Abstract
An assortment of touch receptors innervate the skin and encode different tactile features of the environment. Compared with invertebrate touch and other sensory systems, our understanding of the molecular and cellular underpinnings of mammalian touch lags behind. Two recent breakthroughs have accelerated progress. First, an arsenal of cell-type-specific molecular markers allowed the functional and anatomical properties of sensory neurons to be matched, thereby unraveling a cellular code for touch. Such markers have also revealed key roles of non-neuronal cell types, such as Merkel cells and keratinocytes, in touch reception. Second, the discovery of Piezo genes as a new family of mechanically activated channels has fueled the discovery of molecular mechanisms that mediate and mechanotransduction in mammalian touch receptors.
Introduction
Touch has fascinated philosophers and scientists for more than two millennia. The prevailing view in Aristotle’s time was that touch and taste (then considered a submodality of touch) are inferior senses because they require direct contact and are easily corrupted by our carnal needs for reproduction and sustenance [1]. By contrast, sight, hearing and smell allow action at a distance and, thus, room for contemplation. But Aristotle developed a different view, linking our power of tactile discrimination to human intelligence [2]:
“In the other senses man is inferior to many animals, but in the sense of touch he far surpasses them all in acuity; that is why he also the most intelligent of animals.” De anima, II, 9, 421a 20
Modern neuroscience recognizes the merit of both schools of thought. Touch is one form of mechanotransduction, which is the ability to sense and respond to mechanical disturbances in the environment. In animals, tactile inputs guide fundamental behaviors required for species survival, including mating, obtaining nutrients and avoiding predation. On the other hand, discriminative touch allows us to accomplish uniquely human feats, from playing a concerto to typing a manuscript. Although genetic, behavioral and physiological studies of invertebrate nervous systems have identified basic principles of mechanosensory transduction [3], our understanding of the molecular and cellular basis of mammalian touch reception has been slow to emerge.
Two recent breakthroughs, however, have accelerated progress in the field. First, the development of selective genetic markers in transgenic mouse models has enabled functional dissection of identified classes of touch receptors. Second, the advent of rapid gene silencing technologies has fueled discovery of an entirely new family of mechanotransduction channels, the Piezo family. Here, we will summarize these advances, highlight ensuing progress with a particular focus on the past two years, and discuss open questions.
Getting a genetic grip on touch-receptor diversity
Somatosensory neurons located in trigeminal and dorsal root ganglia (DRG) initiate the senses of touch, proprioception and nociception. Each pseudounipolar neuron possesses a bifurcating sensory afferent that connects the periphery to the spinal cord. Sensory stimuli are transformed into action potentials in peripheral afferents that innervate skin and other target tissues. Central branches transmit these neural signals to the spinal cord and brain, where they are processed to direct behavior.
Somatosensory neurons display an array of sensory modalities, anatomical features and physiological properties (Table 1). Afferents can be categorized as Aβ (thickly myelinated), Aδ (thinly myelinated) or C-fibers (unmeylinated) based on action-potential conduction velocity. They are further classified by somatal diameter, sensory threshold, adaptation and modality. Gentle touch is encoded by low-threshold mechanoreceptors (LTMRs) that fall into Aβ, Aδ or C classes. These include slowly adapting type I (SAI) Aβ afferents, which complex with Merkel cells to form discriminative touch receptors [4]. Rapidly adapting (RA) Aβ LTMRs include corpuscular vibration receptors and hair-follicle afferents. Other hair-follicle afferents include Aδ LTMRs, which are among the most sensitive mammalian touch receptors, and C-LTMRs, which have been proposed to contribute to social touch [5]. Most nociceptors, which respond to noxious mechanical, chemical and/or thermal stimuli, are categorized as Aδ or C-fibers.
Table 1.
