A gene encoding a calcium/proton antiporter is involved in cadmium tolerance and acts to limit oxidative stress.
Abstract
Arabidopsis halleri is a model species for the study of plant adaptation to extreme metallic conditions. In this species, cadmium (Cd) tolerance seems to be constitutive, and the mechanisms underlying the trait are still poorly understood. A previous quantitative trait loci (QTL) analysis performed on A. halleri × Arabidopsis lyrata backcross population1 identified the metal-pump gene Heavy Metal ATPase4 as the major genetic determinant for Cd tolerance. However, although necessary, Heavy Metal ATPase4 alone is not sufficient for determining this trait. After fine mapping, a gene encoding a calcium2+/hydrogen+ antiporter, cation/hydrogen+ exchanger1 (CAX1), was identified as a candidate gene for the second QTL of Cd tolerance in A. halleri. Backcross population1 individuals displaying the A. halleri allele for the CAX1 locus exhibited significantly higher CAX1 expression levels compared with the ones with the A. lyrata allele, and a positive correlation between CAX1 expression and Cd tolerance was observed. Here, we show that this QTL is conditional and that it is only detectable at low external Ca concentration. CAX1 expression in both roots and shoots was higher in A. halleri than in the close Cd-sensitive relative species A. lyrata and Arabidopsis thaliana. Moreover, CAX1 loss of function in A. thaliana led to higher Cd sensitivity at low concentration of Ca, higher sensitivity to methylviologen, and stronger accumulation of reactive oxygen species after Cd treatment. Overall, this study identifies a unique genetic determinant of Cd tolerance in the metal hyperaccumulator A. halleri and offers a new twist for the function of CAX1 in plants.
Pollution by excessive amounts of trace metal elements (TMEs) has become a serious and widespread problem at the global level (Koptsik, 2014). In particular, mining and industrial activities have released large amounts of zinc (Zn), cadmium (Cd), and lead (Pb) into the air, water, and soil. In addition, the application of phosphate fertilizers and sewage sludge contributed to the accumulation of TMEs in agricultural soils and aquatic environments. Cd represents one of the most toxic pollutants released into the environment, and it is recognized as a main threat to human health (Clemens et al., 2013). Moreover, compared with other trace metals, Cd is rapidly absorbed and accumulated in plant tissues, and its transfer to the food chain is a severe consequence of widespread low-level contamination of soil (Clemens et al., 2013). Although plant responses to Cd toxicity are well reported and described in the literature (Lux et al., 2011), the mechanisms underlying Cd toxicity are not completely understood. Cd has been reported to affect the uptake of other minerals from the soil (e.g. Fe, Mg, K, and Ca). At the cellular level, Cd ions lock on many different targets. Cd ions, indeed, can induce mineral deficiencies by competing with the uptake of essential metallic elements, displace other metals from protein active sites (e.g. Fe, Zn, and Ca), and bind sulfhydryl groups of structural proteins and enzymes, leading to misfolding and inhibition of activity (DalCorso et al., 2008; Gallego et al., 2012). Furthermore, Cd can also impair photosynthetic and respiration activities and induce oxidative stress (Heyno et al., 2008). Cd presence inside the cell leads to the arousal of reactive oxygen species (ROS), which can react with lipids, proteins, nucleic acids, and pigments, causing toxic effects and oxidative burst (Sharma and Dietz, 2009; Cuypers et al., 2010). Despite Cd toxicity, a class of plants, referred to as hyperaccumulators, has evolved a combination of mechanisms to reach high levels of tolerance to and accumulation of this element. In these species, some individuals display an extraordinary capacity to accumulate more than 100 µg of Cd g−1 of dry weight in their shoots in their natural habitats (Baker, 1981; Verbruggen et al., 2009; Krämer, 2010). In the last decade, great attention has been given to two Zn and Cd hyperaccumulators, the Brassicaceae Arabidopsis halleri and Noccaea caerulescens. The former is a clonal and self-incompatible plant that is able to grow on both metal-contaminated and noncontaminated soils. In Europe, A. halleri occurs at low altitudes in industrial sites polluted by Zn, Cd, and Pb (e.g. those in northern France, Poland, Germany, and northern Italy among others) and at moderate and high altitudes in soils containing low levels of TMEs. A. halleri exhibits constitutive Zn and Cd tolerance as well as Zn hyperaccumulation, while also maintaining a high natural variability in all of these traits (Bert et al., 2002; Pauwels et al., 2006). Currently, A. halleri is considered a model species for metal accumulation and tolerance studies and also for metal homeostasis because it is a close relative of Arabidopsis thaliana (Al-Shehbaz and O’Kane, 2002). Therefore, the molecular and genetic tools that have been developed for Arabidopsis spp. can also be used for A. halleri. Moreover, because A. halleri is interfertile with Arabidopsis lyrata ssp. petraea, a close phylogenetic relative sensitive to and nonaccumulator of heavy metals, metal tolerance and accumulation can be analyzed in segregating populations.
The genetic basis of Cd tolerance and accumulation has been investigated in backcross population1 (BC1) that was obtained from crosses between A. halleri (metallicolous accession Auby, France) and A. lyrata ssp. petraea (Courbot et al., 2007; Willems et al., 2010). Three chromosomal regions associated with Cd tolerance trait were identified (Courbot et al., 2007). These quantitative trait loci (QTL), named Cadmium Tolerance1 (CdTol1), CdTol2, and CdTol3, explained together 83% of the phenotypic variance (43%, 24%, and 16%, respectively). Another QTL, which was found to be responsible for 21% of the phenotypic variance for Cd accumulation, was identified, and it colocalized with CdTol1 and Heavy Metal ATPase4 (HMA4). HMA4 encodes a Zn/Cd pump that mediates root-to-shoot metal translocation, and it has been validated as a fundamental genetic determinant for Zn/Cd tolerance and accumulation in A. halleri (Hanikenne et al., 2008). Nonetheless, although necessary to modify metal allocation and accumulation, HMA4 alone is not sufficient to explain the high level of Zn and Cd tolerance exhibited by A. halleri. Thus, additional genetic determinants are required for an efficient detoxification mechanism in shoots considering the massive metal flux generated by HMA4 activity (Hanikenne et al., 2008). Results from transcriptomic analyses performed on A. halleri and N. caerulescens suggest that one of the main mechanisms underlying metal tolerance is the constitutive high expression of genes involved in metal transport and detoxification processes (Weber et al., 2006; van de Mortel et al., 2008). Among the genes found to be overexpressed in these tolerant species, those involved in metal uptake (e.g. ZINC-REGULATED TRANSPORTER, IRON-REGULATED TRANSPORTER PROTEIN members), xylem loading (in particular, HMA4 and HMA2), synthesis of ligands (e.g. NICOTIANAMINE SYNTHASE, YELLOW STRIPE-LIKE, and METALLOTHIONEIN members), and vacuolar sequestration and detoxification (e.g. cation/H+ exchanger2 [CAX2], METAL TOLERANCE PROTEIN1 [MTP1], and HMA3) are of particular interest (Verbruggen et al., 2013). In N. caerulescens, several lines of evidence support the key role of elevated transcript levels of the tonoplast Cd transporter HMA3 for the extraordinary vacuolar sequestration capacity of some populations (Ueno et al., 2011). Taken together, these studies suggest that the genes that contribute to hyperaccumulation and hypertolerance are not species specific or novel but rather, differently regulated between tolerant and sensitive species. Nevertheless, apart from HMA4 and HMA3, the molecular mechanisms responsible for Cd tolerance and accumulation in hyperaccumulators still remain elusive. Therefore, the aim of this study was to further analyze CdTol2 QTL to identify unique genetic determinants involved in Cd tolerance. Marker densification in the QTL CdTol2 genomic region resulted in the identification of CAX1 as a possible candidate gene. In Arabidopsis spp., CAX1 encodes a tonoplast Ca2+/H+ exchanger that plays a key role in cellular Ca homeostasis (Cheng et al., 2003; Mei et al., 2007; Conn et al., 2011). By using multiple approaches, we showed that CAX1 is involved in the maintenance of Ca homeostasis, limiting the accumulation of ROS upon Cd stress.
