Fatty acid biosynthesis mutants are temperature sensitive, but mutations in any of five genes of the prokaryotic lipid synthesis pathway suppress this low-temperature phenotype and rescue cold tolerance.
Abstract
The Arabidopsis (Arabidopsis thaliana) fatty acid biosynthesis1 (fab1) mutant has increased levels of the saturated fatty acid 16:0 due to decreased activity of 3-ketoacyl-acyl carrier protein (ACP) synthase II. In fab1 leaves, phosphatidylglycerol, the major chloroplast phospholipid, contains up to 45% high-melting-point molecular species (molecules that contain only 16:0, 16:1-trans, and 18:0), a trait associated with chilling-sensitive plants, compared with less than 10% in wild-type Arabidopsis. Although they do not exhibit typical chilling sensitivity, when exposed to low temperatures (2°C–6°C) for long periods, fab1 plants do suffer collapse of photosynthesis, degradation of chloroplasts, and eventually death. A screen for suppressors of this low-temperature phenotype has identified 11 lines, some of which contain additional alterations in leaf-lipid composition relative to fab1. Here, we report the identification of two suppressor mutations, one in act1, which encodes the chloroplast acyl-ACP:glycerol-3-phosphate acyltransferase, and one in lpat1, which encodes the chloroplast acyl-ACP:lysophosphatidic acid acyltransferase. These enzymes catalyze the first two steps of the prokaryotic pathway for glycerolipid synthesis, so we investigated whether other mutations in this pathway would rescue the fab1 phenotype. Both the gly1 mutation, which reduces glycerol-3-phosphate supply to the prokaryotic pathway, and fad6, which is deficient in the chloroplast 16:1/18:1 fatty acyl desaturase, were discovered to be suppressors. Analyses of leaf-lipid compositions revealed that mutations at all four of the suppressor loci result in reductions in the proportion of high-melting-point molecular species of phosphatidylglycerol relative to fab1. We conclude that these reductions are likely the basis for the suppressor phenotypes.
Many plants that are native to tropical and subtropical regions undergo sharp reductions in photosynthesis and growth at temperatures between 0°C and 12°C, and often exhibit extensive tissue damage after even short exposure to chilling temperatures (Lyons, 1973; Nishida and Murata, 1996; Iba, 2002). Such chilling-sensitive species include many major crops, such as maize (Zea mays), rice (Oryza sativa), and soybean (Glycine max). By contrast, most plants of temperate origin, including Arabidopsis (Arabidopsis thaliana), are able to grow and develop at chilling temperatures and are classified as chilling resistant. Understanding the physical and cellular processes that contribute to chilling sensitivity has long been a goal of plant biology because of the critical importance of crop production across the globe.
The chloroplast membranes of plants host the critical light-harvesting and electron transport reactions of photosynthesis and have a characteristic fatty acid composition. Highly polyunsaturated fatty acids, combinations of 18:3 and 16:3 depending on the type of plant, account for approximately 70% of all thylakoid membrane fatty acids, including over 90% of the fatty acids found in monogalactosyldiacylglycerol (MGD), the most abundant chloroplast lipid (Wada and Murata, 2007). Since polyunsaturated fatty acids increase the fluidity of membranes, it has been inferred that the high levels of fluidity provided by these polyunsaturates is important for chloroplast membrane function, especially at chilling temperatures, when membrane fluidity is reduced (Upchurch, 2008). However, although mutants of Arabidopsis with lower levels of chloroplast unsaturation do show low-temperature phenotypes (Wallis and Browse, 2002), they are still chilling resistant.
A long-standing hypothesis in chilling sensitivity proposes that a phase transition in cellular membranes from liquid-crystalline to gel phase leads to disrupted metabolism in chilled cells and is the underlying cause of injury and death of chilling-sensitive plants. A liquid-crystalline to gel phase transition is not expected for lipids containing polyunsaturated fatty acids, but not all chloroplast acyl lipids are highly unsaturated. One specific model for phase-change effects concerns the major chloroplast phospholipid, phosphatidylglycerol (PG). This glycerolipid is indispensable for both chloroplast development and photosynthetic function in seed plants (Hagio et al., 2002; Xu et al., 2002; Frentzen, 2004), and both of these processes are inhibited by chilling. In particular, PG molecules that contain only saturated or trans-unsaturated fatty acids (16:0, 18:0, and 16:1-trans) at both sn-1 and sn-2 positions of the glycerol backbone, referred to as high-melting-point molecular species, have been correlated with plant chilling sensitivity (Murata and Yamaya, 1984; Wada and Murata, 2007). Typically, chilling-resistant plants contain less than 10% of their leaf PG as high-melting-point molecular species, whereas many chilling-sensitive plants have >30%.
Glycerolipids are synthesized by two distinct pathways in the leaf cells of higher plants. The lipids synthesized in chloroplasts result when glycerol-3-phosphate, synthesized from dihydroxyacetone phosphate through action of the enzyme glycerol-3-phosphate dehydrogenase (GLY1), is combined with fatty acids synthesized as acyl-ACP (for acyl carrier protein) molecules derived from fatty acid synthase. This reaction is mediated by acyl-ACP:glycerol-3-phosphate acyltransferase (ACT1), and the product is lysophosphatidic acid (LPA). The resulting LPA is converted by acyl-ACP:lysophosphatidic acid acyltransferase (LPAT1) to phosphatidic acid. Phosphatidic acid in turn is used either for synthesis of PG, a lipid that in Arabidopsis is synthesized solely in the chloroplast, or for synthesis of diacylglycerol in the pathway leading to MGD and digalactosyldiacylglycerol (DGD). Some of the fatty acids incorporated into PG, MGD, or DGD can be further desaturated by the fatty acid desaturase4 (FAD4), FAD5, and FAD6 desaturases. The similarity of this synthesis to cyanobacterial lipid synthesis led to its description as the prokaryotic pathway.
A complementary eukaryotic pathway relies on hydrolysis of acyl-ACPs, followed by export of 16:0 and 18:1 from the plastid as CoA thioesters with rapid incorporation of these fatty acids into phosphatidylcholine (Bates et al., 2007). Notably, the diacylglycerol moiety of phosphatidylcholine can be returned to the chloroplast envelope and incorporated into thylakoid lipids (Benning, 2009), providing a second route for the synthesis of MGD, DGD, and sulfoquinovosyl diacylglycerol, but not PG. In Arabidopsis and other plants, PG is produced exclusively through the chloroplast pathway (Browse et al., 1986; Wallis and Browse, 2010).
In the fatty acid biosynthesis1 (fab1) mutant of Arabidopsis, a mutation in the gene encoding β-ketoacyl-[ACP] synthase II (KASII) disrupts elongation of 16:0-ACP to 18:0-ACP (Carlsson et al., 2002), producing plants that have increased levels of 16:0 in all membrane glycerolipids (Wu et al., 1994). In particular, fab1 plants contain high-melting-point molecular species of PG at levels similar to many chilling-sensitive plant species (Wu and Browse, 1995). Nevertheless, the fab1 mutant does not show typical symptoms of chilling sensitivity and was unaffected, in comparison with wild-type controls, by a range of chilling treatments that killed chilling-sensitive plants. Instead, fab1 plants only show a collapse of photosynthesis and eventual death after prolonged exposure to low temperatures (Wu and Browse, 1995; Wu et al., 1997).
To examine the relationship between the altered fatty acid composition and collapse of photosynthesis in fab1, we conducted a suppressor screen in the fab1 background (Barkan et al., 2006), mutagenizing fab1 seed and examining the M2 progeny for survival at 2°C. Previously, we have characterized suppressor mutants deficient in the second enzyme of the prokaryotic pathway, lpat1-3, and in the MGD 16:0 Δ7 desaturase (fad5; Barkan et al., 2006; Kim et al., 2010). Here, we use map-based cloning and full genome sequencing to identify the genetic loci of two additional suppressors from our screen in lines S106 and S96. The mutation in S106 is a new hypomorphic allele at the LPAT1 locus, lpat1-4. The suppressor in the S96 line is a hypomorphic mutation in ACT1, which encodes the first enzyme of the prokaryotic pathway, acyl-ACP:glycerol-3P acyltransferase. Because these genes are both important in chloroplast lipid biosynthesis, we hypothesized that mutations of other prokaryotic pathway genes might also suppress fab1. We therefore tested mutations affecting the chloroplast gly1 and the chloroplast 16:1/18:1 fatty acid desaturase (fad6). Both of these mutations also acted as suppressors of the fab1 low-temperature phenotype.