Somatosensory mechanoreceptors in DRG.
| Conduction velocity |
Myelination | Soma | Subclasses | Modality | Molecular marker | Ref. |
|---|---|---|---|---|---|---|
| Aα | Thick | Large | Muscle spindle & Golgi tendon organ |
Proprioception | Runx3+Pvalb+ | [15] |
| Aβ | Thick | Large | SAI (Merkel-cell afferent) |
Touch | transient Slc17a8 (VGLUT3), |
[18] |
| Runx3+Pvalb− | [15] | |||||
| Merkel cells | Touch | Atoh1, Cck, Krt14 | [16,17,29•] | |||
| RA (corpuscles, lanceolates) |
Touch | early Ret+ | [6,7] | |||
| lanceolate ending | Touch | Npy2r | [14••] | |||
| Aδ | Thin | Medium | Aδ LTMR (D-hair) | Touch | Ntrk2 (TrkB) | [14••] |
| A mechanonociceptor | Nociception | |||||
| C | None | Small | C-LTMR | Affective touch? | Slc17a8 (VGLUT3), Th | [10,14••] |
| C tactile-like afferent | Affective touch? | Mrgprb4 | [49] | |||
| Mechanonociceptor & polymodal nociceptor |
Nociception | various | ||||
An important breakthrough has been the development of transgenic mice that selectively express genetic reporters in distinct somatosensory cell types (Table 1) [6–18]. These studies provide an battery of cell-type-specific molecular markers for matching the functional and anatomical properties of sensory neurons [14••]. These markers also allow selective manipulation of neuronal and non-neuronal cell types to determine their roles in tactile encoding (see Cellular Tuning Mechanisms, below).
Piezo2 pushes to the forefront of mechanosensory transduction
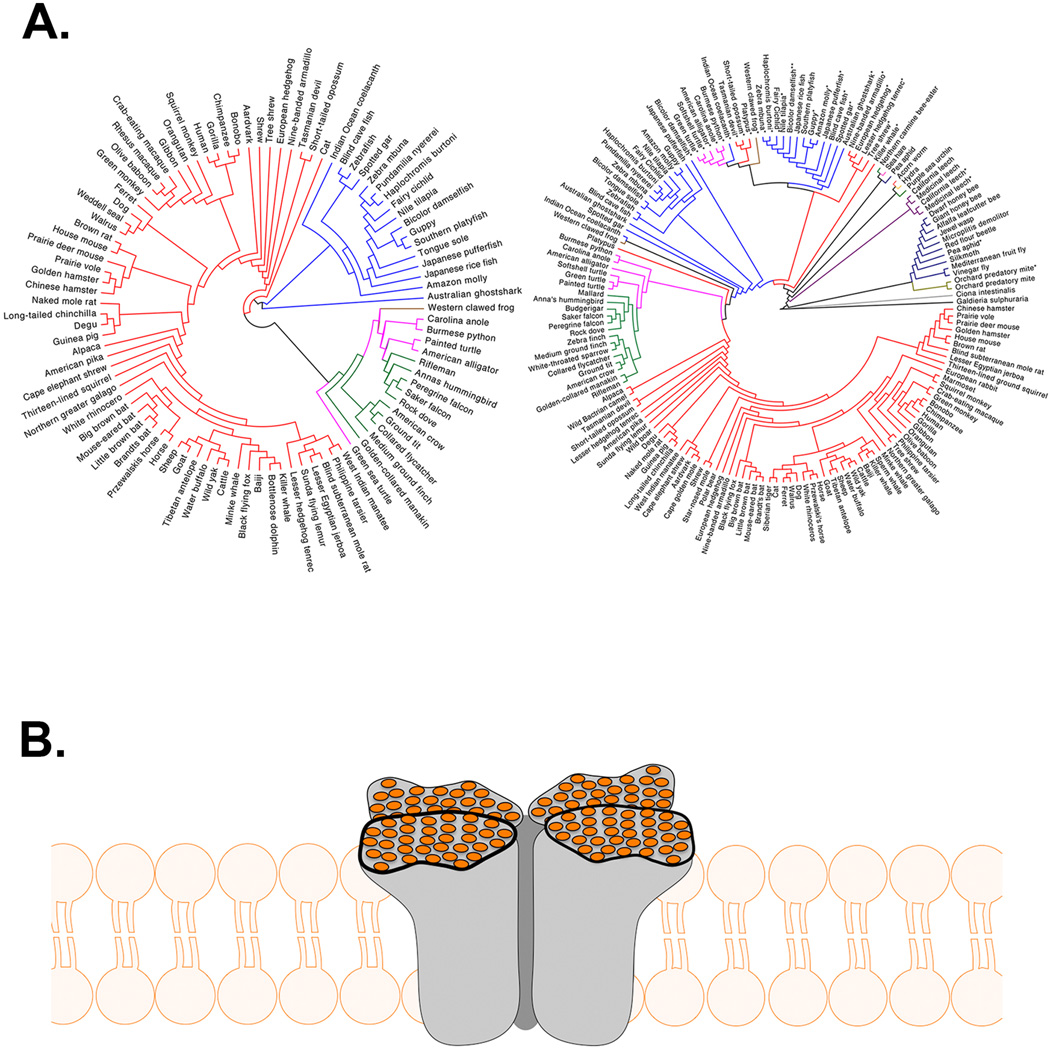
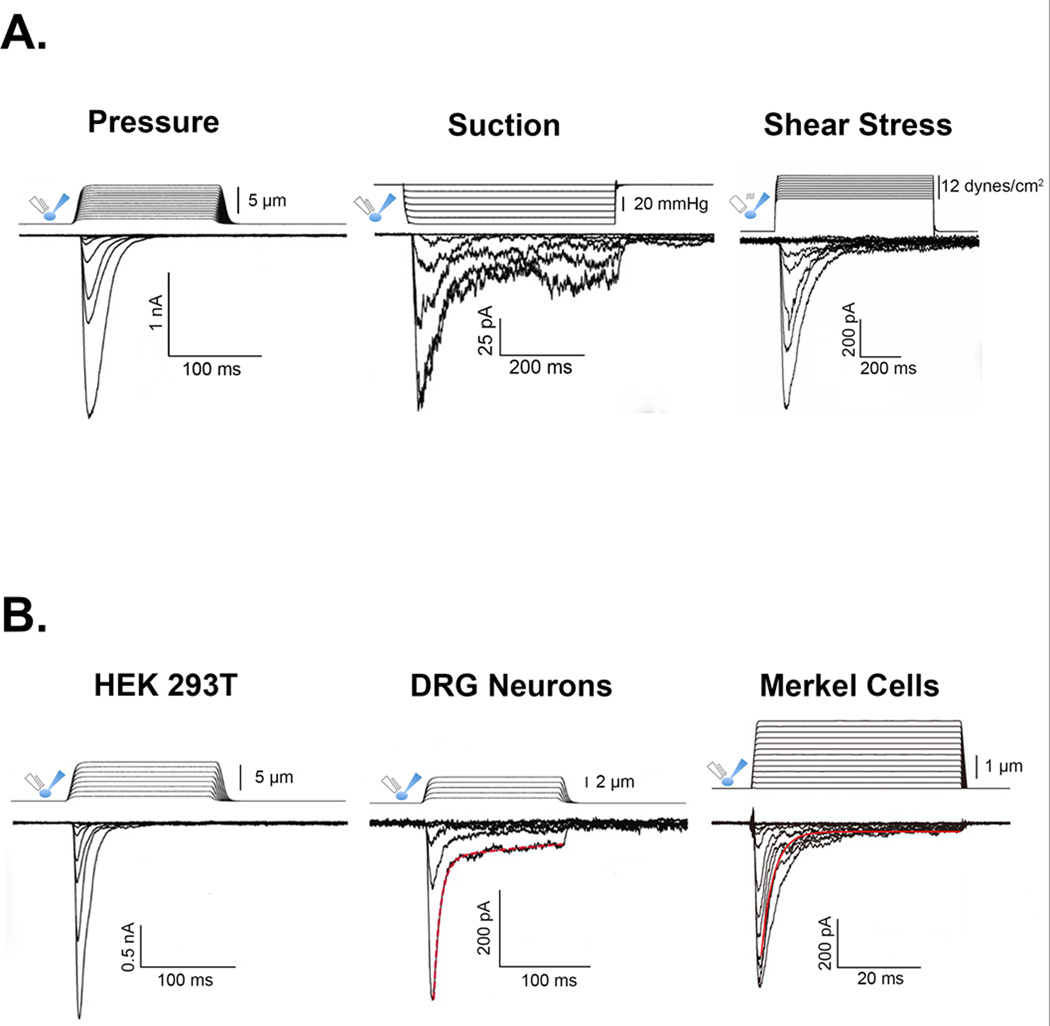
The Piezo proteins are a recently discovered group of mechanically activated ion channels. They are highly evolutionarily conserved (Fig. 1A) and can form homotetramers, with each subunit consisting of over 30 transmembrane domains (Fig. 1B) [19••,20]. These proteins were first implicated in mechanotransduction through an RNA interference screen for mechanically activated