Abstract
Background
A barrier to hepatitis C virus (HCV) treatment among people who inject drugs (PWID) has been a concern that interferon-based HCV treatment may increase injecting risk behaviours. This study evaluated recent (past month) injecting risk behaviours during follow-up among PWID that did and did not receive HCV treatment.
Methods
The Australian Trial in Acute Hepatitis C (ATAHC) was a prospective study of natural history and treatment of recent HCV infection. Analyses were performed using generalized estimating equations.
Results
Among 124 participants with a history of injecting drug use (median age 32 years), 69% were male, and 68% were treated for HCV infection. HCV treatment was not associated with an increase in recent injecting drug use [adjusted odds ratio (aOR) 1.06, 95% CI 0.93, 1.21] or recent used needle and syringe borrowing during follow-up (aOR 0.99, 95% CI 0.89, 1.08). HCV treatment was associated with a decrease in recent ancillary injecting equipment sharing during follow-up (aOR 0.85, 95% CI 0.74, 0.99). Further, among treated participants who remained in follow-up (n=24), ancillary injecting equipment sharing significantly decreased from 54% at enrolment to 17% during follow-up (P=0.012).
Conclusions
HCV treatment was not associated with drug use or used needle and syringe borrowing during follow-up, but was associated with decreased ancillary injecting equipment sharing during follow-up. Programs to enhance HCV assessment and treatment among PWID should be expanded, given that HCV treatment does not lead to increases in injecting risk behaviours and has previously been demonstrated to be safe and effective among PWID.
Keywords: people who inject drugs, injecting drug use, HCV, antiviral therapy
INTRODUCTION
The prevalence of hepatitis C virus (HCV) infection is high among people who inject drugs (PWID), ranging from 64 to 94%, globally (1, 2). HCV infection is a major cause of morbidity and mortality among PWID (3, 4). Interferon-based HCV treatment is safe and effective among people with a history of injecting drug use (5, 6) and those who actively inject drugs (7). International guidelines now recommend HCV treatment for PWID (8–11). However, HCV treatment uptake remains suboptimal among PWID (11–15), due to several barriers at the levels of system, providers and patients (4, 16, 17). Concerns of ongoing drug use or relapse to drug use during interferon-based antiviral therapy among practitioners have contributed to low HCV treatment uptake in this population (16).
A recent meta-analysis among people with a history of injecting drug use has demonstrated similar rates of treatment success, compared to responses obtained in registration trials in the general population (6). Similarly, a recent systematic review among people who reported active drug use has shown acceptable HCV treatment outcomes, high treatment adherence and low treatment discontinuation in this population (7). Earlier studies have shown that HCV treatment is safe among people receiving opioid substitution treatment (OST) and does not increase drug use (18–20). However, recent data on drug use behaviours following initiation of HCV treatment among PWID is scarce. The availability of interferon (IFN)-free direct acting antiviral (DAA) therapies (21) will likely lead to an expansion of treatment among PWID. As such, a better understanding of the impact of HCV treatment on drug use and injecting behaviour is needed to inform clinical decision making in this area. This is particularly important because some clinicians often withhold therapy from PWID, given unfounded concerns that the side effects of interferon-based HCV treatment may mimic opioid withdrawal or lead to depression, thereby leading to a relapse to injecting drug use or increase injecting risk behaviours (22).
The Australian Trial in Acute Hepatitis C (ATAHC) was designed to investigate treatment for recent HCV infection, predominantly in those with injecting drug use-acquired infection. The aim of this study was to evaluate recent injecting risk behaviours during follow-up among people with a history of injecting drug use and recent HCV infection enrolled in the ATAHC study that did and did not receive HCV treatment.
METHODS
Design, setting and participants
ATAHC was a multicentre, prospective cohort study of the natural history and treatment of recent HCV infection, as previously described (23). Study recruitment occurred from June 2004 through February 2008 through an Australian network of tertiary hospitals (n=13) and general practice/primary care clinics (n=3).
Inclusion criteria for the study required recent HCV infection (acute or early chronic HCV infection), defined as first positive anti-HCV antibody within 6 months of enrolment and either acute clinical hepatitis C infection or asymptomatic hepatitis C infection with seroconversion (23). Heavy alcohol intake and active drug use were not exclusion criteria. Participants with a history of injecting drug use formed the study population for this specific analysis.
All participants with detectable HCV RNA were assessed for HCV treatment eligibility and subsequent HCV-related care and treatment provided at the site of study recruitment. From enrolment, participants were followed for up to 12 weeks to allow for spontaneous HCV clearance and if HCV RNA remained detectable were offered treatment (23). All treated and untreated participants had study visits at enrolment and every 12 weeks for up to 144 weeks (unless lost to follow-up).
All study participants provided written informed consent. The study protocol was approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee as well as through local ethics committees at all study sites. The study was registered with clinicaltrials.gov registry (NCT00192569).
HCV treatment
Participants who began HCV treatment received PEG-IFN -α2a 180 micrograms weekly for 24 weeks. Due to non-response at week 12 in the initial two participants with HCV/HIV co-infection, the study protocol was amended to provide PEG-IFN and ribavirin combination therapy for 24 weeks in HIV positive individuals. Ribavirin was prescribed at a dose of 1000–1200 mg for those with genotype 1 infection and 800 mg in those with genotype 2/3.