RESULTS
The Suppressor Mutation in Line S106 Is a New Allele of lpat1
Our screen (Barkan et al., 2006) for mutations that suppress the collapse of photosynthesis and death that are characteristic of fab1 plants exposed to 2°C (Wu and Browse, 1995; Wu et al., 1997) identified 11 suppressor lines, with five of these having additional changes (relative to fab1) in the fatty acid composition of leaf lipids. Plants of the S106 line grew normally at 22°C and, although they were less robust under chilling temperatures than wild-type Arabidopsis, they maintained photosynthetic function and continued to produce new green leaf tissue long after the chilling regimen at 2°C had killed fab1 (Barkan et al., 2006). The changes in leaf fatty acid composition between fab1 and S106 are relatively subtle (Supplemental Figure S1); however, the small but significant decrease in 16:3 from 15.6% to 13.7% provided a clue that the S106 line has a decrease in flux through the prokaryotic pathway of glycerolipid synthesis, which is the pathway responsible for 16:3 production (Browse et al., 1986).
To identify the suppressor mutation, we crossed S106, which is in the Columbia-0 (Col-0) background, to Landsberg erecta. The resulting F1 plants were allowed to self-pollinate to generate an F2 mapping population. Approximately 800 F2 plants were grown at 22°C for 21 d, then transferred to 2°C for 28 d. Both S106 and fab1 plants grew poorly at 2°C and were smaller than the wild type after this 7-week regimen (Supplemental Fig. S2). Larger, robust plants that were either wild type or heterozygous at the fab1 locus were culled from the F2 population, retaining only smaller fab1 and S106 plants, which were cultivated for an additional 3 months at 2°C (Supplemental Fig. S2). At that time, senescent fab1 plants had died, but S106 plants were still green. We selected 50 putative homozygous S106 suppressor plants based on their healthy phenotype and genotyped each candidate with a Cleaved Amplified Polymorphic Sequences (CAPS) marker for fab1 to confirm their homozygosity at the fab1 locus.
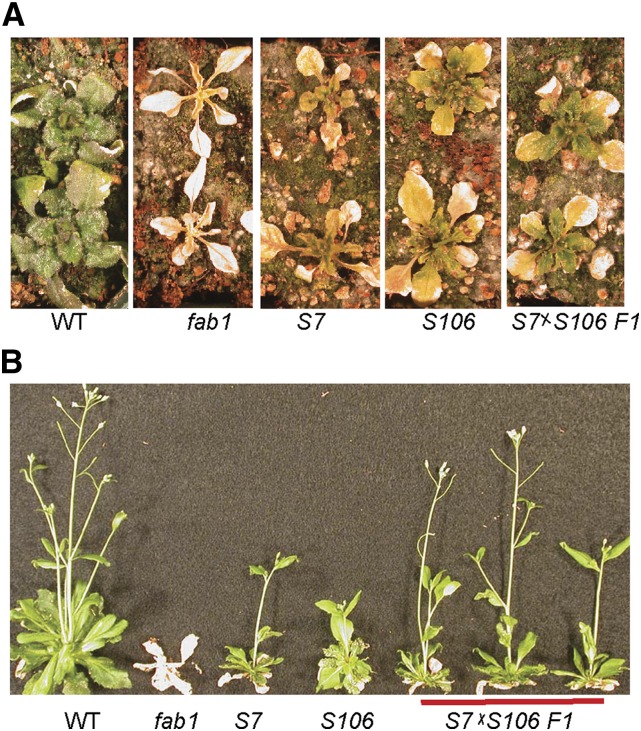
We analyzed these 50 S106 plants using established map-based cloning techniques (Lukowitz et al., 2000; Jander et al., 2002). The analysis indicated that S106 was confined to chromosome IV between 14.55 and 16.7 Mbp. Genomic DNA from the 50 plants was pooled and sequenced. A total of 42 single-nucleotide polymorphisms (SNPs) were identified in the 2-Mbp map window. Twelve of these produced missense mutations in nine proteins (Table I). One confirmed SNP of particular interest was identified in LPAT1 (At4g30580), which encodes the chloroplast LPA acyltransferase (Kim and Huang, 2004). The S106 allele of lpat1 contains a C to T transition at nucleotide 689 of the open reading frame, creating a missense mutation at Pro-230, converting it to Leu (P230L; Table I). We have previously determined that the fab1 suppressor in the S7 line from our screen is a mutation in LPAT1, lpat1-3 (Kim et al., 2010). To determine whether the newly identified P230L mutation, designated lpat1-4, is responsible for suppression of the fab1 low-temperature phenotype, we crossed plants of the S106 and S7 lines. All of the F1 progeny of this cross (fab1/fab1 lpat1-3/lpat1-4) continued to produce green leaves after 75 d at 2°C (Fig. 1A) and, similar to the S7 and S106 parents, were able to grow to maturity and produce seeds following a return to 22°C (Fig. 1B). By contrast, fab1 control plants in this experiment died. Genetic analysis indicates that the suppressor phenotype in both S7 and S106 is recessive (Barkan et al., 2006), so these results demonstrate that the hypomorphic lpat1-4 allele is the suppressor mutation in S106.
Table I. Twelve SNPs that produced missense mutations in the corresponding protein were identified by full genome sequencing within the region determined by mapping the chilling-resistant phenotype of S106.
*, Stop translation codon.
| Locus | Function | Nucleotide Change | Amino Acid Change |
|---|---|---|---|
| AT4G30030 | Aspartic-type endopeptidase | C701T | Thr-234Ile |
| AT4G30120 | P1B-type ATPase zinc transport (HMA3) | C1061T | Pro-354Leu |
| AT4G30580 | Plastidic lysophosphatidic acid acyltransferase (LPAT1) | C689CT | Pro-230Leu |
| AT4G30720 | Photosynthetic oxidoreductase in the chloroplast stroma (PDE327) | T963G | Asp-321Glu |
| AT4G32150 | Soluble N-ethyl-maleimide sensitive factor attachment protein receptor family (VAMP711) | G533A | Arg-178His |
| AT4G33720 | Cys-rich secretory protein | C307T | Gln-103* |
| AT4G34170 | Gal oxidase/kelch repeat superfamily protein | G298A | Gly-100Arg |
| AT4G34280 | Cullin4-RING ubiquitin E3 ligase complex (DHU1) | T197C | Leu-66Ser |
| A203T | Asn-68Ile | ||
| G207T | Glu-69Asp | ||
| A211G | Asn-71Asp | ||
| AT4G35000 | Microsomal ascorbate peroxidase (APX3) | C466G | Leu-156Val |
Figure 1.
The suppressor in line S106 is a new lpat1 allele. A, After 75 d at 2°C, wild-type (WT), S7, S106, and S7×S106 F1 plants had survived, but fab1 plants were dead. B, All plants except fab1 recovered when transferred to 22°C.
Map-Based Cloning of a Lipid Mutation in S96
The S96 suppressor line has not been previously described, but like other fab1 suppressors, it survives growth at 2°C, and like the characterized S31 and S7 lines (Barkan et al., 2006), it has a reduced proportion of 16:3 in the leaf fatty acid profile. In plants grown at 22°C, leaves of S96 plants contained 8.4% 16:3 compared with 16.5% in fab1 and 17.6% in the wild type (Table II). There is also a decrease in 16:0 from 23.0% in fab1 to 15.1% in S96. The reductions in 16:0 and 16:3 are associated with increases in the unsaturated 18-carbon fatty acids (18:1+18:2+18:3), which total 72.9% in S96 compared with 57.3% in fab1. These fatty acid changes indicate that the S96 line has a mutation that affects glycerolipid synthesis by the prokaryotic pathway. We have previously shown that mutations affecting the prokaryotic pathway in S31 (fab1 fad5-2) and S7 (fab1 lpat1-3) lines act to suppress the fab1 low-temperature phenotype (Barkan et al., 2006; Kim et al., 2010). For this reason, we hypothesized that the mutation affecting the prokaryotic pathway in S96 might be the fab1 suppressor and chose to map the locus based on segregation of the low-16:3 phenotype.
Table II. Fatty acid profile from leaves of plants grown at 22°C for 21 d.