currents in vitro. Fam38A (Piezo1) and Fam38B (Piezo2) are necessary for mechanically activated currents in N2A cells and DRG neurons, respectively, and are sufficient to confer mechanically activated currents in heterologous cells [19••]. Interestingly, Piezo-dependent currents can be activated by a variety of mechanical stimuli, including direct touch, membrane suction and shear stress [19••,21••] (Fig. 2A) These findings suggest that Piezo-dependent currents are intrinsically activated by membrane deformation. Although Piezo proteins lack sequence similarity with known ion-channel families, purified Piezo1 protein forms ion-conducting pores when reconstituted in lipid bilayers [20], and human disease-causing mutations slow channel gating kinetics [22–24]. Moreover, recent structure-function studies have narrowed the ion permeation pathway to the C-terminal domain and have identified a residue within a highly conserved motif that controls intrinsic pore properties [25,26]. Together, these findings suggest that Piezo genes encode bona fide ion channels rather than accessory subunits [20]. Both mammalian Piezo isoforms are expressed in tissues replete with mechanosensitive cells [19••]; however, the enrichment of Piezo2 transcripts in DRGs immediately suggested this gene as a prime candidate to encode transduction channels in touch receptors or nociceptors.
Figure 1. Piezo proteins are evolutionarily conserved, widely expressed ion channels.
(A) Unrooted circular dendrogram clustering of Piezo1 and Piezo2 proteins (B) Piezo channels are proposed to be homomeric tetramers. Each subunit is predicted to contain over 30 transmembrance domains (orange circles).
Figure 2. The many modalities of Piezo.
(A) Sample currents demonstrating mechanical activation of Piezo1-transfected HEK293T cells via pressure [19••], suction [19••], and shear stress [50]. (B) Representative touch-evoked Piezo2 currents in transfected HEK293T cells [19••], DRG neurons [19••] and Merkel cells [29•].
