al., 2015). Similarly, GSH moving in the phloem has been described as a suppressor of some S physiological responses (Lappartient et al., 1999), and GSH could negatively interact with ethylene signaling (Chen et al., 2013). Cytokinins have also been described as negative regulators of several physiological responses to nutrient deficiencies (Rubio et al., 2009; Hammond and White, 2011), although their exact relationship with ethylene will require additional research.
Under nutrient deficiency, some signals, such as auxin, NO, ROS, and sugars, have been described as activators of physiological responses to different deficiencies (Graziano and Lamattina, 2007; Kasajima et al., 2007; Lejay et al., 2008; Jung et al., 2009; García et al., 2010, 2011; Wang et al., 2010; Hammond and White, 2011; Kiba et al., 2011; Lei and Liu, 2011; Zhang et al., 2014). Because these signals can interact with ethylene in several ways (see above), it is possible that the roles of all of them on the activation of the responses could be tightly interrelated (Fig. 4; Jung et al., 2009; Romera et al., 2011, 2015).
Under nutrient deficiency, some nutrient-related signals that can move through the phloem, like microRNAs (Buhtz et al., 2010), have also been described as activators of many physiological responses (Zeng et al., 2014; Hu et al., 2015). Many microRNAs increase their expression under nutrient deficiencies: as examples, microRNA399 (miRNA399) is strongly induced under P, Fe, or K deficiency; miR827 is strongly induced under P deficiency; miR395 is strongly induced under S deficiency; miR158 is strongly induced under Fe deficiency; and miR397, miR398, and miR857 are strongly induced under Cu deficiency (Buhtz et al., 2010; Kawashima et al., 2011; Waters et al., 2012; Lin et al., 2013; Zeng et al., 2014; Hu et al., 2015). They usually down-regulate the expression of target genes by posttranscriptional cleavage (Kawashima et al., 2011; Zeng et al., 2014; Hu et al., 2015). The down-regulation of some target genes, such as Arabidopsis Phosphate2 (AtPHO2; encoding an E2 ubiquitin conjugase) or Arabidopsis ATP Sulfurylase4 (AtAPS4; encoding an ATP sulfurylase), caused by miR399 or miR395, respectively, leads to the accumulation of P or S in shoots and the prevention of their transport in the phloem from shoots to roots (Dong et al., 1998; Liang et al., 2010; Kawashima et al., 2011; Zhang et al., 2014). This suggests that some microRNAs could restrict the movement of nutrient-related repressive signals from shoots to roots, thereby positively interacting with ethylene in the activation of the responses (Fig. 4). Moreover, ethylene could potentiate the effects of some microRNAs. As an example, miR395 is induced by S deficiency in a SLIM1(EIL3)-ethylene-dependent manner (Fig. 4; Kawashima et al., 2011).
CONCLUSION
In addition to the participation of ethylene in the regulation of Fe and P deficiency responses, its role has been extended to other deficiencies. This implies that it acts as a general coordinator of many nutrient deficiency responses. Its participation in so many deficiencies suggests that, to confer specificity to the different responses, ethylene should act through different transduction pathways and/or in conjunction with other signals. Its interaction with some of these signals, such as auxin and NO, is partly known. However, its interaction with other signals, such as peptides, microRNAs, and GSH-related compounds moving in the phloem, is practically unknown. Deeper research is required in the near future to clarify the shared steps related to ethylene signaling for the different responses and the signaling steps specific for each response. In addition, it would be necessary to extend research on the role of ethylene to other nutrient deficiency responses not studied yet.
Acknowledgments
We thank Dong Liu (School of Life Sciences, Tsinghua University) for permission to elaborate figure 2 and Brian M. Waters (University of Nebraska) and Jon Shaff (Robert Holley Center for Agriculture and Health) for English correction of the article.
Glossary
- ACC
1-aminocyclopropane-1-carboxylic acid
- GSH
reduced glutathione
- NO
nitric oxide
- ROS
reactive oxygen species
- RSA
root system architecture
- STS
silver thiosulfate
Footnotes
This work was supported by the European Union (European Regional Development Fund), the Ministerio de Economía y Competitividad (project no. AGL2013–40822–R), and the Junta de Andalucía (Research Groups AGR115 and BIO159).
