Abstract
Background
Sleep quality and quantity are severely reduced in critically ill patients receiving mechanical ventilation with potential for adverse consequences. Our objective was to synthesize the randomized controlled trials (RCTs) that measured the efficacy of sleep-promoting interventions on sleep quality and quantity in critically ill patients.
Methods
We included RCTs that objectively measured sleep with electroencephalography or its derivatives and excluded observational studies and those that measured sleep by subjective reports. The research was performed according to PRISMA guidelines.
Results
Of 6,022 studies identified, 13 studies met eligibility criteria involving 296 critically-ill patients. Eight trials looked at different modes of mechanical ventilation as sleep interventions, and the remaining five involved pharmacological, non-pharmacological, or environmental interventions. Meta-analysis of the studies revealed that sleep-promoting interventions improved sleep quantity (pooled standardized mean of differences [SMD] 0.37, 95%CI: 0.05, 0.69; P=0.02) and sleep quality through reduction in sleep fragmentation (SMD −0.31; 95%CI −0.60, −0.01; P=0.04). Subgroup analysis revealed that timed-modes of ventilation improved sleep quantity when compared to spontaneous-modes of ventilation (SMD 0.45, 95%CI 0.10, 0.81; P=0.01). Non-mechanical ventilation interventions tended to improve sleep quantity (SMD 0.65; 95%CI; −0.03, 1.33; P=0.06) and tended to reduce sleep fragmentation (SMD −0.29; 95% CI −0.61, 0.03; P=0.07).
Conclusions
The synthesized evidence suggests that both mechanical ventilation and non-mechanical ventilation-based therapies improve sleep quantity and quality in critically ill patients but the clinical significance is unclear. In the future, adequately-powered multi-center RCTs involving pharmacological interventions to promote sleep in critically ill patients are warranted.
MeSH terms: sleep, critical illness, artificial respiration, Hypnotics and Sedatives, polysomnography, critical care, Positive-Pressure Respiration
Introduction
Sleep quality and quantity are severely reduced in critically ill patients with potential for adverse consequences1–5. In critically ill patients, lack of sleep may contribute to delirium and agitation and in healthy volunteers cause immune dysregulation and negative nitrogen balance4,6–8. In community-dwelling participants, lack of sleep has been associated with all-cause mortality9–15. Although abnormalities of sleep are extremely common in critically ill patients, the mechanisms are not well understood4. Intervention-based studies in critically ill patients can elucidate the mechanistic basis of sleep derangements and are direly needed. However, there is a paucity of such intervention-based mechanistic studies for sleep promotion in critically ill patients due to the arduous nature of conducting such intervention-based experiments; difficulties in surrogate consenting; and collecting electroencephalography signals in an artefact-ridden intensive care unit (ICU) environment4. Even the few randomized controlled trials (RCTs) of sleep in the ICU are limited by small sample size. Nevertheless, they were rigorous in study-design and conduct while exploring the effect of mechanical ventilation, pharmacological, environmental, and other non-pharmacological interventions on sleep in critically ill patients16–19. A meta-analysis, by combining such smaller RCTs could increase the overall power to estimate the efficacy of sleep promoting interventions during critical illness. Such an undertaking could help us better understand the mechanistic underpinnings of sleep derangements during critical illness, and ultimately inform future adequately-powered trials aimed at improving sleep and consequent patient-outcomes in critically ill patients.
Our primary objective was to synthesize the RCTs that measured the efficacy of sleep-promoting interventions on sleep quality and quantity in critically ill patients. Our secondary objective was to understand the treatment effects of sleep-promoting interventions that were categorized by mechanical ventilation versus other interventions.
Methods
Data source and searches
We conducted an electronic search of the literature in Medline, Cochrane central, Dynamed from 1966 to August 2014. We then updated the search in October 2014. We used a combination of MESH subheadings and keywords (sleep, sleep interventions, critical illness, mechanical ventilation, randomized controlled trials). We used “sleep AND critical illness” “sleep AND mechanical ventilation” “sleep interventions AND mechanical ventilation” “sleep interventions AND critical illness” as well the above four combinations with “OR randomized controlled trials” with exploded search terms. We limited the entire list to studies published until October 2014 but there were no limits to age of the studies. We reviewed the bibliographies of the included studies and previous reviews to identify additional citations. The research was guided by an extraction protocol that followed PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)20.
Definitions
Operational definitions of outcome variables were as follows: (a) Sleep quantity was defined as sleep efficiency which is time spent asleep expressed as a percentage of total recording time. (b) Sleep quality was defined as sleep fragmentation measured as arousals and awakenings per hour of sleep. (c) Information of proportion of time spent in various sleep stages were also extracted when available and proportioned into various non-rapid eye movement (stage N1, N2, slow wave sleep) and rapid eye movement (REM) sleep. Explanatory variables were interventions that were categorized into changes (or intervention) made to mechanical ventilation (mode of ventilation), pharmacological therapy (sedatives type or infusion method), environmental (noise reduction or music), non-pharmacological (such as massage) interventions.
Eligibility criteria
We included intervention-based studies if they were RCTs and objectively measured sleep in critically ill patients. We excluded observational studies and those that measured sleep without electroencephalography (EEG) or its derivatives. A priori we decided not to include articles that measured sleep through subjective reports, nursing assessments, or actigraphy due to known reservations about their test characteristics21. We included Bispectral index or fast fourier transformation of EEG signals because such automatically processed signals have good reproducibility characteristics and that there was a paucity of RCTs in this area of study identified through an iterative process22. The search was limited to RCTs that were published in English and studied human subjects.
