An endogenous defensive protein in maize that encodes a Cys protease, provides enhanced resistance to phloem sap-consuming pests through the action of an ethylene-signaling pathway.
Abstract
Signaling networks among multiple phytohormones fine-tune plant defense responses to insect herbivore attack. Previously, it was reported that the synergistic combination of ethylene (ET) and jasmonic acid (JA) was required for accumulation of the maize insect resistance1 (mir1) gene product, a cysteine (Cys) proteinase that is a key defensive protein against chewing insect pests in maize (Zea mays). However, this study suggests that mir1-mediated resistance to corn leaf aphid (CLA; Rhopalosiphum maidis), a phloem sap-sucking insect pest, is independent of JA but regulated by the ET-signaling pathway. Feeding by CLA triggers the rapid accumulation of mir1 transcripts in the resistant maize genotype, Mp708. Furthermore, Mp708 provided elevated levels of antibiosis (limits aphid population)- and antixenosis (deters aphid settling)-mediated resistance to CLA compared with B73 and Tx601 maize susceptible inbred lines. Synthetic diet aphid feeding trial bioassays with recombinant Mir1-Cys Protease demonstrates that Mir1-Cys Protease provides direct toxicity to CLA. Furthermore, foliar feeding by CLA rapidly sends defensive signal(s) to the roots that trigger belowground accumulation of the mir1, signifying a potential role of long-distance signaling in maize defense against the phloem-feeding insects. Collectively, our data indicate that ET-regulated mir1 transcript accumulation, uncoupled from JA, contributed to heightened resistance to CLA in maize. In addition, our results underscore the significance of ET acting as a central node in regulating mir1 expression to different feeding guilds of insect herbivores.
Maize (Zea mays) is among the world’s most important monocot crops and is grown for food, feed, and/or fuel. At the same time, maize is highly confounded by insect pests that can dramatically decrease yields, and there is extensive variation of resistance in this crop against insect pests (McMullen et al., 2009; Meihls et al., 2012). Different feeding guilds of insect pests cause aboveground and belowground damage to the maize crop. Several lepidopteran larvae and phloem sap-feeding aphids attack the aboveground parts of the maize plant, whereas coleopteran larvae feed on the belowground roots, thereby causing considerable damage to the maize plants (Erb et al., 2009a; Dafoe et al., 2011; Christensen et al., 2013; Meihls et al., 2013; Betsiashvili et al., 2015). In addition, chrysomelid beetles (e.g. Diabrotica virgifera) feed from the maize silks, tender kernels, and pollen (Culy et al., 1992; Moeser and Vidal, 2005). Corn leaf aphids (CLA; Rhopalosiphum maidis), similar to other phloem sap-feeding aphids, use their slender stylets present in their piercing/sucking mouthparts to consume nutrients from the phloem sap that otherwise are used by the plants for their normal growth. Furthermore, because the aphid feeds in the phloem of the plant’s vascular system, it acts as a vector for viruses such as maize dwarf mosaic virus and maize leaf fleck virus that cause debilitating diseases in this crop (Thongmeearkom et al., 1976; So et al., 2010). In addition to maize, CLA attack other grasses, including sorghum (Sorghum bicolor), barley (Hordeum vulgare), wheat (Triticum aestivum), and Miscanthus spp. (Carena and Glogoza, 2004; Pointeau et al., 2014).
Plant defenses against insects include both constitutive and preformed factors such as physical barriers (cuticle, trichomes, spines, thorns, etc.) and stored insecticidal compounds. In addition, insect infestation also induces physical defenses in plants. For instance, density of trichomes and spines were significantly increased after insect attack in tomato (Solanum lycopersicum) and horsenettle (Solanum carolinense), respectively (Tian et al., 2012; Kariyat et al., 2013). Some of the insecticidal compounds that provide plant defenses include nicotine, saponin, morphine, cyanogenic glycosides, benzoxazinoids, cardenolides, chlorogenic acid, glucosinolates, and nonprotein amino acids (Wittstock and Gershenzon, 2002; Cortés-Cruz et al., 2003; Geyter et al., 2007; Kim et al., 2008; Meihls et al., 2013; Yan et al., 2015). Moreover, plants produce several other defensive compounds including phenolics, alkaloids, and proteases, which have a direct or indirect effect on the attacking insect herbivores. For instance, a 33-kD Cys protease (Maize insect resistance1-Cys Protease [Mir1-CP]) accumulates rapidly at the site of insect infestation in maize inbred line Mp708 and disrupts the peritrophic matrix (PM) of the attacking caterpillar, which otherwise protects the caterpillar midgut from physical and chemical damage (Pechan et al., 2000, 2002).
Mp708 was developed by classical plant breeding from a cross between the insect-resistant Mp704 and susceptible Tx601 plants (Williams et al., 1990). Both Mp704 and Mp708 have shown significantly enhanced resistance to the aboveground feeding by several lepidopteran pests. Subsequently, several studies have shown that enhanced resistance to caterpillars in Mp708 was due to Mir1-CP accumulation at the site of insect feeding (Pechan et al., 2000, 2002). Furthermore, ectopic expression of mir1 in transgenic maize callus significantly retarded caterpillar growth (Pechan et al., 2000). As mentioned before, Mir1-CP attacks the lepidopteran PM that protects the caterpillar midgut (Pechan et al., 2002). Constitutive low levels of Mir1-CP and mir1 transcripts were detected in Mp708 plants prior to insect attack (Pechan et al., 2000; Harfouche et al., 2006). After caterpillar feeding, Mir1-CP accumulates at elevated levels at the site of insect infestation within 1 h and also in the vascular tissues (Pechan et al., 2000; Lopez et al., 2007). Accumulation of Mir1-CP in the vascular tissues suggested that Mir1-CP can also potentially function as a phloem-mobile protein.
Insect feeding on host plants activates different plant signal transduction pathways, primarily defense pathways mediated by salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) (Mewis et al., 2005; Goggin, 2007; Zarate et al., 2007; Erb et al., 2012; Louis and Shah, 2013). Resistance to chewing group of insects is largely mediated through the JA pathway. Feeding by chewing insects results in the activation of genes involved in the JA signaling pathway and/or JA-dependent antinutritional proteins, including proteinase inhibitors, polyphenol oxidases, and arginase. In maize, it is known that caterpillar feeding increases JA levels both locally and systemically, which results in the activation of a suite of herbivore defense-related genes including genes encoding enzymes in the JA biosynthetic pathway (Erb et al., 2009a; Shivaji et al., 2010). Furthermore, downstream genes that encode direct defense proteins such as proteinase inhibitor, chitinase, and Ribosome-Inactivating Protein2 are induced by caterpillar feeding (Shivaji et al., 2010; Louis et al., 2013; Chuang et al., 2014). Previous studies have shown that Mir1-CP accumulation in maize upon caterpillar feeding is dependent on both JA and ET pathways (Harfouche et al., 2006; Ankala et al., 2009).
The role of aboveground to belowground communication and vice versa is one of the emerging areas of research in the field of plant-insect interactions (Nalam et al., 2013; Soler et al., 2013). Upon foliar insect infestation in tobacco (Nicotiana spp.), the insecticidal compound nicotine is synthesized in the roots and transported to the shoot through the vascular tissues, providing defense to subsequent insect attack (Baldwin et al., 1994; Morita et al., 2009). In Arabidopsis (Arabidopsis thaliana), aphid infestation on the foliage induces the activation of LIPOXYGENASE5 (LOX5)-derived oxylipins in the roots (Nalam et al., 2012). The LOX5-derived oxylipins (e.g. 9-hydoxy-10E, 12Z-octadecadienoic acid) are translocated from the roots to the shoots, where it activates the different defense-signaling genes against aphids (Nalam et al., 2012; Louis and Shah, 2013, 2015). Although there are many reports about long-distance signaling in dicots, little is known about it in the agriculturally important monocot plants. In maize, belowground feeding by corn rootworm increased the amount of the insecticidal hydroxamic acid 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one in the whorl region, primed the production of chlorogenic acid, and subsequently reduced the growth of Egyptian cottonworm (Spodoptera littoralis) larvae (Erb et al., 2009a). Similarly, fall armyworm (Spodoptera frugiperda) infestation in the whorl regions of maize resulted in the accumulation of Mir1-CP in the roots (Lopez et al., 2007). Subsequently, it has been shown that Mir1-CP accumulation in the roots also provides enhanced resistance to root-feeding herbivores (Gill et al., 2011). Although studies related to long-distance defense signaling in plant-insect interactions have gained momentum in recent years, there are still many unknown mechanisms involved in this complex signaling process.
The presence of Mir1-CP in the sieve elements and the capability of Mir1-CP to move through vascular tissues (Luthe et al., 2011) led us to hypothesize that mir1 contributes to defense against CLA. Our results suggest that maize inbred line Mp708 provides both antibiotic- and antixenotic-mediated defenses against CLA. Antibiosis negatively influences insect reproduction, development, and growth on host plants, whereas antixenosis impacts insect behavior and deters insect settling on the host plants (Smith, 2005). Feeding by CLA triggers the accumulation of mir1 transcripts, which encodes a Cys protease. Furthermore, feeding trial bioassays with recombinant Mir1-CP (rMir1-CP) confirm that Mir1-CP provides direct toxicity to insect pests. Finally, our data indicate that long-distance transport of mir1 is critical for providing enhanced resistance to CLA, and mir1 expression in response to CLA infestation is independent of JA pathway but regulated by ET.
