Abstract
HIV penetrates the central nervous system (CNS), and although it is clear that microglia and to a lesser extent astrocytes are infected, whether certain other cell types such as neurons are infected remains unclear. Here, we confirmed the finding that RNAs of both cellular and viral origins are present in native HIV-1 particles and exploited this phenomenon to directly examine HIV-1 infectivity of CNS cell types. Using in vitro transcribed mRNAs that were labeled with a fluorescent dye, we showed that these fluorescent mRNAs were packaged into HIV-1 particles by directly examining infected cells using fluorescence microscopy. Cells in culture infected with these labeled HIV-1 particles showed the fluorescent signals of labeled mRNAs by a distinct pattern of punctate, focal signals within the cells which was used to demonstrate that the CXCR4-tropic NL4-3 strain was able to enter microglia and to a lesser extent astrocytes, but not neurons. The strategy used in the present study may represent a novel approach of simplicity, robustness and reliability for versatile applications in HIV studies, such as the determination of infectivity across a broad range of cell types and within subpopulations of an individual cell type by direct visualization of viral entry into cells.
Keywords: HIV-1, RNA, Fluorescent dye, Viral packaging, Central nervous system
1. Introduction
Retroviruses, including human immunodeficiency virus (HIV), replicate through a DNA proviral intermediate which is integrated into the host cell genome. Transcription of this proviral DNA gives rise to a primary, full-length transcript that acts as genomic RNA for packaging into progeny virions. Retroviruses selectively package two copies of viral genomic RNA per viral particle, which is mediated by the viral structural protein Gag. In addition to the viral genomic RNA, diverse retroviruses have been found to contain RNA species of both cellular and viral origins (Muriaux et al., 2001).
HIV type-1 (HIV-1)-spliced RNAs have been readily detected within infectious, native HIV-1 particles and these transcripts can be reverse-transcribed in both cells and virions (Houzet et al., 2007a,b; Liang et al., 2004). Many cellular RNAs of high abundance such as Y RNAs, 7SK and 7SL RNAs, 5S rRNA, U6 snRNA and GAPDH and β-actin mRNAs have also been found in HIV-1 particles (Tian et al., 2007). These results support the notion that HIV-1 packages various viral and cellular RNAs beyond the genomic viral RNA. There is also evidence that some RNA species such as 7SL and U6 RNAs are packaged into HIV-1 particles at a much higher efficiency than others (Didierlaurent et al., 2011; Tian et al., 2007), which implies HIV-1 uses both selective and passive RNA packaging mechanisms.
It is well known that HIV-1 preferentially infects cells of the human immune system such as CD4+ T cells, macrophages and dendritic cells. However, HIV infection causes diverse neurologic disorders including sensory neuropathy, myelopathy and HIV dementia and cognitive/motor disorder which are getting worldwide attention in HIV patients, collectively termed neuroAIDS (Power et al., 2009). Nonetheless, our understanding on HIV-1 infectivity of particular central nervous system (CNS) cell types has remained elusive. Due to the limited availability of primary human CNS cells, the high degree of phenotypic diversity within individual CNS cell types and the difficulty of maintaining them in culture, the current knowledge on HIV-1 infectivity of CNS cell types has been largely derived using indirect methods for examining the post-mortem brain tissue of HIV patients such as immunohistochemistry. Therefore, the development of methods to directly examine HIV-1 infectivity of limited numbers of individual cell types in culture may provide results that are more clear than those from techniques used to analyze whole human brain tissue. Microglia represent the resident macrophages of the brain and spinal cord, and are thus believed to serve as the principle target of HIV-1 infection in the CNS. Immunohistochemical examination of brain tissue from HIV patients supports that HIV-1 also infects astrocytes, although this cell type is infected at a much lower frequency than microglia and infection of astrocytes may depend more on the cellular environmental conditions (Carroll-Anzinger and Al-Harthi, 2006; Churchill et al., 2006, 2009; Gorry et al., 2003; Li et al., 2011; Messam and Major, 2000). However, it remains controversial whether HIV-1 infects neurons, although it is generally believed that neurons are not infected (reviewed in Kramer-Hammerle et al., 2005; Verma et al., 2010).
HIV entry into the cell is the beginning of the infectious process. In the present study, we first confirmed the diverse RNA packaging phenomenon of HIV-1, and demonstrated that fluorescently-labeled mRNAs from various species were able to be packaged into HIV-1 particles when used to directly examine infected HeLa MAGI-CCR5 cells by fluorescence microscopy. We then employed our strategy to examine HIV-1 infectivity across different primary human CNS cell types in culture by direct visualization of viral entry into these cells. We were able to readily observe fluorescent signals in microglia and astrocytes, but not neurons. We suggest that fluorescently-labeled RNA packaging by HIV-1 for direct visualization of viral entry represents a novel and simple approach to reliably determine infectivity across various cell types and within subpopulations of an individual cell type. Moreover, this method might be further developed to extend to other applications.
2. Materials and methods
2.1. Plasmids, cells and viruses
CXCR4-tropic HIV-1 NL4-3 proviral DNA plasmid was kindly provided by Dr. Fatah Kashanchi (George Mason University; Manassas, VA, USA). HeLa MAGI-CCR5 cells were obtained through the NIH AIDS Research and Reference Reagent Program (catalog #3522), Division of AIDS, NIAID: MAGI-CCR5 from Dr. Julie Overbaugh (Chackerian et al., 1997). Primary human microglia (catalog 1900-f1), astrocytes (catalog 1800) and neurons (catalog #1520) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured according to the manufacturer’s instructions. HIV-1 strain IIIB (HIV-1IIIB) was purchased from Advanced Biotechnologies (Columbia, MD, USA) where the virus was propagated in H9 cells and prepared as: (1) direct pelleted virus (catalog 10-124-000) concentrated from cell culture supernatant by centrifugation without further purification, or (2) purified virus (catalog 10-118-100) clarified from culture supernatant that was concentrated and cleaned of debris by ultracentrifugation and extraction of the viral band from a sucrose density gradient; both of these preparations contain infectious virus.