Acute mechanotransduction
Several studies support the hypothesis that Piezo2 mediates gentle touch in vertebrates. The first behavioral evidence emerged from a study of zebrafish Piezo2b, which is expressed in embryonic Rohon-Beard mechanoreceptors [27]. Morpholino-mediated Piezo2b gene silencing resulted in loss of behavioral responses to tactile stimulation but not to chemical and noxious mechanical stimuli. These findings indicate that Piezo2b is selectively required for touch-evoked behaviors.
Direct evidence that a mammalian Piezo gene is required for mechanotransduction in vivo stemmed from studies of epidermal Merkel cells, which are putative mechanosensory cells innervated by SAI afferents (see Cellular tuning mechanisms, below). Three groups found that rodent Merkel cells express Piezo2 and have mechanically activated currents that resemble Piezo2-dependent currents in other cell types [28•–30•] (Fig. 2B). These touch-evoked currents were abolished in Merkel cells from epidermal-specific Piezo2 knockout mice (driven by Krt14Cre), as well as rat Merkel cells treated with Piezo2-targeted short hairpin RNAs [28•,30•]. Moreover, intact recordings demonstrated that Merkel-cell afferents from Krt14Cre;Piezo2flox/flox mice have truncated firing patterns rather than sustained SAI responses, demonstrating that Merkel-cell Piezo2 is needed for static firing in their associated afferents [29•,30•]. Finally, epidermal-specific Piezo2 knockout mice demonstrated a mild behavioral deficit to gentle touch in vivo [30•]. The subtle nature of this reflex behavioral deficit is not surprising, given the redundancy of mechanoreceptors (see Getting a genetic grip on touch-receptor diversity). Taken together, these data indicate a requirement for Merkel-cell Piezo2 in acute touch reception, particularly for pressure sensation.