References
- Ahlfors R, Brosché M, Kangasjärvi J (2009) Ozone and nitric oxide interaction in Arabidopsis thaliana: a role for ethylene? Plant Signal Behav 4: 878–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch-González M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M (2010) K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot 61: 1139–1145 [DOI] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM (1999) Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ 22: 425–431 [Google Scholar]
- Brumbarova T, Bauer P (2005) Iron-mediated control of the basic helix-loop-helix protein FER, a regulator of iron uptake in tomato. Plant Physiol 137: 1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtz A, Pieritz J, Springer F, Kehr J (2010) Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Huang CS, Huang GJ, Chow TJ, Lin YH (2013) NADPH oxidase inhibitor diphenyleneiodonium and reduced glutathione mitigate ethephon-mediated leaf senescence, H2O2 elevation and senescence-associated gene expression in sweet potato (Ipomoea batatas). J Plant Physiol 170: 1471–1483 [DOI] [PubMed] [Google Scholar]
- Chérel I, Lefoulon C, Boeglin M, Sentenac H (2014) Molecular mechanisms involved in plant adaptation to low K(+) availability. J Exp Bot 65: 833–848 [DOI] [PubMed] [Google Scholar]
- Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16: 3400–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Rengel Z, Delhaize E (1998) Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta 205: 251–256 [DOI] [PubMed] [Google Scholar]
- García MJ, García-Mateo MJ, Lucena C, Romera FJ, Rojas CL, Alcántara E, Pérez-Vicente R (2014) Hypoxia and bicarbonate could limit the expression of iron acquisition genes in Strategy I plants by affecting ethylene synthesis and signaling in different ways. Physiol Plant 150: 95–106 [DOI] [PubMed] [Google Scholar]
- García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R (2010) Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot 61: 3885–3899 [DOI] [PubMed] [Google Scholar]
- García MJ, Romera FJ, Pérez-Vicente R, Lucena C, Alcántara E (2007) Ferric reductase and iron transporter gene expression in different Arabidopsis ethylene mutants. In Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E, eds, Advances in Plant Ethylene Research. Springer, Dordrecht, The Netherlands, pp 401–403 [Google Scholar]
- García MJ, Romera FJ, Stacey MG, Stacey G, Villar E, Alcántara E, Pérez-Vicente R (2013) Shoot to root communication is necessary to control the expression of iron-acquisition genes in Strategy I plants. Planta 237: 65–75 [DOI] [PubMed] [Google Scholar]
- García MJ, Suárez V, Romera FJ, Alcántara E, Pérez-Vicente R (2011) A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant Physiol Biochem 49: 537–544 [DOI] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL (2000) Proteoid root development of phosphorus deficient lupin in mimicked by auxin and phosphonate. Ann Bot 85: 921–928 [Google Scholar]
- Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52: 949–960 [DOI] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163: 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, White PJ (2011) Sugar signaling in root responses to low phosphorus availability. Plant Physiol 156: 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol 98: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Vuylsteke M, Coppens F, Cristescu SM, Harren FJ, Inzé D, Verbruggen N (2010) Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol 187: 132–144 [DOI] [PubMed] [Google Scholar]
- Hernández G, Ramírez M, Valdés-López O, Tesfaye M, Graham MA, Czechowski T, Schlereth A, Wandrey M, Erban A, Cheung F, et al. (2007) Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol 144: 752–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang W, Deng K, Li H, Zhang Z, Zhang L, Chu C (2015) MicroRNA399 is involved in multiple nutrient starvation responses in rice. Front Plant Sci 6: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Khan NA, Nazar R, Teixeira da Silva JA (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 78: 84–90 [Google Scholar]
- Iqbal N, Trivellini A, Masood A, Ferrante A, Khan NA (2013) Current understanding on ethylene signaling in plants: the influence of nutrient availability. Plant Physiol Biochem 73: 128–138 [DOI] [PubMed] [Google Scholar]