RESULTS
Marker Densification in the CdTol2 Region and Identification of CAX1 as a Candidate Gene
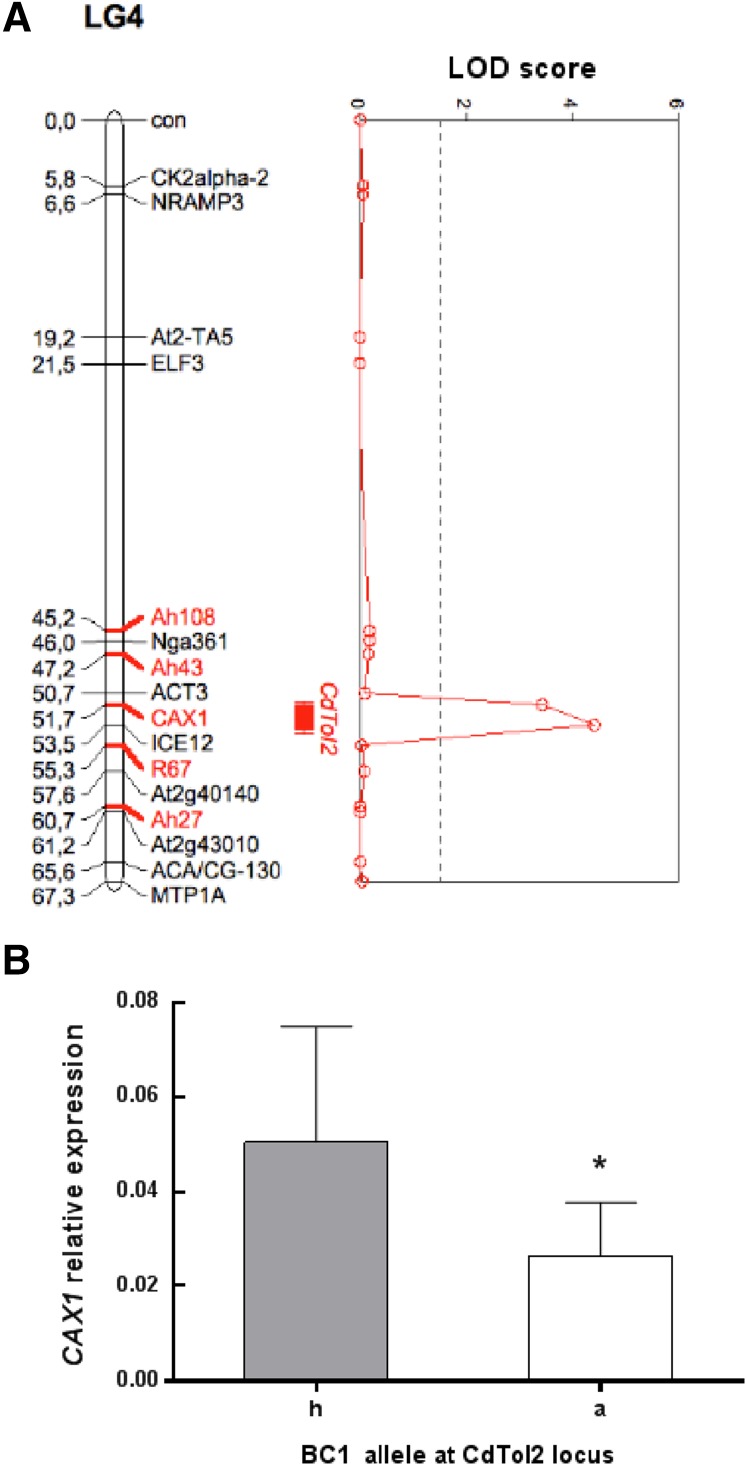
Five markers were added to the Ah × Alp genetic map published by Courbot et al. (2007) to confirm the localization of QTL CdTol2 and narrow down its size. BC1 individuals from the study by Courbot et al. (2007) were genotyped for these markers and used for QTL fine mapping. As a result, the CdTol2 locus was reduced to a 3-centiMorgan region between markers ACTIN3 and At2g40140 (Fig. 1A). In A. thaliana, the region between these markers is approximately 600 kb, and it contains approximately 150 genes. Among them, four genes showed gene ontology annotation associated with Cd response or metal ion transport (Supplemental Table S1). CAX1 was selected for additional studies based on (1) current literature suggesting a role for CAXs in metal transport in the vacuole (Hirschi et al., 2000; Shigaki et al., 2005; Koren’kov et al., 2007; Mei et al., 2007; Wu et al., 2011) and (2) expression analysis of BC1 Ah × Alp individuals displaying different alleles at the CdTol2 locus. Indeed, BC1 individuals with the Ah allele at the CdTol2 locus showed significantly higher CAX1 expression levels in shoots than the ones displaying the Alp allele (Fig. 1B). Furthermore, a second expression analysis identified a positive correlation between CAX1 expression levels in shoots and Cd tolerance in BC1 individuals (n = 20; data not shown).
Figure 1.
Identification of CAX1 as a candidate gene for the QTL CdTol2. A, Densification of the QTL CdTol2. Five additional markers (red) were used to confirm the localization and reduce the CdTol2 locus to a 3-centiMorgan region [for marker descriptions, see Supplemental Table S3; all other markers are defined in Courbot et al. (2007)]. The vertical dashed line represents the LOD score threshold (1.5) for QTL detection at an error level of α = 0.05. Bars indicate the one-LOD (10-fold) support interval, and whiskers (lines extending beyond bars) indicate the two-LOD (100-fold) support intervals. BC1 individuals were phenotyped at 0.5 mm CaNO3 in the study by Courbot et al. (2007). B, CAX1 transcript levels in BC1 individuals displaying the Ah/Alp (h) or Alp/Alp (a) allelic combinations at the CdTol2 locus. At least three cuttings for each BC1 genotype were transferred in hydroponic solution according to the work by Courbot et al. (2007) for 4 weeks. Clones were pooled together, and CAX1 expression levels were assessed through qPCR analysis. Data are means ± sd (n = 9). *, P < 0.05 (Student’s t test).
Cloning of A. halleri CAX1 and in Silico Analyses
In A. thaliana, three CAX1 transcripts are annotated. The At2g38170.3 sequence has been used as a model in all previous studies of AtCAX1, whereas the other two transcripts (At2g38170.1 and At2g38170.2) are predicted as alternative splicing products (The Arabidopsis Information Resource 10). The isolated AhCAX1 transcript exhibited a high level of sequence identity with At2g38170.1 and AlCAX1 (95% and 96%, respectively) and a lower identity with the two other A. thaliana transcripts At2g38170.2 and At2g38170.3 (90% and 85%, respectively). The percentage identities of AhCAX1 at the amino acid level with AlCAX1 and At2g38170.1 are 97% and 95%, respectively. The few differences that exist between the deduced sequence of AhCAX1 and the sequences of AtCAX1 and AlCAX1 all occur outside the annotated functional domains that are the cation selection, exchange, and autoinhibitory regions (Supplemental Fig. S1). These results suggest that the three proteins share a similar function and that substrate specificity of CAX1 transporter is conserved in the three species.
CAX1 Gene Copy Number in A. halleri
Given the high expression of CAX1 in the BC1 individuals displaying the Ah allele (Fig. 1B) and the several reports of gene duplication linked to adaptation to extreme environments (Hanikenne et al., 2008; Ueno et al., 2011; Kondrashov, 2012), the copy number of CAX1 in A. halleri was investigated. In quantitative real-time PCR assays performed with genomic DNA from the Auby population (the grandparent of the BC1 individuals) and the A. thaliana single-copy gene short root (SHR) used as the internal control, it is estimated that there is only one copy of CAX1 in the A. halleri genome (mean = 1.08 ± 0.15; Supplemental Fig. S2).
Study of CAX1 Expression after Cd Treatment at Different Ca Concentrations
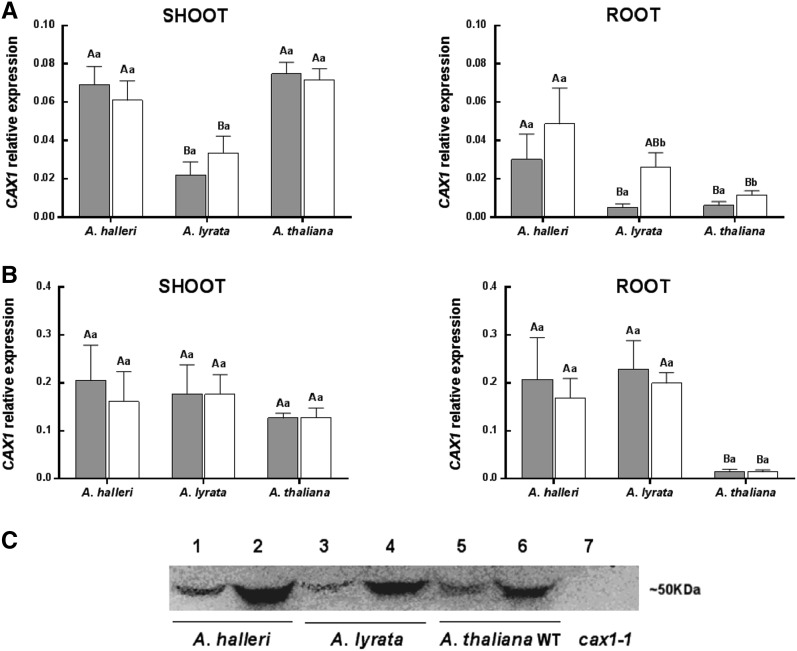
To investigate the impact of Cd treatment on the regulation of CAX1 expression and compare transcript levels in the different species, quantitative real-time reverse transcription (RT)-PCR assays were performed. Because CAX1 is known to have a significant role in Ca homeostasis, levels of CAX1 expression were assayed at two different Ca concentrations. Shoot and root samples were harvested from A. halleri, A. lyrata, and A. thaliana plants grown in a hydroponic system at 0.5 (low Ca concentration; used in the QTL analysis in Courbot et al., 2007) and 2 mm Ca (moderate Ca concentration). A one-half set of samples was further treated with 10 µm CdSO4 for 72 h (Fig. 2, A and B).