Study measurements
Behavioural surveys were administered to all participants at enrolment and every 12 weeks during the first year and every 24 weeks during second and third years. The behavioural survey included sections on demographics (age, sex, ethnicity, education, main source of income and accommodation), history of opioid substitution treatment (including methadone and buprenorphine), injecting drug use, and injecting drug use behaviours. At enrolment, injecting drug use history was collected for lifetime, previous six months and the previous month (recent). Recent (previous month) associated risk behaviours including use of a new sterile needle/syringe for all injections, needle/syringe borrowing and lending, and ancillary injecting equipment sharing (including mixing container, filter and water) were also collected. Follow-up information on injecting drug use and associated risk behaviours in the previous month were used for subsequent longitudinal analyses. Social functioning was measured using the shortened Social Functioning Scale of the Opiate Treatment Index (OTI) (24, 26).
Study outcomes
The primary aim of this analysis from the ATAHC study was to evaluate the impact of HCV treatment on recent (past month) injecting risk behaviour outcomes, measured longitudinally. The injecting risk behaviour outcomes included: 1) injecting drug use; 2) used needle and syringe borrowing; and 3) ancillary injecting equipment sharing. The study population for this aim included all participants with a history of injecting drug use at enrolment. Injecting risk behaviour outcomes from all study visits during follow-up (i.e. all visits after enrolment) were included for analysis.
Given that some participants were lost to follow-up, a secondary aim of this analysis was to evaluate changes in recent injecting risk behaviours among treated and untreated participants with recent injecting drug use at study enrolment who remained in follow-up (indicative of maintained engagement in the study). The injecting risk behaviour outcomes for this aim included: 1) used needle and syringe borrowing; and 2) ancillary injecting equipment sharing. The study population for this analysis included all participants with injecting drug use in the previous month at enrolment and remained in follow-up ≥24 weeks following study enrolment (i.e. for treated individuals, this included end of treatment and/or ≥24 week follow-up visits).
Statistical analyses
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and standard deviation (SD) or median and inter-quartile range (IQR) as appropriate. The proportion of individuals engaging in injecting risk behaviours during follow-up were assessed among treated and untreated individuals. However, to take into account lost to follow-up and adjust for known or suspected confounders of each outcome of interest, additional analyses of the impact of HCV treatment on injecting risk behaviours during follow-up were also assessed using generalized estimating equations (GEE). Injecting risk behaviours were modelled as binary outcome variables. Potential factors hypothesised to be associated with recent injecting risk behaviours during follow-up were determined a priori based on factors previously shown to be associated with injecting risk behaviours or injecting drug use cessation. These factors included age (25, 26), gender (27, 28), owned/renal accommodation (26, 29–31), level of education (32), full-time or part-time employment (29, 30, 33), OST (34), social support (30) and injecting risk behaviour at enrolment (26, 29, 35, 36).
Unadjusted and adjusted GEE models were specified using a binomial family function, a logit link and an autoregressive order one covariance structure, to account for the correlation of consecutive measures of injecting risk behaviours for the same participant. The effect sizes were expressed as odd ratios (ORs) or adjusted Odds Ratios (aOR). All outcome variables satisfied the assumption of linearity via Cochran-Armitage test. In addition to HCV treatment, included in all adjusted models as the primary explanatory variable of interest, other variables significant at the 0.20 level in unadjusted analyses were also initially included in the adjusted analyses. In adjusted analyses, variables were removed in a stepwise fashion using a likelihood-ratio test at each step until all factors significant at a 0.05 level remained in the model.
Changes in injecting risk behaviour outcomes between enrolment and ≥24 weeks of follow-up among treated and untreated participants were compared using the McNemar test (exact binomial probability). Statistically significant differences were assessed at a 0.05 level; p-values were two-sided. All analyses were performed using the Stata v12.0 (StataCorp, College Station, Texas).
RESULTS
Participant characteristics
Among 163 enrolled participants (23), 124 (76%) with a history of injecting drug use were included in this analysis. Compared to those without a history of injecting drug use (n=39), participants with a history of injecting drug use were younger, less often had full-time or part-time employment, had poorer social functioning and less often had HCV/HIV co-infection (Supplementary Table 1).
Among those with a history of injecting drug use (n=124), the median age was 32 years (IQR 25–39 years), the majority were male (69%) and 24% (n=30) had HCV/HIV co-infection. Overall, 82% (n=102) reported injecting drug use during the six months prior to enrolment, with methamphetamine (47%) and heroin (38%) the drugs most commonly injected (Table 1). At enrolment, recent injecting drug use (past month) was reported by 55 (44%) participants (Table 1). Among these participants (n=55), 17% (n=9) had borrowed a used needle and syringe and 52% (n=28) had shared injecting equipment in the month prior to enrolment (Table 1). Compared to those who did not recently inject drugs at enrolment (n=68), participants who had recently injected drugs at enrolment (n=55) more often owned or rented accommodation, more often received methadone or buprenorphine treatment and less often received HCV treatment (Supplementary Table 2).
Table 1.
Characteristics of participants with a history of injecting drug use and recently acquired HCV infection in the ATAHC study (n=124)
| Characteristic, n (%) | Overall n=124 |
|---|---|
| Male gender | 86 (69%) |
| Age, years (median, IQR) | 32 (25–39) |
| Caucasian ethnicity | 113 (91%) |
| Tertiary education or greater | 45 (36%) |
| Owned/rental accommodation¶ | 106 (88%) |
| Full-time or part-time employment¶ | 39 (32%) |
| Social functioning score (median, IQR) ¶ | 15 (9–19) |
| Opioid substitution treatment (methadone or buprenorphine) | |
| Never | 85 (69%) |
| Ever (not current) | 17 (14%) |
| Current | 22 (18%) |
| Age at first injecting drug use, years (median, IQR) | 21 (18–30) |
| Injecting drug use (past six months) | 102 (82%) |
| Injecting frequencyµ | |
| Daily | 35 (34%) |
| Weekly or less than weekly | 67 (66%) |
| Drug most often injectedµ | |
| Methamphetamine | 48 (47%) |
| Heroin | 39 (38%) |
| Methadone and other opiates | 6 (6%) |
| Injecting drug use (past month)¥ | 55 (45%) |
| Used a new sterile needle and syringe for all injections* | 39 (71%) |
| Used needle and syringe borrowing* | 9 (17%) |
| Lending someone else a used needle or syringe* | 9 (17%) |
| Ancillary injecting equipment sharing (mixing container, filter and water)* | 28 (52%) |
| HIV infection¶ | 30 (25%) |
among those with available survey results,
over the past six months, denominator is the number with a history of injecting drug use who reported injecting drug use over the past six months,
one participant (1 of 124) did not have available data on injecting drug use over the past month,
over the past month, denominator is the number with a history of injecting drug use who reported injecting drug use over the past month
HCV treatment uptake
Overall, 68% (n=84) of participants with a history of injecting drug use were treated for HCV infection. Compared to untreated participants (n=40), treated participants were more likely to have full-time or part-time employment, have greater social functioning and not injected drugs in the past month (Supplementary Table 3).