Data extraction and quality assessment
One study team member (CP) reviewed all included papers (n=13) and abstracted all of the relevant data from them into formatted Windows Excel database. To validate the abstraction process, the other two study team members (SGJ, ASK) each reviewed a randomly selected sample so that at least two study members had abstracted each included paper. A third study member (SP) reviewed extracted data from all of the papers in order to identify differences in the abstraction between previous abstractions and resolve discrepancies by consensus. Data were extracted from each selected article using formatted Windows Excel database. Disagreement between the extracting investigators was resolved by consensus. We rated the study quality using United States Preventive Services Task Force (USPTF) criteria (Table 1)23.
Table 1.
Studies Assessing Sleep-promoting Interventions in Critically ill Patients
| Study | Quality scores* | Study design | Participants | Intervention | Control | Outcome measure | Comment |
|---|---|---|---|---|---|---|---|
| Alexopoulou 201326 | I | RCT, cross-over | Intubated, COPD, n=13 |
PAV | PSV | PSG-derived Sleep efficiency Sleep fragmentation index |
PAV failed to improve sleep in mechanically ventilated patients |
| Andrejak 201337 | I | RCT, cross-over | Intubated; n=35 (n=9 discarded) |
PCV | PSV | PSG-derived Sleep efficiency Sleep fragmentation index |
Sleep quantity and quality were significantly improved with PCV compared to low-PSV |
| Bosma 200727 | I | RCT, cross-over | Intubated; n=16 (n=3 discarded) |
PAV | PSV | PSG-derived Sleep efficiency Sleep fragmentation index |
PAV resulted in fewer patient-ventilator asynchronies and better sleep quality. |
| Bourne 200828 | I | RCT | Tracheostomized patients undergoing weaning (n=24) | Melatonin (10 mg) |
Placebo | Bispectral index (time spent <80; sleep efficiency); area under curve (sleep quality) | Melatonin use was associated with increased nocturnal sleep efficiency over 4 nights |
| Cabello 200816 | I | RCT, cross over, 3 arms | Intubated and tracheostomized patients (n=15) | Clinically adjusted PSV (Cabello 2008a) or automatically adjusted PSV (Cabello 2008b) |
ACV | PSG-derived Sleep efficiency Sleep fragmentation index |
The ventilatory mode did not influence sleep pattern, arousals and awakenings. |
| Cordoba-Izquierdo 201330 | I | RCT | Non-invasive ventilation; n=25 (n=1 discarded) |
Dedicated ICU ventilator | Conventional noninvasive ventilator | PSG-derived (Sleep efficiency and Sleep fragmentation index) | There were no observed differences in sleep quality corresponding to the type of ventilator used despite slight differences in patient–ventilator asynchrony. |
| Kondilli 201231 | I | RCT, cross-over | Invasive ventilation; n=13 (n=1 discarded) |
Propofol | No propofol | PSG-derived (Sleep efficiency and Sleep fragmentation index) | In critically ill patients ventilated on assisted modes, propofol administration to achieve the recommended level of sedation suppresses the REM sleep stage and further worsens the poor sleep quality of these patients. |
| Oto 201119 | I | RCT | Invasive ventilation; n=22 | Continuous infusion | Daily interruption of sedation | PSG-derived (Sleep efficiency and Sleep fragmentation index) | In the continuous infusion group, sleep efficiency was greater and sleep fragmentation was lower when compared to group with daily sedation interruption. |
| Parthasarathy 200232 | I | RCT, cross-over | Intubated patients, n=11 |
ACV | PSV | PSG-derived (Sleep efficiency and Sleep fragmentation index) | PSV was associated with sleep fragmentation when compared to ACV |
| Richards 199833 | I | RCT, cross-over, 3 arms | Critically ill men admitted to ICU (n=71); 2 subjects were excluded | Two of 3 arms back massage (n=24) | Usual care (n=17)(Richa rds 1998) or music & relaxation (n=28)(Richa rds 2008b) | PSG-derived (Sleep efficiency and Sleep fragmentation index) | Back massage was useful in promoting sleep in critically ill older men |
| Roche-Campo 201334 | I | RCT, cross-over | Tracheostomized patients undergoing weaning from mechanical ventilation (n=16) | PSV | Spontaneous ventilation (control) | PSG-derived (Sleep efficiency and Sleep fragmentation index) | Sleep quality was similar with or without the ventilator. Sleep quantity was higher during mechanical ventilation. |
| Su 201235 | I | RCT | 28 patients in a medical ICU | Music therapy | Usual care | PSG-derived sleep efficiency (sleep fragmentation was not measured) | Greater amount of slow wave sleep in the music group |
| Toublanc 200736 | I | RCT, cross-over | Intubated patients (n=22)(2 patients were discarded) | ACV | PSV | PSG-derived (Sleep efficiency and Sleep fragmentation index) | ACV was significantly associated with a better sleep quality than those recorded during PSV |
PAV = proportional assist ventilation; PSV = pressure support ventilation; COPD = chronic obstructive pulmonary disease; RCT = randomized controlled trial; PSG = polysomnography; ICU = Intensive care unit. USPTF Hierarchy of research design with range of I, II-1, II-2, II-3, and III (with I being best and defined as, “Evidence obtained from at least one properly randomized controlled trial”).