Figure 2.
Expression analysis of CAX1. CAX1 transcript levels at low (A) and moderate (B) Ca concentration in control condition (gray bars) and upon 72 h of 10 µm CdSO4 treatment (white bars) of A. halleri, A. lyrata ssp. petraea, and A. thaliana. Data are means ± sd (n = 8–12). Lowercase letters are for comparison between conditions in the same species, and capital letters are for comparison between species in the same condition (Kruskall Wallis test). C, Western blot showing relative levels of CAX1 protein in root from A. halleri, A. lyrata, and A. thaliana. Samples have been collected from plants grown at low-Ca concentration in control condition (lanes 1, 3, and 5) or upon 72 h of 10 µm CdSO4 treatment (lanes 2, 4 and 6). As negative control, we loaded total protein fraction extracted from A. thaliana cax1-1 mutant (lane 7). Equal amounts of total protein (35 ng) were loaded onto the gel.
Under noncontaminated condition at low Ca, at least 3-fold higher levels of CAX1 were detected in the shoots and roots of A. halleri compared with those of A. lyrata (Fig. 2A). Between A. halleri and A. thaliana, expression of CAX1 in the shoots was similar. However, in the roots, expression of CAX1 was 5.6-fold higher in A. halleri. After Cd treatment, CAX1 expression increased 5.5- and 2-fold in the roots of the Cd-sensitive species A. lyrata and A. thaliana, respectively. In contrast, CAX1 expression did not increase in the shoots of these two species. An increase in CAX1 transcript levels after Cd treatment was also observed in A. halleri roots, although this difference was not statistically significant (Fig. 2A).
At 2 mm Ca, for all of the conditions tested and all three species, the expression of CAX1 was at least 2-fold higher than the levels detected at low Ca (Fig. 2B). Under noncontaminated condition, CAX1 expression in the shoots was similar in all three species. For the root samples, similar transcript levels were detected in A. halleri and A. lyrata, whereas A. thaliana exhibited 12-fold lower levels of CAX1 transcript than the other two species. In addition, Cd exposure did not modify CAX1 expression in any of the three species at 2 mm Ca (Fig. 2B). The increased expression of CAX1 transcript in roots upon Cd treatment at low Ca concentration was then checked at the protein level by western blot. Increases in levels of CAX1 protein in all three species were confirmed (Fig. 2C).
CdTol2 Is a Ca-Conditional QTL
At moderate Ca concentration, no difference in CAX1 expression was detected between the parental species of the BC1 (Fig. 2B). Therefore, to verify the validity of the candidate gene, individuals from the BC1 progeny were phenotyped for Cd tolerance through a sequential growth test at moderate Ca concentration (2 mm). The broad-sense heritability value that was estimated using at least three clones of each BC1 genotype was relatively high (0.51). In addition, segregation of Cd tolerance for the unique BC1 individuals was bimodal and similar to that observed by Courbot et al. (2007) (data not shown). As a result of the QTL analysis, no QTL were detected on the linkage group 4 (Fig. 3, red line), whereas with the same set of markers and the phenotyping by Courbot et al. (2007) (performed at 0.5 mm Ca), the CdTol2 QTL were detectable (Fig. 3, green line).
Figure 3.
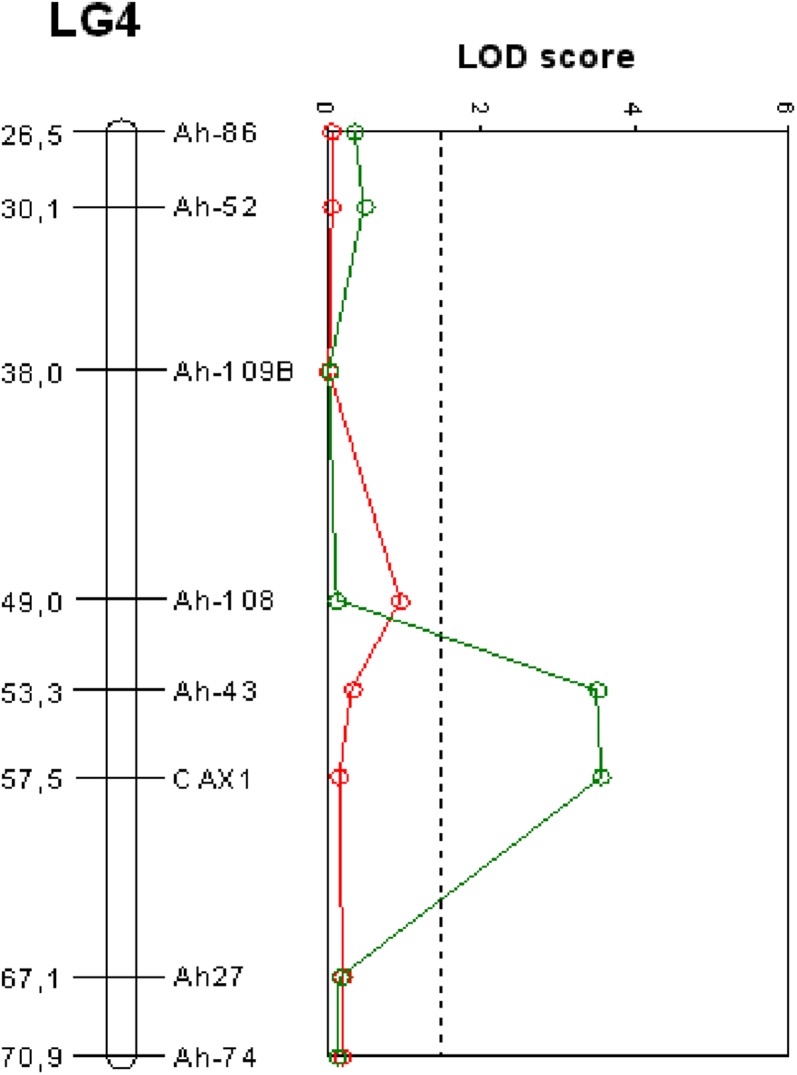
QTL mapping of Cd tolerance in the CdTol2 region at two different Ca concentrations. Red line, LOD score obtained with 125 BC1 individuals phenotyped for Cd tolerance at 2 mm CaNO3 and genotyped for six markers in the CdTol2 region. Green line, LOD score obtained with the phenotyping at 0.5 mm CaNO3 (Courbot et al., 2007) and the six markers. The vertical dashed line represents the LOD score threshold for QTL detection at an error level of α = 0.05. LG4, LINKAGE GROUP4.
Loss of Function in A. thaliana Impairs Cd Tolerance at Low Ca
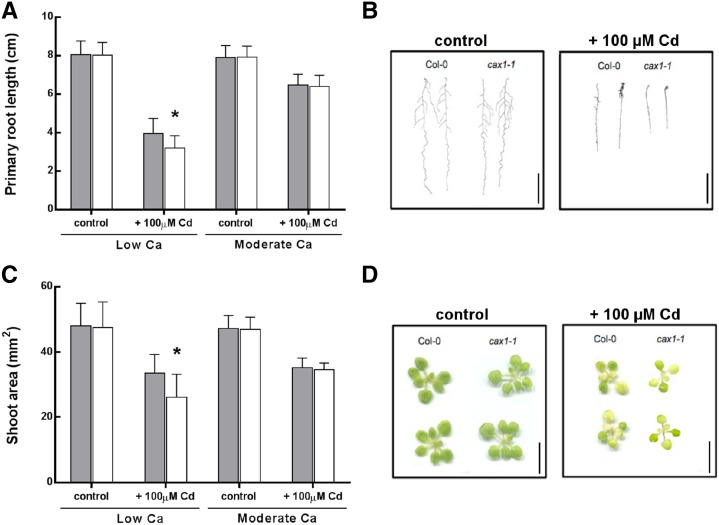
The A. thaliana CAX1 knockout (k.o.) mutant, cax1-1, has been well studied and already characterized for metal tolerance by Cheng et al. (2003). However, no Cd tolerance phenotype has been described for this mutant. Therefore, Cd tolerance of the A. thaliana wild type (Columbia-0 [Col-0] background) and cax1-1 was reassessed at different Ca concentrations using the primary root length and the shoot surface as growth parameters (Fig. 4, A and B). Tests were performed in vitro at low and moderate Ca (0.5 and 2 mm) in the presence or absence of 100 µm Cd for 7 d.