Disrupting Piezo2 in DRG neurons produces more dramatic deficits in touch sensitivity. For example, intrathecal injections of Piezo2 antisense oligonucleotides in mice increased withdrawal thresholds to touch [31]. Moreover, AdvillinCreERT2;Piezo2flox/flox mice, which lack Piezo2 in Merkel cells and DRG neurons, show a profound loss of gentle-touch responses [21••]. Using a battery of assays, the authors demonstrated that behaviors evoked by innocuous touch are almost completely abolished in AdvillinCreERT2;Piezo2flox/flox mice. The ability to respond to noxious mechanical and thermal stimulation remained intact in these mutants, confirming that Piezo2 function is not required for nocifensive responses in healthy animals [21••]. Additionally, in vitro recording from Piezo2-deficient DRG neurons demonstrated that rapidly inactivating currents were severely reduced. In intact recordings, approximately half of all Aβ LTMRs were rendered touch-insensitive, and those mechanoreceptors that remained showed impaired responsiveness. Together, these data indicate that Piezo2 is a principal component of mechanosensory signaling in mouse Aβ LTMRs.
A recent study promisingly indicates that Piezo2 is also required in human LTMRs. A neurotrophin cocktail was used to derive sensory neurons with LTMR properties from human induced pluripotent stem cells (iPSC) [32]. These human iPSC-derived neurons expressed Piezo2 transcripts and displayed mechanically activated inward currents with rapidly inactivating time constants, as in mouse LTMRs. When Piezo2 was ablated in these cells using CRISPR/Cas9 technology, mechanically activated currents were abolished. Along with directly demonstrating that Piezo2 is necessary for mechanotransduction in human LTMRs, this study opens up new avenues for analyzing and genetically correcting, disease-causing Piezo2 mutations in human cell types [22,33,34].
Whether Piezo2 also contributes to acute transduction in Aδ and C afferents is not clear. Piezo2 expression has been reported in medium and small diameter neurons; however, in AdvillinCreERT2;Piezo2flox/flox mice, the proportion of mechanosensitive Aδ LTMRs and A-mechanonociceptors did not differ significantly between mutant and control genotypes [21••]. Aδ LTMRs showed normal sensitivity, but mechanical thresholds were increased in Piezo2 mutant A-mechanonociceptors. Although C-LTMRs were not analyzed in this study, C nociceptors did not differ between genotypes, indicating that Piezo2 is not required for mechanically evoked signaling in these afferents.
Mechanical sensitization
Recent studies also implicate Piezo2 in mechanosensory signaling under conditions of inflammation or injury. For example, intrathecal Piezo2 antisense oligonucleotides reduced mechanical allodynia — a condition in which normally innocuous stimuli are experienced as painful — in two different mouse models of neuropathic pain [31]. Moreover, shRNA-mediated gene silencing of Piezo2 in whisker follicles reduced behavioral responses to whisker deflection after capsaicin-induced sensitization [28•]. At a cellular level, bradykinin (BK), an inflammatory algogen that mediates hyperalgesia, sensitized Piezo2 currents when co-expressed with bradykinin receptor beta 2 in heterologous systems or in a subset of putative nociceptors [35]. These data provide a potential mechanism for mechanical hyperalgesia under conditions of inflammation. Moreover, TRPV1 stimulation inhibited Piezo2 currents in heterologous cells and a subset of DRG neurons, which highlights the potential for crosstalk between Piezo2 and ion channels involved in thermal hyperalgesia [36]. Defining the role of Piezo2 and other transduction channels in mechanical hypersensitivity may point the way toward new therapies for common and debilitating pain conditions.