- Jung JY, Shin R, Schachtman DP (2009) Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 21: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H (2011) Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot 62: 2915–2924 [DOI] [PubMed] [Google Scholar]
- Karve A, Xia X, Moore Bd (2012) Arabidopsis Hexokinase-Like1 and Hexokinase1 form a critical node in mediating plant glucose and ethylene responses. Plant Physiol 158: 1965–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasajima I, Ohkama-Ohtsu N, Ide Y, Hayashi H, Yoneyama T, Suzuki Y, Naito S, Fujiwara T (2007) The BIG gene is involved in regulation of sulfur deficiency-responsive genes in Arabidopsis thaliana. Physiol Plant 129: 351–363 [Google Scholar]
- Kawashima CG, Matthewman CA, Huang S, Lee BR, Yoshimoto N, Koprivova A, Rubio-Somoza I, Todesco M, Rathjen T, Saito K, et al. (2011) Interplay of SLIM1 and miR395 in the regulation of sulfate assimilation in Arabidopsis. Plant J 66: 863–876 [DOI] [PubMed] [Google Scholar]
- Kiba T, Kudo T, Kojima M, Sakakibara H (2011) Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot 62: 1399–1409 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lynch JP, Brown KM (2008) Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ 31: 1744–1755 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Ruzicka D, Shin R, Schachtman DP (2012) The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol Plant 5: 1042–1057 [DOI] [PubMed] [Google Scholar]
- Koprivova A, North KA, Kopriva S (2008) Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol 146: 1408–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D, Römheld V, Landsberg E, Marschner H (1980) Induction of transfer-cell formation by iron deficiency in the root epidermis of Helianthus annuus L. Planta 147: 335–339 [DOI] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B (1999) Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J 18: 89–95 [DOI] [PubMed] [Google Scholar]
- Lee RDW, Cho HT (2013) Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front Plant Sci 4: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Liu D (2011) Sucrose regulates plant responses to deficiencies in multiple nutrients. Plant Signal Behav 6: 1247–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Zhu C, Liu Y, Karthikeyan AS, Bressan RA, Raghothama KG, Liu D (2011) Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol 189: 1084–1095 [DOI] [PubMed] [Google Scholar]
- Lejay L, Wirth J, Pervent M, Cross JMF, Tillard P, Gojon A (2008) Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol 146: 2036–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Gao Y, Tian QY, Shi FL, Li LH, Zhang WH (2011) Stimulation of root acid phosphatase by phosphorus deficiency is regulated by ethylene in Medicago falcata. Environ Exp Bot 71: 114–120 [Google Scholar]
- Li YS, Mao XT, Tian QY, Li LH, Zhang WH (2009) Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environ Exp Bot 67: 172–177 [Google Scholar]
- Liang G, Yang F, Yu D (2010) MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J 62: 1046–1057 [DOI] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Chiou TJ (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Chang CY, Chiou TJ (2009) The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol 12: 312–319 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- Lucena C, Waters BM, Romera FJ, García MJ, Morales M, Alcántara E, Pérez-Vicente R (2006) Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J Exp Bot 57: 4145–4154 [DOI] [PubMed] [Google Scholar]
- Lynch J, Brown KM (1997) Ethylene and plant responses to nutritional stress. Physiol Plant 100: 613–619 [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymiec W. (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29: 177–187 [Google Scholar]