Figure 4.
Analysis of Cd tolerance in the A. thaliana wild type and cax1-1 mutant. Comparison of Cd tolerance between the A. thaliana wild type (Col-0; gray bars) and cax1-1 mutant (white bars) was based on primary root length (A) and total shoot surface (C). Plants were sown in vitro on one-half-strength MS containing 1% Suc solidified with agar. After 1 week, plantlets were transferred to one-half-strength MS agar plates containing low (0.5 mm) or moderate (2 mm) Ca concentrations and 0 or 100 µm CdCl2 for 1 week. Data are means ± sd (n = 58–92 for primary root length measurements and n = 25–47 for shoot surface analysis). *, P < 0.05 (Student’s t test). The representative phenotypes for root (B) and shoot (D) at low Ca are reported. Bars = 2 cm (B; roots) and 1 cm (D; shoots).
Similar root lengths and shoot areas were observed under the control conditions at the two tested Ca concentrations (Fig. 4). In contrast, Cd treatment induced a significant decrease in both growth traits at both concentrations of Ca. However, a more significant decrease was observed in the cax1-1 mutant compared with the wild type at 0.5 mm Ca, with decreases of 19% and 24% observed for primary root length and shoot area, respectively (Fig. 4, A and B). In the presence of 2 mm Ca, no significant differences were detected between the two genotypes (Fig. 4). An in vitro Zn tolerance test was also performed with A. thaliana wild-type and cax1-1 plantlets grown at 0.5 or 2 mm Ca. No difference in either growth parameter was detected between the two genotypes after exposure to 150 µm ZnSO4 (data not shown), thereby excluding a role for CAX1 in Zn tolerance. Shoot tissues were pooled, and full mineral profiles were assayed. No difference in Cd accumulation was observed between wild-type and cax1-1 plants at both Ca concentrations (data not shown).
CAX3 Does Not Seem to Have a Role in Cd Tolerance
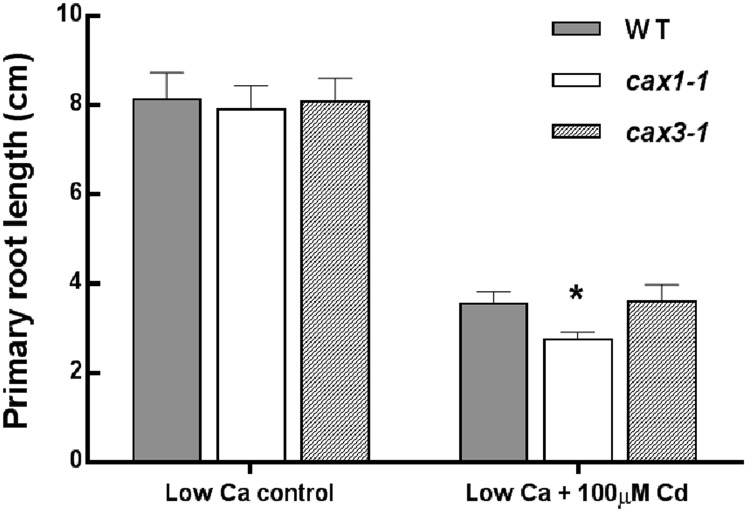
Among the CAX transporters, CAX3 is the closest homolog to CAX1 (77% sequence identity). CAX3 has been shown to have a similar role in Ca homeostasis, and it is able to partially compensate for the loss of CAX1 in the cax1-1 mutant (Cheng et al., 2005; Zhao et al., 2008, 2009; Conn et al., 2011). To investigate whether CAX3 is also involved in the Cd stress response, an in vitro tolerance test was performed for the wild-type A. thaliana and A. thaliana CAX3 k.o. mutant (cax3-1). The test was performed at low and moderate Ca concentrations (0.5 and 2 mm) in control condition or after 100 µm CdCl2 treatment for 7 d. For all of the tested conditions, cax3-1 exhibited similar growth to the A. thaliana wild type. However, after growth of the plants at 0.5 mm Ca and CdCl2 treatment, only cax1-1 mutant exhibited a significant decrease in the primary root length compared with the wild type (Fig. 5).
Figure 5.
Cd tolerance assessed in A. thaliana k.o. mutant cax3-1 at low Ca concentration. Comparison of Cd tolerance was performed between the A. thaliana wild type (WT; gray bars), cax1-1 (white bars), and cax3-1 (striped bars). Tolerance was assessed in vitro by measuring primary root length as a parameter for growth. One week after germination, plantlets were transferred at low (0.5 mm) Ca concentration in control condition or with 100 µm CdSO4 for another 1 week. Data are means ± sd (n = 15–27). *, P < 0.05 (Student’s t test).
CAX1 Limits ROS Accumulation after Cd Treatment
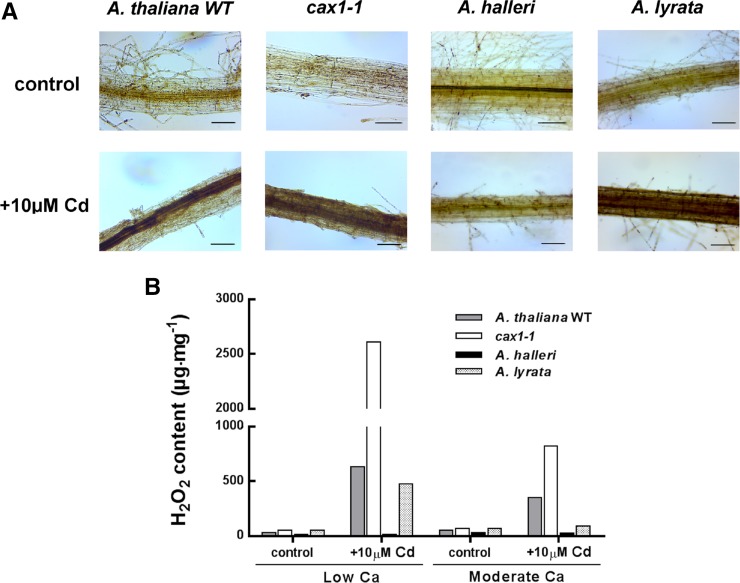
The link between ROS production and stress-induced spikes of cytosolic Ca is well established (Pei et al., 2000; Demidchik et al., 2003; Takeda et al., 2008; Hua et al., 2012). To investigate a potential role for CAX1 in managing ROS accumulation after Cd treatment, levels of hydrogen peroxide (H2O2) and O2− were measured in A. thaliana (the wild type and cax1-1), A. lyrata, and A. halleri plants grown in a hydroponic system and treated with 10 µm CdSO4 for 72 h. Only results for the root samples are presented, because ROS accumulation was not detected in the shoots after 72 h of Cd treatment. At 0.5 mm Ca, all of the root samples presented pale staining. In contrast, A. lyrata and both A. thaliana genotypes, the wild type and cax1-1, exhibited stronger staining after Cd treatment, corresponding to an accumulation of H2O2 (Fig. 6A). Remarkably, cax1-1 roots appeared to accumulate a higher level of ROS than wild-type A. thaliana and A. lyrata (Fig. 6A). Accumulation of H2O2 in A. halleri after Cd treatment was not detected (Fig. 6A). At 2 mm Ca, there was no difference in the staining patterns between the control and the Cd-treated root samples for all of the species and genotypes assayed (data not shown). Quantification of H2O2 accumulation is shown in Figure 6B. After growth at low Ca and Cd treatment, the cax1-1 mutant showed a 4-fold greater accumulation of H2O2 than the A. thaliana wild type. In comparison, H2O2 accumulation after Cd treatment was slightly lower for A. lyrata than for the A. thaliana wild type. Quantification of H2O2 levels allowed differences to be detected after Cd treatment, even when plants were grown with 2 mm Ca. Levels of H2O2 were the highest for the cax1-1 mutant (818.97 µg mg−1) followed by the A. thaliana wild type (350.12 µg mg−1), A. lyrata (91.57 µg mg−1), and A. halleri (26.57 µg mg−1). However, the induction of ROS was at least 2-fold lower at moderate Ca concentrations than at low Ca. For all four genotypes, we observed similar accumulation patterns of O2− (Supplemental Fig. S3).
Figure 6.
H2O2 detection and quantification in DAB-stained samples. A, A. thaliana (the wild type and cax1-1), A. halleri, and A. lyrata roots were stained with DAB reagent in control condition (upper) or after 72 h at 10 µm CdSO4 treatment (lower) at low Ca. Polymerization of DAB is visible as a brown precipitate in the presence of H2O2. Bars = 50 µm. B, The amount of H2O2 was measured in roots of the A. thaliana wild type (WT; gray bars), cax1-1 (white bars), A. halleri (black bars), and A. lyrata (checked bars). H2O2-DAB content in the samples was determined using a standard curve prepared with known amounts of DAB. Plants were grown in hydroponic solution with low or moderate Ca contaminated for 72 h with 0 or 10 µm CdSO4. To obtain sufficient material for H2O2 quantification, roots (n = 3) were pooled before measurements.