Data synthesis and analysis
We conducted a meta-analysis assuming random effects on sleep quantity and quality that provided enough detail to calculate standardized mean differences (SMD; n= 13) (RevMan, Version 5.3.5 Copenhagen, Denmark). Two studies had three study arms each and therefore the comparisons of the experimental groups versus the control or usual group were used and identified as such. Sensitivity analysis by both including and excluding the duplicate representation of the control (or usual care) arms of these two studies were performed. Considering that some of the studies reported medians and inter-quartile range, we calculated standard deviation from the inter-quartile range and used the median as the mean24.
Risk for bias in individual studies was assessed by gauging selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants), detection bias (outcome assessors), attrition bias (incomplete outcome data), and selective reporting or other bias. Risk for bias across studies (publication bias) was performed by making funnel plots and using the Begg and Mazumdar test25 (SPSS version 22, IBM SPSS, Armonk, NY). We also performed meta-analysis of subgroups involving studies that employed mechanical ventilation versus non-mechanical ventilation interventions, timed versus spontaneous mode of ventilation, and performed sensitivity analysis by including and excluding studies with large effect size that may be unduly influencing the meta-analysis.
Results
Of the 6,022 studies that were screened and assessed for eligibility, 29 studies were included in the qualitative analysis that eventually yielded 13 RCTs (with 296 patients) which were then included in the meta-analysis (Figure 1; PRISMA compliant flow-diagram). All of the 13 studies qualified as level I according to USPTF hierarchy of study design23. Eight trials looked at different modes of mechanical ventilations as sleep-promoting interventions, and the remaining five involved pharmacological, non-pharmacological, or environmental interventions16,19,26–37. For each of the 13 RCTs, patient characteristics, intervention, comparators, outcomes, and study design are provided in Table 1. The risk of bias within each study is provided in Table 2.
Figure 1.
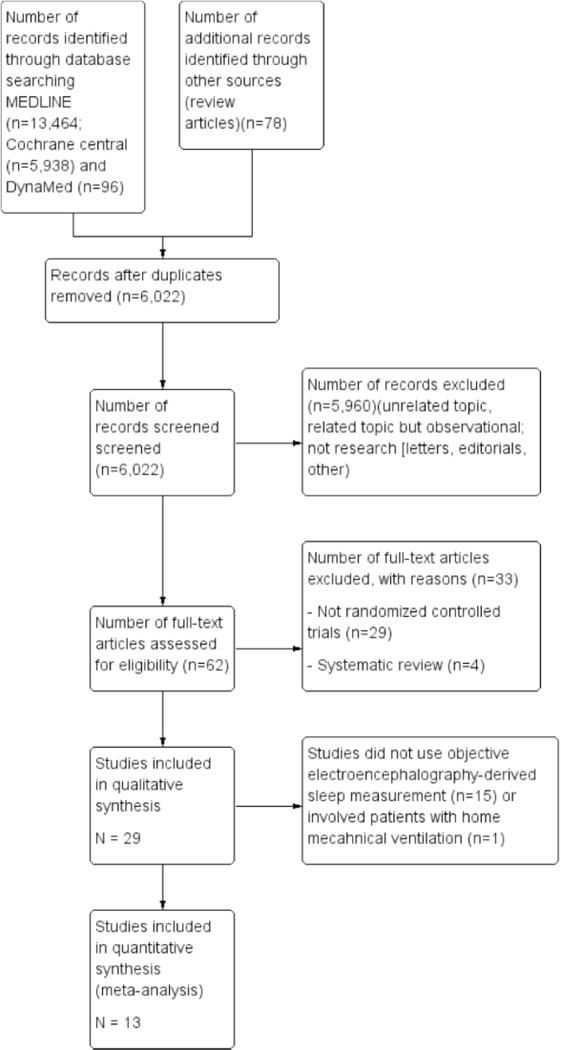
PRISMA compliant flow chart summarizing the number of abstracts and papers reviewed and the reasons for excluding them from the meta-analysis.
Table 2.
Risk of Bias Summary
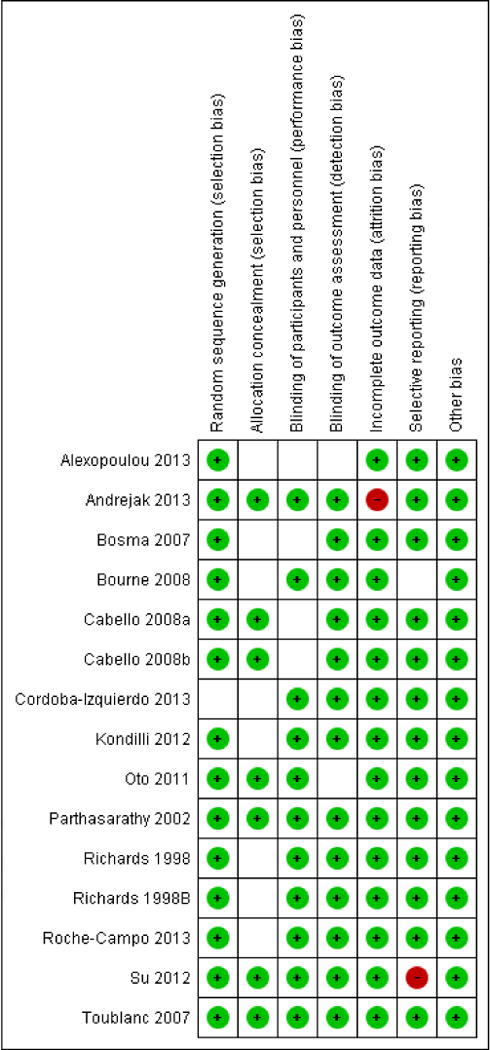
|
Green symbols signify low risk for bias, blank spaces signify unclear risk for bias, and red symbols signify high risk for bias. Last name of first author and year of publication are provided. See table 1 for PICOS information and reference number for the publications.
