Abstract
Atherosclerosis is the most life-threatening pathology worldwide. Its major clinical complications, stroke, myocardial infarction, and heart failure, are on the rise in many regions of the world—despite considerable progress in understanding cause, progression, and consequences of atherosclerosis. Originally perceived as a lipid-storage disease of the arterial wall (Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. August Hirschwald Verlag Berlin, [1871]), atherosclerosis was recognized as a chronic inflammatory disease in 1986 (New Engl J Med 314:488-500, 1986). The presence of lymphocytes in atherosclerotic lesions suggested autoimmune processes in the vessel wall (Clin Exp Immunol 64:261–268, 1986). Since the advent of suitable mouse models of atherosclerosis (Science 258:468–471, 1992; Cell 71:343-353, 1992; J Clin Invest 92:883–893, 1993) and the development of flow cytometry to define the cellular infiltrate in atherosclerotic lesions (J Exp Med 203:1273–1282, 2006), the origin, lineage, phenotype, and function of distinct inflammatory cells that trigger or inhibit the inflammatory response in the atherosclerotic plaque have been studied. Multiphoton microscopy recently enabled direct visualization of antigen-specific interactions between T cells and antigen-presenting cells in the vessel wall (J Clin Invest 122:3114–3126, 2012). Vascular immunology is now emerging as a new field, providing evidence for protective as well as damaging autoimmune responses (Int Immunol 25:615–622, 2013). Manipulating inflammation and autoimmunity both hold promise for new therapeutic strategies in cardiovascular disease. Ongoing work (J Clin Invest 123:27–36, 2013; Front Immunol 2013; Semin Immunol 31:95–101, 2009) suggests that it may be possible to develop antigen-specific immunomodulatory prevention and therapy—a vaccine against atherosclerosis.
Keywords: Atherosclerosis, Macrophage, Monocyte, T cell, Autoimmunity, Vaccine
Monocytes—precursors of most macrophages and dendritic cells
It has been shown over the last decades by a plethora of experiments that macrophages—tissue-resident cells of myeloid origin that figure in a variety of physiological and pathological conditions—are the main effector cells in the atherosclerotic plaque [13, 14]. In a simplified view, macrophages represent promiscuous phagocytes that can engulf a plethora of pathogens and debris in a variety of tissues, including modified and native lipids in the vessel wall [15]. While fulfilling their role as the main scavengers of the innate immune system, macrophages transform, become lipid-laden foam cells and drive the inflammatory milieu in the atherogenic vessel wall by secretion of cytokines and chemokines, and their interaction with other immune cells in the plaque [16], although this view has recently been challenged [17]. It has been proposed that lesional macrophage burden and phenotype may correlate with clinical outcome. This as yet unproven hypothesis has led to the terms stable (thick cap, few inflammatory cells) and unstable (macrophage and lipid-rich plaque) plaque [18]. Unstable plaques are more likely to give rise to life-threatening complications, such as myocardial infarction and stroke [19]. Destabilization of the atherosclerotic plaque is thought to occur as result of matrix metalloproteinases that are secreted by macrophages and other cells and can eventually destruct the plaque’s extracellular matrix [20, 21].
Originally, it was believed that macrophages solely arise from monocytes [22]. However, many tissues are seeded by primitive hematopoietic precursor cells prior to the emergence of definitive hematopoiesis [22, 23] and self-renew in tissues [24]. Consistent with this, macrophages are found in the aorta even in the absence of atherosclerosis [7]. However, monocyte invasion, in particular of the pro-inflammatory, CCR2+ Ly6Chigh (Gr-1+) monocyte subset occurs through all stages of disease [25, 26], an observation that has led to the assumption that plaque macrophages originate from monocytes [27, 28]. A number of findings supports that circulating monocytes contribute to plaque macrophages: Numbers of monocytes in the periphery are elevated in atherosclerosis-prone ApoE−/− mice and correlate with disease severity [25–27]. Homing studies using latex beads to label monocyte populations [26] suggest that both Ly-6Chi and Ly-6Clo monocytes access atherosclerotic lesions, but monocyte transendothelial migration and differentiation to macrophages have not been observed directly. Recent development of multiphoton intravital microscopy in atherosclerotic arteries [29] holds promise that this may become possible soon. This would establish whether monocytes enter from the lumen as is currently believed, or whether vasa vasorum is an additional source of monocytes.
Novel mechanisms that explain monocytosis during atherosclerosis have recently been identified: Some of them are located in the bone marrow, where hyperglycemia [30] or modulation of reverse cholesterol transport in hematopoietic stem cells (HSPCs) [31] regulates monocyte generation and their efflux in the periphery. Interestingly, activation of the sympathetic nervous tone by pain, anxiety, stress, and circadian events can drive adrenergic signaling in the bone marrow niche and generation of Ly6Chigh pro-inflammatory monocytes [32–34]. Notably, monocyte progenitors can also populate alternative niches, such as the spleen, and can be released into circulation [35, 36]. Taken together, monocytes infiltrating into the plaque are one origin of plaque macrophages.
The plaque macrophage—a stem cell within the artery?