CAX1 Involvement in Oxidative Stress
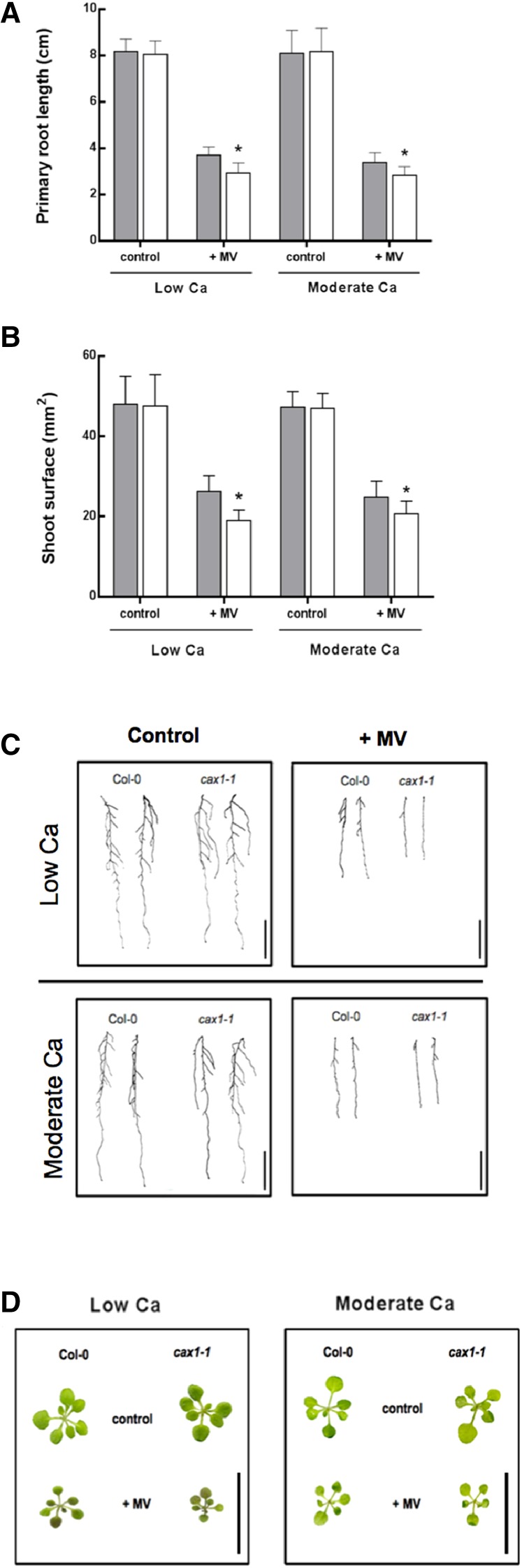
To investigate whether the role for CAX1 in ROS accumulation was Cd specific or general for response to oxidative stress, we performed in vitro tolerance test using methylviologen (MV). This herbicide, which affects electron-transducing reactions in chloroplast and mitochondria, has been widely used to study ROS-mediated damage in plants. For the tolerance test, A. thaliana wild-type and cax1-1 plantlets were grown in the presence of 0.5 and 2 mm Ca as control conditions and then, treated for 7 d with 0.1 µm MV. The cax1-1 plants exhibited higher sensitivity to MV than the wild type at the two Ca concentrations. Indeed, cax1-1 plants exhibited approximately 20% and 17% reduction in primary root growth and shoot surface area, respectively, compared with A. thaliana wild-type plants (Fig. 7).
Figure 7.
In vitro tolerance to ROS-inducing reagent MV. Comparison between the A. thaliana wild type (Col-0; gray bars) and cax1-1 mutant (white bars) was based on primary root length (A) and total shoot surface (B). Plants were grown at low (0.5 mm) and moderate (2 mm) Ca concentrations in control condition or with 0.1 µm MV. Data are means ± sd (n = 20–30). *, P < 0.05 (Student’s t test). Pictures of A. thaliana wild-type and cax1-1 roots and shoots are reported in C and D, respectively. Bars = 2 cm.
DISCUSSION
To identify unique genetic determinants of Cd tolerance in the metal hyperaccumulator A. halleri, a fine-mapping analysis was performed on the A. halleri × A. lyrata petraea BC1 progeny previously studied by Courbot et al. (2007). This analysis allowed the identification of CAX1 as a candidate gene at the locus of the QTL CdTol2, the second major QTL for Cd tolerance, explaining 23.7% of the phenotypic variance observed. In support of CAX1 as a candidate gene for CdTol2 QTL, a positive correlation between CAX1 expression and the tolerance level of individuals of the BC1 progeny was observed. In addition, individuals with the highest levels of CAX1 expression displayed the A. halleri allele at the CdTol2 locus. In A. thaliana, CAX1 encodes a Ca2+/H+ exchanger that localizes to the vacuolar membrane (Cheng et al., 2003; Conn et al., 2011). Plant CAXs are members of a multigene family that have the capacity to transport a broad range of cations (Manohar et al., 2011). In Arabidopsis spp., there are six CAX genes, and CAX1 is a major tonoplast Ca2+ transporter (Cheng et al., 2003; Shigaki and Hirschi, 2006). Other members of the CAX family display the capability to transport Cd into the vacuole than Ca (e.g. CAX2 and CAX4; Hirschi et al., 2000; Mei et al., 2009). Furthermore, a mutant of an activated N-terminal form of AtCAX1 that differs by one amino acid in the selectivity domain, named CAXcd, showed high apparent Cd transport (Shigaki et al., 2005), and petunia (Petunia hybrida) plants expressing this CAXcd exhibited greater Cd accumulation than control plants along with fewer toxicity symptoms (Wu et al., 2011).
In this study, the expression of CAX1 was found to be more than 3-fold higher in the shoots and roots of A. halleri compared with the Cd-sensitive species A. lyrata and 5-fold higher in the A. halleri roots compared with the A. thaliana ones. One possible explanation for the high levels of CAX1 expression observed in A. halleri is copy number expansion, a common strategy that has evolved in plants to survive and adapt to unfavorable environments (Flagel and Wendel, 2009; Kondrashov, 2012). Indeed, some of the genes involved in metal tolerance (e.g. HMA4 and MTP1) have multiple copies in the A. halleri genome (Talke et al., 2006; Hanikenne et al., 2008; Shazad et al., 2013). Our data suggest that AhCAX1 is a single-copy gene in A. halleri and that differential expression between species can be attributed to cis- or transregulation. High levels of AhCAX1 expression could have evolved in ancestral nonmetallicolous populations from which metalicolous populations derived (Pauwels et al., 2005). Indeed, nonmetallicolous populations in several parts of Europe are found in calcareous areas (P. Saumitou-Laprade, personal communication), and levels of CAX1 expression were found to be dependent on the external Ca concentration (Hirschi, 1999; this study). Other than that, a link between Ca concentration in the soil and level of CAX1 expression was previously suggested for soils with low levels of Ca. The loss of function of CAX1 in Arabidopsis spp. conferred better growth in culture conditions similar to serpentine soils, which are characterized by a low Ca to Mg ratio (Bradshaw, 2005). CAX1 transcript levels were also found to vary according to the exposure of Cd. At low Ca concentration, CAX1 was induced by Cd treatment in the roots of all three species. To further investigate the possible role of CAX1 in Cd tolerance, a k.o. mutant of Arabidopsis spp. was studied. In the in vitro tolerance tests that were performed, loss of CAX1 in A. thaliana significantly affects the Cd tolerance phenotype at low Ca but not at moderate concentrations. As a result, significant reductions in primary root length and shoot surface area were observed compared with the A. thaliana wild type. The metal sensitivity of the cax1-1 mutant has been studied, but no particular phenotype linked to Cd was identified before, most probably because of the Ca level used during the phenotype assays performed (Cheng et al., 2003).
Previous studies on cax1-1 mutant have reported very subtle phenotypes after Ca2+ stress caused by functional compensation by CAX3 (Cheng et al., 2005; Conn et al., 2011). Therefore, in this study, Cd sensitivity of the A. thaliana cax3-1 k.o. mutant was evaluated. The k.o. of CAX3 did not affect Cd sensitivity compared with the wild-type phenotype. These results suggest a specific role of CAX1 in response to Cd stress.