2.2. RT-PCR
Total RNA was isolated using the miRNeasy Mini Kit (Qiagen; Valencia, CA, USA). Following DNase treatment of RNA, reverse transcription was conducted using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Foster City, CA, USA). RT-PCR reactions were performed in a total volume of 20 μL containing SensiMix™ SYBR qPCR reagents (Bioline USA; Tauton, MA, USA) using a Corbett Rotor-Gene 6000 real-time PCR system (Qiagen). Sequences of the primer sets used for RT-PCR are listed in Table 1, and these primers were designed to span introns whenever possible. For detection of packaged RNAs in native HIV-1 particles (Fig. 1), RT-PCR conditions consisted of an initial hold step at 95 °C for 10 min, followed by 5 cycles (95 °C for 5 s, 62 °C/−1 °C each cycle for 10 s, 72 °C for 20 s) and 25 cycles (95 °C for 5 s, 58 °C for 10 s, 72 °C for 20 s). For detection of HIV-1 genomic RNA in supernatants from HEK-293T cells transfected with proviral DNA plasmid with and without fluorescently-labeled mRNAs (Fig. 2B), RT-PCR conditions consisted of an initial hold step at 95 °C for 10 min, followed by 5 cycles (95 °C for 5 s, 62 °C/−1 °C each cycle for 10 s, 72 °C for 20 s) and 35 cycles (95 °C for 5 s, 58 °C for 10 s, 72 °C for 20 s). The specificity of the amplified products was determined by melting curve analysis followed by agarose gel electrophoresis and sequencing. The agarose gels were stained with ethidium bromide, and images were taken using a Kodak Image Station 440 (Kodak; Rochester, NY, USA).
Table 1.
Primer sequences used for RT-PCR and cDNA cloning.
| Target RNA | Forward primer | Reverse primer | Amplicon (bp) |
|---|---|---|---|
| HIV-1 gRNA | 5′-TGTGTGCCCGTCTGTTGTGT-3′ | 5′-GAGTCCTGCGTCGAGAGAGC-3′ | 143 |
| HIV-1 Tat 1-86 | 5′-GGAAGCATCCAGGAAGTCAGCCTA-3' | 5′-GGGTTGGGAGGTGGGTTGCTT-3' | 170 |
| 7SL | 5′-ATCGGGTGTCCGCACTAAG-3′ | 5′-CACCCCTCCTTAGGCAACCT-3′ | 81 |
| Y4 | 5′-AAGCCAGTCAAATTTAGCAGTGGG-3′ | 5′-AAGCCAGTCAAATTTAGCAGTGGG-3′ | 93 |
| Y1 | 5′-GGCTGGTCCGAAGGTAGTGA-3′ | 5′-AAAAGACTAGTCAAGTGCAGT-3′ | 113 |
| GAPDH | 5′-CATGGCACCGTCAAGGCTGAGAA-3′ | 5′-CAGTGGACTCCACGACGTACTCA-3' | 130 |
| β-Actin | 5′-CTGGCACCACACCTTCTACAATGA-3′ | 5′-GCTGGGGTGTTGAAGGTCTCAAA-3′ | 137 |
| EEF2 | 5′-ATCCGCGCCATCATGGACAAGAA-3' | 5′-TCGTCCTTCCGGGTATCAGTGAA-3' | 164 |
| EndoG | 5′-AGGCCATGGACGACACGTTCTAC-3′ | 5'-GAAGAGTGGCCCTGTGCAGACATA-3' | 146 |
| CPSF100 | 5′-GCCATCAGCTCATAAGACGAAGCAT-3′ | 5′-AGTAGC TTGAAGCTCTGGCACTAAG-3' | 187 |
| aHIV-1 Tat1-86 (full-length) | 5′-ATGGAGCCAGTAGATCCTAGA-3′ | 5′-CTATTCCTTCGGGCCTGTC-3′ | 261 |
| amEndoG | 5′-CACCATGGACGACACCTTCTACC-3′ | 5′-CTTGCTGCCAGCAGTGATAGC-3′ | 432 |
| (C-terminal) |
Primers for cDNA cloning.
Fig. 1.
RT-PCR detection of RNAs packaged within native HIV-1 particles. Total RNA was isolated from pelleted and purified native HIV-1 infectious particles, where viral particles (equivalent of ~650 ng p24) were also treated with RNase in the presence or absence of Triton X-100 for 1 h at 37 ° C in a total volume of 100 μL prior to RNA extractions as indicated. Total RNA was then reverse-transcribed using random hexamer primers to generate cDNA templates used for RT-PCR detection of the indicated RNAs, which included HIV-1 genomic RNA (gRNA), fully-spliced viral mRNA (Tat) and cellular small RNAs and mRNAs. Primer sequences and RT-PCR product sizes are listed in Table 1. RT-PCR products were detected using 2% agarose gels stained with ethidium bromide. For RT-PCR reaction controls, reverse transcription of DNase-treated RNA without the addition of reverse transcriptase was input to serve as a negative control (NTC, no cDNA template control), and HIV-1IIIB Tat1-86 full-length cDNA plasmid (pMax-Tat) and cDNA from primary human astrocytes were used to serve as positive controls for the amplification of Tat and cellular RNAs, respectively. Marker (M) indicates 100 base pair (bp) DNA ladder marker where the 100, 200 and 300 bp markers are shown.
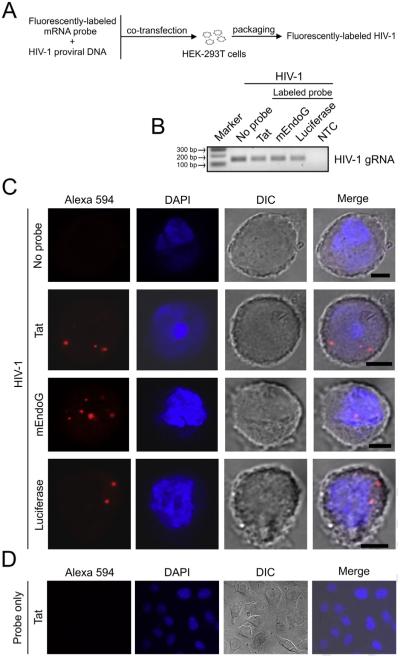
Fig. 2.