Sleep-promoting interventions, that was mediated by changes to mechanical ventilation or other sleep promotion therapies (environmental, pharmacological, or non-pharmacological), increased sleep quantity as measured by sleep efficiency (figure 2). There was high heterogeneity among these studies (I2 = 62%; P=0.0007). Sensitivity analysis performed by removal of study by Oto 201119 removed the heterogeneity (I2=26%; P=0.17) but did not materially change the results (SMD 0.26, 95% CI 0.03, 0.49; P=0.02). Sleep-promoting interventions improved sleep quality by reducing sleep fragmentation (figure 3). There was moderate heterogeneity among these studies (I2 =49%; P=0.02). Sensitivity analysis performed by removal of study by Parthasarathy 200232 significantly reduced heterogeneity (I2=0%; P=0.98), but did not materially change the results (SMD −0.19, 95% CI −0.39, 0.02; P=0.08).
Figure 2.
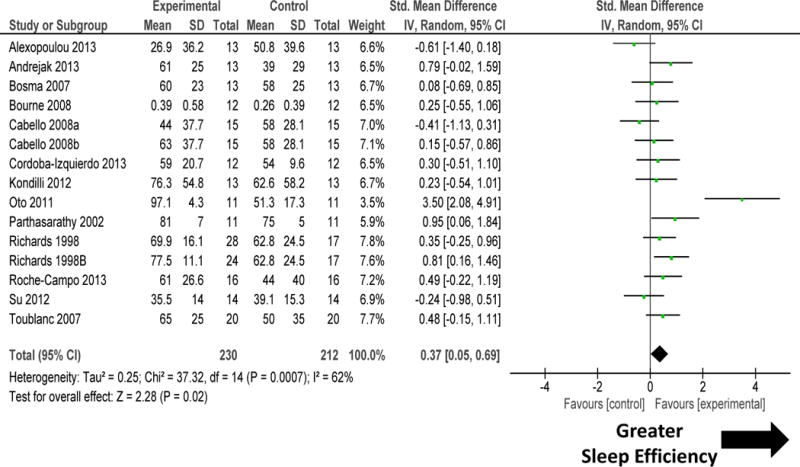
Forest plot for sleep efficiency during sleep-promoting intervention (mechanical ventilation and non-mechanical ventilation). The size of the box reflects the study’s relative weight based on the standard error. The diamond indicates the 95 percent confidence interval of the summary estimate. Sleep promotion interventions improved sleep efficiency in the 13 randomized controlled trials. There was high heterogeneity among these studies (I2 =62%). Sensitivity analysis performed by removal of study by Oto 201119 did not materially change the results (SMD 0.26, 95% CI 0.03, 0.49; P=0.02) but significantly reduced heterogeneity (I2=26%).
Figure 3.
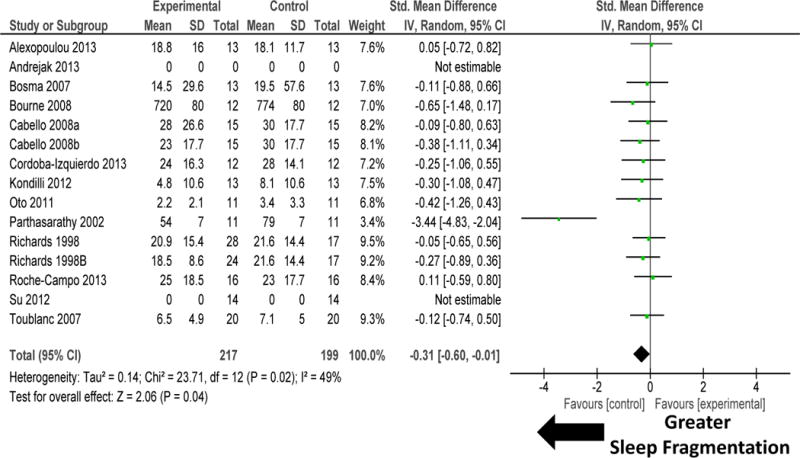
Forest plot for sleep fragmentation (arousals and awakenings) during sleep-promoting intervention (mechanical ventilation and non-mechanical ventilation). Sleep promotion interventions improved sleep quality through reduction in sleep fragmentation in the 13 randomized controlled trials. There was moderate heterogeneity among these studies (I2 = 49%). Sensitivity analysis performed by removal of study by Parthasarathy 200232 did not materially change the results (SMD −0.19, 95% CI −0.39, 0.02; P=0.08) but significantly reduced heterogeneity (I2=0%). Explanation of symbols is provided in legend of figure 2.
Subgroup and sensitivity analysis
Change to mechanical ventilation modes in eight RCTs tended to improve sleep quantity by increasing sleep efficiency (figure 4). There was no heterogeneity among these studies (P=0.11). Change in mechanical ventilation modes tended to improve sleep quality by decreasing sleep fragmentation (figure 5). There was significant heterogeneity among these studies (I2=68%). Sensitivity analysis performed by removal of study by Parthasarathy 2002 significantly reduced heterogeneity (I2=0%; P=0.98) but made the results for improvement in sleep quality non-significant (P=0.43).
Figure 4.