RESULTS
Maize Inbred Line Mp708 Provides Enhanced Resistance to CLA
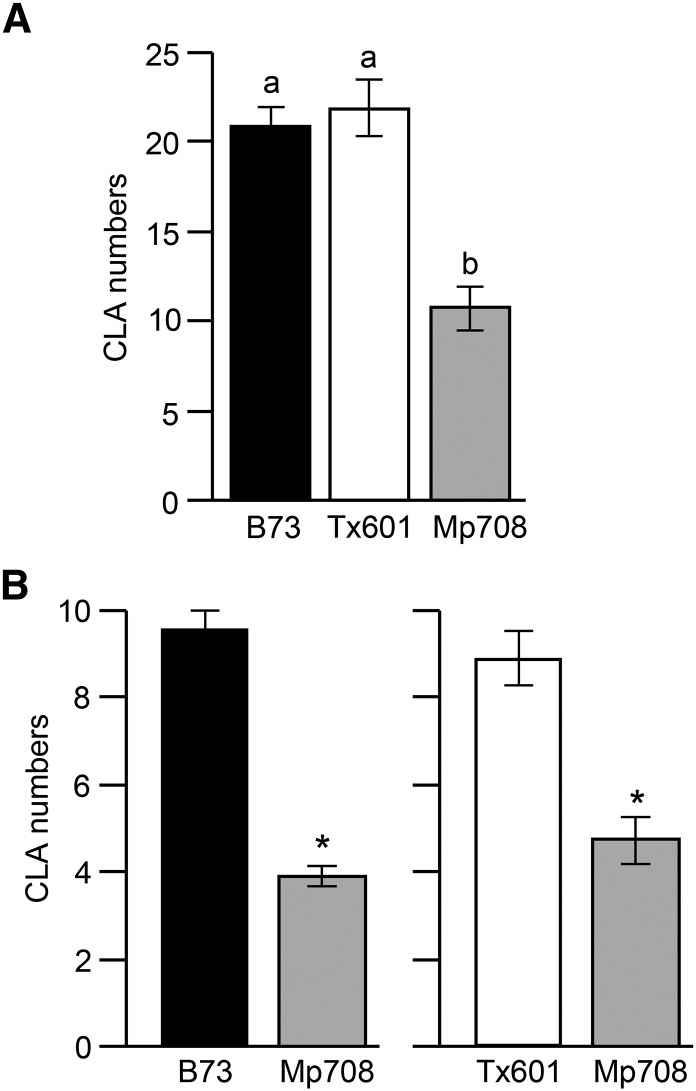
Previously, it was shown that Mp708 provides enhanced resistance to the aboveground feeding by several lepidopteran pests (Pechan et al., 2000, 2002; Lopez et al., 2007; Shivaji et al., 2010). Here, we examined whether Mp708 provides enhanced resistance to the piercing-sucking insect CLA. To evaluate this, we conducted a no-choice assay to determine antibiosis. In the no-choice assay, five adult apterous CLA were released on each maize line, and the CLA population was monitored 2 and 7 d postinfestation. Our results show that CLA counts were lower on the Mp708 plants compared with maize inbred lines Tx601 and B73 (Fig. 1A; Supplemental Fig. S1). To determine whether antixenotic factors contribute to Mp708’s resistance to CLA, we performed a choice assay by releasing 15 adult apterous CLA at the center of the pot containing both plants (B73 and Mp708 or Tx601 and Mp708). The number of adult CLA that had settled on each plant was counted after 24 h. When given a choice between B73 and Mp708 plants, CLA had a strong preference for the B73 plants (Fig. 1B). Similarly, when given a choice between Tx601 and Mp708 plants, CLA preferred to settle on Tx601 plants (Fig. 1B). These data indicate that Mp708 provides both antixenotic- and antibiotic-mediated defenses against CLA.
Figure 1.
Maize inbred line Mp708 provides enhanced antibiotic- and antixenotic-mediated resistance to CLA. A, Total number of CLA adults and nymphs recovered (antibiosis; no-choice assay) 7 d after infestation of V3 maize plants (defined in Ritchie et al., 1993) with five adult apterous aphids per plant (n = 12). B, In the choice test to determine antixenosis, 15 adult CLA were released at the center of a pot containing one plant of each indicated maize line. The number of adult aphids that settled on each plant was counted after 24 h (n = 10). For both A and B, values are the mean ± se of aphid numbers on a minimum of 12 plants of each genotype for no-choice assay (n = 12) and 10 plants of each genotype for choice assay (n = 10). Aphid bioassays were repeated two times with similar results with at least eight plants per genotype. Different letters or asterisks above the bars indicate values that are significantly different from each other (P < 0.05).
CLA Feeding Rapidly Induces mir1 Accumulation in Mp708
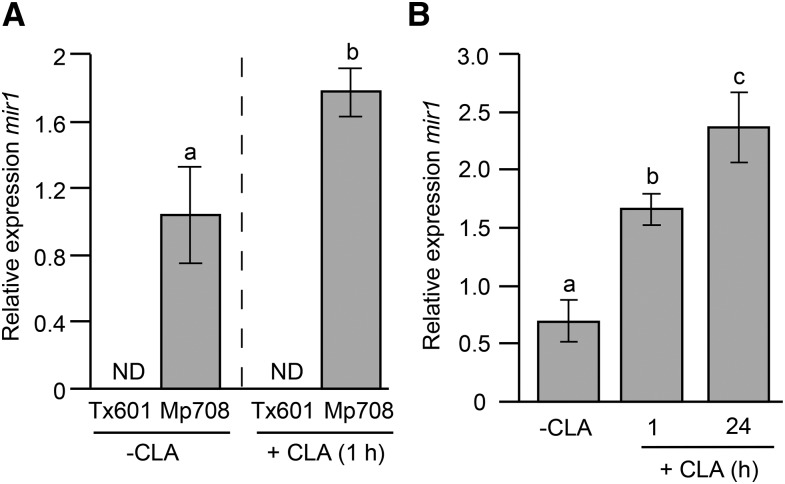
Mp708 provides resistance to caterpillars by rapidly accumulating Mir1-CP at the site of insect infestation (Pechan et al., 2000). To examine whether CLA feeding also induces mir1 expression, quantitative real-time (qRT)-PCR was used to compare mir1 transcript levels in CLA-uninfested and -infested leaves of Tx601 and Mp708 maize plants. As shown in Figure 2A, CLA feeding induces the rapid accumulation of mRNA transcripts encoding Mir1-CP within 1 h in the leaves of Mp708 compared with Tx601 plants. We did not detect mir1 expression in the Tx601 maize inbred line before or after CLA infestation (Fig. 2A), supporting our previous observation that mir1 is not expressed in Tx601 maize lines (Harfouche et al., 2006). Furthermore, the expression of mir1 remained at elevated levels through 24 h of CLA infestation in Mp708 plants (Fig. 2B), suggesting that CLA feeding-induced mir1 accumulation potentially contributes to Mp708 resistance to CLA.
Figure 2.
CLA feeding induces the rapid accumulation of mir1 transcripts in Mp708. A, qRT-PCR analysis of mir1 transcripts in uninfested (–CLA) and CLA-infested leaves (+CLA) for 1 h on V3 stage Tx601 and Mp708 maize plants. B, Time course analysis of mir1 transcript accumulation in the Mp708 (V3 stage) whorl in response to CLA infestation. For experiments A and B, n = 4. Different letters above the bars indicate values that are significantly different from each other (P < 0.05). Error bars represent ± se. ND, Not detected.