Customizing Piezo function
The biophysical properties of mechanotransduction channels are important for setting the speed, sensitivity and dynamic range of mechanosensory signaling. Mechanisms that govern these biophysical properties in different types of touch receptors are under active investigation.
To evaluate the dynamic range of mechanically activated channels in DRG neurons, a novel elastomeric pillar assay was developed to deliver cellular deflections as small as 10 nm [37•]. This assay was used to analyze mechanical thresholds in neurons lacking stomatin-like protein 3 (STOML3), which is required for normal touch sensitivity in mice [38]. Mechanical activation thresholds of rapidly inactivating Piezo2 currents were increased by an order of magnitude in a subset of Stoml3 mutant DRG neurons. Conversely, co-expressing Piezo1 or Piezo2 with STOML3 in HEK293 dramatically lowered the stimulus threshold for mechanically evoked currents [37•]. Thus, STOML3 is capable of broadening the dynamic range of Piezo channels by reducing their activation thresholds.
The mechanical sensitivity of transduction channels is another important determinant of sensory signaling. A recent study of trigeminal sensory neurons from tactile foraging ducks showed that their mechanically activated currents have unusually steep displacement-response relations and that a high proportion of their trigeminal neurons express Piezo2 compared with other species [39]. By contrast, the ultra-tactile-sensitive star organ of the star-nose mole is primarily innervated by trigeminal neurons highly enriched with Piezo1, whereas DRG neurons express primarily Piezo2 [40]. Future studies of these and other tactile-specialist species hold promise for uncovering new mechanisms of tactile sensation [41–43].
Cellular tuning mechanisms
In touch receptors, firing patterns are thought to be shaped by terminal specializations that comprise sensory afferents juxtaposed to non-neuronal cell types. Recent studies have taken advantage of cell-type-specific transgenic technology to dissect how anatomical specializations and non-neuronal cell types contribute to distinctive firing properties in LTMRs.
In mice, hair follicles are innervated by RA Aβ LTMRs, Aδ-LTMRs and/or C-LTMRs, whose innervation patterns have been recently reviewed [5,44]. These neurons form similar lanceolate endings; however, they differ in their physiological responses [14••]. In most cases, different classes of LTMRs innervate the same hair follicle, forming interdigitating endings enwrapped by a common terminal Schwann cell [45]. This observation suggests that functional differences between these touch receptors reflect intrinsic neuronal mechanisms, such as distinct transduction machinery or ion channels that set membrane excitability. Interestingly, Piezo2 is required for mechanically evoked firing in RA and SA Aβ LTMRs, and set the mechanical thresholds observed in A-mechanonociceptors. How one channel can contribute to distinct mechanical properties in these diverse cell types remains to be determined.
Many lanceolate endings polarize to the caudal side of hair follicles [46]. These include TrkB-expressing Aδ LTMRs, which preferentially respond to hair movements in the caudal-to-rostral direction [47•]. Interestingly, keratinocytes located on the caudal side of hair follicles express the TrkB ligand BDNF, and epidermal-specific BDNF knockout disrupts the caudal polarization of Aδ LTMR endings [47•]. Individual Aδ LTMRs displayed directional selectivity in BDNF mutant mice; however, the orientation of their selectivity vectors did not show a strong rostral preference. Together, these findings suggesting that BDNF-independent mechanisms confer polarity in Aδ LTMRs, and that keratinocyte-derived BDNF properly orients this polarity.
Merkel cells are another epidermal cell type that governs the firing properties of LTMRs (see Acute mechanotransduction, above). Whether Merkel cells actively function in sensory transduction has long been debated. To address this question, optogenetic tools were used to selectively excite or silence Merkel-cell signaling in the intact skin [29•]. When the skin was illuminated to activate Merkel cells, SAI afferents produced sustained volleys of action potentials that mimicked the response to static pressure. Conversely, touch-evoked SAI firing was reversibly inhibited when Merkel cells were optogenetically silenced. Thus, Merkel cells are both necessary and sufficient to confer sustained firing in SAI afferents. Moreover, electrophysiological analysis of mutant mice devoid of Merkel cells demonstrated that Merkel-cell afferents are capable of transducing dynamic touch, but with markedly reduced activity. This finding suggests that Merkel cells facilitate high-frequency firing during dynamic touch. Together with studies of Merkel-cell Piezo2 summarized above, these findings indicate that the Merkel cell-neurite complex is a compound touch receptor. Future studies are needed to identify neurotransmitters and receptors that mediate signaling between Merkel cells and sensory afferents.