Participant loss to follow-up
Among those with a history of injecting drug use at enrolment (n=124), participants were followed for a median of 1.8 years [interquartile range (IQR) 1.1–2.7], with shorter follow-up observed among untreated participants (1.6 years, IQR 1.0–2.0) as compared to those treated (2.1 years, IQR 1.2–2.8). Following enrolment, 62% (n=77) of participants remained in ≥24 week follow-up (Supplementary Figure 1). Compared to participants remaining in ≥24 week follow-up (n=77), participants who were lost to study follow-up (n=47) were younger at the time of enrolment, younger at the time of first injecting drug use and had poorer social functioning (Supplementary Table 4).
Injecting drug use during follow-up
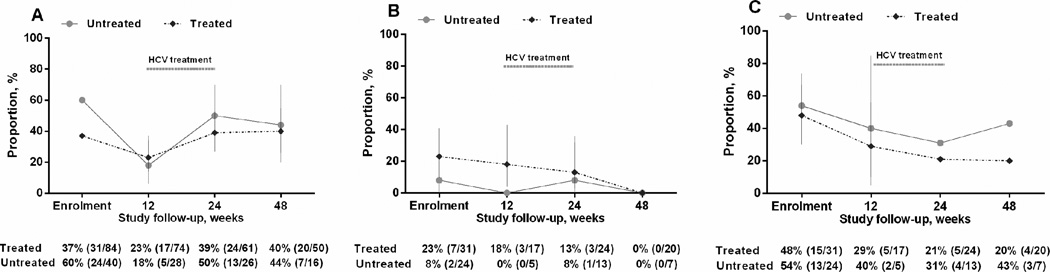
Injecting drug use in the past 30 days among treated and untreated participants during study follow-up were assessed (Figure 1). Among treated participants with a history of injecting drug use, the proportion reporting recent injecting drug use was 37% (31 of 84) at enrolment, 39% (24 of 61) at 24 weeks post-enrolment and 40% (20 of 50) at 48 weeks post-enrolment. Among untreated participants with a history of injecting drug use, the proportion reporting recent injecting drug use was 60% (24 of 40) at enrolment, 50% (13 of 26) at 24 weeks post-enrolment and 44% (7 of 16) at 48 weeks post-enrolment (Figure 1).
Figure 1.
Injecting drug use behaviour among participants with recently acquired HCV infection in the ATAHC study (n=124); A) injecting drug use, B) used needle and syringe borrowing, C) ancillary injecting equipment sharing
In unadjusted GEE analysis, recent injecting drug use during follow-up was not associated with HCV treatment (Table 2). In adjusted analysis, recent injecting drug use during follow-up was associated with poorer social functioning [aOR 1.22, 95% CI 1.02, 1.45] and no used needle and syringe borrowing at enrolment (aOR 0.79, 95% CI 0.67, 0.93), but not HCV treatment (aOR 1.06, 95% CI 0.93, 1.21) (Table 2).
Table 2.
Unadjusted and adjusted factors associated with injecting drug use during follow-up, among participants with recently acquired HCV infection in the ATAHC study (n=124)
| Characteristic | OR | 95% CI | P | aOR¶ | 95% CI | P |
|---|---|---|---|---|---|---|
| HCV treatment (vs. none) | 0.93 | 0.84, 1.03 | 0.139 | 1.06 | 0.93, 1.21 | 0.365 |
| Age (per 10 year increase) | 0.98 | 0.93, 1.02 | 0.387 | - | - | - |
| Gender | ||||||
| Male | 1.00 | - | - | 1.00 | - | - |
| Female | 0.93 | 0.84, 1.03 | 0.169 | 0.88 | 0.77, 1.01 | 0.069 |
| Transgender | 1.22 | 0.82, 1.82 | 0.326 | - | - | - |
| Owned/rental accommodation (vs. none) | 1.13 | 0.98, 1.32 | 0.103 | - | - | - |
| Tertiary education or greater (vs. none) | 1.04 | 0.95, 1.14 | 0.412 | - | - | - |
| Full-time or part-time employment (vs. none) | 0.87 | 0.79, 0.96 | 0.005 | - | - | - |
| Social functioning | ||||||
| 0–9 | 1.00 | - | - | 1.00 | - | - |
| ≥10–16 | 1.23 | 1.09, 1.39 | 0.001 | 1.13 | 0.95, 1.35 | 0.168 |
| >16 | 1.27 | 1.13, 1.43 | <0.001 | 1.22 | 1.02, 1.45 | 0.032 |
| Injecting drug use (vs. none)* | 1.89 | 1.76, 2.03 | <0.001 | - | - | - |
| Use of a sterile needle and syringe for all injections (vs. none) * | 1.13 | 1.00, 1.27 | 0.047 | - | - | - |
| Used needle and syringe borrowing (vs. none)* | 0.84 | 0.73, 0.98 | 0.022 | 0.79 | 0.67, 0.93 | 0.005 |
| Lending someone else a used needle or syringe (vs. none)* | 0.97 | 0.84, 1.10 | 0.623 | - | - | - |
| Ancillary injecting equipment sharing (vs. none)* | 0.94 | 0.84, 1.05 | 0.287 | - | - | - |
| Current opiate substitution treatment (vs. none) | 0.69 | 0.37, 1.26 | 0.225 | - | - | - |
adjusted odds ratio,
injecting drug use and injecting drug use behaviours at enrolment, defined over the one month prior to enrolment
Given that some people were lost to follow-up during the study, the impact of HCV treatment on cessation of injecting drug use was also assessed among people with recent injecting drug use at enrolment and who remained in ≥24 week follow-up (40 of 55, 73%) (Supplementary Figure 1). There were no differences with respect to demographic characteristics between participants who did and did not remain in ≥24 week follow-up (Supplementary Table 5). Among the 40 participants who reported injecting drug use at enrolment and remained in ≥24 week follow-up, 60% (n=24) and 40% (n=16) did and did not receive treatment for HCV infection, respectively. Among treated participants (n=24), 21% (n=5) ceased injecting drug use during ≥24 week follow-up. Among untreated participants (n=16), 19% (n=3) ceased injecting drug use during ≥24 week follow-up (Figure 2).