CLA-Infested Vascular Sap of Mp708 Contributes to Enhanced Resistance to Aphids
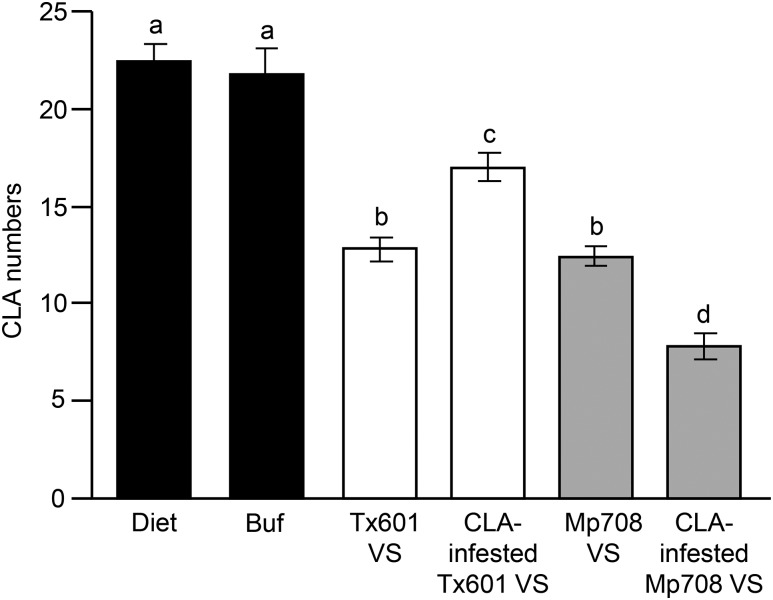
Because Mir1-CP is expressed in the vascular tissues, we also collected the vascular sap exudates from maize plants and examined whether mir1 or mir1-dependent factors present in the sap provide any toxicity to these phloem-feeding insects. When we tested vascular sap exudates by including it in their diet, the number of aphids recovered from diet containing vascular sap collected from uninfested plants was comparable between Mp708 and Tx601 plants (Fig. 3). However, if plants were infested with CLA prior to sap collection, Mp708 vascular sap significantly reduced the number of aphids compared with Tx601, suggesting that CLA feeding-induced accumulation of toxic factors may contribute to defense against aphids (Fig. 3). Interestingly, we also observed that CLA performed better on Tx601 vascular sap mixed with aphid diet that had prior infestation with CLA. The CLA infestation-promoting activity in the vascular sap of Tx601 plants suggests that CLA are capable of suppressing host plant defenses and can alter the physiology of Tx601 plants to make it more suitable for its proliferation (Fig. 3). This result, taken together with the no-choice bioassay in which the CLA population was significantly higher on Tx601 compared to Mp708 plants (Fig. 1A), confirms that CLA feeding-induced mir1 expression may contribute to defense against CLA.
Figure 3.
CLA feeding-induced accumulation of antibiosis factors in vascular sap (VS) of Mp708 contributes to enhanced aphid resistance. Three adult CLA were allowed to feed on artificial diet, diet supplemented with the buffer (Buf) used to collect the vascular sap, or artificial diet containing vascular sap collected from aphid-uninfested or -infested Tx601 and Mp708 maize plants. Four days later, the numbers of aphids (adults plus nymphs) in each chamber were counted (n = 12). This experiment was conducted twice with similar results. Different letters above the bars indicate values that are significantly different from each other (P < 0.05). Error bars represent ± se.
rMir1-CP Provides Direct Toxicity to CLA
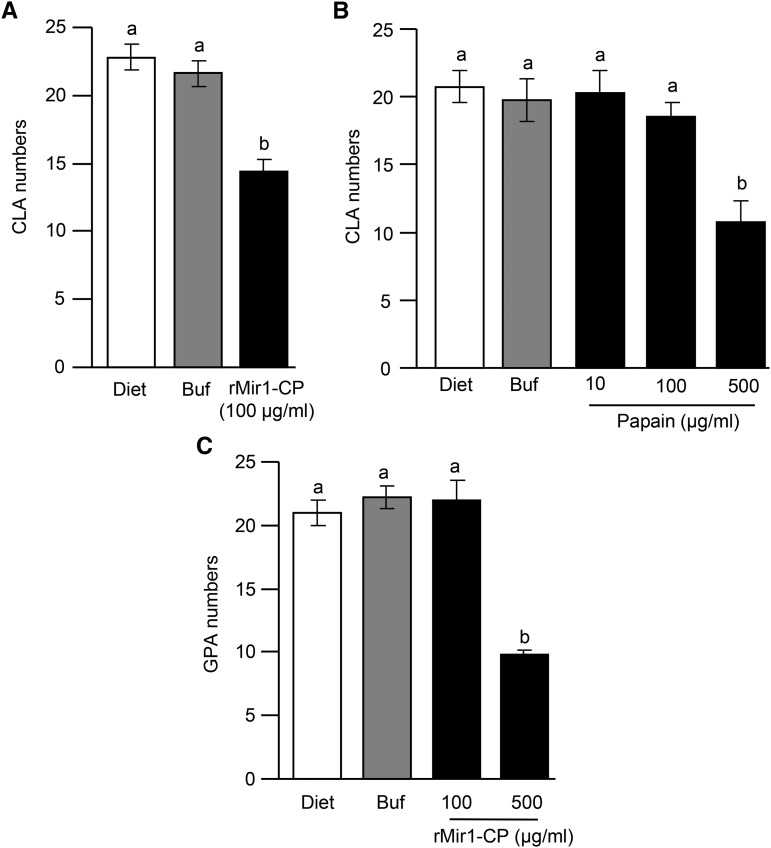
We further examined whether Mir1-CP has any direct toxic effect on CLA growth, development, and reproduction. To assess this, CLA were allowed to feed on an artificial diet mixed with rMir1-CP protein. Compared with the diet alone and the diet mixed with the phosphate-buffered saline (PBS) buffer, which was used as a solvent for the rMir1-CP, the aphid diet containing a concentration of 100 µg mL–1 rMir1-CP significantly impacted CLA proliferation (Fig. 4A). We also tested the effect of papain, a plant-derived Cys protease, against CLA. In feeding trial bioassays, we did not observe any effect of papain at the same concentration as of rMir1-CP (100 µg mL–1; Fig. 4B). However, at a higher concentration of papain (500 µg mL–1), we observed a 40% reduction in CLA growth compared with controls. Furthermore, at a higher concentration of rMir1-CP (500 µg mL–1) in feeding trial bioassays, it significantly affected the growth, development, and reproduction of green peach aphid (GPA; Myzus persicae; Fig. 4C), a polyphagous insect that has a wide range of hosts of more than 50 plant families (Blackman and Eastop, 2000).
Figure 4.
rMir1-CP exhibits direct toxicity to CLA. Feeding trial bioassays: A, Comparison of CLA numbers on an artificial diet, the diet containing the buffer (Buf) used to dissolve the rMir1-CP, and the diet containing rMir1-CP, or B, the diet containing different concentrations of papain. C, Comparison of GPA numbers on an artificial diet, the diet containing the buffer used to dissolve the rMir1-CP, and the diet containing rMir1-CP. Three adult aphids were introduced into each feeding chamber and allowed to feed on the diet, and the total numbers of aphids in each chamber were counted after 4 d (n = 4–5). This experiment was conducted three times with similar results. Error bars represent ± se. Different letters above the bars indicate values that are significantly different (P < 0.05) from each other.
Aboveground-Belowground Signaling Is Critical For CLA Feeding-Induced mir1 Expression
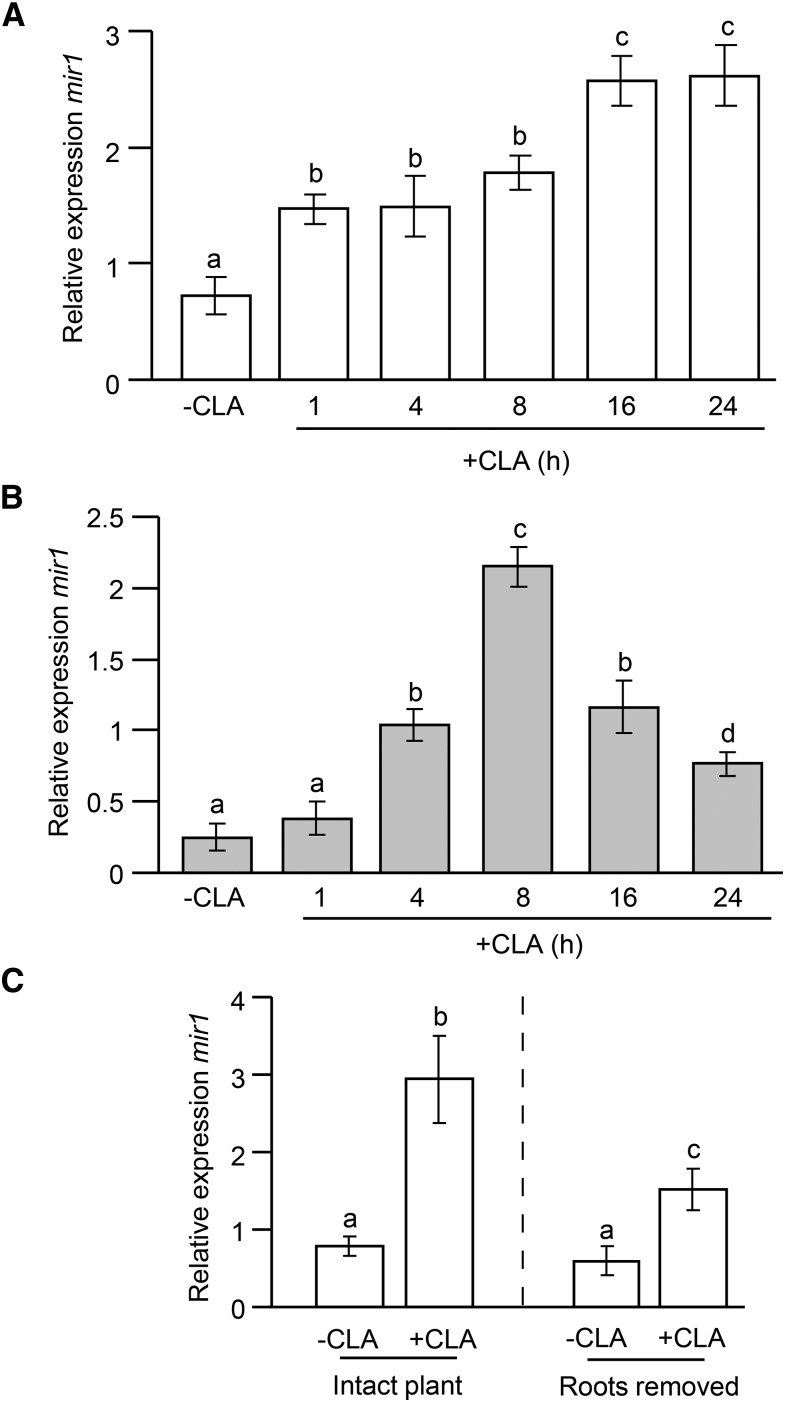
Because Mir1-CP is expressed in the vascular tissues (Lopez et al., 2007), we further investigated whether the CLA feeding in the whorl sends any signal to the roots through the vascular system. Using qRT-PCR, a time course analysis of mir1 expression in both shoots and roots was monitored before and after foliar infestation of CLA. As shown in Figure 5A, CLA feeding rapidly induced the expression of mir1 in shoots within 1 h and sustained at elevated levels until 8 h in Mp708 plants. mir1 expression was further significantly elevated in shoots 16 h postinfestation (hpi) of CLA and maintained at higher levels until 24 hpi (Fig. 5A). In contrast to the shoots, foliar infestation of CLA did not significantly induce the mir1 expression in roots within 1 h in Mp708 plants (Fig. 5B). However, we did observe a significantly higher expression of mir1 after 4 h in roots. The expression of mir1 in roots was highest at 8 hpi but dropped significantly past 16 hpi of CLA on shoots (Fig. 5B). Interestingly, simultaneously, we found a significant increase in the expression of mir1 in shoots 16 hpi of CLA (Fig. 5A), suggesting that mRNA transcripts encoding Mir1-CP contribute to intraplant defense signaling in Mp708 genotype.