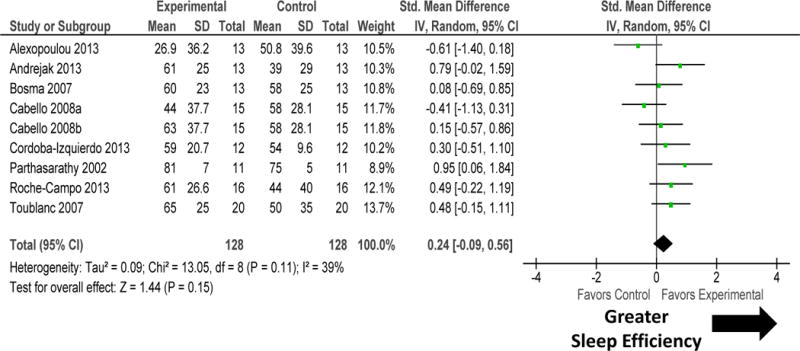
Forest plot for sleep efficiency during intervention accomplished by adjusting mechanical ventilation modality. Change in mechanical ventilation modes tended to improve sleep quantity by increasing sleep efficiency. There was no heterogeneity among these studies. Explanation of symbols is provided in legend of figure 2.
Figure 5.
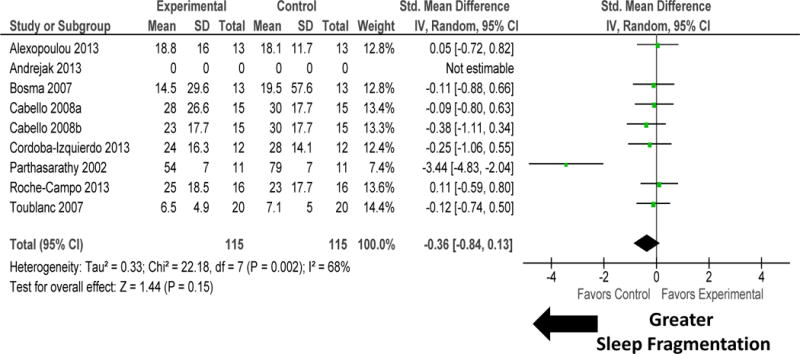
Forest plot for sleep fragmentation during intervention accomplished by adjusting mechanical ventilation modality. Change in mechanical ventilation modes tended to improve sleep quality by decreasing sleep fragmentation. There was significant heterogeneity among these studies. Sensitivity analysis performed by removal of study by Parthasarathy 200232 made the results non-significant (P=0.43) and significantly reduced heterogeneity (I2=0%; P=0.98). Explanation of symbols is provided in legend of figure 2.
Subgroup comparison of four RCTs that compared timed versus spontaneous modes of ventilation was undertaken (figure 6 and 7). Timed-mode of mechanical ventilation improved sleep quantity when compared to spontaneous mode of ventilation (figure 6). There was no heterogeneity among these studies (I2=12%; P=0.3). Timed-mode of mechanical ventilation did not influence sleep quality measured as sleep fragmentation (figure 7). There was significant heterogeneity among these studies (I2 = 87%; P<0.0001). Sensitivity analysis performed by removal of one study Parthasarathy 200232 did not change the results materially.
Figure 6.

Forest plot for sleep efficiency during intervention accomplished by timed versus spontaneous modes of mechanical ventilation. Timed-mode of mechanical ventilation improved sleep quantity when compared to spontaneous mode of ventilation. There was no heterogeneity among these studies (I2 =12%). Explanation of symbols is provided in legend of figure 2.
Figure 7.
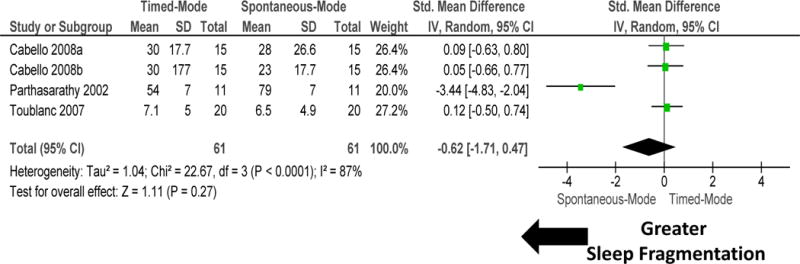
Forest plot for sleep fragmentation during intervention accomplished by timed versus spontaneous modes of mechanical ventilation. Timed-mode of mechanical ventilation did not improve sleep quality measured as sleep fragmentation. There was significant heterogeneity among these studies (I2 =87%). Sensitivity analysis performed by removal of one study by Parthasarathy 200232 did not change the results materially. Explanation of symbols is provided in legend of figure 2.
Subgroup comparison of five RCTs that compared environmental, pharmacological or non-pharmacological methods to promote sleep was undertaken (figure 8 and 9; Table 1). Non-mechanical ventilation-based interventions improved sleep quantity. There was significant heterogeneity among these studies (figure 8). Removal of one study Oto 201119 removed the heterogeneity (I2=10%; P=0.35), but did not change the results significantly (SMD 0.32, −0.01, 0.65; P=0.06). Non-mechanical ventilation-based interventions tended to improve sleep quality (figure 9). There was significant heterogeneity among these studies.
Figure 8.