- Martín-Rejano EM, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Rexach J, Navarro-Gochicoa MT, González-Fontes A (2011) Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol Plant 142: 170–178 [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18: 3235–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Lee J, Gong Q, Ma S, Jin JB, Yoo CY, Miura T, Sato A, Bohnert HJ, Hasegawa PM (2011) SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiol 155: 1000–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Djordjevic MA, Imin N (2013) Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Front Plant Sci 4: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko G, Skoneczny M, Zientara-Rytter K, Wawrzyńska A, Głów D, Cristescu SM, Harren FJM, Sirko A (2013) Tobacco LSU-like protein couples sulphur-deficiency response with ethylene signalling pathway. J Exp Bot 64: 5173–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Nagarajan VK, Smith AP (2012) Ethylene’s role in phosphate starvation signaling: more than just a root growth regulator. Plant Cell Physiol 53: 277–286 [DOI] [PubMed] [Google Scholar]
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33: 633–650 [DOI] [PubMed] [Google Scholar]
- O’Rourke JA, Charlson DV, Gonzalez DO, Vodkin LO, Graham MA, Cianzio SR, Grusak MA, Shoemaker RC (2007) Microarray analysis of iron deficiency chlorosis in near-isogenic soybean lines. BMC Genomics 8: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JA, Yang SS, Miller SS, Bucciarelli B, Liu J, Rydeen A, Bozsoki Z, Uhde-Stone C, Tu ZJ, Allan D, et al. (2013) An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol 161: 705–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah M, Jain A, Raghothama KG (2014) ETHYLENE RESPONSE FACTOR070 regulates root development and phosphate starvation-mediated responses. Plant Physiol 164: 1484–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E (2004) Ethylene involvement in the regulation of Fe-deficiency stress responses by Strategy I plants. Funct Plant Biol 31: 315–328 [DOI] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E (1994) Iron-deficiency stress responses in cucumber (Cucumis sativus L.) roots (a possible role for ethylene?). Plant Physiol 105: 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E, de la Guardia MD (1999) Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by Strategy I plants. Ann Bot 83: 51–55 [Google Scholar]
- Romera FJ, García MJ, Alcántara E, Pérez-Vicente R (2011) Latest findings about the interplay of auxin, ethylene and nitric oxide in the regulation of Fe deficiency responses by Strategy I plants. Plant Signal Behav 6: 167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Lucena C, Alcántara E (2007) Plant hormones influencing iron uptake in plants. In Barton LL, Abadía J, eds, Iron Nutrition in Plants and Rhizospheric Microorganisms. Springer, Dordrecht, The Netherlands, pp 251–278 [Google Scholar]
- Romera FJ, Lucena C, García MJ, Alcántara E, Pérez-Vicente R (2015) The role of ethylene and other signals in the regulation of Fe deficiency responses by dicot plants. In Sarwat M, ed, Stress Signaling in Plants: Genomics and Proteomics Perspectives, Vol 2 Springer, Dordrecht, The Netherlands [Google Scholar]
- Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69: 361–373 [DOI] [PubMed] [Google Scholar]
- Sauter M, Moffatt B, Saechao MC, Hell R, Wirtz M (2013) Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem J 451: 145–154 [DOI] [PubMed] [Google Scholar]
- Schikora A, Schmidt W (2002) Formation of transfer cells and H(+)-ATPase expression in tomato roots under P and Fe deficiency. Planta 215: 304–311 [DOI] [PubMed] [Google Scholar]
- Schmidt W, Schikora A (2001) Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiol 125: 2078–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Schikora A, Pich A, Bartels M (2000a) Hormones induce an Fe-deficiency-like root epidermal cell pattern in the Fe-inefficient tomato mutant fer. Protoplasma 213: 67–73 [Google Scholar]
- Schmidt W, Tittel J, Schikora A (2000b) Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 122: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel SN, Wang X, Binder BM, Schaller GE (2013) Mechanisms of signal transduction by ethylene: overlapping and non-overlapping signalling roles in a receptor family. AoB Plants 5: plt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP (2005) Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol 46: 1350–1357 [DOI] [PubMed] [Google Scholar]
- Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101: 8827–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B. (2014) The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci 5: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari RK, Kumar P, Sharma PN (2006) Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci Hortic (Amsterdam) 108: 7–14 [Google Scholar]