Figure 5.
Roots of maize inbred Mp708 line act as a potential source of mir1 transcript accumulation in response to foliar feeding by CLA. Time course qRT-PCR analysis of mir1 in Mp708 (V3 stage) shoots (A) and roots (B) before (–) and after (+) CLA infestation. C, qRT-PCR analysis of mir1 in Mp708 (V3 stage) whorls of intact plants and those with roots removed prior to CLA infestation for 24 h. For all experiments, n = 3. This experiment was conducted twice with similar results. Different letters above the bars indicate values that are significantly different from each other (P < 0.05). Error bars represent ± se.
In addition, we tested the hypothesis that the roots act as a pool for mir1 synthesis in providing enhanced resistance to foliar feeding by CLA. Root removal prior to CLA feeding greatly reduced the accumulation of mir1 in the whorl region (Fig. 5C), thus confirming that roots act as a reservoir of mir1 during the initial stages of aphid attack. Collectively, these results suggest that aboveground to belowground (and vice versa) signaling interactions in Mp708 act as a critical component in modulating maize defense against the phloem sap-feeding CLA.
ET, Not JA or SA, Contributes to mir1-Dependent Defense against CLA in Mp708
Prior to herbivory, Mp708 plants had elevated levels of endogenous JA compared with the susceptible Tx601 plants (Shivaji et al., 2010). We further examined whether these elevated levels of JA contribute to enhanced resistance to CLA. qRT-PCR was used to monitor the expression of LOX1 and 12-oxo-phytodienoic acid reductase8 (OPR8), two key genes involved in JA biosynthesis in maize (Kim et al., 2003; Yan et al., 2012). Previously, it was shown that fall armyworm feeding significantly induced the expression of JA biosynthesis genes in Mp708 compared with the Tx601 plants (Shivaji et al., 2010). By contrast, expression of LOX1 and OPR8 genes was comparable between Mp708 and Tx601 plants after CLA infestation (Supplemental Fig. S2, A and B), suggesting that Mp708’s resistance to CLA might be operating through a defense pathway aside from JA. In fact, CLA feeding suppressed the expression of LOX1 in Mp708 and was comparable to the Tx601 susceptible inbred line (Supplemental Fig. S2A). To further rule out the requirement of JA in maize defense against CLA, Mp708 plants were pretreated with ibuprofen (IBU) or nordihydroguaiaretic acid (NDGA) that blocks the JA pathway, and CLA performance and mir1 expression were monitored before and after CLA infestation. Several studies have shown that the pretreatment of plants with JA inhibitors suppressed the activity of LOX, JA biosynthesis, and/or JA-dependent downstream products (Staswick et al., 1991; Oikawa et al., 2001; Zhang et al., 2009; Mandal et al., 2015). Our results also demonstrate that JA inhibitor treatment significantly suppressed the expression of LOX1 and Proteinase Inhibitor in Mp708 plants and were similar to a level found in the Tx601 maize susceptible inbred line (Supplemental Fig. S3, A and B). Mp708 plants pretreated with JA inhibitors did not affect CLA proliferation in a no-choice bioassay (Supplemental Fig. S3C) and mir1 transcript expression after CLA infestation (Supplemental Fig. S3D), thus confirming our notion that JA is not crucial for providing defense against CLA in Mp708.
Next, we examined whether SA contributes to mir1-mediated defense against CLA. Though CLA feeding on Mp708 plants significantly induced the expression of the SA marker gene Pathogenesis-related Protein1 (Supplemental Fig. S4A), plants that were pretreated with SA 24 h prior to release of aphids did not adversely affect CLA population size (Supplemental Fig. S4B). Furthermore, mir1 transcript expression after CLA infestation in Mp708 plants was comparable between plants that were pretreated with or without SA (Supplemental Fig. S4C). Previously, it was shown that Mp708 plants have significantly reduced levels of SA relative to Tx601 plants, supporting the fact that there is a mutual antagonism occurring between the JA and SA pathways (Shivaji et al., 2010). Hence, we also pretreated Mp708 plants with methyl jasmonate (MeJA) and monitored CLA performance. Pretreatment of Mp708 plants with MeJA did not alter CLA performance compared with Mp708 control plants (Supplemental Fig. S4D). These findings demonstrate that in addition to JA, SA may not be required for mir1-mediated defense against CLA.
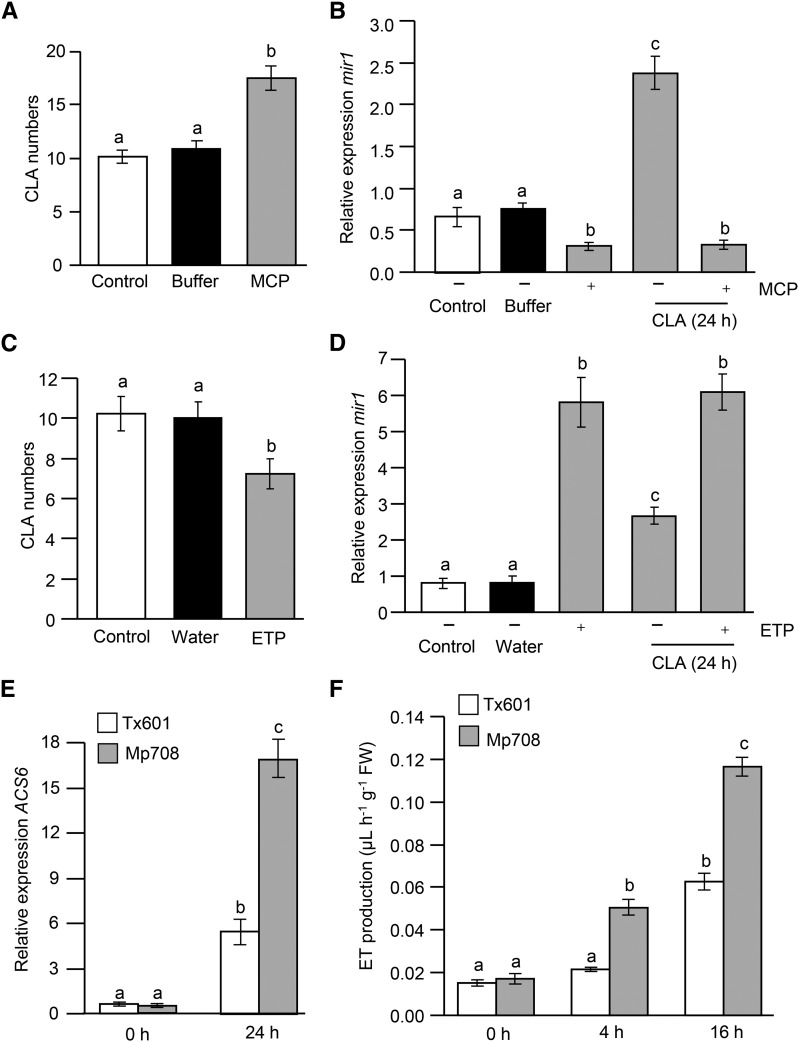
Previous studies with fall armyworm indicated that JA functions upstream of ET in the Mir1-CP pathway, but both are required for Mir1-CP accumulation in response to caterpillar feeding (Harfouche et al., 2006; Ankala et al., 2009). To further determine the role of ET in contributing to mir1-mediated defense against CLA, Mp708 plants were pretreated with 1-methylcyclopropane (MCP; commercially available as Ethylbloc) and ethephon (ETP), which block and activate the ET pathway, respectively. Mp708 plants treated with MCP prior to CLA infestation had increased aphid numbers in a no-choice bioassay (Fig. 6A) and suppressed mir1 transcript accumulation (Fig. 6B). The mir1 expression was comparable between plants that were treated with MCP alone and both with MCP treatment and CLA infestation. Plants that were not treated with MCP, as expected, induced higher levels of mir1 expression in Mp708 after CLA infestation for 24 h (Fig. 6B). By contrast, Mp708 plants pretreated with ET-generating compound ETP provided enhanced resistance to CLA (Fig. 6C) and elevated mir1 transcript expression (Fig. 6D). Furthermore, mir1 expression remained at elevated levels in Mp708 plants after ETP treatment and CLA infestation (Fig. 6D), suggesting that ET plays a key role in mir1-mediated defense against CLA. Plants that were treated with buffer or water to dissolve MCP and ETP, respectively, were used as the controls. These results are different from those with caterpillar infestation, where both JA and ET are indispensable for mir1 transcript and Mir1-CP protein accumulation (Harfouche et al., 2006; Ankala et al., 2009). Taken together, no-choice bioassays in conjunction with qRT-PCR analysis of mir1 transcript expression indicate that CLA feeding-induced expression of mir1 is independent of JA but dependent on the ET pathway.