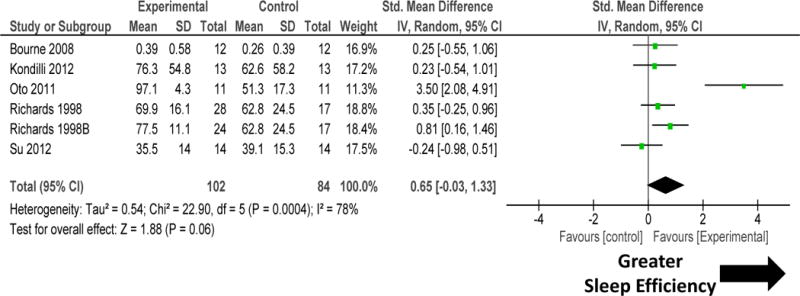
Forest plot for sleep quantity during non-mechanical ventilation-based interventions that included pharmacological, non-pharmacological, or environmental interventions. Non-mechanical ventilation-based interventions improved sleep quantity. There was significant heterogeneity among these studies. Explanation of symbols is provided in legend of figure 2.
Figure 9.
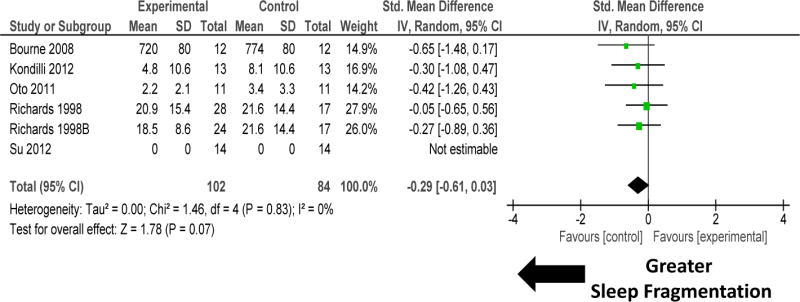
Forest plot for sleep fragmentation during non-mechanical ventilation-based interventions that included pharmacological, non-pharmacological, or environmental interventions. Non-mechanical ventilation-based interventions improved sleep quality by reducing sleep fragmentation. There was no heterogeneity among these studies (I2 = 0%). Explanation of symbols is provided in legend of figure 2.
There were two studies (Cabello 2008 and Richards 1998) that had three arms in the RCT16,33. In the presented comparisons, for each study, the control arms were included twice. Sensitivity analysis was performed by removing these studies from the meta-analysis. Such sensitivity analysis did not materially change the results. Furthermore, stratification of results by proportion of time spent in various sleep stages (stage N1, N2, slow wave sleep, REM) revealed small effects on slow wave sleep (web-only supplementary material [eTable 1]).
Assessment of bias
By a priori design, we only chose RCTs for this meta-analysis. Therefore the within study bias was minimal (Table 2). Bias across studies was assessed by funnel plots (figure 10). There was no evidence for publication bias assessed by Begg and Mazumdar’s test25 (Kendall’s tau b=0.12; P=0.52).
Figure 10.
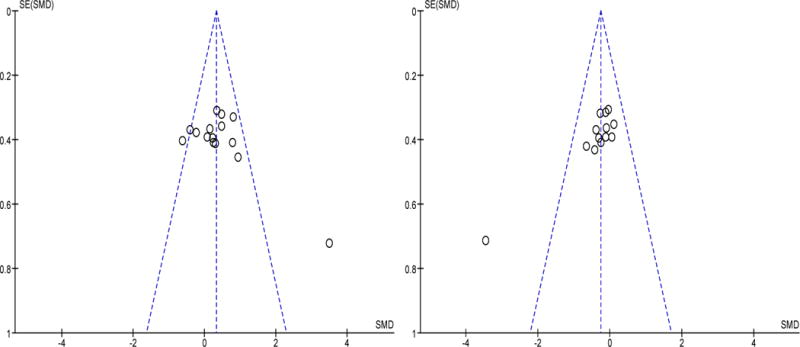
Funnel plots for sleep efficiency (left panel) and sleep fragmentation (right panel) during sleep-promoting interventions. There was no evidence for publication bias.
Discussion
To our knowledge this is the first meta-analysis that assessed the efficacy of sleep-promotion in critically ill patients. Several general observations of the main findings can be made. First, sleep-promoting interventions improved both sleep quantity and quality in critically ill patients, but the effect size was small with heterogeneity across studies. Second, both mechanical ventilation-based as well as non-mechanical ventilation-based interventions improved sleep quantity and quality in critically ill patients, but the effect size of non-mechanical ventilation appeared to be larger than that of mechanical ventilation-based interventions. Third, timed-mode of mechanical ventilation improved sleep quantity when compared to spontaneous mode of ventilation. Fourth, the sleep-promoting interventions were heterogeneous in nature requiring us to collapse the interventions by mechanistic approach (such as mechanical ventilation, environmental, pharmacological and non-pharmacological interventions). Fifth, although the studies were of good quality with low risk for bias within such studies (table 2), these studies were limited by small sample size and generalization was limited due to lack of any multi-center studies. Sixth, although sleep quality and quantity are important outcomes, these RCTs did not uniformly focus on the effect of sleep on other important patient outcomes – such as delirium, duration of hospitalization, or mortality – despite the existence of sound rationale for such a potential impact9–14,18,38–40. Lastly, there was no evidence for publication bias across studies (figure 10).