- Thirugnanasambantham K, Durairaj S, Saravanan S, Karikalan K, Muralidaran S, Islam VIH (2015) Role of Ethylene Response Transcription Factor (ERF) and its regulation in response to stress encountered by plants. Plant Mol Biol Rep 33: 347–357 [Google Scholar]
- Tian QY, Sun P, Zhang WH (2009) Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytol 184: 918–931 [DOI] [PubMed] [Google Scholar]
- Wang BL, Tang XY, Cheng LY, Zhang AZ, Zhang WH, Zhang FS, Liu JQ, Cao Y, Allan DL, Vance CP, et al. (2010) Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol 187: 1112–1123 [DOI] [PubMed] [Google Scholar]
- Wang F, Cui X, Sun Y, Dong CH (2013) Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep 32: 1099–1109 [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Y, Yang J, Ma C, Zhang Y, Ge T, Qi Z, Kang Y (2015) Arabidopsis ROOT HAIR DEFECTIVE3 is involved in nitrogen starvation-induced anthocyanin accumulation. J Integr Plant Biol 57: 708–721 [DOI] [PubMed] [Google Scholar]
- Wang L, Dong J, Gao Z, Liu D (2012) The Arabidopsis gene hypersensitive to phosphate starvation 3 encodes ethylene overproduction 1. Plant Cell Physiol 53: 1093–1105 [DOI] [PubMed] [Google Scholar]
- Wang S, Lu B, Wu T, Zhang X, Xu X, Han Z, Wang Y (2014a) Transcriptomic analysis demonstrates the early responses of local ethylene and redox signaling to low iron stress in Malus xiaojinensis. Tree Genet Genomes 10: 573–584 [Google Scholar]
- Wang Z, Straub D, Yang H, Kania A, Shen J, Ludewig U, Neumann G (2014b) The regulatory network of cluster-root function and development in phosphate-deficient white lupin (Lupinus albus) identified by transcriptome sequencing. Physiol Plant 151: 323–338 [DOI] [PubMed] [Google Scholar]
- Waters BM, Blevins DG (2000) Ethylene production, cluster root formation, and localization of iron(III) reducing capacity in Fe deficient squash roots. Plant Soil 225: 21–31 [Google Scholar]
- Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, Alcántara E, Pérez-Vicente R (2007) Ethylene involvement in the regulation of the H(+)-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45: 293–301 [DOI] [PubMed] [Google Scholar]
- Waters BM, McInturf SA, Stein RJ (2012) Rosette iron deficiency transcript and microRNA profiling reveals links between copper and iron homeostasis in Arabidopsis thaliana. J Exp Bot 63: 5903–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyńska A, Lewandowska M, Sirko A (2010) Nicotiana tabacum EIL2 directly regulates expression of at least one tobacco gene induced by sulphur starvation. J Exp Bot 61: 889–900 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ou B, Zhang J, Si W, Gu H, Qin G, Qu LJ (2014) The Arabidopsis Mediator subunit MED16 regulates iron homeostasis by associating with EIN3/EIL1 through subunit MED25. Plant J 77: 838–851 [DOI] [PubMed] [Google Scholar]
- Zaid H, El Morabet R, Diem HG, Arahou M (2003) Does ethylene mediate cluster root formation under iron deficiency? Ann Bot (Lond) 92: 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Wang G, Hu X, Wang H, Du L, Zhu Y (2014) Role of microRNAs in plant responses to nutrient stress. Plant Soil 374: 1005–1021 [Google Scholar]
- Zhang YJ, Lynch JP, Brown KM (2003) Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J Exp Bot 54: 2351–2361 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liao H, Lucas WJ (2014) Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J Integr Plant Biol 56: 192–220 [DOI] [PubMed] [Google Scholar]
- Zhao W, Yang X, Yu H, Jiang W, Sun N, Liu X, Liu X, Zhang X, Wang Y, Gu X (2015) RNA-Seq-based transcriptome profiling of early nitrogen deficiency response in cucumber seedlings provides new insight into the putative nitrogen regulatory network. Plant Cell Physiol 56: 455–467 [DOI] [PubMed] [Google Scholar]
- Zheng D, Han X, An YI, Guo H, Xia X, Yin W (2013) The nitrate transporter NRT2.1 functions in the ethylene response to nitrate deficiency in Arabidopsis. Plant Cell Environ 36: 1328–1337 [DOI] [PubMed] [Google Scholar]
- Zuchi S, Cesco S, Varanini Z, Pinton R, Astolfi S (2009) Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta 230: 85–94 [DOI] [PubMed] [Google Scholar]