Data are means ± se, n = 4.
| Genotype | Leaf Fatty Acids as Percentage of Total |
||||||
|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |
| Wild type | 13.2 ± 0.2 | 1.3 ± 0.0 | 17.6 ± 0.4 | 0.2 ± 0.0 | 1.9 ± 0.5 | 10.8 ± 0.4 | 54.6 ± 0.9 |
| fab1 | 23.0 ± 1.6 | 2.6 ± 0.3 | 16.5 ± 0.9 | 0.3 ± 0.0 | 3.6 ± 0.4 | 5.7 ± 0.9 | 48.0 ± 1.3 |
| S96 (fab1 act1-6) | 15.1 ± 0.1 | 3.3 ± 0.1 | 8.4 ± 0.5 | 0.1 ± 0.0 | 7.4 ± 0.7 | 10.1 ± 0.4 | 55.4 ± 1.1 |
| fab1 gly1 | 18.1 ± 0.4 | 3.1 ± 0.0 | 3.6 ± 0.3 | 0.2 ± 0.0 | 8.7 ± 0.4 | 10.6 ± 0.6 | 55.6 ± 0.4 |
| fab1 fad6 | 21.2 ± 0.7 | 10.7 ± 0.3 | 0.3 ± 0.0 | 0.3 ± 0.1 | 16.9 ± 1.3 | 11.0 ± 1.2 | 38.3 ± 3.1 |
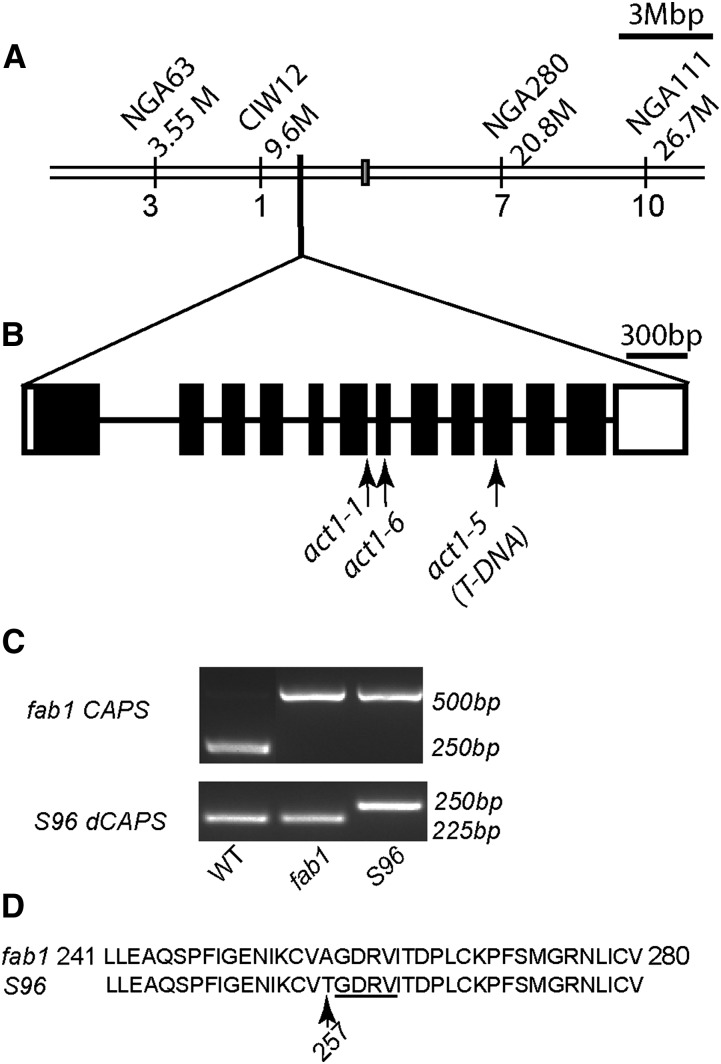
We crossed S96 to Landsberg erecta to create an F2 mapping population of approximately 100 plants. We measured the fatty acid composition of leaves from emerging plants and selected 24 plants with 16:3 less than 10% of total fatty acids. The remaining plants had 16:3 greater than 13% of total leaf fatty acids. The 24 selected plants were genotyped using established PCR protocols to identify simple sequence length polymorphism (SSLP) markers (Lukowitz et al., 2000). The low-16:3 trait found in S96 mapped close to ciw12 on chromosome 1 (Fig. 2A), with only a 4.2% recombination frequency separating the two loci. The ACT1 (At1g32200) gene that encodes the first enzyme of the prokaryotic pathway (acyl-ACP:glycerol-3-phosphate acyltransferase) is close to ciw12 (Lukowitz et al., 2000), and the reduced 16:3 and 16:0 in S96 leaves is consistent with a hypomorphic act1 mutation (Kunst et al., 1988; Xu et al., 2006). We first tested the hypothesis that S96 might be a new allele of ACT1 by amplifying the complementary DNA (cDNA) sequence of the ACT1 gene from S96. The analysis detected a single nucleotide change in the eighth exon of the open reading frame, from G to A at nucleotide 1768 in the genomic sequence, which created an Ala-257 to Thr missense alteration (A257T) in the predicted amino acid sequence (Fig. 2B). We designed a derived Cleaved Amplified Polymorphic Sequence (dCAPS) marker, dCAPS1768, to facilitate tracking this mutation (Fig. 2C). The mutation occurs in a recognized conserved glycerol-3-phosphate acyltransferase-acyl-ACP:lysophosphatidic acid acyltransferase domain (Turnbull et al., 2001; Tamada et al., 2004) and is immediately adjacent to a putative glycerol-3-phosphate binding-pocket motif (Fig. 2D). To confirm that the altered leaf composition of S96 plants is caused by an act1 mutation, we crossed S96 to the act1-1 mutant (Kunst et al., 1988). The F1 progeny of the cross all had a reduced proportion of 16:3 and increased 18-carbon fatty acids relative to wild-type and fab1 controls. We conclude that the differences in leaf fatty acid composition between S96 and fab1 are caused by the mutation in act1 that we designate act1-6.
Figure 2.
Identifying the S96 gene locus by map-based cloning. A, The S96 gene locus was closely linked to the ciw12 marker on chromosome I. B, The ACT1 coding region showing the location of the act1-6 mutation identified in line S96. Locations of the act1-1 and act1-5 mutations are also shown. Black boxes indicate exons, and lines indicate introns. C, Genotyping the S96 mutant by the fab1 CAPS and S96 dCAPS markers. D, The act1-6 mutation causes an amino acid change (A257T) in a conserved domain of the ACT1 protein, immediately adjacent to the putative glycerol-3-phosphate binding pocket motif (underlined). T-DNA, Transfer DNA; WT, wild type.
Mutations in ACT1 Suppress the fab1 Low-Temperature Phenotype
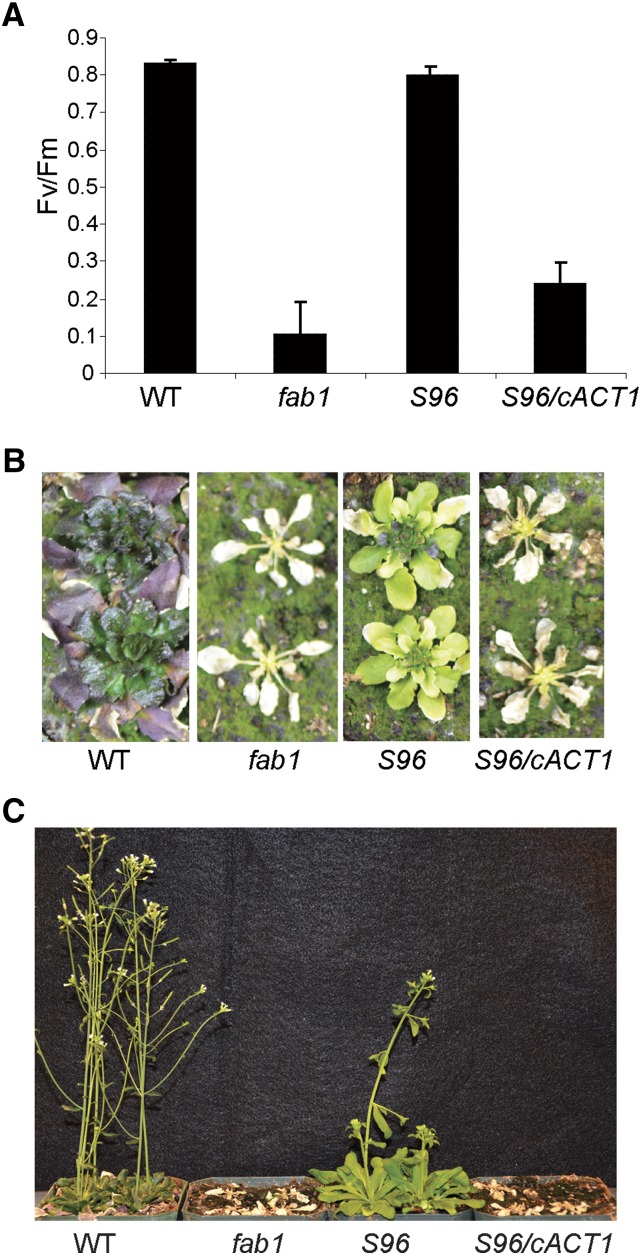
To test whether the act1-6 mutation in S96 is responsible for suppression of the fab1 chilling phenotype, we transformed S96 plants with an ACT1 cDNA under control of the Cauliflower mosaic virus 35S promoter. Three independent transformants (termed S96/cACT1) all showed complementation of the leaf fatty acid composition to that of the fab1 parental line. The fab1 act1-6 genotype of these three lines was confirmed using the fab1 CAPS and act1-6 dCAPS1768 markers. Plants of these three S96/cACT1 lines were grown alongside S96, fab1, and wild-type plants for 21 d before transfer to 2°C. After 38 d at 2°C, we used fluorescence analysis to measure the potential quantum yield of PSII (ratio of variable fluorescence to maximal fluorescence [Fv/Fm]) of the plants. Like other fab1 suppressor lines (Barkan et al., 2006), S96 maintains Fv/Fm close to the wild type (0.80 ± 0.01 and 0.83 ± 0.01, respectively; Fig. 3A). However, both fab1 and S96/cACT1 plants had an Fv/Fm < 0.3, indicating a collapse of photosynthesis that is characteristic of the fab1 phenotype (Wu et al., 1997). These results demonstrate that expression of wild-type ACT1 restored low-temperature sensitivity to the S96 plants. Cold treatment was continued to a total of 79 d, at which time both fab1 and S96/35S-act1 plants appeared dead (Fig. 3B), and indeed neither recovered when transferred to growth conditions at 22°C, whereas both wild-type and S96 plants recovered and set seed (Fig. 3C).