Figure 6.
mir1-dependent resistance to CLA requires ET. Total number of CLA adults and nymphs recovered 4 d after infestation of V3 Mp708 plants that were pretreated with MCP (A) or ETP (C) for 24 h. For experiments A and C, Mp708 plants were infested with three adult apterous aphids per plant after 24 h of MCP or ETP treatment. Values are the mean ± se of aphid numbers on a minimum of 12 Mp708 plants (n = 12). This experiment was replicated two times with similar results with at least eight Mp708 plants. qRT-PCR analysis of mir1 transcripts in V3 Mp708 plants with (+) and without (–) prior treatment of MCP and CLA infestation (B) or ETP and CLA infestation (D). Plants that were treated with buffer or water to dissolve MCP or ETP, respectively, were used as the controls. E, qRT-PCR analysis of ACS6 transcripts in V3 Tx601 and Mp708 plants before and after (24 h) CLA infestation. For experiments B, D, and E, n = 4. F, Profiles of ET emission in V3 Tx601 and Mp708 maize plants at different time points before and after CLA infestation (n = 5). Different letters above the bars indicate values that are significantly different from each other (P < 0.05). Error bars represent ± se. FW, Fresh weight.
Moreover, we monitored the expression of maize aminocyclopropane-1-carboxylic acid synthase6 (ACS6), which is involved in the biosynthesis of ET production in maize (Young et al., 2004). CLA infestation significantly induced the expression of ACS6 in Mp708 compared with Tx601 plants (Fig. 6E). As CLA feeding induces ACS6, we also monitored ET production in CLA-infested leaves to determine whether maize responds to CLA feeding by elevating ET levels. There was no significant difference in the production of ET in uninfested leaves of both Tx601 and Mp708 plants. CLA feeding induced ET production in both maize lines 4 hpi, albeit at significantly lower levels in Tx601 plants. Because CLA feeding-induced expression of mir1 in Mp708 foliage was significantly higher at 16 hpi (Fig. 5A), we also monitored ET production 16 hpi of CLA to assess whether mir1 expression parallels ET production in Mp708 plants. As shown in Figure 6F, an ET burst was observed in Mp708 plants after 16 hpi of CLA compared with the Tx601 plants. These data further augment our hypothesis that ET acts as a critical component in mir1-mediated maize defense against CLA.
DISCUSSION
mir1, which encodes a Cys protease, was identified as single copy in the maize genome that maps on chromosome 6, bin 6.02 (Jiang et al., 1995; Brooks et al., 2007). Our study suggests that CLA feeding-induced expression of mir1 contributes to enhanced defense in the Mp708 maize inbred line. Mir1-CP accumulation in response to lepidopteran larvae requires combined actions of JA and ET pathways (Harfouche et al., 2006; Ankala et al., 2009). However, surprisingly, CLA feeding-induced mir1 expression is dependent only on the ET pathway in providing defense against the phloem sap-sucking aphids (Fig. 6). Furthermore, we observed comparable levels of JA biosynthetic genes LOX1 and OPR8 in both Mp708 and Tx601 plants after CLA infestation, suggesting that JA may not be a key component in Mp708’s resistance to CLA (Supplemental Fig. S2). Based on the evidence provided, we propose a unique mechanism that involves the differential regulation of mir1 by the ET pathway upon CLA infestation. Studies have shown that aphid infestation-induced ET pathway-related genes in resistant varieties of melon (Cucumis melo) and tomato contributed to plant defenses that curtail the insect proliferation (Anstead et al., 2010; Wu et al., 2015). Furthermore, it has been shown that the Brown planthopper induced008a gene in rice (Oryza sativa) interacts with the ET pathway in activating plant immune responses against brown planthopper, a phloem sap-feeding insect (Hu et al., 2011). Collectively, our experiments and previous studies underscore the significance of the ET pathway and its interaction with defense gene networks for evoking defensive mechanisms against phloem-feeding insects in various crop plants.
In addition to the food/nutrient storage and resource acquisition, roots may also act as a site for toxin synthesis in response to aboveground herbivory (Erb et al., 2009b; Nalam et al., 2013). Our data suggest that aboveground feeding by CLA transduced yet-to-be-discovered signal(s) to the roots that triggered belowground accumulation of mir1 (Fig. 5). However, the dynamic responses of roots in response to aboveground herbivory and the signal(s) that trigger the long-distance signaling in maize are largely unknown. Transcriptomic profiling and/or metabolomics approaches could be utilized to establish the extent of systemic activation of defense genes or metabolites in response to aboveground herbivory. Several studies have shown that JA is critical for its involvement in long-distance signaling mechanisms in plant-insect interactions (Ankala et al., 2009; Shivaji et al., 2010; Wu and Baldwin, 2010; Erb et al., 2012). However, our results suggest that JA is dispensable for mir1 accumulation upon CLA infestation.
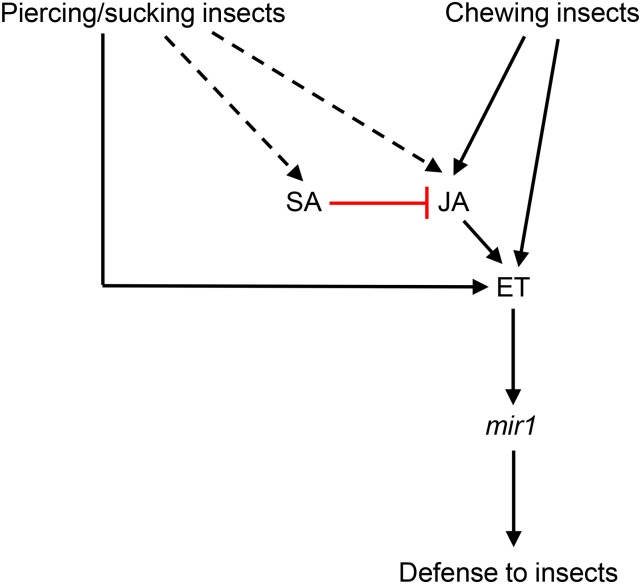
In this study, we unravel the unique role of the ET pathway and its interaction with the mir1 defensive gene to provide enhanced resistance to phloem-feeding aphids in Mp708 plants. As depicted in Figure 7, ET acts as a central node in providing mir1-mediated defense to different feeding guilds of insect pests. Although CLA feeding altered the expression of JA and SA defense-related gene markers, pharmacological studies have confirmed that mir1-dependent defense against CLA is uncoupled from both JA and SA pathways (Supplemental Figs. S2–S4). Previously, it was shown that feeding by silverleaf whitefly (Bemisia tabaci), another phloem sap-sucking pest, activates SA signaling on its host plant to suppress the activation of JA signaling (Zarate et al., 2007; Walling, 2008). Similarly, CLA feeding might be activating the SA signaling pathway to antagonize the activation of the JA pathway, which, in turn, activates the downstream defense-signaling components, including mir1. Moreover, plants that were pretreated with SA suppressed the expression of mir1 (Supplemental Fig. S4C), most likely through the inactivation of JA and/or JA-dependent signaling components. However, we cannot rule out the possibility that CLA feeding-induced SA also directly modulates the ET activity independent of the JA pathway. JA inhibitor treatments and expression of JA biosynthesis genes after CLA infestation suggest the uncoupling of the JA pathway in Mp708’s resistance to CLA. However, because the concentration of JA in maize leaves after CLA infestation is not known, the potential role for JA in Mp708’s resistance to CLA cannot be completely ruled out. At best, the data indicate a significantly reduced or negligible contribution from the JA pathway compared with that reported with chewing insects (Supplemental Fig. S3; Harfouche et al., 2006; Ankala et al., 2009). Previous studies and the data presented in this work are in agreement with the model that ET acts as a central signaling node in linking mir1 expression with enhanced resistance to different feeding styles of insect pests (Fig. 7; Harfouche et al., 2006; Ankala et al., 2009).