Labeling of HIV-1 particles via packaging of fluorescent mRNAs and the pattern within infected cells. (A) Flow diagram showing the steps for preparing labeled HIV-1 by packaging of fluorescent mRNA probes. Probes were prepared by in vitro transcription using plasmid cDNAs and the resulting mRNAs were labeled by covalent conjugation with the Alexa Fluor® 594 red-fluorescent dye. Fluorescently-labeled mRNA probes and a proviral DNA plasmid encoding the HIV-1 NL4-3 clone were co-transfected into HEK-293T cells, resulting in the packaging of labeled mRNAs into progeny virions during HIV-1 particle assembly. (B) RT-PCR detection of HIV-1 genomic RNA (gRNA) in the supernatants of cells transfected as described in A. HIV-1 particles were generated with or without labeled mRNA probes (no probe). Three labeled mRNA probes of different origins were made and packaged into virus: full-length HIV-1 Tat1-86 mRNA, and mRNAs from the C-terminal portions of mouse EndoG (mEndoG) and firefly luciferase. For an RT-PCR reaction control, reverse transcription of DNase-treated RNA without the addition of reverse transcriptase was input to serve as a negative control (NTC, no cDNA template control). Marker indicates 100 base pair (bp) DNA ladder marker where the 100, 200 and 300 bp markers are shown. (C) Confocal images of Z-stack sections showing a distinct fluorescent pattern of punctate, focal signals within HeLa MAGI-CCR5 cells infected by the indicated probe-labeled (red) or unlabeled HIV-1 particles. Representative cells are shown. DAPI (blue) staining indicates cell nuclei. DIC (differential interference contrast) images show cell shape. Note that the size of the fluorescent spots does not correspond to the diameter of a viral particle but reflects scattering of photons in the detector. Scale bar = 5 μm. (D) Representative field in which labeled Tat mRNA alone was added to HeLa MAGI-CCR5 cells for 24 h in culture imaged at 63× magnification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. In vitro transcription and labeling of mRNA
The full-length coding cDNA fragment (261 bp) of HIV-1IIIB Tat1-86 mRNA and the C-terminal coding cDNA fragment (432 bp) of mouse EndoG mRNA were prepared by PCR with the primers indicated in Table 1, while the C-terminal coding cDNA fragment (423 bp) of firefly luciferase mRNA was provided with the pGEM-T-easy vector system (Promega; Madison, WI, USA); these cDNA fragments were cloned into the pGEM-T-easy vector. The insert and direction in the resulting plasmids were confirmed by sequencing, and plasmid DNA was prepared using the Hipure Plasmid Filter Midiprep Kit (Invitrogen; Grand Island, NY, USA). Plasmids were linearized by restriction enzyme digestion, and in vitro transcription was performed to produce sense messenger RNA followed by covalently conjugating the Alexa Fluor® 594 fluorescent dye to the mRNAs using the FISH Tag™ RNA Red Kit (Invitrogen; catalog F32954) according to the manufacturer’s instructions.
2.4. Packaging of fluorescently-labeled RNA into HIV-1 particles
HEK-293T cells (GenHunter; Nashville, TN, USA; catalog Q401) were co-transfected with HIV-1 NL4-3 proviral DNA plasmid and each fluorescently-labeled mRNA at a ratio of 1 μg to 0.6 μg, respectively, corresponding to molar ratios of 1:65 (Tat), 1:39 (EndoG) and 1:40 (luciferase), using Lipofectamine 2000 reagent (Invitrogen). After 48 h, the cell culture supernatants containing virus were collected, subjected to 1 freeze-thaw cycle and centrifuged at 13,200 r.p.m. for 5 min to remove cells and debris. Virus-containing supernatants were stored in aliquots at −80 °C.
2.5. HIV-1 treatments and fluorescence microscopy
Cells were cultured in 4-well glass chamber slides coated with poly-l-lysine. After overnight culture, cells were incubated with fluorescent mRNA-labeled HIV-1 for 24 h at 37 °C (24 h was chosen as the time point to ensure that all cell types had an ample opportunity to infect). Cells were then fixed in 3.7% paraformaldehyde/PBS for 15 min. After additional washing in PBS, coverslips were mounted onto the chamber slides using ProLong® Gold antifade reagent containing DAPI (Invitrogen). Samples were imaged using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss; Thornwood, NY, USA). Images were edited with ImageJ (NIH; Bethesda, MD, USA) and Adobe Photoshop (Adobe Systems Incorporated; San Jose, CA, USA) software. For a control experiment, fluorescently-labeled Tat mRNA alone was added to cells grown in 4-well glass chamber slides at an amount of 0.1 μg per well and incubated for 24 h at 37 °C, followed by fixation and image recording using a Zeiss Axio Observor Z1 microscope system (Carl Zeiss). For co-detection of labeled mRNA in HIV-1-infected cells, treated cells were fixed with 3.7% paraformaldehyde/PBS and detection of infection was performed using the QuantiGene® ViewRNA ISH Cell Assay (Affymetrix; Santa Clara, CA, USA; catalog QVC0001) according to the manufacturer’s instructions. Probes were designed to target mRNA within the coding region of gag from the HIV-1 pNL4-3 clone (Affymetrix; catalog VF4-14763) and FITC was used as the label for detection. Following cell nuclei staining with DAPI, samples were imaged using a Zeiss LSM 700 laser scanning confocal microscope. For capturing enlarged fields of cells, tile scans were used. Images were collected with ZEN 2009 Light Edition software (Carl Zeiss) and edited using Adobe Photoshop software (Adobe Systems Incorporated).
2.6. Immunocytochemistry
For immunolabeling of CNS cell-type-specific markers, cells cultured in 4-well glass chamber slides were fixed with 3.7% paraformaldehyde/PBS, permeabilized using 0.5% Triton X-100, immunolabeled and cell nuclei stained with DAPI. Primary antibodies were directed to GFAP (Millipore; Billerica, MA, USA; catalog AB5804), Iba1 (Wako; Richmond, VA, USA; catalog 019-19741) and MAP2 (Abcam; Cambridge, MA, USA; catalog ab32454), all used at a 1:200 dilution. Primary antibodies were visualized with an Alexa Fluor® 488 dye-conjugated secondary antibody (Invitrogen; catalog A-11034) used at a 1:500 dilution. Samples were imaged using a Zeiss LSM 700 laser scanning confocal microscope. Images were collected with ZEN 2009 Light Edition software (Carl Zeiss) and edited using Adobe Photoshop software (Adobe Systems Incorporated).