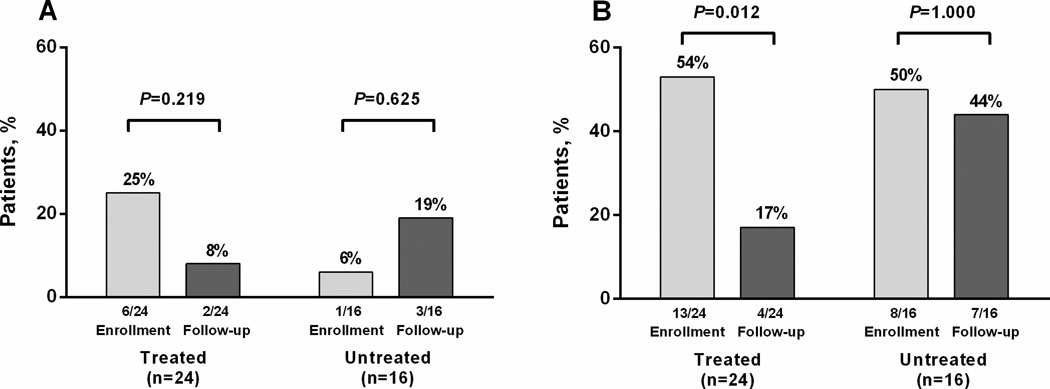
Figure 2.
Injecting drug use behaviour among participants with recently acquired HCV infection in the ATAHC study (n=40); A) used needle and syringe borrowing, B) ancillary injecting equipment sharing
Impact of HCV treatment on needle and syringe borrowing during follow-up
Needle and syringe borrowing in the past 30 days among treated and untreated participants during study follow-up were assessed (Figure 1). Among treated participants who had recently injected drugs, the proportion reporting recent needle and syringe borrowing was 23% (7 of 31) at enrolment, 13% (3 of 24) at 24 weeks post-enrolment and 0% (0 of 20 ) at 48 weeks post-enrolment. Among untreated participants who had recently injected drugs, the proportion reporting recent needle and syringe borrowing was 8% (2 of 24) at enrolment, 8% (1 of 13) at 24 weeks post-enrolment and 0% (0 of 7) at 48 weeks post-enrolment (Figure 1).
In unadjusted analysis, recent needle and syringe borrowing during follow-up was not associated with HCV treatment (Table 3). In adjusted analysis, recent needle and syringe borrowing during follow-up was associated with used needle and syringe borrowing at enrolment (aOR 1.52, 95% CI 1.33, 1.75), but not HCV treatment (aOR 0.99, 95% CI 0.89, 1.07). Female gender was marginally associated with used needle and syringe borrowing (aOR 1.11, 95% CI 1.00, 1.24) (Table 3).
Table 3.