Figure 7.
Model depicting the action of ET-dependent mir1-mediated defense to insect pests. Feeding by both piercing-sucking and chewing insects induce mir1 accumulation in Mp708 plants through different signaling mechanisms. Phloem sap-sucking insects rapidly induce the mir1 expression independent of JA but dependent on the ET-signaling pathway. Previous studies with chewing insects have demonstrated that JA acts upstream of ET in activating mir1-dependent defenses in maize. In addition, feeding by chewing insects activates the ET pathway independently of JA, thus contributing to mir1-mediated resistance in maize (Ankala et al., 2009). CLA feeding also induces the expression of SA and JA defense-related marker genes. However, pharmacological studies indicate that mir1-mediated enhanced resistance to maize is independent of both JA and SA pathways. Furthermore, plants that were pretreated with SA suppressed the mir1 expression, presumably through the inactivation of JA and/or JA-dependent signaling cascades. However, available evidence does not rule out the possibility of SA directly modulating the activity of ET. Nonetheless, our data clearly suggest that ET acts a central node for mir1-dependent resistance to different feeding guilds of insect pests. (Positive effects are denoted with black lines ending in arrows, constitutive expression is represented with broken black lines ending in arrows, and a red line that terminates with a perpendicular bar suggests a suppressive effect).
One major difference between feeding by lepidopteran larvae and the phloem-feeding aphid is the rapidity of mir1 transcript accumulation in the whorl region of Mp708 plants. In the case of the lepidopteran insect, mir1 transcripts do not significantly increase until at least 24 h after the initial feeding, whereas CLA feeding triggers a significant mir1 transcript level within 1 h (Fig. 2A; Ankala et al., 2009). One possible explanation is that lepidopteran larvae tend to avoid feeding on vascular tissues until all of the other tissue is eaten, while the CLA immediately probes and feeds in the maize vascular tissues. Furthermore, previous studies have shown that feeding by chewing and piercing-sucking insects alter host defenses through different phytohormone signaling pathways, most likely due to the release of unique salivary signals arising from these herbivore pests (Musser et al., 2002; Howe and Jander, 2008; Mutti et al., 2008; Walling, 2008; Erb et al., 2009a; Pitino et al., 2011; Tian et al., 2012; Felton et al., 2014). Aphid feeding can also alter host physiology, possibly by the action of aphid salivary effectors (Sandström et al., 2000; Dinant et al., 2010; Hogenhout and Bos, 2011; Wilson et al., 2011; Nalam et al., 2012). We observed that the vascular sap obtained from Tx601 plants that were previously infested with aphids had less antibiotic factors, suggesting that CLA were able to suppress defenses in Tx601 plants. By contrast, vascular sap from Mp708 plants that had prior exposure to aphids revealed elevated levels of defense-promoting factors, possibly due to mir1 and/or mir1-dependent antibiosis factors against CLA (Fig. 3). However, how plants perceive the salivary signals and transduce the early signaling events remains to be determined.
It is highly likely that mir1 provides direct toxicity to the aphids, because our feeding trial bioassays with rMir1-CP demonstrate strong antibiotic effect by curtailing aphid reproduction, growth, and development (Fig. 4A). Previously, it was shown that Mir1-CP disrupts the PM of caterpillars (Pechan et al., 2002). Although the mode of action of Mir1-CP on aphids remains unknown, one possible mechanism could be the binding of Mir1-CP to the brush border membrane vesicles of the aphid midgut, as has been shown in the case of garlic (Allium sativum) leaf lectin binding to the brush border membrane vesicles of the mustard aphid (Lipaphis erysimi; Bandyopadhyay et al., 2001). Alternatively, Mir1-CP may bind to the head region, thereby blocking the expression of several salivary proteins that are essential for the aphid’s feeding and/or survival.
Unlike lepidopteran and coleopteran pests, Bacillus thuringiensis (Bt) toxins exhibit little toxicity against sap-sucking hemipteran pests (Raps et al., 2001; Gatehouse, 2008; Chougule and Bonning, 2012). Furthermore, Bt maize supports higher aphid populations compared with non-Bt maize (Faria et al., 2007), suggesting that better management practices are required to control hemipteran sap-sucking pests. Our results suggest that the aphid feeding-induced mir1 or mir1-dependent mechanisms could be effectively used to improve resistance against aphids. Furthermore, rMir1-CP provides toxicity to GPA (Fig. 4C), the most damaging pest of many crop plants that causes a sharp decline in yields (Blackman and Eastop, 2000). Hence, deploying Mir1-CP transgenic crops can be successfully used to combat GPA attack, thereby augmenting both the level and durability of plant resistance against phloem-feeding aphids. The possibility of using Mir1-CP singly or in combination with Bt toxins can be explored in the future to develop unique pest management strategies for controlling phloem-feeding insect pests.
In summary, we have uncovered that the ET-regulated mir1 is responsible for providing enhanced resistance to aphids in maize. Furthermore, we provided a molecular framework to further examine the role of mir1 in providing defense to different feeding styles of insect pests. Determining the signaling partner(s) of mir1 that is involved in long-distance defense-signaling mechanisms is essential to effectively enhance plant’s innate immunity against insect pests.
MATERIALS AND METHODS
Plant and Aphid Growth Conditions
Maize (Zea mays) plants were grown in field soil in growth chambers with a 14-h-light/10-h-dark photoperiod, 160 μE m–2 s –1, 25°C, and 50% to 60% relative humidity. All plants for the experiments were used at the V2 to V3 stage (approximately 2 weeks; Ritchie et al., 1993). Except for the choice assays, all maize plants were grown in 3.8- × 21.0-cm plastic Cone-tainers (Hummert International). For choice assays, maize plants were grown in 15- × 15-cm pots. A CLA colony was started with aphids obtained from Frederick Gildow (Pennsylvania State University) that were originally collected from Pennsylvania State University and maintained on barley (Hordeum vulgare). In our laboratory, CLA was reared on a mixture of moderately susceptible to susceptible barley seed in a Percival growth chamber with a 14-h-light/10-h-dark photoperiod, 140 μE m–2 s–1, 23°C, and 50% to 60% relative humidity. A GPA colony was obtained from Gary Thompson (Pennsylvania State University) and was maintained on cabbage (Brassica oleracea) plants in the growth chambers with the above-mentioned growth conditions.
Aphid Bioassays
Both no-choice and choice aphid bioassays were performed as described previously (Meihls et al., 2013). For no-choice assays, five adult apterous aphids were introduced on each plant, and the total number of aphids (adults plus nymphs) was recorded 7 d after initial release of aphids. For choice assays, 15 adult apterous aphids were released at the center of the pot, equidistant from both genotypes. The number of adult aphids settled on each plant was recorded after 24 h. For no-choice and choice aphid bioassays, we used eight to 12 maize plants per genotype (n = 8–12) and at least 10 maize plants per genotype (n = 10), respectively. All of the no-choice and choice aphid bioassays were replicated two times with at least eight maize plants per genotype.
Artificial-Diet Feeding Trial Bioassays
Artificial diet for CLA was prepared as described previously (Meihls et al., 2013). Aphid feeding trial bioassays were conducted according to a previously described method (Louis et al., 2010, 2012). Briefly, three adult CLA were introduced into each feeding chamber and allowed to feed on the diet, and the total numbers of aphids (adults plus nymphs) in each chamber was counted after 4 d.
Vascular Sap Collection
Collection of vascular sap from maize plants enriched in phloem sap was performed using the EDTA-based method as previously described (King and Zeevaart, 1974; Raps et al., 2001; Marti et al., 2013). Aphids were removed from the plants prior to sap collection from aphid-infested maize plants. A minimum of 24 plants of each genotype per treatment (25 aphids per plant for aphid-infested plants) was used to collect the vascular sap.
RNA Extraction and qRT-PCR
Maize leaf tissue (50–100 mg) was used for RNA extraction. qRT-PCR was performed with SYBR Green Master Mix (Roche Applied Science) on a 7500 Fast Real-Time PCR System (Applied Biosystems; Peiffer et al., 2009; Louis et al., 2013). The qRT-PCR primers specific for genes of interest can be found in Supplemental Table S1. Primer efficiencies and relative expression levels were calculated as described previously (Peiffer et al., 2009). At least three independent leaf samples were included in each qRT-PCR run, and each sample contained three technical replicates.
rMir1-CP and Papain
rMir1-CP was prepared as described previously (Mohan et al., 2006). Papain for feeding trial bioassays was obtained from Sigma-Aldrich. Both rMir1-CP and papain were mixed with PBS buffer, and the aphid diet mixed with PBS buffer was used as the control for artificial-diet feeding trial bioassays.