Figure 3.
Expression of the ACT1 coding sequence complements the phenotype of S96. A, After 38 d at 2°C, wild-type (WT) and the S96 suppressor plants maintain high Fv/Fm, but fab1 and S96 plants expressing a wild-type ACT1 cDNA (S96/cACT1) suffer dramatic loss of Fv/Fm. Data are the means ± se for four replicates. B, After 79 d at 2°C, wild-type and S96 plants survived, but fab1 and S96/cACT1 plants had died. C, When returned to 22°C, only wild-type and S96 plants recovered.
We also tested the effects of act1-1 and act1-5 alleles (Xu et al., 2006) on the fab1 low-temperature phenotype by crossing these into the fab1 line. When grown at 22°C, both fab1 act1-1 and fab1 act1-5 double-homozygous mutants were slightly chlorotic and smaller than fab1 act1-6 plants (Supplemental Fig. S3). After transfer to 2°C for 65 d, all of the fab1 act1 double mutants were alive, and they recovered and set seed after being returned to 22°C. By contrast, fab1 control plants died in the cold (Supplemental Fig. S3). Plants of the fab1 act1-6 line generally appeared more healthy than fab1 act1-1 and fab1 act1-5 plants in the cold and produced more seeds after returning to 22°C (Supplemental Fig. S3).
gly1 and fad6 Mutations Also Suppress fab1 Chilling Sensitivity
The fab1 suppressors that we have identified to date are mutations in genes that encode enzymes of the chloroplast-localized prokaryotic pathway of glycerolipid synthesis. For this reason, we next tested mutations in two other genes, gly1, encoding chloroplast glycerol-3-phosphate dehydrogenase (Miquel et al., 1998; Kachroo et al., 2004), and fad6, encoding the chloroplast 16:1/18:1 fatty acid desaturase (Browse et al., 1989), as potential fab1 suppressors. Each of these mutants has a fatty acid phenotype in its leaves. Plants mutant in gly1 have low levels of 16:3 in leaf lipids, and fad6 plants have higher levels of 18:1 and 16:1 fatty acids than the wild type.
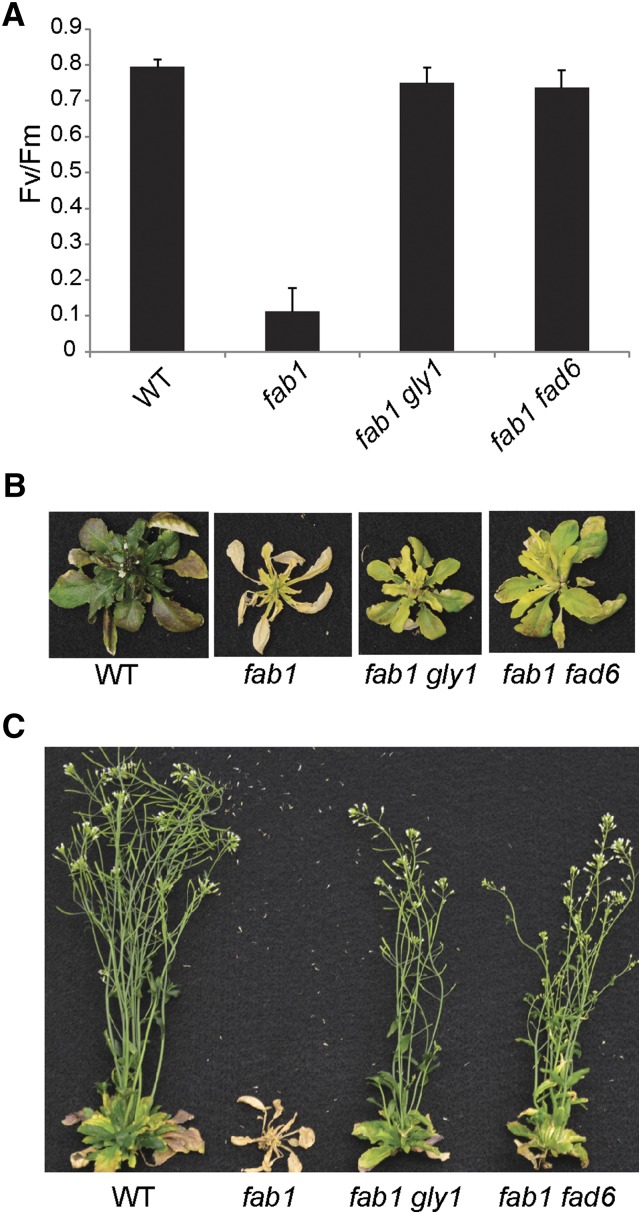
To test the suppressor hypothesis, we generated plant lines doubly mutant in fab1 gly1 and in fab1 fad6, confirming the presence of mutant alleles by analysis of leaf fatty acids (Table II). To assess chilling sensitivity, these double-mutant plants together with fab1 and wild-type controls were grown at 22°C for 21 d and then transferred to 2°C. As expected, after 30 d at 2°C, the Fv/Fm of wild-type plants was approximately 0.8, but only 0.1 for fab1. Plants harboring either gly1 or fad6 mutation in the fab1 background exhibited a Fv/Fm near 0.8, similar to the wild-type plants (Fig. 4A). After 65 d at 2°C, fab1 leaves were completely senescent, but wild-type, fab1 gly1, and fab1 fad6 plants survived (Fig. 4B). After plants were moved back to 22°C, the fab1 plants died, but fab1 gly1 and fab1 fad6 mutant plants recovered, flowered, and set seed (Fig. 4C). These results demonstrate that these additional mutants in the prokaryotic lipid synthesis pathway also suppress the fab1 low-temperature phenotype.
Figure 4.
The fab1 phenotype is suppressed in fab1 gly1 and fab1 fad6 double mutants. A, Photosynthetic Fv/Fm of wild-type (WT), fab1, fab1 gly1, and fab1 fad6 plants were analyzed after 30 d at 2°C. Data are the means ± se for four replicates. B, Appearance of plants after 65 d at 2°C. C, The same plants transferred to 22°C for 1 week. Wild-type, fab1 gly1, and fab1 fad6 double mutants all recovered, but fab1 mutants died.
The act1, gly1, and fad6 Suppressor Mutations All Reduce Levels of High-Melting-Point PG
To investigate changes in fatty acid and lipid composition, we first analyzed total leaf fatty acids of fab1 act1-6, fab1 gly1, and fab1 fad6 plants grown for 21 d at 22°C, and then again after a further 20 d at 2°C. At 22°C, the 16:0 content of total leaf lipids in fab1 was almost 10 percentage points higher than in the wild type (Table II). However, the 16:0 level of each of the suppressors was lower than that of fab1 but higher than wild-type plants. The three suppressor lines also have substantially lower 16:3 and increased levels of 18-carbon unsaturated fatty acids compared with fab1. After 20 d at 2°C, the 16:0 proportion of total fatty acids increased in all lines as compared with 22°C, but all three suppressors maintained substantially lower 16:0 than fab1 (Table III).
Table III. Fatty acid profile from leaves of plants grown at 22°C for 21 d followed by cultivation at 2°C for 20 d.