Unadjusted and adjusted factors associated with used needle and syringe borrowing during follow-up, among participants with recently acquired HCV infection in the ATAHC study (n=124)
| Characteristic | OR | 95% CI | P | aOR¶ | 95% CI | P |
|---|---|---|---|---|---|---|
| HCV treatment (vs. none) | 1.01 | 0.92, 1.10 | 0.877 | 0.99 | 0.89, 1.07 | 0.655 |
| Age (per 10 year increase) | 0.98 | 0.93, 1.03 | 0.473 | - | - | - |
| Gender | ||||||
| Male | 1.00 | - | - | - | - | - |
| Female | 1.08 | 0.98, 1.19 | 0.115 | 1.11 | 1.00, 1.24 | 0.064 |
| Transgender | 0.92 | 0.69, 1.24 | 0.589 | - | - | - |
| Owned/rental accommodation (vs. none) | 1.04 | 0.89, 1.22 | 0.598 | - | - | - |
| Tertiary education or greater (vs. none) | 0.97 | 0.90, 1.06 | 0.511 | - | - | - |
| Full-time or part-time employment (vs. none) | 0.94 | 0.86, 1.04 | 0.232 | - | - | - |
| Social functioning | ||||||
| 0–9 | 1.00 | - | - | - | - | - |
| ≥10–16 | 1.08 | 0.95, 1.22 | 0.228 | - | - | - |
| >16 | 1.16 | 1.03, 1.31 | 0.015 | - | - | - |
| Injecting drug use (vs. none)* | 1.05 | 0.95, 1.15 | 0.356 | - | - | - |
| Use of a sterile needle and syringe for all injections (vs. none) * | 0.84 | 0.76, 0.94 | 0.002 | - | - | - |
| Used needle and syringe borrowing (vs. none)* | 1.49 | 1.31, 1.70 | <0.001 | 1.52 | 1.33, 1.75 | <0.001 |
| Lending someone else a used needle or syringe (vs. none)* | 1.21 | 1.07, 1.37 | 0.002 | - | - | - |
| Ancillary injecting equipment sharing (vs. none)* | 1.12 | 1.02, 1.24 | 0.023 | - | - | - |
| Current opiate substitution therapy (vs. none) | 0.63 | 0.36, 1,03 | 0.068 | 0.77 | 0.53, 1.16 | 0.399 |
adjusted odds ratio,
injecting drug use and injecting drug use behaviours at enrolment, defined over the one month prior to enrolment
The impact of HCV treatment on used needle and syringe borrowing was also assessed among people with recent injecting drug use at enrolment and who remained in ≥24 week follow-up (n=40). Among treated participants (n=24), used needle and syringe borrowing decreased from 25% (n=6) at enrolment to 8% (n=2, P=0.219) during ≥24 week follow-up. Among untreated participants (n=16), used needle and syringe borrowing increased from 6% (n=1) at enrolment to 19% (n=3, P=0.625) during ≥24 week follow-up (Figure 2).
Impact of HCV treatment on ancillary injecting equipment sharing during follow-up
Ancillary injecting equipment sharing in the past 30 days among treated and untreated participants during study follow-up were assessed (Figure 1). Among treated participants who had recently injected drugs, the proportion reporting ancillary injecting equipment sharing was 48% (15 of 31) at enrolment, 21% (5 of 24) at 24 weeks post-enrolment and 20% (4 of 20) at 48 weeks post-enrolment. Among untreated participants who had recently injected drugs, the proportion reporting ancillary injecting equipment sharing was 54% (13 of 24) at enrolment, 31% (4 of 13) at 24 weeks post-enrolment and 43% (3 of 7) at 48 weeks post-enrolment (Figure 1).
In unadjusted analysis, HCV treatment was associated with a reduction in recent ancillary injecting equipment sharing during follow-up [odds ratio (OR) 0.80, 95% CI 0.70, 0.92] and the association remained significant in the adjusted analysis (aOR 0.85, 95% CI 0.74, 0.99) (Table 4). Further, in adjusted analysis, ancillary injecting equipment sharing at enrolment was associated with ancillary injecting equipment sharing during follow-up (aOR 1.52, 95% CI 1.34, 1.74). Full-time or part-time employment was also marginally associated with reduced recent ancillary injecting equipment sharing during follow-up (aOR 0.86, 95% CI 0.73, 1.00) (Table 4).
Table 4.
Unadjusted and adjusted factors associated with ancillary injecting equipment sharing (mixing container, filter and water) during follow-up, among participants with recently acquired HCV infection in the ATAHC study (n=124)
| Characteristic | OR | 95% CI | P | aOR¶ | 95% CI | P |
|---|---|---|---|---|---|---|
| HCV treatment (vs. none) | 0.80 | 0.70, 0.92 | 0.001 | 0.85 | 0.74, 0.99 | 0.030 |
| Age (per 10 year increase) | 1.03 | 0.95, 1.11 | 0.494 | - | - | - |
| Gender | ||||||
| Male | 1.00 | - | - | - | - | - |
| Female | 1.05 | 0.90, 1.22 | 0.523 | - | - | - |
| Transgender | 1.21 | 0.76, 1.92 | 0.426 | - | - | - |
| Owned/rental accommodation (vs. none) | 1.17 | 0.92, 1.49 | 0.195 | 0.83 | 0.64, 1.07 | 0.152 |
| Tertiary education or greater (vs. none) | 0.93 | 0.81, 1.06 | 0.252 | - | - | - |
| Full-time or part-time employment (vs. none) | 0.85 | 0.73, 0.98 | 0.023 | 0.86 | 0.73, 1.00 | 0.056 |
| Social functioning | ||||||
| 0–9 | ||||||
| ≥10–16 | 1.37 | 1.13, 1.67 | 0.002 | - | - | - |
| >16 | 1.23 | 1.02, 1.48 | 0.033 | - | - | - |
| Injecting drug use (vs. none)* | 1.04 | 0.89, 1.21 | 0.611 | - | - | - |
| Use of a sterile needle and syringe for all injections (vs. none) * | 0.77 | 0.66, 0.91 | 0.002 | 0.88 | 0.72, 1.07 | 0.209 |
| Used needle and syringe borrowing (vs. none)* | 1.19 | 0.96, 1.48 | 0.111 | 0.90 | 0.69, 1.16 | 0.414 |
| Lending someone else a used needle or syringe (vs. none)* | 1.01 | 0.84, 1.22 | 0.891 | - | - | - |
| Ancillary injecting equipment sharing (vs. none)* | 1.51 | 1.31, 1.73 | <0.001 | 1.52 | 1.34, 1.74 | <0.001 |
| Current opiate substitution therapy (vs. none) | 1.04 | 0.33, 3.27 | 0.953 | - | - | - |
adjusted odds ratio,
injecting drug use and injecting drug use behaviours at enrolment, defined over the one month prior to enrolment
The impact of HCV treatment on ancillary injecting equipment sharing was also assessed among people with recent injecting drug use at enrolment and who remained in ≥24 week follow-up (n=40). Among treated participants (n=24), ancillary injecting equipment sharing significantly decreased from 54% (n=13) at enrolment to 17% (n=4, P=0.012) during ≥24 week follow-up (Figure 2). Among untreated participants (n=16), ancillary injecting equipment sharing remained stable from 50% (n=8) at enrolment to 44% (n=7, P=1.000) during ≥24 week follow-up (Figure 2).