Treatment with Phytohormone Inducers and Inhibitors
Ethylbloc (MCP) to block the ET perception in maize was obtained from Floralife. The Ethylbloc solution was prepared at a final concentration of approximately 1 mg L–1 by dissolving 2,000 mg of the powder in 10 mL of the releasing buffer supplied by the manufacturer. Two plants each of the same genotype and a beaker containing the solution were placed in a 40- × 40- × 120-cm plexiglass chamber. Control plants were placed in a plexiglass chamber having similar dimensions, and a beaker that contained the buffer used to dissolve MCP was placed in the chamber. The effect of exogenous ET was determined by spraying maize plants with ETP as described previously (Ankala et al., 2009). To block the JA biosynthesis genes, plants that were sprayed with 5 mm IBU were used as described previously (Ankala et al., 2009). Plants that were sprayed with water to dissolve IBU were used as the controls. An additional JA inhibitor, NDGA, was used in this study. One millimolar NDGA dissolved in 0.1% (v/v) methanol (MeOH) diluted in water was sprayed on maize plants. To activate or block the SA pathway, 250 µm sodium salicylate or 500 µm MeJA dissolved in 0.1% (v/v) MeOH or 0.1% (v/v) Tween, respectively, was sprayed on maize plants. Control plants were treated with 0.1% (v/v) MeOH or 0.1% (v/v) Tween. All of the chemicals, except MCP, were purchased from Sigma-Aldrich.
ET Measurement
For ET determination, maize plants were infested with 25 adult apterous CLA on each plant. Plants that were not infested with CLA were used as the controls. The second true leaf from each maize plant was excised before and after CLA infestation at the indicated times. The collected leaf samples were placed in 20-mL glass vials and immediately sealed with a rubber stopper. Following 1 h of sealing, 1 mL of headspace was sampled with a gas-tight syringe and immediately injected manually into a Hewlett-Packard gas chromatograph (HP-5890 GC) equipped with a single flame ionization detector, Porapak Q 80/100 column, helium carrier gas flow of 40 mL min–1, and an injector temperature of 70°C. Tissue fresh weight was recorded for each sample.
Statistical Analyses
For all the data sets, ANOVA was performed using PROC GLIMMIX (SAS 9.3, SAS Institute). Both normality and homogeneity of data sets were checked. For aphid bioassays and feeding trial bioassays, when significant, means were separated using the lsd procedure. Multiple comparisons of qRT-PCR data were carried out using Dunnett’s test (SAS 9.3). Pairwise comparison among treatments irrespective of maize genotypes utilizing a two-sample Student’s t test was used for ET emission analysis.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AF019145 (mir1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Mp708 provides enhanced resistance to CLA.
Supplemental Figure S2. mir1-dependent maize resistance to CLA is independent of the JA pathway.
Supplemental Figure S3. JA inhibitor treatments did not affect CLA performance on Mp708 plants.
Supplemental Figure S4. SA is not required for mir1-mediated defense to CLA in maize.
Supplemental Table S1. Primers used for qRT-PCR study.
Acknowledgments
We thank Paul Ayayee for assistance with ET measurements, Kathleen M. Brown for access to analytical instrumentation, and Gautam Sarath for comments on the draft of the article.
Glossary
- ET
ethylene
- JA
jasmonic acid
- CLA
corn leaf aphid
- PM
peritrophic matrix
- SA
salicylic acid
- qRT
quantitative real-time
- hpi
hours postinfestation
- IBU
ibuprofen
- NDGA
nordihydroguaiaretic acid
- MEJA
methyl jasmonate
- MCP
1-methylcyclopropane
- ETP
ethephon
- Bt
Bacillus thuringiensis
- GPA
green peach aphid
- MeOH
methanol
- PBS
phosphate-buffered saline
Footnotes
This work was supported at different times by the University of Nebraska, Lincoln (start-up research funds and a faculty seed grant to J.L.) and the U.S. Department of Agriculture (National Institute of Food and Agriculture grant nos. 2010–65105–20639 and 2011–67013–30352 to G.W.F. and D.S.L.).
Articles can be viewed without a subscription.
References
- Ankala A, Luthe DS, Williams WP, Wilkinson JR (2009) Integration of ethylene and jasmonic acid signaling pathways in the expression of maize defense protein Mir1-CP. Mol Plant Microbe Interact 22: 1555–1564 [DOI] [PubMed] [Google Scholar]
- Anstead J, Samuel P, Song N, Wu CJ, Thompson GA, Goggin FL (2010) Activation of ethylene-related genes in response to aphid feeding on resistant and susceptible melon and tomato plants. Entomol Exp Appl 134: 170–181 [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE (1994) Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J Chem Ecol 20: 2139–2157 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Roy A, Das S (2001) Binding of garlic (Allium sativum) leaf lectin to the gut receptors of homopteran pests is correlated to its insecticidal activity. Plant Sci 161: 1025–1033 [Google Scholar]
- Betsiashvili M, Ahern KR, Jander G (2015) Additive effects of two quantitative trait loci that confer Rhopalosiphum maidis (corn leaf aphid) resistance in maize inbred line Mo17. J Exp Bot 66: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman RL, Eastop VF (2000) Aphids on the World’s Crops: An Identification and Information Guide, Ed 2 John Wiley, Chichester, UK [Google Scholar]
- Brooks TD, Bushman BS, Williams WP, McMullen MD, Buckley PM (2007) Genetic basis of resistance to fall armyworm (Lepidoptera: Noctuidae) and southwestern corn borer (Lepidoptera: Crambidae) leaf-feeding damage in maize. J Econ Entomol 100: 1470–1475 [DOI] [PubMed] [Google Scholar]
- Carena MJ, Glogoza P (2004) Resistance of maize to the corn leaf aphid: a review. Maydica 49: 241–254 [Google Scholar]
- Chougule NP, Bonning BC (2012) Toxins for transgenic resistance to hemipteran pests. Toxins (Basel) 4: 405–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SA, Nemchenko A, Borrego E, Murray I, Sobhy IS, Bosak L, DeBlasio S, Erb M, Robert CAM, Vaughn KA, et al. (2013) The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J 74: 59–73 [DOI] [PubMed] [Google Scholar]
- Chuang WP, Herde M, Ray S, Castano-Duque L, Howe GA, Luthe DS (2014) Caterpillar attack triggers accumulation of the toxic maize protein RIP2. New Phytol 201: 928–939 [DOI] [PubMed] [Google Scholar]
- Cortés-Cruz M, Snook M, McMullen MD (2003) The genetic basis of C-glycosyl flavone B-ring modification in maize (Zea mays L.) silks. Genome 46: 182–194 [DOI] [PubMed] [Google Scholar]
- Culy MD, Edwards CR, Cornelius JR (1992) Effect of silk feeding by western corn rootworm (Coleoptera: Chrysomelidae) on yield and quality of inbred corn in seed corn production fields. J Econ Entomol 85: 2440–2446 [Google Scholar]
- Dafoe NJ, Huffaker A, Vaughan MM, Duehl AJ, Teal PE, Schmelz EA (2011) Rapidly induced chemical defenses in maize stems and their effects on short-term growth of Ostrinia nubilalis. J Chem Ecol 37: 984–991 [DOI] [PubMed] [Google Scholar]
- Dinant S, Bonnemain JL, Girousse C, Kehr J (2010) Phloem sap intricacy and interplay with aphid feeding. C R Biol 333: 504–515 [DOI] [PubMed] [Google Scholar]
- Erb M, Flors V, Karlen D, de Lange E, Planchamp C, D’Alessandro M, Turlings TCJ, Ton J (2009a) Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J 59: 292–302 [DOI] [PubMed] [Google Scholar]
- Erb M, Lenk C, Degenhardt J, Turlings TCJ (2009b) The underestimated role of roots in defense against leaf attackers. Trends Plant Sci 14: 653–659 [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria CA, Wäckers FL, Pritchard J, Barrett DA, Turlings TCJ (2007) High susceptibility of Bt maize to aphids enhances the performance of parasitoids of lepidopteran pests. PLoS One 2: e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Chung SC, Estrada-Hernańdez MG, Louis J, Peiffer M, Tian D (2014) Herbivore oral secretions are the first line of protection against plant induced defenses. Ann Plant Rev 47: 37–76 [Google Scholar]
- Gatehouse JA. (2008) Biotechnological prospects for engineering insect-resistant plants. Plant Physiol 146: 881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyter ED, Lambert D, Geelen G, Smagghe G (2007) Novel advances with plant saponins as natural insecticides to control pest insects. Pers Technol 1: 96–105 [Google Scholar]
- Gill TA, Sandoya G, Williams P, Luthe DS (2011) Belowground resistance to western corn rootworm in lepidopteran-resistant maize genotypes. J Econ Entomol 104: 299–307 [DOI] [PubMed] [Google Scholar]
- Goggin FL. (2007) Plant-aphid interactions: molecular and ecological perspectives. Curr Opin Plant Biol 10: 399–408 [DOI] [PubMed] [Google Scholar]
- Harfouche AL, Shivaji R, Stocker R, Williams PW, Luthe DS (2006) Ethylene signaling mediates a maize defense response to insect herbivory. Mol Plant Microbe Interact 19: 189–199 [DOI] [PubMed] [Google Scholar]
- Hogenhout SA, Bos JIB (2011) Effector proteins that modulate plant-insect interactions. Curr Opin Plant Biol 14: 422–428 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Hu J, Zhou J, Peng X, Xu H, Liu C, Du B, Yuan H, Zhu L, He G (2011) The Bphi008a gene interacts with the ethylene pathway and transcriptionally regulates MAPK genes in the response of rice to brown planthopper feeding. Plant Physiol 156: 856–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Siregar U, Willeford KO, Luthe DS, Williams WP (1995) Association of 33-kD cysteine found in corn callus with the inhibition of fall armyworm larval growth. Plant Physiol 108: 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyat RR, Balogh CM, Moraski RP, De Moraes CM, Mescher MC, Stephenson AG (2013) Constitutive and herbivore-induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am J Bot 100: 1014–1021 [DOI] [PubMed] [Google Scholar]