After cloning AhCAX1, the deduced amino acid sequences were analyzed considering the different variants issued from alternative splicing and compared with the proteins of A. thaliana and A. lyrata. AhCAX1 shares 97% and 95% identity at the amino acid level with AlCAX1 and AtCAX1, respectively. Moreover, the annotated functional domains are all entirely conserved among A. lyrata, A. halleri, and A. thaliana, including the selectivity domain. Therefore, it could be hypothesized that AlCAX1 and AhCAX1 have the same substrate specificity as AtCAX1 and that the role of CAX1 in Cd tolerance is probably not linked to Cd transport. Furthermore, given that Cd accumulation is similar between the cax1-1 mutant and the A. thaliana wild type, it is unlikely that CAX1 participates in the sequestration of Cd in the vacuole. It was further investigated how CAX1 could impact Cd sensitivity. Cd treatment is known to induce oxidative stress (Sandalio et al., 2001; Cuypers et al., 2010; Gallego et al., 2012). It has been reported that an increase of cytosolic ROS level is responsible for the activation of Ca channels in pollen, guard cells, and root cells (Pei et al., 2000; Demidchik et al., 2003; Hua et al., 2012; Wudick and Feijó, 2014) and that Ca accumulation in the cytosol seems to enhance the cellular accumulation of ROS (Potocký et al., 2007; Takeda et al., 2008). A positive feedback loop was accordingly shown between ROS production and stress-induced cytosolic Ca spikes (Pei et al., 2000; Demidchik et al., 2003; Takeda et al., 2008; Hua et al., 2012). Given that CAX1 has a major role in Ca homeostasis, a possible role for CAX1 in limiting ROS production and oxidative burst in response to Cd stress by sequestration of Ca in the vacuole was investigated in this study. Loss of CAX1 function in A. thaliana led to an enhanced accumulation of ROS after Cd treatment. The patterns of ROS accumulation in roots also correlated with the expression profile of CAX1 in the different species examined. Indeed, the genotypes that resulted in null or low levels of CAX1 expression showed a higher augmentation of ROS after Cd treatment. The strongest accumulation was observed in the cax1-1 mutant (ROS accumulation was 4-fold higher than in the A. thaliana wild type). The only genotype that did not accumulate ROS after exposure to Cd was A. halleri, which well fits the exceptional Cd tolerance of that species. The absence of H2O2 accumulation in the A. halleri root samples could be caused by (1) the efficient transfer of Cd to the upper part of the plant as a result of high levels of HMA4 expression, (2) the constitutive high levels of CAX1 expression that lead to a stable maintenance of Ca homeostasis in the cytosolic compartment, or (3) a synergistic action of these mechanisms.
On the whole, the evidence collected suggests that CAX1 expression may play an important role in maintaining cytosolic Ca levels to avoid uncontrolled ROS accumulation. Furthermore, the high sensitivity to ROS-generating reagent of the cax1-1 mutant suggests that CAX1 plays a role not limited to Cd tolerance but also, in the general response to oxidative stress. In addition, we cannot exclude that changes of CAX1 expression may have an impact on downstream targets responsive to Cd stress. For example, Catala et al. (2003) showed that CAX1 negatively regulates the expression of the C-REPEAT BINDING FACTOR/DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN1 transcription factor gene, which plays a role in adaptation to freezing after cold acclimation.
In this study, the impact of CAX1 on Cd tolerance could only be observed at low Ca supply in the external medium, in the CAX1 loss of function in Arabidopsis spp., and in the genetic analysis of Cd tolerance architecture in A. halleri. The apparent discrepancy in the observations at low and moderate Ca can be explained by two different sides of plant response to Cd and Ca supplies. First, the sensitivity of the cax1-1 mutant to Cd was only detected at a low level of Ca in the medium, whereas, its sensitivity to MV-induced oxidative stress was independent of Ca concentration in the medium. These contrasting results may be caused by stronger Ca/Cd competition when Ca levels in the medium are increased. Correspondingly, it has previously been reported in the literature that high external Ca mediates a protection effect against Cd stress caused by competition at Ca-binding motifs of structural proteins and enzymes (Rivetta et al., 1997; Perfus-Barbeoch et al., 2002; Heyno et al., 2008; Rodríguez-Serrano et al., 2009; Lu et al., 2010; Tian et al., 2011; Zorrig et al., 2012). Second, the CdTol2 QTL was only detectable at low Ca concentration (Courbot et al., 2007). The absence of the CdTol2 detection at moderate Ca concentration is probably caused by the absence of differences in CAX1 expression between Cd-sensitive species and the Cd-tolerant A. halleri under those conditions. The fact that CdTol2 is Ca conditional further strongly supports CAX1 as the candidate gene for this QTL in A. halleri.
It will be interesting to further investigate the role of CAX1 in the Cd hypertolerance of A. halleri in its natural environment. This will contribute to a better understanding of the evolution of naturally selected tolerance to extreme conditions in plants. Overall, this study identifies a unique genetic determinant of Cd tolerance in the metal hyperaccumulator A. halleri and offers a unique twist for the function of CAX1 in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
For expression analysis and ROS detection experiments, seeds of Arabidopsis halleri ecotype Auby (Bert et al., 2000), Arabidopsis lyrata ssp. petraea (Unhost; Macnair et al., 1999), Arabidopsis thaliana ecotype Col-0, and A. thaliana cax1 k.o. mutant (cax1-1; Cheng et al., 2003) were used. Seeds were sown on humid vermiculite, and 4 weeks after germination, seedlings were transferred to hydroponic culture. The hydroponic solution was a modified Murashige and Skoog culture (Courbot et al., 2007): K2SO4 (0.88 mm), KH2PO4 (0.25 mm), NaCl (10 µm), CaCl2 (0.5 or 2 mm), MgNO4 (1 mm), Fe-ethylenediamine-N,N-bis(2-hydroxyphenylacetic acid; 20 µm), H3BO3 (10 µm), ZnSO4 (1 µm), MnSO4 (0.6 µm), CuSO4 (0.1 µm), and (NH4)6Mo7O24 (0.01 µm) adjusted to pH 5.7 with KOH and buffered with 0.25 mm MES. Plants were cultivated in a climate-controlled growth chamber (20°C/17°C day-night cycle; 8-h-light [100 µmol photons m−2 s−1]/16-h-dark cycle; 60% humidity), and the hydroponic solution was changed every 1 week. After 4 weeks in noncontaminated solution, 10 µm CdSO4 was supplied to one-half of the plants for 72 h.
QTL mapping at 2 mm Ca was performed using [Ah × Alp] × Alp BC1 individuals generated from a batch of BC1 seeds from the same original cross described by Courbot et al. (2007). This unique set of individuals was produced with the same parental genotypes, because BC1 individuals used for the previous QTL mapping (Courbot et al., 2007) died before starting our experiment. QTL analysis was performed using three cuttings from 125 genotypes of the unique BC1 progeny. Cuttings were grown on sand for 4 weeks in greenhouses, and rooted ones were then moved to hydroponic culture. Tolerance was assessed through a sequential growth test (Courbot et al., 2007). The range of CdSO4 concentrations tested was 10, 25, 50, 75, 100, 150, and 250 µm. The composition of the hydroponic solution was the same as reported in the work by Courbot et al. (2007), except that 2 mm CaCl2 was used instead of 0.5 mm CaCl2.
In vitro Cd tolerance test was performed by using 7-d-old plantlets previously grown on one-half-strength Murashige and Skoog medium (MS; Murashige and Skoog, 1962) containing 1% (w/v) Suc and 0.8% (w/v) agar. Cd concentration was chosen after preliminary assays were performed with one-half-strength MS plates supplemented with 75, 100, and 150 µm CdCl2. The concentration (100 µm Cd) inhibiting root length of the wild type by 50% 7 d after the transfer was selected. This concentration is higher than concentration used in hydropony because of the presence of agar, which is known to bind cations.
The same transfer procedure was used to test the tolerance of Col-0 and cax1-1 to the ROS-inducing reagent MV (0.1 µm; Sigma-Aldrich Co.). All plates were incubated in a growth chamber (22°C under an 8-h-light/16-h-dark illumination cycle and 100 µmol photons m−2 s−1 of irradiance).
Marker Densification of the QTL CdTol2
To confirm localization of the QTL CdTol2 on linkage group 4 and decrease its size, five markers (three microsatellite markers and two single-nucleotide polymorphism [SNP] markers; Supplemental Table S1) were added to the genetic map by Courbot et al. (2007). These markers were selected to cover the CdTol2 region according to their position in the A. thaliana genome. The unique microsatellite marker was developed as described by Frérot et al. (2010). Resequencing data were used to design the two SNP markers. Illumina paired-end sequencing reads from six A. halleri populations (P. Saumitou-Laprade, unpublished data) were aligned to the Araly1 assembly of the A. lyrata lyrata genome. Within regions of interest, the criteria used for the selection of SNPs were (1) frequency of the polymorphism in A. halleri and A. lyrata equal to one, (2) 50 base flanking regions with low polymorphism within A. halleri and between A. halleri and A. lyrata, and (3) read depth >20. BC1 individuals from the study by Courbot et al. (2007) were genotyped for the microsatellite according to the work by Frérot et al. (2010) and the SNPs using competitive allele-specific PCR Kompetitive Allele Specific PCR genotyping system (KASP) chemistry (LGC Genomics). KASP assays were performed in a final reaction volume of 8 μL containing 4 µL of KASP Master Mix V2 Low ROX (LGC Genomics), 0.125 μL of KASP Mix Assay (LGC Genomics), and approximately 100 ng of genomic DNA. The PikoReal Real-Time PCR System (Thermo Scientific) was used with the following cycling conditions: 15 min at 94°C; 10 touchdown cycles of 20 s at 94°C and 60 s at 61°C to 55°C (the annealing temperature for each cycle being reduced by 0.6°C per cycle); and 26 cycles of 20 s at 94°C and 60 s at 55°C. Fluorescence detection was performed at the end of each cycle, and the data were analyzed using the allelic discrimination relative fluorescence units-based method of the PikoReal software 2.1.