2.7. Statistics
Statistical analysis was performed by two-way ANOVA with Bonferroni’s post-hoc test using GraphPad Prism 5 (GraphPad Software; La Jolla, CA, USA). A value of P < 0.05 was considered significant.
3. Results
3.1. Detection of fully-spliced Tat and cellular RNAs in native HIV-1 infectious particles
HIV-1 has been recently reported to package spliced viral and cellular RNAs into virions, in addition to the two copies of viral genomic RNA (Didierlaurent et al., 2011; Houzet et al., 2007b; Tian et al., 2007). To confirm these findings, we extracted total RNA from pelleted and purified native HIV-1 infectious particles, and performed PCR detection after reverse transcription (RT-PCR). As listed in Table 1, we used a pair of primers designed from the non-coding region of HIV-1 genomic RNA to detect viral genomic RNA (gRNA); a pair of primers designed to the cross-splicing junctions of HIV-1 Tat1-86 mRNA to detect fully-spliced Tat mRNA; and a set of primer pairs to detect cellular RNAs including abundant Pol III-transcribed 7SL, Y1 and Y4 RNAs and Pol II-transcribed GAPDH, β-actin, EEF2 (eukaryotic translation elongation factor 2), EndoG (endonuclease G) and CPSF100 (cleavage and polyadenylation specificity factor 100 kDa subunit) mRNAs with different abundances in cells. These RNAs, except EndoG and CPSF100 (not shown) mRNAs, were readily detected in both pelleted and purified native HIV-1 particles (Fig. 1, lanes 1 and 7; Y1 not shown).
To better examine RNAs that are packaged within virions, native viral particles were also subjected to RNase treatment to eliminate RNAs outside of virions, and RNase treatment with the addition of the non-ionic detergent Triton X-100 to disrupt the viral membrane prior to total RNA extractions as reported previously (Tian et al., 2007). Under regimens of 10 μg/mL or 1000 μg/mL RNase treatment most of these RNAs remained detectable (Fig. 1, lanes 2, 3, 8 and 9), suggesting their resistance to degradation by RNase, and attributed to inclusion within virions and protection by the viral membrane. In contrast, under regimens of RNase treatment plus the addition of Triton X-100 essentially all of these RNAs, including HIV-1 genomic RNA, were eliminated from the viral particles supporting their residence within virions (Fig. 1, lanes 4, 5, 6, 10 and 11), and which seemed to occur more readily with purified than pelleted virus possibly due to less interfering substances present from the purification process. However, RNAs of viral origin (HIV-1 gRNA and HIV-1 Tat) and host 7SL RNA seemed more resistant to RNase treatment. As these viral RNAs and host 7SL transcripts have been reported to be components of native HIV-1 particles that are packaged in a non-random manner and Gag is a major contributor to their packaging selectivity (Didierlaurent et al., 2011; Tian et al., 2007), the binding of Gag protein to these RNAs could increase their resistance to RNase treatment. Overall, these results support prior findings that packaging of RNAs other than the viral genomic RNA occurs commonly in HIV-1.
3.2. Packaging and delivery of fluorescently-labeled RNAs by HIV-1 infection
HIV entry into the cell may mark the capability of infection for a specific cell type. To investigate HIV-1 infectivity across individual cell types, we prepared mRNAs labeled with the Alexa Fluor® 594 red-fluorescent dye and then incorporated these fluorescent probes into HIV-1 particles using the phenomenon of viral RNA packaging by co-transfecting labeled mRNAs and HIV-1 NL4-3 proviral DNA plasmid into HEK-293T cells (Fig. 2A). These mRNAs were derived from widely different species, including the C-terminal parts of mouse EndoG and firefly luciferase, as well as HIV-1 Tat. Virus production was verified by RT-PCR detection of HIV-1 genomic RNA (gRNA) in the supernatants of transfected cells (Fig. 2B), while supernatant from mock-transfected cells produced no detectable signal (data not shown). These labeled virions were then used to infect cells in culture and directly examine whether the mRNA probes were delivered into cells by fluorescence microscopy. We first tested the ability of these labeled virions to infect HeLa MAGI-CCR5 cells which are established as being infectable by HIV-1 and are engineered to express the HIV-1 receptor CD4 and the CCR5 coreceptor (Deng et al., 1996; Kimpton and Emerman, 1992), as well as endogenously expressing CXCR4 (Sakamoto et al., 2003). Infected cells were fixed and confocal microscopy was used to examine the fluorescent pattern by 3-dimensional analysis of optical sections through the cell body. Similar distinct punctate, focal patterns of fluorescence within the cell body were observed for HIV-1 infection using all three viruses labeled with fluorescent mRNAs from the different species (Fig. 2C, bottom three panels). In contrast, we did not observe fluorescent signals in HeLa MAGI-CCR5 cells which were infected by HIV-1 NL4-3 generated in parallel without the co-transfection of any labeled mRNA (Fig. 2C, top panel), or in HEK-293 cells (which do not express the HIV-1 receptors) treated with labeled virus (data not shown). Furthermore, addition of labeled Tat mRNA alone to HeLa MAGI-CCR5 cells produced no signal (Fig. 2D), indicating that these mRNAs are unable to enter cells independently of virus. These results demonstrate the packaging and delivery of labeled RNAs by HIV-1 during infection and that RNAs of different origins can be successfully packaged into viral particles.
3.3. Labeled RNA delivered by HIV-1 infection is found in cells with viral mRNA
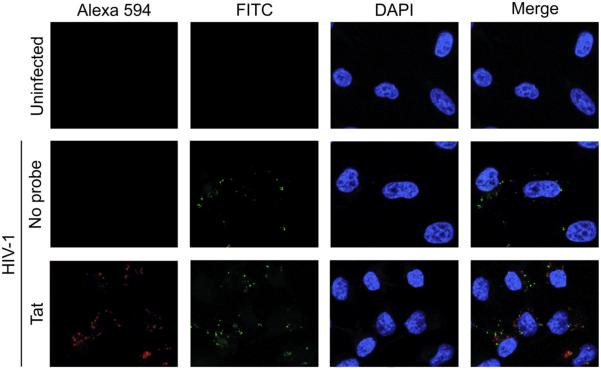
To further characterize whether the fluorescent signals that we observed from labeled virions were associated with infected cells, we performed a fluorescence in situ hybridization assay to co-detect the presence of Gag mRNA from HIV-1 NL4-3 in HeLa MAGI-CCR5 cells treated with the fluorescent Tat mRNA probe-labeled virus. As expected, no fluorescent signals were observed in uninfected cells while cells infected with unlabeled virus only showed signals associated with Gag mRNA that were detected using FITC green-fluorescent probes (Fig. 3). However, cells infected with labeled virus in which the fluorescent Tat mRNA was observed also showed signals from Gag. These results demonstrate that the fluorescent signals from our labeled viruses occur in cells that are HIV-1-infected.