Sleep quality and quantity are severely reduced in critically ill patients with potential for adverse consequences1–5. In community-dwelling participants, poor sleep quantity and quality due to chronic insomnia has been independently associated with all-cause and cardiopulmonary mortality9–14. Some of the mechanistic basis for such an association may be mediated by systemic inflammation14. Such a mechanistic pathway is supported by controlled experiments in healthy volunteers that revealed elevation in pro-inflammatory cytokines following sleep loss41. Although, there is rich evolving body of work on the effect of sleep quality and quantity on well-being in ambulatory patients and population-based studies, there is a paucity of sleep research in critically ill patients. Conceivably, critically ill patients may be even more susceptible to the harmful effects of poor sleep than ambulatory patients and community-dwelling participants. In our meta-analysis, we were able to find only 13 small RCTs conducted prior to October 2014 that undertook rigorous sleep measurement methodology without any time restrictions to the age of these studies. A paucity of such intervention-based mechanistic studies for sleep promotion in critically ill patients may be due to the arduous nature of conducting such intervention-based studies in critically ill patients4. Even these RCTs of sleep in the ICU were limited by small sample size in individual studies with a maximum of 24 patients per study arm. Nevertheless, they were rigorous in study-design and measurement methodology while they explored the effect of mechanical ventilation, pharmacological, environmental, and other non-pharmacological interventions on sleep in critically ill patients16–19. Our meta-analysis, by combining 13 such smaller RCTs increased the overall power to estimate the efficacy of sleep-promoting interventions during critical illness with a cumulative sample size of 296 critically ill patients. Such an undertaking was quite revealing and we discuss the findings here along with caveats and other limitations.
Sleep-promoting interventions improved both sleep quantity and quality in critically ill patients, but the effect size was small and heterogeneous. We performed sensitivity analysis to reduce the heterogeneity and found that sleep quantity and to some extent sleep quality is indeed modifiable in critically ill patients. We noticed that the effect size although small, was relatively larger for non-mechanical ventilation-based interventions than mechanical ventilation-based approaches for promoting sleep, with pharmacological interventions manifesting the greatest effect size. We were limited by the heterogeneous nature of the sleep-promoting interventions but handled this problem by performing subgroup analysis by mechanistic approach of the sleep interventions. This is a limitation of our study, but again highlights the need for a uniform intervention in a larger adequately powered study. An additional observation was the lack of sufficient head-to-head studies of sedative agents in improving sleep quantity and quality. Additionally, the critically ill patient sub-populations were heterogeneous and variably involved patients with exacerbations of chronic obstructive pulmonary disease, heart failure, Acute Respiratory Distress Syndrome, or pneumonia. Considering the greater effect of non-mechanical ventilation-based interventions, the lack of head-to-head studies of pharmacological agents, and inhomogeneous patient population and interventions, there is clearly an identifiable knowledge gap for performing RCTs with pharmacological interventions (with active control group) to promote sleep quality and quantity in a homogenous group of critically ill patients.
In subgroup analysis, timed mode of ventilation was better than spontaneous mode of ventilation in improving sleep quantity and quality in critically ill patients (figures 6 and 7). This is in line with findings in ambulatory patients with sleep-disordered breathing who manifested better sleep quality with a back-up respiratory rate due to reduction in respiratory events and improvement in pattern of breathing29. Despite the small number of studies in this sub-group analysis, the findings were homogeneous. Future studies that test other non-mechanical ventilation based interventions need to control for such a potential confounder.
In conclusion, sleep promoting-interventions, both timed mode of mechanical ventilation and non-mechanical ventilation-based therapies, can improve sleep quantity and quality in critically ill patients but the effect size was small and heterogeneous with unclear clinical significance. We believe that these findings provide rationale for performing larger, multi-center, adequately-powered trials for promoting sleep in critically ill patients. Specifically, there is identifiable knowledge gap for performing a pharmacological intervention (with active control group) to promote sleep quality and quantity in a homogenous group of critically ill patients. Such studies should be adequately powered to measure important patient-outcomes that are mechanistically downstream to sleep – such as delirium, systemic inflammation, duration of hospitalization, or even mortality – while carefully measuring the mediating effects of sleep.
Supplementary Material
Clinical Significance.
Sleep-promoting interventions improved sleep quantity in critically ill patients.
Timed-modes improved sleep quantity when compared to spontaneous-modes of ventilation
Effect size of sleep promotion interventions was small and heterogeneous in the critically ill.
Effect size of non-mechanical ventilation was larger than mechanical ventilation-based interventions.
Acknowledgments
Dr. Parthasarathy reports grants from NIH/NHLBI, grants from Patient Centered Outcomes Research Institute, grants from US Department of Defense, grants from NIH (National Cancer Institute) NCI, grants from US Department of Army, grants from Johrei Institute, personal fees from American Academy of Sleep Medicine, personal fees from American College of Chest Physicians, non-financial support from National Center for Sleep Disorders Research of the NIH (NHLBI), personal fees from USMLEWorld Inc., personal fees from UpToDate Inc., personal fees from Philips-Respironics, Inc., grants from Younes Sleep Technologies, Ltd., grants from Niveus Medical Inc., grants from Philips-Respironics, Inc., outside the submitted work; In addition, Dr. Parthasarathy has a patent UA 14-018 U.S.S.N. 61/884,654; PTAS 502570970 (Home breathing device) pending. The above-mentioned conflicts including the patent are unrelated to the topic of this paper.
Funding support: This work was supported by the National Institutes of Health (NIH) Grants (5R01HL095748) and PCORI grant (IHS-1306-2505) to S.P. During the writing of this manuscript S.P was supported by NIH grants HL095799 and CA184920. The funding institutions did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Drs. Chithra Poongkunran, Santosh G. John, Arun S. Kannan, Safal Shetty, MD and Christian Bime, MD do not have any conflicts of interest to disclose.