Data are means ± se, n = 5.
| Genotype | Leaf Fatty Acids as Percentage of Total | ||||||
|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |
| Wild type | 20.5 ± 0.2 | 1.1 ± 0.1 | 10.1 ± 0.2 | 0.9 ± 0.1 | 2.7 ± 0.3 | 15.9 ± 0.6 | 48.6 ± 0.8 |
| fab1 | 28.5 ± 0.2 | 3.6 ± 0.1 | 11.4 ± 0.1 | 1.1 ± 0.1 | 4.1 ± 0.2 | 6.9 ± 0.2 | 43.7 ± 0.4 |
| S96 (fab1 act1-6) | 20.4 ± 0.2 | 4.3 ± 0.1 | 6.9 ± 0.1 | 1.0 ± 0.1 | 5.8 ± 0.2 | 12.5 ± 0.3 | 48.4 ± 0.5 |
| fab1 gly1 | 21.1 ± 0.2 | 4.2 ± 0.1 | 5.2 ± 0.1 | 1.0 ± 0.0 | 5.2 ± 0.1 | 11.6 ± 0.2 | 51.3 ± 0.3 |
| fab1 fad6 | 22.7 ± 0.3 | 12.1 ± 0.4 | 0.8 ± 0.0 | 0.6 ± 0.1 | 17.6 ± 0.3 | 11.1 ± 0.3 | 33.9 ± 1.1 |
We next separated leaf glycerolipids by thin-layer chromatography (TLC) and determined the fatty acid composition of individual membrane lipids (Table IV). As reported previously (Wu et al., 1997), all membrane glycerolipids in fab1 leaves had higher 16:0 content than found in the wild type, and 16:3 was increased in MGD. All three suppressor mutations resulted in reductions of 16:0 in all of the major leaf glycerolipids, sometimes to levels comparable with the wild type, but more often to values intermediate between the wild type and fab1 (Table IV). The reductions in 16:0 content of PG are particularly noteworthy because molecular species of PG containing saturated (16:0 or 18:0) and trans-unsaturated (16:1-trans) fatty acids at both the sn-1 and sn-2 positions of the glycerol backbone have been associated with low-temperature damage and death, both in chilling-sensitive plant species (Murata and Yamaya, 1984; Nishida and Murata, 1996) and in the fab1 mutant (Wu et al., 1997).
Table IV. Fatty acid compositions of leaf lipids from wild-type and mutant Arabidopsis grown at 22°C.
Data are the average of four replicates. DGD, Digalactosyldiacylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SQ, sulfoquinovosyl diacylglycerol.
| Lipid Class | Genotype | Percentage of Total Polar Lipids | Fatty Acid as Percentage of Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| MGD | Wild type | 25.3 | 5.1 | 3.2 | 26.6 | 2.0 | 3.8 | 4.8 | 51.6 |
| fab1 | 29.8 | 8.8 | 4.0 | 31.0 | 1.1 | 3.6 | 2.6 | 46.4 | |
| fab1 act1-6 | 30.1 | 5.1 | 2.7 | 14.8 | 0.9 | 4.8 | 3.1 | 67.4 | |
| fab1 gly1 | 35.3 | 4.5 | 1.9 | 15.4 | 0.1 | 3.0 | 2.4 | 71.6 | |
| fab1 fad6 | 38.6 | 4.4 | 29.0 | 0 | 0.2 | 32.2 | 1.1 | 31.6 | |
| PG | Wild type | 15.6 | 30.6 | 23.1 | 3.6 | 4.0 | 7.9 | 9.3 | 21.5 |
| fab1 | 19.4 | 48.5 | 21.2 | 3.3 | 3.1 | 5.5 | 4.5 | 13.9 | |
| fab1 act1-6 | 13.2 | 31.0 | 28.1 | 2.6 | 2.8 | 5.5 | 7.9 | 22.9 | |
| fab1 gly1 | 12.6 | 33.6 | 26.1 | 0.9 | 1.2 | 5.0 | 9.3 | 24.0 | |
| fab1 fad6 | 14.2 | 40.3 | 24.4 | 0 | 1.0 | 27.6 | 3.4 | 3.4 | |
| DGD | Wild type | 17.6 | 24.0 | 0.4 | 3.1 | 3.7 | 2.8 | 6.9 | 58.5 |
| fab1 | 12.4 | 38.6 | 1.9 | 3.8 | 3.9 | 3.6 | 4.2 | 43.0 | |
| fab1 act1-6 | 14.4 | 24.5 | 2.3 | 2.1 | 2.8 | 5.8 | 3.7 | 58.2 | |
| fab1 gly1 | 13.8 | 21.4 | 1.7 | 1.2 | 1.0 | 2.9 | 2.0 | 69.4 | |
| fab1 fad6 | 13.7 | 24.0 | 6.0 | 0 | 1.5 | 15.1 | 1.2 | 52.2 | |
| SQ, PI | Wild type | 7.9 | 40.9 | 0.4 | 3.2 | 8.6 | 3.9 | 15.1 | 27.3 |
| fab1 | 8.7 | 53.7 | 1.6 | 2.9 | 5.6 | 3.6 | 10.8 | 21.8 | |
| fab1 act1-6 | 9.0 | 43.3 | 1.6 | 1.3 | 4.0 | 5.0 | 16.3 | 27.4 | |
| fab1 gly1 | 7.9 | 50.1 | 0.7 | 0.1 | 3.3 | 3.5 | 14.4 | 27.9 | |
| fab1 fad6 | 7.7 | 49.6 | 2.9 | 0.2 | 3.1 | 13.2 | 12.2 | 18.8 | |
| PE | Wild type | 14.7 | 28.9 | 0.4 | 3.4 | 7.6 | 4.8 | 30.2 | 23.6 |
| fab1 | 13.4 | 38.2 | 3.0 | 1.3 | 4.8 | 5.6 | 23.7 | 23.4 | |
| fab1 act1-6 | 14.2 | 33.4 | 1.6 | 1.2 | 3.1 | 10.0 | 34.2 | 16.2 | |
| fab1 gly1 | 11.0 | 34.4 | 0.8 | 0 | 2.4 | 8.5 | 33.9 | 19.7 | |
| fab1 fad6 | 10.0 | 36.9 | 2.2 | 0.3 | 3.2 | 8.3 | 31.8 | 16.0 | |
| PC | Wild type | 15.5 | 25.4 | 0.4 | 0.9 | 4.8 | 8.4 | 33.9 | 26.1 |
| fab1 | 16.3 | 35.3 | 4.0 | 1.8 | 5.1 | 6.4 | 20.7 | 26.3 | |
| fab1 act1-6 | 19.2 | 26.3 | 2.8 | 1.1 | 2.8 | 13.9 | 30.9 | 22.0 | |
| fab1 gly1 | 19.5 | 26.7 | 2.3 | 0.5 | 2.2 | 11.3 | 29.5 | 27.4 | |
| fab1 fad6 | 15.8 | 29.5 | 6.0 | 0 | 2.8 | 10.0 | 30.0 | 21.6 | |
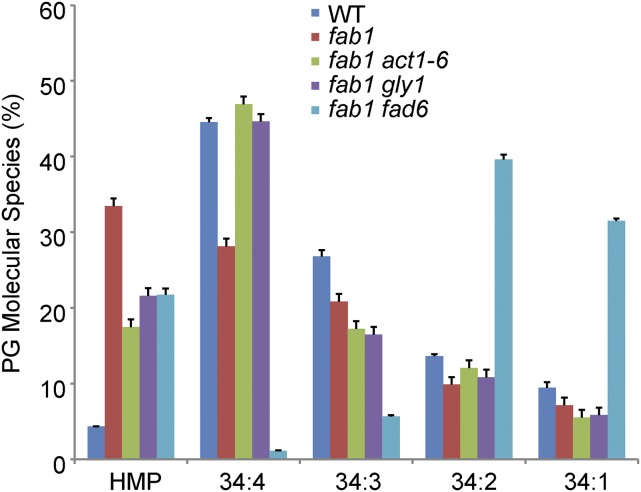
To directly quantify the molecular species of PG and other glycerolipids, we submitted samples from fab1 act1-6, fab1 gly1, and fab1 fad6 plants, along with wild-type and fab1 controls, for lipidomics analysis by mass spectrometry. The results for PG indicate that the PG high-melting-point molecular species (PG 32:1 + PG 32:0 + PG 34:0) amount to 4.3% of total PG in the wild type but 33.5% in the fab1 mutant (Fig. 5). In each of the three suppressor lines, the high-melting-point species of PG are considerably lower than in fab1: 17.5% for fab1 act1-6, 21.6% for fab1 gly1, and 21.7% for fab1 fad6. A spreadsheet containing the lipidomics data for all of the leaf glycerolipids analyzed in the five lines is included as Supplemental Table S1.
Figure 5.
Three mutations reduce levels of high-melting-point (HMP) molecular species of PG. High-melting-point molecules are the sum of 18:0/16:0, 16:0/16:1, and 16:0/16:0 fatty acid combinations; see “Materials and Methods” for details. Data are means ± se for four replicates.
DISCUSSION
Many plants that are native to tropical and subtropical habitats suffer tissue damage and reduced growth after even short exposure to temperatures between 0°C and 12°C. This chilling sensitivity and the associated reductions in growth and yield can have severe effects on world food supplies (Vinocur and Altman, 2005; Thakur et al., 2010). Chilling-sensitive crops include maize, rice, soybean, and cotton (Gossypium hirsutum; Lyons, 1973). Understanding what produces sensitivity to chilling and what will alleviate it has the potential to ameliorate chilling damage by environmental control, by traditional breeding, and by molecular-genetic manipulation of plants (Vinocur and Altman, 2005).