Fig. 3.
Viral mRNA is present in cells infected with labeled HIV-1. HeLa MAGI-CCR5 cells were infected using HIV-1 packaged with the fluorescently-labeled Tat mRNA probe (red) or no probe, or left uninfected. The presence of HIV-1 Gag mRNA was detected by a fluorescence in situ hybridization assay using FITC-conjugated probes (green). Representative fields imaged at 63× magnification are shown. DAPI (blue) staining indicates cell nuclei. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Characterization of CNS cell types
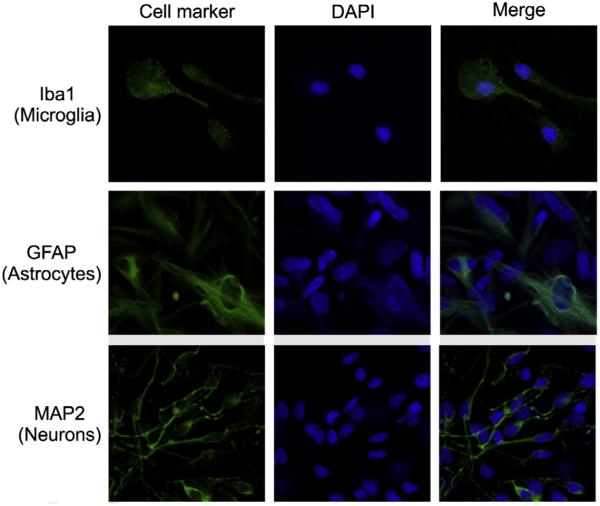
The identity and purity of primary human CNS cells in culture were confirmed by immunocytochemistry using antibodies recognizing cell-type-specific markers: ionized calcium binding adapter molecule 1 (Iba1) for microglia (Imai et al., 1996), glial fibrillary acidic protein (GFAP) for astrocytes (Eng et al., 2000) and microtubule-associated protein 2 (MAP2) for neurons (Izant and McIntosh, 1980). Characteristic patterns of expression were observed with the respective markers for all three cell types (Fig. 4). Secondary antibody alone was used as a negative control to subtract background noise and cross-reactivity experiments for each primary antibody with the other cell types did not produce any signals (data not shown).
Fig. 4.
CNS cell-type characterization. Primary human microglia, astrocytes and neurons were obtained commercially, and cell identity and purity were further determined in culture by immunocytochemistry using antibodies to cell-type-specific markers (green) which were Iba1, GFAP and MAP2 for microglia, astrocytes and neurons, respectively. Representative fields imaged at 63× magnification are shown. DAPI (blue) staining indicates cell nuclei. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Examination of HIV-1 infection across CNS cell types using virions containing labeled RNA
Having established our labeled RNA packaging strategy for the determination of HIV-1 infection using HeLa MAGI-CCR5 cells, we next examined whether our method would easily provide direct evidence of HIV-1 infectivity across primary CNS cell types. As microglia are the principle target of HIV infection in the CNS, this cell type should provide results similar to the engineered HeLa MAGI-CCR5 cells. As expected, a distinct punctate, focal pattern of fluorescence was observed within microglial cells infected by the fluorescent Tat mRNA probe-labeled virus (Fig. 5, bottom three panels). The bottom three panels are continuous sections of a Z-stack image from an infected cell, clearly showing fluorescent signals within the cell body due to entry or internalization of labeled virus. No fluorescent signals were observed within microglia infected by unlabeled virus (Fig. 5, top panel). Similar patterns of fluorescence were observed within microglial cells infected by viruses labeled with the fluorescent non-viral mouse EndoG and firefly luciferase mRNA probes (data not shown).
Fig. 5.
Infection of microglia by HIV-1 labeled via packaging of fluorescent mRNA probes. HIV-1 packaged with the fluorescently-labeled Tat mRNA probe (red), or no probe, were used to infect primary human microglia in culture, followed by cell fixing and confocal imaging. Representative cells are shown. DAPI (blue) staining indicates cell nuclei. DIC (differential interference contrast) images show cell shape. To demonstrate that the fluorescent signals were located inside cells, three continuous images of a Z-stack series for a single infected cell are presented. Scale bar = 5 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
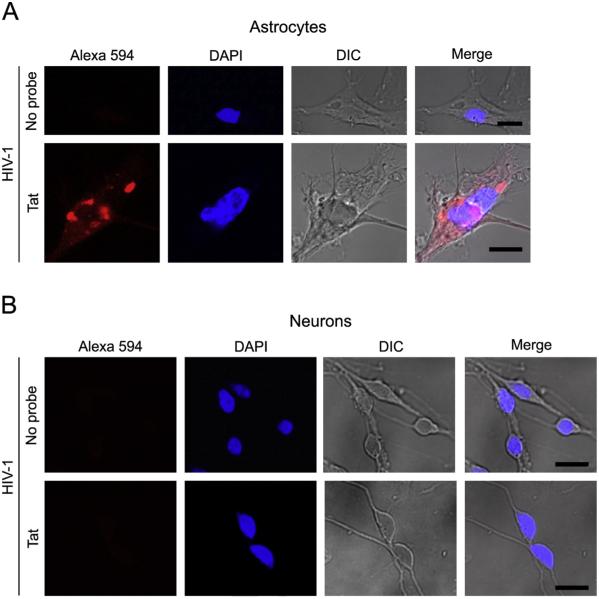
Next, we treated astrocytes with the fluorescent Tat mRNA probe-labeled virus and obtained a signal pattern of fluorescence similar to the infected microglia, indicating HIV-1 infection of astrocytes (Fig. 6A). Similar fluorescent patterns were also observed in astrocytes upon treatment with viruses labeled using the fluorescent mouse EndoG and firefly luciferase mRNA probes (data not shown). In contrast, we did not observe any neurons harboring fluorescent signals when treated with the fluorescent Tat mRNA probe-labeled virus (Fig. 6B), or fluorescent mouse EndoG and firefly luciferase mRNA probe-labeled viruses (data not shown), indicating that HIV-1 did not gain entry into neurons.