Conclusions and pressing questions
Recent studies have made significant progress in our understanding of the mechanisms underlying mammalian somatosensory transduction. The identification of Piezo proteins and the demonstration that Piezo2 can account for mechanotransduction in most mammalian touch receptors, answers a long-standing question in the field of mechanotransduction. Likewise, the identification of molecular markers for distinct subtypes of touch receptors enables the selective disruption of candidate genes and the ability to temporally manipulate activity in individual classes of sensory neurons. Such approaches can now be used to understand how different neuronal classes contribute to encoding of tactile stimuli. Nonetheless, a number of key questions remain to be addressed. First, how intrinsic neuronal mechanisms differ between functionally distinct somatosensory neurons remains enigmatic. A recent tour de force used single-cell RNA sequencing to identify unique transcriptional profiles for different classes of somatosensory neurons [48]. These datasets provide an excellent starting point for defining such intrinsic mechanisms. Second, there is a need for new motivated tactile behavioral tasks (rather than reflexive tasks) to assay the function of discriminative touch receptors. Third, the ion channels that transduce noxious mechanical stimuli have yet to be identified. Finally, whether Piezo2 contributes to human tactile disorders or sensory dysfunction remains largely unknown. The significant strides in our understanding of mammalian touch over the past few years suggest that answers to these questions are within reach.
Highlights.
Selective genetic markers allow functional dissection of identified touch receptors
Piezo genes encode mechanically activated ion channels
Piezo2 is a principal component of mechanotransduction in mammalian touch receptors
Cellular and molecular mechanisms tune the mechanosensory function of touch receptors
Acknowledgements
We thank Maurizio Pellegrino and Shannan McClain for comments on the manuscript, and members of the Lumpkin and Bautista laboratories for helpful discussions. The authors are supported by the National Institutes of Health (R01NS073119 to EAL and AR059385 and DOD007123A to DMB) and a Schaefer Scholar Award (to EAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nothing to declare.
References
- 1.Massie P. Touching, thinking, being: the sense of touch in Aristotle's De anima and its implications. Minerva - an internet journal of philosophy. 2013;17:74–101. [Google Scholar]
- 2.Viano C. Aristotle and the starting point of moral development: The notion of natural virtue. In: Stern-Gillet S, Corrigan K, editors. Reading ancient texts volume II: Aristotle and Neoplatonism; Essays in Honor of Denis O’Brien. Koninslijki Brill NV; 2008. p. 280. vol II.] [Google Scholar]
- 3.Geffeney SL, Goodman MB. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron. 2012;74:609–619. doi: 10.1016/j.neuron.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa H, Wang F. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2-hPLAP mice. J Comp Neurol. 2008;511:543–556. doi: 10.1002/cne.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 10.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 12.Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 14. Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. This paper showed that three types of hair follicles that comprise the mouse coat are differentially innervated by unique combinations of Ab, Ad and C-LTMRs and that the group of afferents that innervate a given hair follicle form columnar projections in the dorsal spinal cord.
- 15.de Nooij JC, Doobar S, Jessell TM. Etv1 inactivation reveals proprioceptor subclasses that reflect the level of NT3 expression in muscle targets. Neuron. 2013;77:1055–1068. doi: 10.1016/j.neuron.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. This study used an unbiased screen to identify Piezo1 and Piezo2 as candidate transduction channels proteins that necessary and sufficient for mechanically activated currents in cultured cell lines and somatosensory neurons.
- 20.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. Analysis of a tissue-specific Piezo2 knockout mice showed that this channel is required for normal sensitivity to a variety of light touch stimuli, but not for noxious mechanical responses in mice.