The unique Ah × Alp linkage map was constructed with the Joinmap 3.0 Program (Van Ooijen et al., 2002). Markers along each linkage group were ordered using the sequential method implemented in Joinmap 3.0. The mapping function by Kosambi (1944) was used to translate recombination frequencies into map distances. Detection of QTLs was performed using the MapQTL 4.0 software (Van Ooijen et al., 2002). A multiple QTL model analysis was performed every centiMorgan, in which markers close to detected QTLs (by interval mapping) were selected as cofactors to take over the role of the nearby QTLs in the approximate multiple QTL models used in the subsequent multiple QTL model analysis. The calculated LOD scores were compared with an LOD score threshold obtained by a permutation test (1,000 permutations), which corresponds to a linkage group-wide empirical significance threshold at the 5% level (Churchill and Doerge, 1994).
QTL CdTol2 Detection at 2 mm Ca
The unique BC1 progeny (n = 125) was genotyped for eight markers covering one-half of the linkage group 4 of the Ah × Alp map. Four of these markers are described in the previous paragraph. The four other microsatellite markers (Supplemental Table S1) were developed and genotyped according to the work by Frérot et al. (2010). For map construction and QTL mapping, we used the same procedure as above.
Cloning of the A. halleri CAX1 Sequence
To clone the coding sequence of AhCAX1, A. halleri tissues were homogenized in liquid nitrogen, and RNA was extracted with the Aurum Total RNA Mini Kit (BIO-RAD Laboratories) according to the manufacturer’s instructions. Complementary DNA (cDNA) was then synthesized using the GoScript Reverse Transcription Kit (Promega). The primers used for the cloning were designed on conserved regions of AtCAX1 (At2g38170) and AlCAX1 (fgenesh2_kg.4__1885__AT2G38170.1 on Araly assembly) and are reported in Supplemental Table S2. To obtain the full-length AhCAX1 transcript sequence, 5′− and 3′−RACE PCRs were performed (Scotto-Lavino et al., 2006a, 2006b). The deduced amino acid sequence of AhCAX1 (GeneBank accession no. KT156755) was obtained using the cDNA translation tool on Expasy (http://web.expasy.org/translate/).
Real-Time RT-PCR Analysis
For CAX1 transcript quantification by quantitative RT-PCR, leaf and root tissues of A. halleri, A. thaliana, and A. lyrata were separately homogenized in liquid nitrogen, and RNA was extracted as described above. Extraction quality and quantity were checked through nanodrop detector. cDNA was then synthesize, and real-time PCR was performed in 96-well plates with the PikoReal Real-Time PCR System (Thermo Scientific). In each 10-µL reaction, there were 2.5 µL of cDNA, 5 µL of SYBR Mastermix (VeriQuest Fast SYBR Green qPCR Master Mix; Affymetrix), 2 µL of water, and 0.5 µm each primer. In total, three technical repeats were run per cDNA and primer pair combination. The quantitative PCR (qPCR) reaction thermal profile was preincubation at 95°C for 3 min, 40 cycles at 95°C for 30 s, and 60°C for 1 min. Relative transcript levels were calculated by normalization to ELONGATION FACTOR1α as a constitutively expressed reference gene (Talke et al., 2006; Deinlein et al., 2012).
For gene copy number analysis, genomic DNA of A. halleri and A. thaliana species was extracted using the GeneJET Genomic DNA Purification Kit (Thermo Scientific). qPCR was performed using the single-copy gene SHR (At4g37650) as internal control (Ueno et al., 2011; Craciun et al., 2012). Reactions were carried out as described above.
All primers were designed in conserved genomic and transcript regions of A. thaliana and A. lyrata using Primer 3 (http://primer3.sourceforge.net/) and checked on Beacon-Free software (Premier Biosoft) and mfold (Zuker, 2003). Amplicons were sequenced, and reaction efficiencies were determined for each primer pair (Supplemental Tables S2 and S3).
Protein Extraction and Immunoblotting
Proteins were extracted from plants grown in hydroponic culture for 4 weeks (under the same conditions used for the plants analyzed by real-time RT-PCR analysis). Root samples were homogenized in liquid nitrogen and then resuspended in 0.15 m Tris (pH 7.5). After centrifugation, the supernatants were recovered, and the total protein content of each sample was estimated using the Bradford Protein Assay (Quick Start Bradford Protein Assay; BIO-RAD Laboratories). As a negative control, the total protein fraction extracted from cax1-1 mutant was used. Proteins were separated at 4°C by SDS-PAGE (12% polyacrylamide gel) in SDS-Tris-Gly buffer (25 mm Tris, 0.192 m Gly, and 0.1% SDS). Western blotting was then performed by transferring proteins to a nitrocellulose membrane. CAX1 protein was detected using a CAX1-specific antibody (sc-27240; Santa Cruz Biotechnology). Bound antibodies were visualized using Lumi-Phos WB Chemiluminescent Substrate for AP (Thermo Scientific).
Analysis of in Vitro Tolerance and Mineral Analysis
After 1 week of growth in one-half-strength MS supplied with different Ca concentrations (under control or Cd treatment conditions as described above), plates were scanned, and images were analyzed using the RootNav platform (Pound et al., 2013) to calculate primary root length. At the same time, shoot surface area was measured using ImageJ software (Abràmoff et al., 2004) and used as an important parameter of plant growth in response to stress conditions (Claeys et al., 2014). Shoot tissues were then harvested, dried at 60°C, and pooled to have sufficient weight, and full mineral profiles of the bulks were assayed by inductively coupled plasma optical emission spectrometry. Roots were not analyzed because of their insufficient weight. Tolerance level of plants grown on MV-containing plates was assessed by measuring primary root length and shoot surface.
ROS Detection and Quantification
In situ detection of H2O2 and O2− was carried out for leaves and roots of Arabidopsis (A. halleri, A. lyrata, the A. thaliana wild type, and A. thaliana cax1-1 mutant) plants according to protocols reported by Daudi et al. (2012) and Ramel et al. (2009). To quantify the H2O2 content after 3,3′-diaminobenzidine (DAB) staining, samples were frozen in liquid nitrogen, ground to a powder, resuspended in 0.2 m HClO4, and centrifuged for 10 min at 12,000g. A450 values were immediately measured and compared with a standard curve obtained from known amounts of DAB resuspended in HClO4 (Ramel et al., 2009).
For O2− quantification after nitroblue tetrazolium staining, samples were homogenized in liquid nitrogen and resuspended in 2 m KOH:dimethyl sulfoxide (1:1.16, v/v). After centrifugation (10 min at 12,000g), A630 was measured and plotted on a standard curve.
Sequence data from this article can be found in the GenBank data library under accession number KT156755 (AhCAX1 sequence).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Alignment of the amino-acid sequences of AtCAX1, AlCAX1 and the predicted sequence of AhCAX1.
Supplemental Figure S2. Genomic copy number of CAX1 in A. thaliana and A. halleri.
Supplemental Figure S3. Detection and quantification of superoxide anion (O2.−).
Supplemental Table S1. List of genes with gene ontology annotations for cadmium response or metal ion transport in the refined CdTol2 QTL.
Supplemental Table S2. Primer sequences.
Supplemental Table S3. List of markers used for the fine-mapping and the QTL mapping at 2 mM CaCl2.
Acknowledgments
We thank Simon J. Conn for cax1-1 and cax3-1 seeds, Susanne Reiner and Nicolaus Von Wirén for mineral analysis, Eugeniusz Małkowski and the Groupement de Recherche International Locomet Network for interesting discussions, and Cecile Godé and Sophie Galina for technical assistance on design and genotyping of molecular markers.
Glossary
- cDNA
complementary DNA
- Col-0
Columbia-0
- DAB
3,3′-diaminobenzidine
- H2O2
hydrogen peroxide
- k.o.
knockout
- MS
Murashige and Skoog medium
- MV
methylviologen
- qPCR
quantitative PCR
- QTL
quantitative trait loci
- ROS
reactive oxygen species
- RT
reverse transcription
- SNP
single-nucleotide polymorphism
- TME
trace metal element
Footnotes
This work was supported by the Fonds National de la Recherche Scientifique (grant no. PDR T.0206.13 and fellowships to C.B. and C.-L.M.).