Fig. 6.
Treatment of astrocytes and neurons with HIV-1 labeled via packaging of fluorescent mRNA probes. HIV-1 packaged with the fluorescently-labeled Tat mRNA probe (red), or no probe, were used to treat primary human (A) astrocytes and (B) neurons in culture, followed by cell fixing and confocal imaging. Representative cells are shown. DAPI (blue) staining indicates cell nuclei. DIC (differential interference contrast) images show cell shape. Images were from Z-stack sections. Scale bar = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Efficiency of labeled HIV-1 infection across cell types
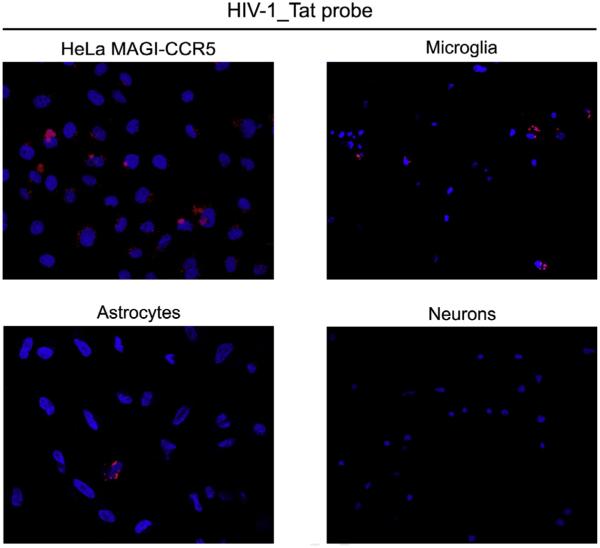
To assess the infection efficiency across the cell types examined, we evaluated enlarged fields of cells that were treated with the fluorescent Tat mRNA probe-labeled virus (Fig. 7), and the fluorescent mouse EndoG and firefly luciferase mRNA probe-labeled viruses (data not shown). The fields demonstrated that the rate of infection was consistent among multiple cells of a given cell type. Manual counting from these fields was used to quantify the number of infected cells (Table 2). We found for the CNS cell types where infection was observed that there was an expected difference in which the average infection rate of astrocytes with all three labeled viruses was much lower (~ 9-fold) and significantly different (P < 0.001 for Tat, P < 0.01 for mEndoG and P < 0.001 for luciferase) compared to microglia (Table 2). These data support that our labeling strategy can be used to determine differences in HIV-1 infectivity across individual cell types.
Fig. 7.
Enlarged fields of cell types treated with labeled HIV-1. Fields of the indicated cell types treated with the fluorescent Tat mRNA probe-labeled virus (red) are shown that were captured using tile scans at 63× magnification. DAPI (blue) staining indicates cell nuclei. Images are representative of those used to generate the data presented in Table 2. The infection rates were ~9% for HeLa MAGI-CCR5 cells, ~22% for microglia and ~2% for astrocytes while no fluorescent signals were observed in any neurons (Table 2). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Fluorescently-labeled HIV-1 infection rates of HeLa and primary CNS cell types.
| Cell type | HIV-1 infection % labeled with various fluorescent mRNAsa |
||
|---|---|---|---|
| Tat (%) | mEndoG (%) | Luciferase (%) | |
| HeLa MAGI-CCR5 | 9.3 ± 4.0 | 11.2 ± 3.3 | 17.2 ± 5.9 |
| Microglia | 21.8 ± 9.1 | 21.1 ± 6.8 | 23.7 ± 2.0 |
| Astrocytes | 1.7 ± 0.9 | 2.5 ± 0.6 | 2.9 ± 1.3 |
| Neurons | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Data are the average infection percentage ± the standard deviation for two independent experiments consisting of two lots of primary human cells from different individuals where each lot was treated with a separate preparation of each labeled virus. At least 75 cells were counted for neurons, and at least 100 cells were counted for all other cell types in each replicate. F(3,12) = 37.98, P < 0.0001 for the effect between cell types; F(2,12) = 1.04, P = 0.3835 for the effect between labeled viruses; and F(6,12) = 0.42, P = 0.8487 for the interaction between cell types and labeled viruses.
4. Discussion
In the present study, we verified and employed the phenomenon of RNA packaging into retroviruses to produce fluorescently-labeled HIV-1 particles that were used to examine HIV infectivity across cell types by direct visualization of viral entry into cells. We demonstrated that HIV-1 labeled by packaging of various fluorescent mRNAs from different species entered into HeLa MAGI-CCR5 cells, resulting in distinct punctate, focal patterns of fluorescence within this cell line in culture. We then examined whether these labeled virions could clearly discern HIV-1 infectivity across primary human microglia, astrocytes and neurons from the CNS in culture. Our results were in agreement with previous reports that indirectly found HIV-1 infection in macrophage-lineage cells as well as astrocytes (which are not macrophage lineage), but not neurons, from the brain tissue of human subjects with neuroAIDS using p24 immunohistochemistry and PCR detection of HIV-1 DNA (Churchill et al., 2006; Churchill et al., 2009).
HIV entry into the cell may serve as the key rate-limiting step of infection. Therefore, developing strategies to directly visualize viral entry is of tremendous importance for characterizing the infectivity of different cell types in culture, and especially primary human cells with limited availability. Additionally, techniques to assess the heterogeneity of infected cells within the population of an individual cell type require the ability to directly examine and sort cells at the single-cell level, which could not be achieved using current methods to determine HIV-1 infection in whole cell populations such as ELISAs, reverse transcriptase assays, and PCR detection and quantification. Previously, co-transfection of HEK-293T cells with HIV-1 NL4-3 proviral DNA plasmid and an expression vector encoding a Vpr-GFP fusion protein has been employed to generate GFP-labeled virions for studying HIV-1 binding and entry in infection (El-Hage et al., 2011; Schaeffer et al., 2001, 2004). Per our own practice of using GFP-labeled virus, however, autofluorescence similar to GFP signals is a common issue within cells which may require careful discrimination of signals. For example, microscopes equipped with multispectral imaging readily permit separation of GFP and autofluorescent signals in living cells, but are less widely available in laboratories studying infectious HIV-1. Furthermore, it is unknown how the size of the 27 kDa GFP molecule could interfere with native virion assembly, structure and/or biological function. In comparison to fluorescent proteins, a variety of small fluorescent dyes can be selectively used to label RNAs with a high degree of signal specificity, and we were able to obtain distinct fluorescent patterns of punctate, focal signals with little background noise within infected cells.