DISCUSSION
In this study of PWID with recently acquired HCV, interferon-based HCV treatment was not associated with an increase in injecting drug use or needle and syringe borrowing during follow-up. However, HCV treatment was associated with a reduction in ancillary injecting equipment sharing during follow-up. These results should address concerns by some practitioners that HCV treatment may lead to an increase in drug use risk behaviours (16, 37). Programs to enhance HCV assessment and treatment among PWID should be expanded, given that HCV treatment, does not lead to increases in injecting risk behaviours, is safe and effective among PWID and reduces HCV-related morbidity and mortality.
HCV treatment was not associated with an increase in injecting drug use or needle and syringe borrowing during follow-up. This is consistent with previous work suggesting that HCV treatment does not lead to increases in injecting drug use (18–20). In one study of HCV treatment among people receiving OST in Australia, compared to baseline, there was no impact of HCV treatment on drug use behaviours at the end of treatment or at 24 weeks following treatment (20). This is important because the side effects of interferon-based therapy mimic opioid withdrawal and a major concern among some clinicians is that treatment might lead to an increase in drug use or relapse to substance use (16, 37). The finding that HCV treatment did not increase needle and syringe borrowing during follow-up is novel. In fact, although it was not significant, decreases in needle and syringe borrowing during ≥24 week follow-up (25% to 8%) were observed among treated participants remaining in ≥24 week follow-up, which is in contrast to an increase observed among untreated participants remaining in ≥24 week follow-up (6% to 19%).Given that HCV treatment did not lead to increases in injecting drug use or needle and syringe borrowing, this should not be used as a reason for withholding HCV treatment for PWID.
HCV treatment was associated with a reduction in ancillary injecting equipment sharing during follow-up. Further, among treated participants who remained in post-week 24 follow-up, ancillary injecting equipment sharing significantly decreased between enrolment and post-week 24 follow-up (decrease from 54% to 17%). These data are of interest, considering there are little prospective data on the impact of HCV treatment on injecting risk behaviours. Given that the sharing of ancillary injecting equipment contributes to the risk of HCV transmission (38), reductions in ancillary injecting equipment sharing is important. These data from the ATAHC study are consistent with a small observational study of HCV treatment among current and former PWID (n=14) in Canada, where decreases in injecting risk behaviours were observed following HCV treatment initiation (39). Despite the potential for social desirability bias, it is likely that ongoing therapeutic relationships and harm reduction education provided by various members of the multidisciplinary team (including physicians, nurses, counsellors and other allied health provides) to patients may contribute to reductions in injecting risk behaviours. As such, enhanced engagement of PWID into HCV treatment programs may provide an important opportunity to improve knowledge about injecting-associated risks among PWID (particularly ancillary injecting equipment sharing), having implications for potentially reducing HCV transmission.
This study has a number of limitations. The ATAHC study is not a random sample of the eligible population of PWID, especially because members of the cohort have acute HCV infection and are encouraged to access preventative health services through their involvement in the clinical trial. As a result, the findings may not be generalizable to other urban or remote/rural settings where drug use is common. Further, the sample size of participants who remained in ≥24 week follow-up was small and may have precluded the detection of significant changes in injecting risk behaviours between enrolment and ≥24 week follow-up. Analyses of injecting risk behaviours relied on self-reported data, which are prone to response bias and may promote socially desirable responses, particularly with respect to injecting risk behaviours. Specifically, this may have led to an under-reporting of needle and syringe borrowing and ancillary injecting equipment sharing in this study. Finally, in the evaluation of factors associated with recent injecting risk behaviours during follow-up, variables other than those measured in this study could have also been associated with the outcome.
In conclusion, in this study of PWID treated for recent HCV infection, HCV treatment was not associated with an increase in injecting drug use or needle and syringe borrowing during follow-up. However, HCV treatment was associated with a reduction in ancillary injecting equipment sharing during follow-up. There is now considerable data demonstrating that HCV treatment is safe and effective among people with a history of injecting drug use (5, 6) and those who actively inject drugs (7), and international guidelines now recommend HCV treatment for PWID (8–11). The data in this study provide further support of the expansion of programs aimed at enhancing assessment and treatment for HCV among PWID. Treatment should not be withheld based on unfounded concerns that treatment will lead to increases in drug use or injecting equipment sharing. Further, the findings of this study illustrate the importance of incorporating harm reduction education by health care providers into HCV treatment programs and potential benefit in reducing ancillary injecting equipment sharing. This finding has important prevention implications and may inform studies evaluating HCV treatment as prevention (4). This is also particularly important given the potential risk for HCV reinfection following HCV treatment. Although the reported rates of HCV reinfection to date have been low (7, 40), continued risk reduction education will be important as interferon-free regimens are expanded to populations of PWID who might be at greater risk for re-exposure.
The impending interferon-free era will certainly be an exciting time with the potential to substantially impact on HCV-related disease burden and work towards control of HCV among PWID (4). However, further studies will be needed to evaluate the impact of HCV therapy on risk behaviours in the interferon-free era, particularly given the importance of the risk of HCV reinfection.