Articles can be viewed without a subscription.
References
- Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11: 36–41 [Google Scholar]
- Al-Shehbaz IA, O’Kane SL Jr (2002) Taxonomy and phylogeny of Arabidopsis (Brassicaceae). Arabidopsis Book 1: e0001, doi/10.1199/tab.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM. (1981) Accumulators and excluders: strategies in the response of plants to heavy metals. J Plant Nutr 3: 643–654 [Google Scholar]
- Bert V, Bonnin I, Saumitou-laprade P, De Laguérie P, Petit D (2002) Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol 155: 47–57 [DOI] [PubMed] [Google Scholar]
- Bert V, Macnair MR, De Laguerie P, Saumitou-Laprade P, Petit D (2000) Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol 146: 225–233 [DOI] [PubMed] [Google Scholar]
- Bradshaw HD., Jr (2005) Mutations in CAX1 produce phenotypes characteristic of plants tolerant to serpentine soils. New Phytol 167: 81–88 [DOI] [PubMed] [Google Scholar]
- Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J (2003) Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15: 2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15: 347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys H, Van Landeghem S, Dubois M, Maleux K, Inzé D (2014) What is stress? Dose-response effects in commonly used in vitro stress assays. Plant Physiol 165: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18: 92–99 [DOI] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AA, et al. (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, Verbruggen N (2007) A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol 144: 1052–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun AR, Meyer CL, Chen J, Roosens N, De Groodt R, Hilson P, Verbruggen N (2012) Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation. J Exp Bot 63: 4179–4189 [DOI] [PubMed] [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, et al. (2010) Cadmium stress: an oxidative challenge. Biometals 23: 927–940 [DOI] [PubMed] [Google Scholar]
- DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50: 1268–1280 [DOI] [PubMed] [Google Scholar]
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinlein U, Weber M, Schmidt H, Rensch S, Trampczynska A, Hansen TH, Husted S, Schjoerring JK, Talke IN, Krämer U, et al. (2012) Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 24: 708–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183: 557–564 [DOI] [PubMed] [Google Scholar]
- Frérot H, Faucon MP, Willems G, Godé C, Courseaux A, Darracq A, Verbruggen N, Saumitou-Laprade P (2010) Genetic architecture of zinc hyperaccumulation in Arabidopsis halleri: the essential role of QTL x environment interactions. New Phytol 187: 355–367 [DOI] [PubMed] [Google Scholar]
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83: 33–46 [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U (2008) Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453: 391–395 [DOI] [PubMed] [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179: 687–699 [DOI] [PubMed] [Google Scholar]
- Hirschi KD. (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11: 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol 124: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov FA. (2012) Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc Biol Sci 279: 5048–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koptsik GN. (2014) Problems and prospects concerning the phytoremediation of heavy metal polluted soils: a review. Eurasian Soil Sci 47: 923–939 [Google Scholar]
- Koren’kov V, Park S, Cheng NH, Sreevidya C, Lachmansingh J, Morris J, Hirschi K, Wagner GJ (2007) Enhanced Cd2+ -selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225: 403–411 [DOI] [PubMed] [Google Scholar]
- Kosambi DD. (1944) The estimation of map distances from recombination values. Ann Eugen 12: 172–175 [Google Scholar]
- Krämer U. (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61: 517–534 [DOI] [PubMed] [Google Scholar]
- Lu L, Tian S, Zhang M, Zhang J, Yang X, Jiang H (2010) The role of Ca pathway in Cd uptake and translocation by the hyperaccumulator Sedum alfredii. J Hazard Mater 183: 22–28 [DOI] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62: 21–37 [DOI] [PubMed] [Google Scholar]
- Macnair MR, Bert V, Huitson SB, Saumitou-Laprade P, Petit D (1999) Zinc tolerance and hyperaccumulation are genetically independent characters. Proc Biol Sci 266: 2175–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M, Shigaki T, Hirschi KD (2011) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol (Stuttg) 13: 561–569 [DOI] [PubMed] [Google Scholar]
- Mei H, Cheng NH, Zhao J, Park S, Escareno RA, Pittman JK, Hirschi KD (2009) Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol 183: 95–105 [DOI] [PubMed] [Google Scholar]
- Mei H, Zhao J, Pittman JK, Lachmansingh J, Park S, Hirschi KD (2007) In planta regulation of the Arabidopsis Ca(2+)/H(+) antiporter CAX1. J Exp Bot 58: 3419–3427 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Pauwels M, Frérot H, Bonnin I, Saumitou-Laprade P (2006) A broad-scale analysis of population differentiation for Zn tolerance in an emerging model species for tolerance study: Arabidopsis halleri (Brassicaceae). J Evol Biol 19: 1838–1850 [DOI] [PubMed] [Google Scholar]
- Pauwels M, Saumitou-Laprade P, Holl AC, Petit D, Bonnin I (2005) Multiple origin of metallicolous populations of the pseudometallophyte Arabidopsis halleri (Brassicaceae) in central Europe: the cpDNA testimony. Mol Ecol 14: 4403–4414 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32: 539–548 [DOI] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174: 742–751 [DOI] [PubMed] [Google Scholar]
- Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T (2013) RootNav: navigating images of complex root architectures. Plant Physiol 162: 1802–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetta A, Negrini N, Cocucci M (1997) Involvement of Ca2+ -calmodulin in Cd 2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ 20: 600–608 [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150: 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52: 2115–2126 [DOI] [PubMed] [Google Scholar]
- Scotto-Lavino E, Du G, Frohman MA (2006a) 3′ end cDNA amplification using classic RACE. Nat Protoc 1: 2742–2745 [DOI] [PubMed] [Google Scholar]
- Scotto-Lavino E, Du G, Frohman MA (2006b) 5′ end cDNA amplification using classic RACE. Nat Protoc 1: 2555–2562 [DOI] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14: 43–50 [DOI] [PubMed] [Google Scholar]
- Shazad Z, Gosti F, Frérot H, Lacombe E, Roosens N, Saumitou-Laprade P, Berthomieu P (2013) The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri. PLoS Genet 6: e1000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T, Barkla BJ, Miranda-Vergara MC, Zhao J, Pantoja O, Hirschi KD (2005) Identification of a crucial histidine involved in metal transport activity in the Arabidopsis cation/H+ exchanger CAX1. J Biol Chem 280: 30136–30142 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD (2006) Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol (Stuttg) 8: 419–429 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Talke IN, Hanikenne M, Krämer U (2006) Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol 142: 148–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84: 63–69 [DOI] [PubMed] [Google Scholar]
- Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Ma JF (2011) Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66: 852–862 [DOI] [PubMed] [Google Scholar]
- van de Mortel JE, Schat H, Moerland PD, Ver Loren van Themaat E, van der Ent S, Blankestijn H, Ghandilyan A, Tsiatsiani S, Aarts MGM (2008) Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 31: 301–324 [DOI] [PubMed] [Google Scholar]
- Van Ooijen JW, Boer M, Jansen R, Maliepaard C (2002) MapQTL 4.0: software for the calculation of QTL positions on genetic maps. Plant Research International, Wageningen, The Netherlands [Google Scholar]
- Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181: 759–776 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Juraniec M, Baliardini C, Meyer CL (2013) Tolerance to cadmium in plants: the special case of hyperaccumulators. Biometals 26: 633–638 [DOI] [PubMed] [Google Scholar]
- Weber M, Trampczynska A, Clemens S (2006) Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd(2+)-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ 29: 950–963 [DOI] [PubMed] [Google Scholar]
- Willems G, Frérot H, Gennen J, Salis P, Saumitou-Laprade P, Verbruggen N (2010) Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytol 187: 368–379 [DOI] [PubMed] [Google Scholar]
- Wu Q, Shigaki T, Williams KA, Han JS, Kim CK, Hirschi KD, Park S (2011) Expression of an Arabidopsis Ca2+/H+ antiporter CAX1 variant in petunia enhances cadmium tolerance and accumulation. J Plant Physiol 168: 167–173 [DOI] [PubMed] [Google Scholar]
- Wudick MM, Feijó JA (2014) At the intersection: merging Ca2+ and ROS signaling pathways in pollen. Mol Plant 7: 1595–1597 [DOI] [PubMed] [Google Scholar]
- Zhao J, Barkla BJ, Marshall J, Pittman JK, Hirschi KD (2008) The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227: 659–669 [DOI] [PubMed] [Google Scholar]
- Zhao J, Shigaki T, Mei H, Guo YQ, Cheng NH, Hirschi KD (2009) Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J Biol Chem 284: 4605–4615 [DOI] [PubMed] [Google Scholar]
- Zorrig W, Shazad Z, Abdelly C, Berthomieu P (2012) Calcium enhances cadmium tolerance and decreases cadmium accumulation in lettuce (Lactuca sativa). Afr J Biotechnol 11: 8441–8448 [Google Scholar]
- Zuker M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31: 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]