- Kim ES, Choi E, Kim Y, Cho K, Lee A, Shim J, Rakwal R, Agrawal GK, Han O (2003) Dual positional specificity and expression of nontraditional lipoxygenase induced by wounding and methyl jasmonate in maize seedlings. Plant Mol Bio lnteract 52: 1203–1213 [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee BW, Schroeder FC, Jander G (2008) Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J 54: 1015–1026 [DOI] [PubMed] [Google Scholar]
- King RW, Zeevaart JAD (1974) Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol 53: 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L, Camas A, Shivaji R, Ankala A, Williams P, Luthe D (2007) Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta 226: 517–527 [DOI] [PubMed] [Google Scholar]
- Louis J, Gobbato E, Mondal HA, Feys BJ, Parker JE, Shah J (2012) Discrimination of Arabidopsis PAD4 activities in defense against green peach aphid and pathogens. Plant Physiol 158: 1860–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J, Leung Q, Pegadaraju V, Reese J, Shah J (2010) PAD4-dependent antibiosis contributes to the ssi2-conferred hyper-resistance to the green peach aphid. Mol Plant Microbe Interact 23: 618–627 [DOI] [PubMed] [Google Scholar]
- Louis J, Peiffer M, Ray S, Luthe DS, Felton GW (2013) Host-specific salivary elicitor(s) of European corn borer induce defenses in tomato and maize. New Phytol 199: 66–73 [DOI] [PubMed] [Google Scholar]
- Louis J, Shah J (2013) Arabidopsis thaliana-Myzus persicae interaction: shaping the understanding of plant defense against phloem-feeding aphids. Front Plant Sci 4: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J, Shah J (2015) Plant defence against aphids: the PAD4 signalling nexus. J Exp Bot 66: 449–454 [DOI] [PubMed] [Google Scholar]
- Luthe DS, Gill T, Zhu L, Lopéz L, Pechanova O, Shivaji R, Ankala A, Williams WP (2011) Aboveground to belowground herbivore defense signaling in maize: a two-way street? Plant Signal Behav 6: 126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Upadhyay S, Wajid S, Ram M, Jain DC, Singh VP, Abdin MZ, Kapoor R (2015) Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 25: 345–357 [DOI] [PubMed] [Google Scholar]
- Marti G, Erb M, Boccard J, Glauser G, Doyen GR, Villard N, Robert CA, Turlings TC, Rudaz S, Wolfender JL (2013) Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ 36: 621–639 [DOI] [PubMed] [Google Scholar]
- McMullen M, Frey M, Degenhardt J (2009) Genetics and biochemistry of insect resistance in maize. In Bennetzen JL, Hake S, eds. Handbook of Maize: Its Biology. Springer, New York, 271–290 [Google Scholar]
- Meihls LN, Handrick V, Glauser G, Barbier H, Kaur H, Haribal MM, Lipka AE, Gershenzon J, Buckler ES, Erb M, et al. (2013) Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 25: 2341–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meihls LN, Kaur H, Jander G (2012) Natural variation in maize defense against insect herbivores. Cold Spring Harb Symp Quant Biol 77: 269–283 [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC (2005) Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser J, Vidal S (2005) Nutritional resources used by the invasive maize pest Diabrotica virgifera virgifera in its new Southeast-European distribution range. Entomol Exp Appl 114: 55–63 [Google Scholar]
- Mohan S, Ma PWK, Pechan T, Bassford ER, Williams WP, Luthe DS (2006) Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J Insect Physiol 52: 21–28 [DOI] [PubMed] [Google Scholar]
- Morita M, Shitan N, Sawada K, Van Montagu MCE, Inzé D, Rischer H, Goossens A, Oksman-Caldentey KM, Moriyama Y, Yazaki K (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci USA 106: 2447–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: Caterpillar saliva beats plant defences. Nature 416: 599–600 [DOI] [PubMed] [Google Scholar]
- Mutti NS, Louis J, Pappan LK, Pappan K, Begum K, Chen MS, Park Y, Dittmer N, Marshall J, Reese JC, et al. (2008) A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci USA 105: 9965–9969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam VJ, Keeretaweep J, Sarowar S, Shah J (2012) Root-derived oxylipins promote aphid performance on Arabidopsis thaliana foliage. Plant Cell 24: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam VJ, Shah J, Nachappa P (2013) Emerging role of roots in plant responses to above ground insect herbivory. Insect Sci 20: 286–296 [DOI] [PubMed] [Google Scholar]
- Oikawa A, Ishihara A, Hasegawa M, Kodama O, Iwamura H (2001) Induced accumulation of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves. Phytochemistry 56: 669–675 [DOI] [PubMed] [Google Scholar]
- Pechan T, Cohen A, Williams WP, Luthe DS (2002) Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Acad Sci USA 99: 13319–13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechan T, Ye L, Chang Y, Mitra A, Lin L, Davis FM, Williams WP, Luthe DS (2000) A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other Lepidoptera. Plant Cell 12: 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer M, Tooker JF, Luthe DS, Felton GW (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 184: 644–656 [DOI] [PubMed] [Google Scholar]
- Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA (2011) Silencing of aphid genes by dsRNA feeding from plants. PLoS One 6: e25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointeau S, Jaguenet E, Couty A, Dubois F, Rambaud C, Ameline A (2014) Differential performance and behavior of the corn leaf aphid, Rhopalosiphum maidis, on three species of the biomass crop miscanthus. Ind Crops Prod 54: 135–141 [Google Scholar]
- Raps A, Kehr J, Gugerli P, Moar WJ, Bigler F, Hilbeck A (2001) Immunological analysis of phloem sap of Bacillus thuringiensis corn and of the nontarget herbivore Rhopalosiphum padi (Homoptera: Aphididae) for the presence of Cry1Ab. Mol Ecol 10: 525–533 [DOI] [PubMed] [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO (1993) How a corn plant develops. Special Report Number 48. Iowa State University of Science and Technology, Cooperative Extension Service, Ames, IA [Google Scholar]
- Sandström J, Telang A, Moran NA (2000) Nutritional enhancement of host plants by aphids: a comparison of three aphid species on grasses. J Insect Physiol 46: 33–40 [DOI] [PubMed] [Google Scholar]
- Shivaji R, Camas A, Ankala A, Engelberth J, Tumlinson JH, Williams WP, Wilkinson JR, Luthe DS (2010) Plants on constant alert: elevated levels of jasmonic acid and jasmonate-induced transcripts in caterpillar-resistant maize. J Chem Ecol 36: 179–191 [DOI] [PubMed] [Google Scholar]
- Smith CM (2005) Plant Resistance to Arthropods: Molecular and Conventional Approaches. Springer, Dordrecht, The Netherlands [Google Scholar]
- So YS, Ji HC, Brewbaker JL (2010) Resistance to corn leaf aphid (Rhopalosiphum maidis Fitch) in tropical corn (Zea mays L.). Euphytica 172: 373–381 [Google Scholar]
- Soler R, Erb M, Kaplan I (2013) Long distance root-shoot signalling in plant-insect community interactions. Trends Plant Sci 18: 149–156 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Huang JF, Rhee Y (1991) Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol 96: 130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongmeearkom P, Ford RE, Jedlinski H (1976) Aphid transmission of maize dwarf mosaic virus strains. Phytopathology 66: 332–335 [Google Scholar]
- Tian D, Peiffer M, Shoemaker E, Tooker J, Haubruge E, Francis F, Luthe DS, Felton GW (2012) Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS One 7: e36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling LL. (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W, Davis FM, Windham GL (1990) Registration of Mp708 germplasm line of maize. Crop Sci 30: 757 [Google Scholar]
- Wilson ACC, Sternberg LdaS, Hurley KB (2011) Aphids alter host-plant nitrogen isotope fractionation. Proc Natl Acad Sci USA 108: 10220–10224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U, Gershenzon J (2002) Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr Opin Plant Biol 5: 300–307 [DOI] [PubMed] [Google Scholar]
- Wu C, Avila CA, Goggin FL (2015) The ethylene response factor Pti5 contributes to potato aphid resistance in tomato independent of ethylene signalling. J Exp Bot 66: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44: 1–24 [DOI] [PubMed] [Google Scholar]
- Yan J, Lipka AE, Schmelz EA, Buckler ES, Jander G (2015) Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress. J Exp Bot 66: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Christensen S, Isakeit T, Engelberth J, Meeley R, Hayward A, Emery RJ, Kolomiets MV (2012) Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24: 1420–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Meeley RB, Gallie DR (2004) ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J 40: 813–825 [DOI] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yin XR, Li X, Yang SL, Ferguson IB, Chen KS (2009) Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. J Agric Food Chem 57: 2875–2881 [DOI] [PubMed] [Google Scholar]