Supplementary Material
There is a concern that HCV treatment may increase injecting risk behaviours
HCV treated and untreated participants were followed for a median of 1.8 years
Association between HCV treatment and injecting risk behaviours was assessed
HCV treatment was not associated with injecting drug use or used needle and syringe borrowing
HCV treatment was associated with decreased ancillary injecting equipment sharing
Acknowledgments
Funding
The Kirby Institute is funded by the Australian Government Department of Health, under the agreement ID number 2-D3X513. The views expressed in this publication do not necessarily represent the position of the Australian Government. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award R01DA015999. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Roche supplied financial support for pegylated IFN-alfa-2a/ribavirin. Gregory Dore and Paul Haber were supported through a National Health and Medical Research Council Practitioner Research Fellowship. Jason Grebely was supported through a National Health and Medical Research Council Career Development Award. Margaret Hellard was supported through a National Health and Medical Research Council Research Fellowship. John Kaldor was supported through a National Health and Medical Research Council Research Fellowship.
Gail Matthews has received travel grants and research support from Roche, Gilead and MSD. Gail Matthews has been on advisory board for Gilead. Gail Matthews has received travel support from BMS. Gail Matthews has received research support from Janssen. Paul Haber is on an advisory board for Lundbeck Pharmaceuticals. Paul Haber has been paid by Roche to give presentations. Barbara Yeung has received travel grants from Roche. Kathy Petoumenos has received a travel grant from ViiV Healthcare. Kathy Petoumenos’ research program has received support from Gilead Sciences. John Kaldor has received research support from Roche. Gregory Dore is a member of advisory board, on the speaker’s bureau, has received travel grants and research support from Roche, Merck and Gilead. Gregory Dore is a member of advisory board, has received travel grants and research support from Bristol-Myers Squibb and Abbvie. Gregory Dore is a consultant/advisor for Merck, Tibotec, and Abbvie. Gregory Dore is a member of advisory board for Janssen. Gregory Dore is on the speaker’s bureau for Janssen. Gregory Dore has received research support from Vertex. Margaret Hellard has received research support from Gilead Sciences and Abbvie. Jason Grebely is a member of an advisory board for Merck.
ATAHC Study Group
Protocol Steering Committee members:
John Kaldor (Kirby Institute), Gregory Dore (Kirby Institute), Gail Matthews (Kirby Institute), Pip Marks (Kirby Institute), Andrew Lloyd (UNSW), Margaret Hellard (Burnet Institute, VIC), Paul Haber (University of Sydney), Rose Ffrench (Burnet Institute, VIC), Peter White (UNSW), William Rawlinson (UNSW), Carolyn Day (University of Sydney), Ingrid van Beek (Kirketon Road Centre), Geoff McCaughan (Royal Prince Alfred Hospital), Annie Madden (Australian Injecting and Illicit Drug Users League, ACT), Kate Dolan (UNSW), Geoff Farrell (Canberra Hospital, ACT), Nick Crofts (Nossal Institute, VIC), William Sievert (Monash Medical Centre, VIC), David Baker (407 Doctors).
Kirby Institute ATAHC Research Staff:
John Kaldor, Gregory Dore, Gail Matthews, Pip Marks, Barbara Yeung, Jason Grebely, Brian Acraman, Kathy Petoumenos, Janaki Amin, Carolyn Day, Anna Doab, Therese Carroll.
Burnet Institute Research Staff:
Margaret Hellard, Oanh Nguyen, Sally von Bibra.
Immunovirology Laboratory Research Staff:
UNSW Pathology - Andrew Lloyd, Suzy Teutsch, Hui Li, Alieen Oon, Barbara Cameron.
SEALS – William Rawlinson, Brendan Jacka, Yong Pan.
Burnet Institute Laboratory, VIC – Rose Ffrench, Jacqueline Flynn, Kylie Goy.
Clinical Site Principal Investigators:
Gregory Dore, St Vincent’s Hospital, NSW; Margaret Hellard, The Alfred Hospital, Infectious Disease Unit, VIC; David Shaw, Royal Adelaide Hospital, SA; Paul Haber, Royal Prince Alfred Hospital; Joe Sasadeusz, Royal Melbourne Hospital, VIC; Darrell Crawford, Princess Alexandra Hospital, QLD; Ingrid van Beek, Kirketon Road Centre; Nghi Phung, Nepean Hospital; Jacob George, Westmead Hospital; Mark Bloch, Holdsworth House GP Practice; David Baker, 407 Doctors; Brian Hughes, John Hunter Hospital; Lindsay Mollison, Fremantle Hospital; Stuart Roberts, The Alfred Hospital, Gastroenterology Unit, VIC; William Sievert, Monash Medical Centre, VIC; Paul Desmond, St Vincent’s Hospital, VIC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The other authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nature Reviews Gastroenterology Hepatology. 2013;10(9):553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. [Review]. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dore GJ, Grebely J. What is killing people with hepatitis C virus infection? Seminars in Liver Disease. 2011;31(4):331–339. doi: 10.1055/s-0031-1297922. [DOI] [PubMed] [Google Scholar]
- 4.Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral research. 2014;104:62–72. doi: 10.1016/j.antiviral.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clinical Infectious Diseases. 2009;49(4):561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 6.Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clinical Infectious Diseases. 2013;56(6):806–816. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clinical Infectious Diseases. 2013;57(suppl 2):S80–S89. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 8.Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55(2):245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Robaeys G, Grebely J, Mauss S, Bruggmann P, Moussalli J, De Gottardi A, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clinical Infectious Diseases. 2013;57(suppl 2):S129–S137. doi: 10.1093/cid/cit302. [DOI] [PubMed] [Google Scholar]
- 11.Grebely J, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. International Journal of Drug Policy. 2015 doi: 10.1016/j.drugpo.2015.07.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. Journal of Viral Hepatitis. 2009;16(5):352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, et al. Limited uptake of hepatitis C treatment among injection drug users. Journal of Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alavi M, Raffa JD, Deans GD, Lai C, Krajden M, Dore GJ, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents Liver International. 2014;34(8):1198–1206. doi: 10.1111/liv.12370. [DOI] [PubMed] [Google Scholar]
- 15.National Centre in HIV Epidemiology and Clinical Research. [cited Sep 2014];Australian NSP Survey National Data Report 2005 – 2009 [Internet] Available from: http://kirby.unsw.edu.au/sites/default/files/hiv/resources/ANS.2005_2009.pdf.