- 22.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110:E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae C, Gottlieb PA, Sachs F. Human PIEZO1: removing inactivation. Biophys J. 2013;105:880–886. doi: 10.1016/j.bpj.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal regoin. Nat Commun. 2015 doi: 10.1038/ncomms8223. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prole DL, Taylor CW. Identification and analysis of putative homologues of mechanosensitive channels in pathogenic protozoa. PLoS One. 2013;8:e66068. doi: 10.1371/journal.pone.0066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faucherre A, Kissa K, Nargeot J, Mangoni ME, Jopling C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica. 2014;99:70–75. doi: 10.3324/haematol.2013.086090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. By using whole-cell recordings from epidermal cells in semi-intact rat whisker follicles, this study demonstrated that Merkel cells have Peizo2-like mechanically activated currents in situ.
- 29. Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. In the first use of optogenetics in skin cells, this study showed that Merkel-cell activity is both necessary and sufficient for sustained firing in SAI afferents.
- 30. Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. This study demonstrated that Merkel-cell mechanotransduction is mediated by Piezo2 and is required for normal tactile sensitivity in vivo.
- 31.Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun. 2013;4:1682. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, Utikal J, Lewin GR, Lechner SG, Siemens J. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat Neurosci. 2015;18:10–16. doi: 10.1038/nn.3894. [DOI] [PubMed] [Google Scholar]
- 33.McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, Aracena MI, Aylsworth AS, Bitoun P, Carey JC, et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet. 2014;94:734–744. doi: 10.1016/j.ajhg.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo M, Fujita A, Saito Y, Komaki H, Ishiyama A, Takeshita E, Kojima E, Koichihara R, Saito T, Nakagawa E, et al. A family of distal arthrogryposis type 5 due to a novel PIEZO2 mutation. Am J Med Genet A. 2015;167:1100–1106. doi: 10.1002/ajmg.a.36881. [DOI] [PubMed] [Google Scholar]
- 35.Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2012;2:511–517. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal. 2015;8:ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat Commun. 2014;5:3520. doi: 10.1038/ncomms4520. Using a novel mechanical stimulation technique, this study revealed that Piezos can be activated by nanometer displacements and that STOML3 modulates piezo-mediated currents.
- 38.Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 39.Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Natl Acad Sci U S A. 2014;111:14941–14946. doi: 10.1073/pnas.1413656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerhold KA, Pellegrino M, Tsunozaki M, Morita T, Leitch DB, Tsuruda PR, Brem RB, Catania KC, Bautista DM. The star-nosed mole reveals clues to the molecular basis of mammalian touch. PLoS One. 2013;8:e55001. doi: 10.1371/journal.pone.0055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catania KC. The neurobiology and behavior of the American water shrew (Sorex palustris) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199:545–554. doi: 10.1007/s00359-012-0781-7. [DOI] [PubMed] [Google Scholar]
- 42.Leitch DB, Catania KC. Structure, innervation and response properties of integumentary sensory organs in crocodilians. J Exp Biol. 2012;215:4217–4230. doi: 10.1242/jeb.076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall KL, Chadha M, deSouza LA, Sterbing-D'Angelo SJ, Moss CF, Lumpkin EA. Somatosensory substrates of flight control in bats. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.04.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346:950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Ginty DD. The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife. 2014;3:e01901. doi: 10.7554/eLife.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Williams J, Nathans J. Morphologic diversity of cutaneous sensory afferents revealed by genetically directed sparse labeling. Elife. 2012;1:e00181. doi: 10.7554/eLife.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rutlin M, Ho CY, Abraira VE, Cassidy C, Bai L, Woodbury CJ, Ginty DD. The cellular and molecular basis of direction selectivity of Adelta-LTMRs. Cell. 2014;159:1640–1651. doi: 10.1016/j.cell.2014.11.038. This study showed that the ability to detect the direction of mechanical stimuli by Adelta low-threshold mechanosensory nerve fibers is conferred through their asymmetric association with hair follicles.
- 48.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 49.Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013;493:669–673. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]