Retroviruses package non-genomic viral RNAs which are believed to be important for retroviral particle assembly (Campbell and Vogt, 1995; Muriaux et al., 2001). Native HIV-1 particles have been recently found to also contain various cellular RNAs that are present with higher abundances in cells including Pol III-transcribed RNAs (7S, Y and U6) and Pol II-transcribed GAPDH and β-actin mRNAs (Houzet et al., 2007b; Tian et al., 2007). Consistent with these findings, we confirmed the presence of 7SL, Y1 (not shown) and Y4 RNAs, as well as GAPDH and β-actin mRNAs in native HIV-1 particles. In addition, we were able to detect mRNA for EEF2, a protein involved in translation, but not for EndoG, which is a nuclear-encoded mitochondrial protein, and CPSF100 (not shown), a protein functioning in mRNA 3’-end processing. As GAPDH and β-actin mRNAs were reported to be packaged into native HIV-1 particles less efficiently than other RNA species such as 7SL RNA, their presence in virions has been attributed to random or passive packaging by the virus (Tian et al., 2007). It was also shown that 7SL and U6 RNAs, but not GAPDH mRNA, were selectively encapsidated into native HIV-1 particles (Houzet et al., 2007b). HIV-1 Gag polyproteins interact with nucleic acids (Tian et al., 2007), supporting a more selective packaging mechanism of one RNA species over another due to differences in RNA sequence and/or structure. Therefore, both the abundance and selective packaging efficiency of RNAs may contribute to the extent that an RNA species is packaged into HIV-1. In the present study, we used co-transfection to initiate RNA packaging of fluorescently-labeled mRNAs into HIV-1, by which we can control the abundance of labeled mRNA and/or make a selection of mRNA sequence. Interestingly, all three fluorescently-labeled mRNAs, which were prepared by in vitro transcription of genes from widely different species (mouse, firefly and HIV-1), were successfully packaged into HIV-1 and visualized after infection of HeLa MAGI-CCR5 cells. While there were differences among the three mRNAs with respect to their sequence context and length, we did not observe any obvious difference between them for the purpose of labeling virus. As a relatively large amount of labeled mRNA was used for transfection, the high abundance may override the selective packaging efficiency attributed to differences in RNA sequence and/or structure. Overall, our results provide clear evidence that mRNAs of various origins can be packaged into HIV-1 during viral particle assembly. These findings also suggest that the cell milieu under varying conditions may differentially influence the complement of RNAs that are packaged into assembled, infectious HIV-1.
In native HIV-1 particles, low but significant amounts of singly- and fully-spliced viral mRNAs have been detected (Houzet et al., 2007a). In addition, spliced viral mRNAs are reverse transcribed as efficiently as the viral genomic RNA, both in cells and virions, which may contribute to viral DNA recombination within host cells beyond that of the intact HIV genome (Houzet et al., 2007a). HIV-1 fully-spliced mRNAs represent the majority of spliced transcripts, and these so-called early transcripts encode the viral regulatory proteins Tat and Rev which are essential for viral gene expression and infection (Houzet et al., 2007a). While we confirmed that fully-spliced Tat mRNA was present in native HIV-1 particles, packaged Tat mRNA seemed highly resistant to RNase treatment in the presence of Triton X-100, implying the existence of a protection mechanism possibly due to its binding with Gag and/or other unknown proteins within the virion. HIV injects its contents into host cells after membrane binding/fusion and viral entry. Thus, fully-spliced Tat mRNA contained in virions may be delivered into the cytoplasm of host cells right after viral entry for immediate translation of Tat protein. Tat is essential for HIV-1 replication and viral gene transcription, and it is also a potent transcriptional activator for many cellular genes (Johri et al., 2011). In this regard, packaging of fully-spliced Tat mRNA into viral particles may be of significant biological importance for the early stages of HIV-1 infection and worth further investigation.
HIV penetrates the CNS, leading to HIV-associated dementia (HAD) and encephalitis (HIVE) (Kolson et al., 1998). To address these complications, it is necessary to understand the infectivity of HIV-1 on different cell types and within sub-populations of an individual cell type in the CNS. Microglia have been reported to be immuno-positive for HIV in infected patients, and HIV replication in primary microglial cells has also been demonstrated in vitro (reviewed in Verma et al., 2010). While earlier studies concluded that astrocytes are latently infected by HIV-1 and do not produce progeny virus (Churchill et al., 2006; Gorry et al., 2003), a more recent report found astrocyte infection is extensive in subjects with HAD, occurring in up to 19% of GFAP-positive cells (Churchill et al., 2009), implying the extent of HIV infection in astrocytes can be different for patients under various conditions associated with the disease. It is still somewhat controversial regarding HIV infection of neurons, which is at least partly attributed to some inherent limits to demonstrate infection of this cell type, such as that neurons are unable to regenerate and tend to die in response to adverse stimuli. Nonetheless, so far neurons have not been found to be permissive for HIV infection (reviewed in Kramer-Hammerle et al., 2005; Verma et al., 2010). Thus, the current notion is that HIV does not infect neurons. Overall, these findings are in agreement with our results using fluorescent mRNA probe-labeled virus which showed HIV-1 infection of microglia and astrocytes, but not neurons.