One model of chilling sensitivity proposes that damage originates from a change in the physical state of membrane lipids, from the liquid-crystalline phase of normal temperatures to a gel phase produced when temperatures drop (Lyons, 1973). A version of this model specific to the chloroplast (plastid) lipids proposes that molecular species of the major chloroplast phospholipid, PG, containing only saturated and trans-unsaturated fatty acids (16:0, 18:0, and 16:1-trans) at both the sn-1 and sn-2 of the glycerol backbone (high-melting-point molecular species) confer chilling sensitivity (Murata, 1983; Nishida and Murata, 1996). Results supporting this model include a broad correlation across plant species between the severity of chilling sensitivity and the proportion of high-melting-point PG in their leaf lipids (Murata, 1983; Roughan, 1985) and the detection of liquid-crystalline to gel phase transitions in PG isolated from some chilling-sensitive plants (Murata and Yamaya, 1984). Transgenic approaches that alter PG composition also support a role for high-melting-point PG in plant chilling responses (Murata et al., 1992; Wolter et al., 1992; Moon et al., 1995; Ishizaki-Nishizawa et al., 1996).
Wild-type Arabidopsis plants are chilling tolerant and contain high-melting-point PG as less than 10% of the total PG molecular species (Wu and Browse, 1995; Fig. 5). The fab1 mutation causes a Leu-337-Phe substitution in KASII, the 3-ketoacyl-ACP synthase responsible for elongation of 16:0-ACP during fatty acid synthesis (Carlsson et al., 2002). This results in a 40% reduction in KASII activity and increased proportions of 16:0 in all major leaf glycerolipids of fab1 plants (Wu et al., 1994). In particular, high-melting-point molecular species are increased to 35% to 45% of the total PG, equivalent to values typical of many chilling-sensitive plants (Murata, 1983; Roughan, 1985). Nevertheless, fab1 plants survived a range of chilling treatments that quickly damaged and killed chilling-sensitive plants (Wu and Browse, 1995). fab1 plants only begin to show a decline in photosynthetic capacity (as indicated by the fluorescence parameter Fv/Fm) starting 10 d after transfer to 2°C. The mutant plants will eventually die at 2°C, but they succumb only after 2 to 3 months (Barkan et al., 2006; Figs. 3 and 4); before this, they retain a substantial capacity for recovery (Wu et al., 1997). These findings and results from other studies of chilling-sensitive plants (Wu and Browse, 1995; Jones et al., 1998; Thakur et al., 2010) suggest that an elevated level of high-melting-point PG is one of several traits that evolved in tropical and subtropical plants (possibly conferring selective advantage) but that are incompatible with growth in periodically cold climates (Wu and Browse, 1995). It is important to note that our observation of elevated high-melting-point PG in fab1 does not establish this as the cause of damage and death of fab1 plants at 2°C. For example, the proportion of MGD is greatly increased in both the fab1 gly1 (35.3% MGD) and fab1 fad6 (38.6%) suppressors (Table IV). This and other differences in fatty acid and lipid composition between fab1 and the wild type (Table IV; Supplemental Table S1) may contribute to the phenotype.
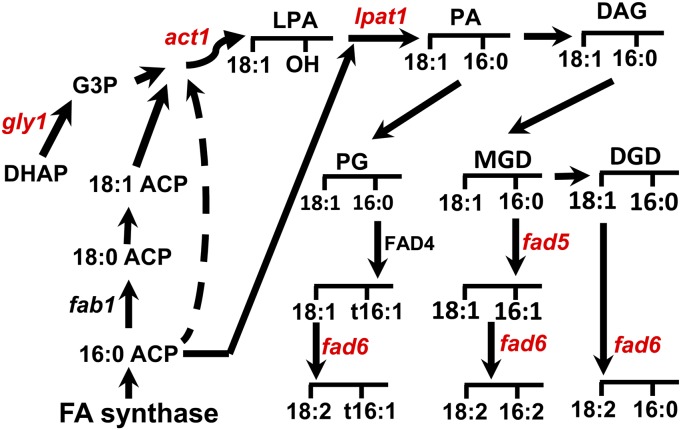
To better understand the changes in lipid composition that contribute to the fab1 phenotype and the effects they have on the photosynthesis machinery, we conducted a screen for mutations that suppress the collapse of photosynthesis and death of fab1 plants that occur at low temperature (Barkan et al., 2006). Two of the suppressors have been described previously, and both are mutations in genes encoding enzymes in the chloroplast-localized prokaryotic pathway of glycerolipid synthesis, one in fad5 (Barkan et al., 2006) and one in lpat1 (Kim et al., 2010). Figure 6 shows the main reactions of the prokaryotic pathway together with the five mutations (in red) that act as suppressors of the fab1 low-temperature phenotype. In the research reported here, we characterized two additional suppressor lines. One of these, S106, contains a recessive, hypomorphic mutation in lpat1, and we demonstrate that this mutation, lpat1-4, is the suppressor by a test cross to the previously characterized fab1 lpat1-3 (S7) suppressor line. Plants of the S96 line contain a hypomorphic mutation at the act1 locus, act1-6. The ACT1 gene encodes the first enzyme of the prokaryotic pathway of glycerolipid synthesis, the chloroplast acyl-CoA:glycerol-3-phosphate acyltransferase (Kunst et al., 1988). Complementation of the act1-6 lipid phenotype by expression of a 35S:ACT1 transgene in S96 plants also reestablished the fab1 phenotype, reduced Fv/Fm, and induced death of plants at 2°C (Fig. 3). When we generated fab1 act1-1 and fab1 act1-5 double mutants, these plants survived at 2°C but were not quite as robust as fab1 act1-6 (S96) plants. These results establish that mutations in act1 suppress the fab1 phenotype and suggest that the relatively weak act1-6 allele is a more effective suppressor than the stronger act1-1 and act1-6 alleles.
Figure 6.
Prokaryotic pathway of glycerolipid synthesis. The normal synthetic pathway is indicated by solid arrows; the dotted line indicates the overloading of 16:0-ACP as a substitute for 18:1-ACP that occurs in the fab1 mutant. FAD4 is responsible for trans-16:1 synthesis; mutations at all the other indicated loci (in red) suppress the fab1 mutation to some degree and are described in the text. DHAP, Dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; PA, phosphatidic acid; DAG, diacylglycerol; FA, fatty acid.
Our identification of mutations in three genes affecting the prokaryotic pathway of chloroplast glycerolipid synthesis, act1, lpat1, and fad5, prompted us to test two additional genes involved in the same pathway. The gly1 mutant has reduced lipid synthesis by the prokaryotic pathway (Miquel et al., 1998) because it lacks the chloroplast glycerol-3-phosphate dehydrogenase that supplies substrate to the ACT1 enzyme (Kachroo et al., 2004). The fad6 mutant is deficient in the chloroplast 16:1/18:1 desaturase, and this deficiency is also associated with a modest reduction of glycerolipid synthesis by the prokaryotic pathway (Browse et al., 1989). When we generated double mutants, plants of both the fab1 gly1 and the fab1 fad6 lines retained photosynthetic capacity and were able to survive, recover, and set seed after 65 d of exposure to 2°C (Fig. 4). These results indicate that mutations at gly1 and fad6 also act as suppressors of the fab1 low-temperature phenotype.
The suppression of the fab1 phenotype by these mutations in the prokaryotic pathway of lipid synthesis does not provide a complete recovery of wild-type growth at 2°C. After 65 d at 2°C, the suppressors all appeared weaker than wild-type plants. The expanding leaves of fab1 act1-6, fab1 gly1, and fab1 fad6 mutants are chlorotic and the older leaves are paler green than the wild type (Figs. 3B and 4B). This phenotype is unlike that of fab1 lpat1-3 and fab1 lpat1-4; in those double mutants, older leaves senesce prematurely but new leaves remain green in chilling conditions (Kim et al., 2010; Fig. 1A). Other differences in fatty acid and lipid composition between fab1 and the wild type may also contribute to the phenotype and may not be compensated by the suppressor mutations (Table IV).
The mutations in five genes of the prokaryotic pathway (Fig. 6) that each suppress the fab1 phenotype have disparate effects on the fatty acid and lipid compositions of leaf tissue, relative to the fab1 parent (Tables I, II, and IV; Barkan et al., 2006; Kim et al., 2010). Relative to fab1 (and the wild type), all of the suppressors have reduced levels of 16:3, which is only produced via the prokaryotic pathway (Browse et al., 1986) and is found predominantly on MGD, the major thylakoid lipid. We considered the possibility that the lower levels of 16:3 in MGD might be responsible for suppression. The 16:3 in MGD is largely replaced by 16:0 and 16:1 in the fab1 fad5 and fab1 fad6 lines, respectively. However, the fad5 and fad6 mutants (in a wild-type, FAB1 background) both have low-temperature phenotypes (Hugly and Somerville, 1992), suggesting that reduced 16:3 in these mutants is not the basis for suppression of fab1. In other mutants, decreased 16:3 has also been correlated with reduced photosynthetic performance at low temperatures (Routaboul et al., 2000). In the fab1 gly1, fab1 act1, and fab1 lpat1 lines, the loss of 16:3 from MGD is substantially replaced by increases in 18:3 (Table IV; Kim et al., 2010). Our current understanding of the biophysics of glycerolipids and membranes regards these two trienoic fatty acids as substantially equivalent, so again, the reduction in 16:3 does not readily explain suppression of fab1.