- 16.Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. Journal of viral hepatitis. 2014;21(3):198–207. doi: 10.1111/jvh.12129. [DOI] [PubMed] [Google Scholar]
- 17.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking Down the Barriers to Hepatitis C Virus (HCV) Treatment Among Individuals With HCV/HIV Coinfection: Action Required at the System, Provider, and Patient Levels. Journal of Infectious Diseases. 2013;207(suppl 1):S19–S25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grebely J, Petoumenos K, Matthews G, Haber P, Marks P, Lloyd A, et al. Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: The ATAHC Study. Drug and alcohol dependence. 2010;107(2):244–249. doi: 10.1016/j.drugalcdep.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40(1):120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 20.Van Thiel DH, Anantharaju A, Creech S. Response to treatment of hepatitis C in individuals with a recent history of intravenous drug abuse. The American Journal of Gastroenterology. 2003;98(10):2281–2288. doi: 10.1111/j.1572-0241.2003.07702.x. [DOI] [PubMed] [Google Scholar]
- 21.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of "perfectovir". Clin Infect Dis. 2015 Jun 15;60(12):1829–1836. doi: 10.1093/cid/civ197. Epub 2015 Mar 11. [DOI] [PubMed] [Google Scholar]
- 22.Sasadeusz JJ, Dore G, Kronborg I, Barton D, Yoshihara M, Weltman M. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977–984. doi: 10.1111/j.1360-0443.2010.03347.x. [DOI] [PubMed] [Google Scholar]
- 23.Myles A, Mugford GJ, Zhao J, Krahn M, Wang PP. Physicians’ attitudes and practice toward treating injection drug users infected with hepatitis C virus: Results from a national specialist survey in Canada. Canadian Journal of Gastroenterology. 2011;25(3):135–139. doi: 10.1155/2011/810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138(1):123–135. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multidimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British Journal of Addiction. 1992;87(5):733–742. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 26.Horyniak D, Dietze P, Degenhardt L, Higgs P, McIlwraith F, Alati R, et al. The relationship between age and risky injecting behaviours among a sample of Australian people who inject drugs. Drug and alcohol dependence. 2013;132(3):541–546. doi: 10.1016/j.drugalcdep.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Debeck K, Kerr T, Marshall BD, Simo A, Montaner J, Wood E. Risk factors for progression to regular injection drug use among street-involved youth in a Canadian setting. Drug and alcohol dependence. 2013;133(2):468–472. doi: 10.1016/j.drugalcdep.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher L, Jalaludin B, Chant KG, Jayasuriya R, Sladden T, Kaldor JM, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101(10):1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 29.Evans MJL, Hahn JA, Page-Shafer MK, Lum MPJ, Stein MES, Davidson MPJ, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) Journal of Urban Health. 2003;80(1):137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug and Alcohol Dependence. 2006;83(2):147–156. doi: 10.1016/j.drugalcdep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Cox J, Maurais E, Hu L, Moodie EE, Law S, Bozinoff N, et al. Correlates of drug use cessation among participants in the Canadian HIV–HCV Co-infection Cohort. Drug and Alcohol Dependence. 2014;137:121–128. doi: 10.1016/j.drugalcdep.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Linton SL, Celentano DD, Kirk GD, Mehta SH. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug and Alcohol Dependence. 2013;132(3):457–465. doi: 10.1016/j.drugalcdep.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramaniam GA, Stitzer ML, Woody G, Fishman MJ, Kolodner K. Clinical characteristics of treatment-seeking adolescents with opioid versus cannabis/alcohol use disorders. Drug and Acohol Dpendence. 2009;99(1):141–149. doi: 10.1016/j.drugalcdep.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed C, Bliss C, Stuver SO, Heeren T, Tumilty S, Horsburgh CR, Jr, et al. Predictors of Active Injection Drug Use in a Cohort of Patients Infected With Hepatitis C Virus. American Journal of Public Health. 2013;103(1):105–111. doi: 10.2105/AJPH.2012.300819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 36.Bruneau J, Brogly SB, Tyndall MW, Lamothe F, Franco EL. Intensity of drug injection as a determinant of sustained injection cessation among chronic drug users: the interface with social factors and service utilization. Addiction. 2004;99(6):727–737. doi: 10.1111/j.1360-0443.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 37.Evans JL, Hahn JA, Lum PJ, Stein ES, Page K. Predictors of injection drug use cessation and relapse in a prospective cohort of young injection drug users in San Francisco, CA (UFO Study) Drug and Alcohol Dependence. 2009;101(3):152–157. doi: 10.1016/j.drugalcdep.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer M, Sarkar R, Diez-Quevedo C. Management of mental health problems prior to and during treatment of hepatitis C virus infection in patients with drug addiction. Clinical infectious diseases. 2013;57(suppl 2):S111–S117. doi: 10.1093/cid/cit266. [DOI] [PubMed] [Google Scholar]
- 39.Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction. 2012;107(6):1057–1065. doi: 10.1111/j.1360-0443.2011.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman AI, Beckstead S, Beking D, Finch S, Knorr T, Lynch C, et al. Treatment of chronic hepatitis C infection among current and former injection drug users within a multidisciplinary treatment model at a community health centre. Canadian Journal of Gastroenterology. 2013;27(4):217. doi: 10.1155/2013/515636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57(Suppl 2):S105–S110. doi: 10.1093/cid/cit301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.