HIV infection requires the expression of a receptor and/or coreceptor(s) on the cell surface. CD4 is the primary attachment receptor for the HIV-1 NL4-3 strain (Stauber et al., 1999), and the initial binding of HIV-1 to this receptor on CD4+ T lymphocytes has been found to be required for viral entry in both the fusion and endocytosis pathways (Schaeffer et al., 2004). HIV-1 envelope-mediated cell membrane fusion, which is a highly cooperative process depending on receptor density, conformation and envelope-receptor affinities (Doms, 2000), promotes the insertion of HIV cores into the cytoplasm and often leads to productive infection (Binley and Moore, 1997; Chan and Kim, 1998; Doms and Trono, 2000). In contrast, the endocytic pathway generally leads to non-productive infection due to virus inactivation in acidified endosomes or degradation in lysosomes (Marechal et al., 1998; Schaeffer et al., 2004; Stein et al., 1987). However, endocytosis may not uniformly lead to virus inactivation and degradation (Schaeffer et al., 2004). Interestingly, it was found that even latent infection resulted in global changes to astrocyte gene expression (Wang et al., 2004). Thus, we intend to suggest that internalization of HIV-1 into cells, either via the fusion or endocytosis pathways, should be appropriate to serve as a marker for the characterization of infection. Previous studies indicated that the CD4 antigen showed clear expression on microglia, and only negligible expression on astrocytes, but no expression on neurons (reviewed in Verma et al., 2010). We did not identify any neurons containing fluorescent signals after exposure to labeled virus, suggesting that neurons do not allow for internalization of HIV-1 by any entry pathway including fusion and endocytosis, most likely due to the lack of CD4 expression. Because we only used the CXCR4-tropic NL4-3 clone in our study, we recognize it is possible that different HIV-1 stains and tropisms could show varying effects on the infection rate of an individual cell type. The labeling method presented here should be applicable for use in combination with any proviral plasmid DNA, regardless of strain and tropism, for the future examination of these effects.
The utility of this labeling method may be further exploited to include studies on HIV entry and assembly, as well as determining infectivity using a wide variety of cell types, both inside and outside the CNS, under various conditions of growth, development and differentiation. In addition, this strategy may be optimized for high-throughput screening of drug resistance to HIV infection and possibly similar applications with other retroviruses. It may also extend to the development of clinical applications for delivering therapeutic RNAs to cells as well as labeling other membrane-bound structures released from cells that contain RNA such as exosomes.
5. Conclusions
In the present study, we developed a strategy to label HIV-1 from viral packaging of fluorescent mRNAs to show infection by direct visualization of virus entry into cells. This labeling method may be of great potential use for a series of applications in HIV studies due to its simplicity of production, robust signal discrimination and reliability across cell types.
Acknowledgements
We thank Pamela E. Knapp and Kurt F. Hauser for discussions regarding the data and assistance with editing the manuscript as well as for use of existing laboratory supplies and equipment from which part of the data were generated. NIH funding to support this study provided by F32 DA033898 to SMD and R01 DA036154 to NEH is gratefully acknowledged. Part of the confocal microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant 5P30 NS047463. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH or endorsement by any other individuals.
References
- Binley J, Moore JP. HIV-cell fusion. The viral mousetrap. Nature. 1997;387:346–348. doi: 10.1038/387346a0. [DOI] [PubMed] [Google Scholar]
- Campbell S, Vogt VM. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J. Virol. 2006;80:541–544. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian B, Long EM, Luciw PA, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Didierlaurent L, Racine PJ, Houzet L, Chamontin C, Berkhout B, Mougel M. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res. 2011;39:8915–8927. doi: 10.1093/nar/gkr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology. 2000;276:229–237. doi: 10.1006/viro.2000.0612. [DOI] [PubMed] [Google Scholar]
- Doms RW, Trono D. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 2000;14:2677–2688. doi: 10.1101/gad.833300. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host defenses. J. Virol. 2011;85:11601–11614. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr. HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Houzet L, Morichaud Z, Mougel M. Fully-spliced HIV-1 RNAs are reverse transcribed with similar efficiencies as the genomic RNA in virions and cells, but more efficiently in AZT-treated cells. Retrovirology. 2007a;4:30. doi: 10.1186/1742-4690-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Paillart JC, Smagulova F, Maurel S, Morichaud Z, Marquet R, Mougel M. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acids Res. 2007b;35:2695–2704. doi: 10.1093/nar/gkm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Izant JG, McIntosh JR. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc. Natl. Acad. Sci. U.S.A. 1980;77:4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri MK, Mishra R, Chhatbar C, Unni SK, Singh SK. Tits and bits of HIV Tat protein. Expert Opin. Biol. Ther. 2011;11:269–283. doi: 10.1517/14712598.2011.546339. [DOI] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J. Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Hu J, Russell RS, Kameoka M, Wainberg MA. Spliced human immunodeficiency virus type 1 RNA is reverse transcribed into cDNA within infected cells. AIDS Res. Hum. Retroviruses. 2004;20:203–211. doi: 10.1089/088922204773004923. [DOI] [PubMed] [Google Scholar]
- Marechal V, Clavel F, Heard JM, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Major EO. Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue. J. Neurovirol. 2000;6(Suppl. 1):S90–S94. [PubMed] [Google Scholar]
- Muriaux D, Mirro J, Harvin D, Rein A. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5246–5251. doi: 10.1073/pnas.091000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Boisse L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can. J. Neurol. Sci. 2009;36:285–295. doi: 10.1017/s0317167100007009. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Ushijima H, Okitsu S, Suzuki E, Sakai K, Morikawa S, Muller WE. Establishment of an HIV cell–cell fusion assay by using two genetically modified HeLa cell lines and reporter gene. J. Virol. Methods. 2003;114:159–166. doi: 10.1016/j.jviromet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E, Soros VB, Greene WC. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 2004;78:1375–1383. doi: 10.1128/JVI.78.3.1375-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber RH, Rulong S, Palm G, Tarasova NI. Direct visualization of HIV-1 entry: mechanisms and role of cell surface receptors. Biochem. Biophys. Res. Commun. 1999;258:695–702. doi: 10.1006/bbrc.1999.0511. [DOI] [PubMed] [Google Scholar]
- Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Tian C, Wang T, Zhang W, Yu XF. Virion packaging determinants and reverse transcription of SRP RNA in HIV-1 particles. Nucleic Acids Res. 2007;35:7288–7302. doi: 10.1093/nar/gkm816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AS, Singh UP, Dwivedi PD, Singh A. Contribution of CNS cells in NeuroAIDS. J. Pharm. Bioallied Sci. 2010;2:300–306. doi: 10.4103/0975-7406.72129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J. Neurovirol. 2004;10(Suppl. 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]