Lipid profiling indicates that the lpat1, gly1, act1, and fad6 mutations in the fab1 background all reduce the proportion of high-melting-point PG in leaf lipids from the elevated values found in fab1 to values that are intermediate between fab1 and the wild type (Fig. 5; Kim et al., 2010). Given the evidence discussed above implicating high-melting-point PG in low-temperature responses of plants, we consider these reductions as the likely basis for suppression of the fab1 phenotype. However, our analysis of the fab1 fad5-2 (S31) line indicated that there is no substantial change in PG composition in this line relative to fab1. For this suppressor, we proposed that a change in the molecular shape of MGD molecules within the thylakoid membrane may compensate for changes in the fatty acid composition of other glycerolipids within the membrane (Barkan et al., 2006).
We have previously proposed that the fab1 phenotype is due to compromised assembly or maintenance of photosynthetic complexes, specifically at low temperatures (Wu and Browse, 1995; Wu et al., 1997). We have now characterized five genetic loci where mutations are found that suppress the fab1 low-temperature phenotype by providing changes in lipid composition that compensate for the damage to photosynthetic machinery at low temperatures caused by the defect in KASII enzyme activity. At least six other suppressor lines, S3, S11, S22, S23, S101, and S107, do not have changes in leaf fatty acid composition. We hypothesize that each of these suppressors may contain mutations that alleviate the fab1 defects by introducing a compensatory alteration in the structure of one of the photosystem proteins. Identification of the suppressor mutations in these lines by map-based cloning and whole-genome sequencing is now underway. Of course, some of these suppressors may be in genes affecting other chloroplast or cellular functions (or in genes of unknown function), but these will also provide new possibilities for understanding the relationship between thylakoid lipid structure and photosynthetic function.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The wild-type control was Arabidopsis (Arabidopsis thaliana) ecotype Col-0. For cultivation, seeds were sown directly on soil, transferred to 4°C for 48 h, then cultivated at 22°C with 16-h light at 100 to 150 µE/m2. For chilling treatment, plants were grown at 2°C in growth chambers that provided continuous light at 100 to 150 µE/m2 for as long as 12 weeks. For the generation of mapping populations, Col-0-derived mutants were crossed with Arabidopsis Landsberg erecta mutant (Alonso-Blanco et al., 1998). Plant lines mutant in acyl-ACP:glycerol-3-phosphate acyltransferase (act1-1 and act1-5; Xu et al., 2006), glycerol-3-phosphate synthase (gly1-1; Miquel et al., 1998; Kachroo et al., 2004), and the chloroplast 16:1/18:1 desaturase (fad6-1; Browse et al., 1989) were also used in this study.
Suppressor Screening
The functional suppressor screen of the fab1 mutant has been described (Barkan et al., 2006). In brief, the M2 progeny of homozygous fab1 gl1 seeds that had been mutagenized with ethyl methanesulfonate were sown in petri dishes using Gamborg’s B5 medium, germinated under normal growth conditions, then incubated at 2°C under a 16-h light regimen for 4 weeks, a procedure that produces chlorotic fab1 plants. Suppressors identified by the production of green, nonchlorotic leaves were transferred to normal 22°C growth conditions for recovery. Seeds of the putative S106 and S96 suppressors were germinated and rescreened to confirm suppression of the fab1 chilling phenotype. Homozygous plants of each suppressor line were backcrossed to fab1, and segregation of each suppressor indicated a single, recessive mutation based on chilling sensitivity in the F2 population.
Identifying the S106 Suppressor
Homozygous S106 plants were genetically crossed with Landsberg erecta. The F1 plants were grown to maturity and allowed to self-pollinate, and the F2 seed was collected. A portion of these seeds sufficient to produce more than 800 plants were sown, and after growth at 22°C for 21 d, the plants were transferred to 2°C for 4 more weeks. Homozygous fab1 mutant plants were expected to be smaller and weaker than the wild type under these growth conditions, so all but 250 diminutive plants were culled, and the remainder were grown at 2°C for another 2 months. The 60 healthiest surviving plants were genotyped using a fab1 CAPS marker (Supplemental Table S2), and 50 fab1 homozygous plants with the S106 suppressor phenotype were identified. Twenty of these plants were used for initial mapping using SSLP markers (Lukowitz et al., 2000). In these S106 plants, two mapping linkages were identified, one close to nga111, as is the fab1 locus, and a second linkage close to ciw7 on chromosome IV. Design and testing of additional gene markers (Supplemental Table S2) narrowed the location of S106 to the region between 14.55 and 16.57 Mbp in chromosome IV.
To identity the specific location of the mutation creating S106, genomic DNA was purified from all 50 double-homozygous plants from the S106×Landsberg erecta F2 mapping population (DNase Plant Mini kit, Qiagen). Portions of the resulting DNA samples were mixed and analyzed by the Washington State University genomics facility. The genomic DNA was treated with RNase, chloroform extracted, and starch fractionally precipitated away from the nucleic acid with 20% ethanol. One microgram of the resulting genomic DNA was sonicated to an average size of 250 bp with a Bioruptor 300 sonicator (Diagenode). A sequencing library was constructed using the Ion Plus fragment library kit (Life Technologies) and quantified by real-time PCR. Sequencing beads were made with Ion OneTouch 200 reagents and loaded into two Ion 318 semiconductor sequencing chips, then sequenced on an Ion Torrent PGM. A total of 9 × 106 reads were generated with an average read length of 228 bp. Mapping with the CLC Bio Genomics workbench placed 71% of the reads on the five chromosomes for 12-fold coverage. Variants were called from the mapping and included 12 variants resulting in amino acid substitutions. The mutations that occurred in the mapped interval were detected using established techniques (Austin et al., 2011).
Identifying the S96 Suppressor
To identify the locus of the S96 suppressor, plants homozygous for the suppressor were genetically crossed with Landsberg erecta. The F1 plants were grown to maturity and allowed to self-pollinate and the F2 seed was collected. A portion of these seeds were planted on soil, and after growth at 22°C for 20 d, leaves were collected for fatty acid analysis by gas chromatography (Wu et al., 1994). From a population of 96 F2 plants, 24 were selected based on their significantly reduced 16:3 fatty acid levels, a characteristic of the homozygous S96 suppressor. Initial mapping characterization relied on PCR analysis of DNA isolated from leaf tissue of these 24 plants, using well-characterized SSLP markers (Lukowitz et al., 2000). Because the S96 suppressor was so closely linked to the ciw12 marker on chromosome I, we hypothesized that it might be a mutation in act1; act1 mutants have a fatty acid phenotype similar to S96 (Kunst et al., 1988). After PCR and sequence analysis confirmed the presence of a mutation in act1, we developed a dCAPS marker, dCAPS1768 (Neff et al., 1998; Supplemental Table S2), for genotyping the act1 mutation in S96.
Transgenic and Complementation Experiment
An act1 cDNA was cloned by reverse transcription of RNA isolated from Col-0 leaves (RNase mini kit, Qiagen) followed by PCR, using primers listed in Supplemental Table S2, which were designed to simplify further manipulation. The PCR product was cloned into pENTR-TOPO (Invitrogen), and the cDNA was confirmed by sequence analysis. The cDNA was transferred by the LR Clonase reaction (Invitrogen) to PB2GW7 (Karimi et al., 2002), a Gateway plant expression vector that uses the 35S-Cauliflower mosaic virus promoter to express the inserted cDNA. Following transformation of the construct into Agrobacterium tumefaciens GV3101, whole S96 plants were transformed using established techniques (Clough and Bent, 1998), followed by selection for Basta resistance.
Lipid Extraction and Analysis
The overall fatty acid compositions of leaf tissues were determined using established methods (Wu et al., 1994; Roston et al., 2011). Membrane glycerolipids were analyzed by TLC using activated ammonium sulfate-impregnated silica gel TLC plates (Si250PA, Mallinckrodt or Silica Gel HL, 20 × 20 cm, 250 microns, Analytic), with a solvent system of acetone/toluene/water (91/30/7, vol/vol; Wang and Benning, 2011; Li et al., 2012). Lipids were rendered visible under UV light by exposure to 0.005% (w/v) primulin in 80% (v/v) acetone, after which the individual lipid classes were collected and their fatty acid content determined by gas chromatography. For lipidomics analysis, lipids were extracted according to the instructions provided by the Kansas Lipidomics Research Center (http://www.k-state.edu/lipid/lipidomics/), and PG molecular species were quantified by analysis performed at that facility (Esch et al., 2007). Lipid molecular species for the single lipids were reanalyzed, and the content of all molecular species in specific lipids was set as 100%. Data were collected from four independent samples.