Authorship credit: Conceived and designed the experiments (CP, SGJ, ASK, SS, SP), Analyzed the data (CP, SGJ, ASK, SS, SP), Interpretation of data (CP, SGJ, ASK, SS, CB, SP), contributed reagents/materials/analysis tools (CP, SGJ, ASK, SS, CB, SP), drafted the article or revised it critically for important intellectual content (CP, SGJ, ASK, SS, CB, SP), final approval of the version to be published (CP, SGJ, ASK, SS, CB, SP). Sairam Parthasarathy, MD [Corresponding author] had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Drouot X, Cabello B, d’Ortho MP, et al. Sleep in the intensive care unit. Sleep Med Rev. 2008;12:391–403. doi: 10.1016/j.smrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Weinhouse GL, Watson PL. Sedation and sleep disturbances in the ICU. Crit Care Clin. 2009;25:539–549. doi: 10.1016/j.ccc.2009.04.003. ix. [DOI] [PubMed] [Google Scholar]
- 3.Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29:707–716. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Med. 2004;30:197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AB, Gabor JY, Hanly PJ. Sleep in the critically ill patient. Semin Respir Crit Care Med. 2001;22:153–164. doi: 10.1055/s-2001-13829. [DOI] [PubMed] [Google Scholar]
- 6.Dinges DF, Douglas SD, Hamarman S, et al. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- 7.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scrimshaw NS, Habicht JP, Pellet P, et al. Effects of sleep deprivation and reversal of diurnal activity on protein metabolism of young men. Am J Clin Nutr. 1966;19:313–319. doi: 10.1093/ajcn/19.5.313. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–141. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300–309. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida OP, Alfonso H, Yeap BB, et al. Complaints of difficulty to fall asleep increase the risk of depression in later life: the health in men study. J Affect Disord. 2011;134:208–216. doi: 10.1016/j.jad.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Zhang X, Winkelman JW, et al. The Association between Insomnia Symptoms and Mortality: A Prospective Study of US Men. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent Insomnia Is Associated With Mortality Risk. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 16.Cabello B, Thille AW, Drouot X, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36:1749–1755. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 17.Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136:284–294. doi: 10.1378/chest.08-1546. [DOI] [PubMed] [Google Scholar]
- 18.Kamdar BB, Needham DM, Collop NA. Sleep Deprivation in Critical Illness: Its Role in Physical and Psychological Recovery. J Intensive Care Med. 2011 doi: 10.1177/0885066610394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oto J, Yamamoto K, Koike S, et al. Effect of daily sedative interruption on sleep stages of mechanically ventilated patients receiving midazolam by infusion. Anaesth Intensive Care. 2011;39:392–400. doi: 10.1177/0310057X1103900309. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 21.Beecroft JM, Ward M, Younes M, et al. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34:2076–2083. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 22.Ambrogio C, Koebnick J, Quan SF, et al. Assessment of sleep in ventilator-supported critically III patients. Sleep. 2008;31:1559–1568. doi: 10.1093/sleep/31.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2011 Version 5.1.0. [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 26.Alexopoulou C, Kondili E, Plataki M, et al. Patient-ventilator synchrony and sleep quality with proportional assist and pressure support ventilation. Intensive Care Med. 2013;39:1040–1047. doi: 10.1007/s00134-013-2850-y. [DOI] [PubMed] [Google Scholar]
- 27.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 28.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contal O, Adler D, Borel JC, et al. Impact of different backup respiratory rates on the efficacy of noninvasive positive pressure ventilation in obesity hypoventilation syndrome: a randomized trial. Chest. 2013;143:37–46. doi: 10.1378/chest.11-2848. [DOI] [PubMed] [Google Scholar]
- 30.Cordoba-Izquierdo A, Drouot X, Thille AW, et al. Sleep in hypercapnic critical care patients under noninvasive ventilation: conventional versus dedicated ventilators. Crit Care Med. 2013;41:60–68. doi: 10.1097/CCM.0b013e31826764e3. [DOI] [PubMed] [Google Scholar]
- 31.Kondili E, Alexopoulou C, Xirouchaki N, et al. Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study. Intensive Care Med. 2012;38:1640–1646. doi: 10.1007/s00134-012-2623-z. [DOI] [PubMed] [Google Scholar]
- 32.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 33.Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. Am J Crit Care. 1998;7:288–299. [PubMed] [Google Scholar]
- 34.Roche-Campo F, Thille AW, Drouot X, et al. Comparison of sleep quality with mechanical versus spontaneous ventilation during weaning of critically III tracheostomized patients. Crit Care Med. 2013;41:1637–1644. doi: 10.1097/CCM.0b013e318287f569. [DOI] [PubMed] [Google Scholar]
- 35.Su CP, Lai HL, Chang ET, et al. A randomized controlled trial of the effects of listening to non-commercial music on quality of nocturnal sleep and relaxation indices in patients in medical intensive care unit. J Adv Nurs. 2013;69:1377–1389. doi: 10.1111/j.1365-2648.2012.06130.x. [DOI] [PubMed] [Google Scholar]
- 36.Toublanc B, Rose D, Glerant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–1154. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 37.Andrejak C, Monconduit J, Rose D, et al. Does using pressure-controlled ventilation to rest respiratory muscles improve sleep in ICU patients? Respir Med. 2013;107:534–541. doi: 10.1016/j.rmed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9:464–468. [PubMed] [Google Scholar]
- 39.Sanders RD, Maze M. Contribution of sedative-hypnotic agents to delirium via modulation of the sleep pathway. Can J Anaesth. 2011;58:149–156. doi: 10.1007/s12630-010-9421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacas S, Degos V, Feng X, et al. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