Measurements of Chlorophyll Fluorescence
Chlorophyll fluorescence from leave tissues was analyzed by a Fluorescence Monitoring System (Hansatech). Fv/Fm, representing the potential quantum yield of PSII, was measured after leaves on intact plants were dark adapted at 22°C for 30 min (Barkan et al., 2006).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Fatty acid profiles in leaves of the wild type, fab1, and the S106 suppressor.
Supplemental Figure S2. Identification of S106 putative mutants in the F2 mapping population.
Supplemental Figure S3. An act1 mutation in line S96 (fab1 act1-6) suppresses the fab1 phenotype.
Supplemental Table S1. Lipidomics data from analysis of wild-type, fab1, and fab1 suppressors.
Supplemental Table S2. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Shuangyi Bai for helpful discussions and Deirdre Fahy for correcting language in the writing process. Dr. Mark R. Wilding helped us analyze the genome sequencing data and detect mutations. We are grateful to the Kansas State University Lipidomics Research Center for analyses.
Glossary
- MGD
monogalactosyldiacylglycerol
- PG
phosphatidylglycerol
- LPA
lysophosphatidic acid
- DGD
digalactosyldiacylglycerol
- Col-0
Columbia-0
- SNP
single-nucleotide polymorphism
- cDNA
complementary DNA
- TLC
thin-layer chromatography
- Fv/Fm
ratio of variable fluorescence to maximal fluorescence
Footnotes
This work was supported by the U.S. National Science Foundation (grant no. IOS–1258799) and the Agricultural Research Center at Washington State University.
J.G. and J.B. conceived the research plan; J.G. performed the experiments; J.G., J.G.W., and J.B. wrote the article.
Articles can be viewed without a subscription.
References
- Alonso-Blanco C, Peeters AJ, Koornneef M, Lister C, Dean C, van den Bosch N, Pot J, Kuiper MT (1998) Development of an AFLP based linkage map of Ler, Col and Cvi Arabidopsis thaliana ecotypes and construction of a Ler/Cvi recombinant inbred line population. Plant J 14: 259–271 [DOI] [PubMed] [Google Scholar]
- Austin RS, Vidaurre D, Stamatiou G, Breit R, Provart NJ, Bonetta D, Zhang J, Fung P, Gong Y, Wang PW. , et al. (2011) Next-generation mapping of Arabidopsis genes. Plant J 67: 715–725 [DOI] [PubMed] [Google Scholar]
- Barkan L, Vijayan P, Carlsson AS, Mekhedov S, Browse J (2006) A suppressor of fab1 challenges hypotheses on the role of thylakoid unsaturation in photosynthetic function. Plant Physiol 141: 1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282: 31206–31216 [DOI] [PubMed] [Google Scholar]
- Benning C. (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25: 71–91 [DOI] [PubMed] [Google Scholar]
- Browse J, Kunst L, Anderson S, Hugly S, Somerville C (1989) A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol 90: 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR (1986) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem J 235: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AS, LaBrie ST, Kinney AJ, von Wettstein-Knowles P, Browse J (2002) A KAS2 cDNA complements the phenotypes of the Arabidopsis fab1 mutant that differs in a single residue bordering the substrate binding pocket. Plant J 29: 761–770 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Esch SW, Tamura P, Sparks AA, Roth MR, Devaiah SP, Heinz E, Wang X, Williams TD, Welti R (2007) Rapid characterization of the fatty acyl composition of complex lipids by collision-induced dissociation time-of-flight mass spectrometry. J Lipid Res 48: 235–241 [DOI] [PubMed] [Google Scholar]
- Frentzen M. (2004) Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: anionic membrane lipids and phosphate regulation. Curr Opin Plant Biol 7: 270–276 [DOI] [PubMed] [Google Scholar]
- Hagio M, Sakurai I, Sato S, Kato T, Tabata S, Wada H (2002) Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol 43: 1456–1464 [DOI] [PubMed] [Google Scholar]
- Hugly S, Somerville C (1992) A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol 99: 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53: 225–245 [DOI] [PubMed] [Google Scholar]
- Ishizaki-Nishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T (1996) Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nat Biotechnol 14: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129: 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL, Tucker DE, Ort DR (1998) Chilling delays circadian pattern of sucrose phosphate synthase and nitrate reductase activity in tomato. Plant Physiol 118: 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P (2004) Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc Natl Acad Sci USA 101: 5152–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kim HU, Huang AHC (2004) Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol 134: 1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HU, Vijayan P, Carlsson AS, Barkan L, Browse J (2010) A mutation in the LPAT1 gene suppresses the sensitivity of fab1 plants to low temperature. Plant Physiol 153: 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA 85: 4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Gao J, Benning C, Sharkey TD (2012) Characterization of photosynthesis in Arabidopsis ER-to-plastid lipid trafficking mutants. Photosynth Res 112: 49–61 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM. (1973) Chilling injury in plants. Annu Rev Plant Physiol 24: 445–466 [Google Scholar]
- Miquel M, Cassagne C, Browse J (1998) A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol 117: 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon BY, Higashi S, Gombos Z, Murata N (1995) Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA 92: 6219–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. (1983) Molecular species composition of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Cell Physiol 24: 81–86 [Google Scholar]
- Murata N, Ishizaki-Nishizawa Q, Higashi S, Hayashi H, Tasaka Y, Nishida I (1992) Genetically engineered alteration in the chilling sensitivity of plants. Nature 356: 710–713 [Google Scholar]
- Murata N, Yamaya J (1984) Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol 74: 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J 14: 387–392 [DOI] [PubMed] [Google Scholar]
- Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47: 541–568 [DOI] [PubMed] [Google Scholar]
- Roston R, Gao J, Xu C, Benning C (2011) Arabidopsis chloroplast lipid transport protein TGD2 disrupts membranes and is part of a large complex. Plant J 66: 759–769 [DOI] [PubMed] [Google Scholar]
- Roughan PG. (1985) Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol 77: 740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J (2000) Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol 124: 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Feese MD, Ferri SR, Kato Y, Yajima R, Toguri T, Kuroki R (2004) Substrate recognition and selectivity of plant glycerol-3-phosphate acyltransferases (GPATs) from Cucurbita moscata and Spinacea oleracea. Acta Crystallogr D Biol Crystallogr 60: 13–21 [DOI] [PubMed] [Google Scholar]
- Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 67: 429–443 [Google Scholar]
- Turnbull AP, Rafferty JB, Sedelnikova SE, Slabas AR, Schierer TP, Kroon JTM, Simon JW, Fawcett T, Nishida I, Murata N. , et al. (2001) Analysis of the structure, substrate specificity, and mechanism of squash glycerol-3-phosphate (1)-acyltransferase. Structure 9: 347–353 [DOI] [PubMed] [Google Scholar]
- Upchurch RG. (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30: 967–977 [DOI] [PubMed] [Google Scholar]
- Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16: 123–132 [DOI] [PubMed] [Google Scholar]
- Wada H, Murata N (2007) The essential role of phosphatidylglycerol in photosynthesis. Photosynth Res 92: 205–215 [DOI] [PubMed] [Google Scholar]
- Wallis JG, Browse J (2002) Mutants of Arabidopsis reveal many roles for membrane lipids. Prog Lipid Res 41: 254–278 [DOI] [PubMed] [Google Scholar]
- Wallis JG, Browse J (2010) Lipid biochemists salute the genome. Plant J 61: 1092–1106 [DOI] [PubMed] [Google Scholar]
- Wang Z, Benning C (2011) Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC). J Vis Exp 49: 2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter FP, Schmidt R, Heinz E (1992) Chilling sensitivity of Arabidopsis thaliana with genetically engineered membrane lipids. EMBO J 11: 4685–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Browse J (1995) Elevated levels of high-melting-point phosphatidylglycerols do not induce chilling sensitivity in an Arabidopsis mutant. Plant Cell 7: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, James DW Jr, Dooner HK, Browse J (1994) A mutant of Arabidopsis deficient in the elongation of palmitic acid. Plant Physiol 106: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lightner J, Warwick N, Browse J (1997) Low-temperature damage and subsequent recovery of fab1 mutant Arabidopsis exposed to 2°C. Plant Physiol 113: 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Härtel H, Wada H, Hagio M, Yu B, Eakin C, Benning C (2002) The pgp1 mutant locus of Arabidopsis encodes a phosphatidylglycerolphosphate synthase with impaired activity. Plant Physiol 129: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yu B, Cornish AJ, Froehlich JE, Benning C (2006) Phosphatidylglycerol biosynthesis in chloroplasts of Arabidopsis mutants deficient in acyl-ACP glycerol-3-phosphate acyltransferase. Plant J 47: 296–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.