The dogma that every monocyte turns into a macrophage has been challenged in the last years. Firstly, it was shown that Ly6Chigh monocytes that enter inflamed tissue do not necessarily turn into macrophages, but instead serve as short-lived antigen-presenting cells that are capable of presenting antigen on MHC-II and traveling to lymph nodes without significant differentiation [37]. Secondly, it was observed that blocking CCR2, a chemokine receptor highly efficient in recruiting Ly6Chigh monocytes to inflammatory sites, did not alter atherosclerotic lesion development [38]. Similarly, diphtheria-toxoid-guided depletion of leukocytes bearing the integrin Mac-1, including neutrophils, monocytes, and macrophages [39], only affected de novo atherosclerosis, but not established disease [40]. These findings have called into question whether monocyte influx is always required for atherosclerosis.
An alternative idea is that atherosclerotic plaque may be populated with macrophages by self-renewal and proliferation of tissue-resident cells. Notably, proliferation signals have been detected in the atherosclerotic plaque, especially in the macrophage-rich fatty streaks [41, 42], where they co-localized with foam cells [43]. Also, macrophages in other locations have been shown to proliferate in response to inflammation, presumably by innate immune signaling [44], and in steady state [45]. A study by Robbins et al. demonstrated that plaque macrophages proliferate in situ [46]. The authors of this study have used an elegant experimental approach called parabiosis to address this challenging question: The skin of two mice expressing distinct reporter genes is stitched together to establish a shared blood circulation. Thus, migration of cells traveling from one mouse into the circulation and organs of the other mouse allows fate-mapping studies. By testing parabiosis, the authors demonstrated that the macrophage pool in the plaque actively incorporated BrdU—a surrogate marker for replicating DNA and hence cellular turnover. Influx of circulating monocytes contributed little to plaque macrophages. These data indicate that plaque macrophages—under some circumstances and likely in the later stages of disease—proliferate locally. The authors conclude that proliferation accounts for about 90 % of macrophage accumulation in established disease. Notably, proliferation was supported by LDL-uptake into macrophages by the scavenger receptor SR-A (Msr1) in this study. The role of SR-A in atherosclerosis is controversial: While one study shows that Msr1−/− mice develop normal atherosclerotic lesions [47], other studies suggest a role for Msr1 in late atherosclerotic, necrotic lesions [48]. A gene-silencing approach also suggested a pro-atherogenic role of Msr1 [49].
After myocardial infarction, the heart muscle is infiltrated by macrophages [35]. Interestingly, fate mapping of macrophages residing in the myocardium demonstrated that proliferation occurred in macrophages that preferentially take up bacteria, suggesting a role in immune-surveillance [50]. On the contrary, in an inflammatory milieu during myocardial infarction, the proliferating fraction of macrophages died or disappeared from the heart, while newly recruited macrophages dominated. These data unveil that the function of proliferating macrophages in heart and atheromata may be distinct [51].
It is clear from these results that macrophages in the plaque and in the heart share properties with self-renewing stem cells, although their lineage potential may be very narrow. Tissue-resident macrophages have clearly been shown to self-renew in the peritoneum, liver (Kupffer-Cells), brain (microglia), and the skin (Langerhans cells). These cells originate from primitive hematopoietic cells in the yolk sac and are placed in tissues during embryogenesis [23, 24, 52–56]. Such macrophages are also found in the heart and their contribution to tissue regeneration, antigen sampling, and efferocytosis, rather than to inflammation has been proposed [57, 58]. Whether such yolk sac-derived macrophages are also present in the arterial wall and whether they proliferate locally remains to be tested.
Transdifferentiation—an alternative source of plaque macrophages?
The different origins of plaque macrophages may be expanded by one old idea that has gained new attention in the last years: that non-myeloid cells may give rise to macrophage-like phagocytes in the plaque. It has been proposed by some studies that vascular smooth muscle cells (VSMCs) can acquire some of the prototypic functions of a macrophage, such as phagocytosis of lipids [59] or phenotypic conversion toward the monocyte lineage [60]. A recent study by Feil et al. has provided new evidence supporting this concept. By employing a genetic fate-mapping approach, the authors suggest that VCMCs residing in the media aortic wall before disease initiation are mobilized into the intima during atherosclerosis [61, 62]. Loss of typical markers of contractile smooth muscle cells (SMC), such as smooth muscle actin (SMA), clonal expansion of the transdifferentiated cells, and expression of myeloid cell markers, such as CD68 and Mac-2, supports the author’s hypothesis that SMCs convert in macrophage-like cells. However, despite their phenotypic similarities, it is unclear whether these transdifferentiated cells harbor the same functions as classical plaque macrophages. This will have to be clarified in future studies on gene expression, signaling, and function—an evaluation beyond the expression of some more or less specific myeloid cell markers. In this regard, one recent study proposed that cholesterol signaling skews the phenotype of SMCs toward macrophage-like cells in vitro [63], which would support a specific role for transdifferentiation in atherosclerotic plaques that are rich of native and modified cholesterol.
The removal of plaque macrophages
In the context of plaque inflammation, it has been proposed that myeloid cells are capable of leaving the plaque and thus facilitate resolution of inflammation [64, 65]. This idea has partially been based on the observation that statins—potent anti-inflammatory drugs developed to lower cholesterol by blocking endogenous cholesterol synthesis—resulted in regression of atherosclerotic plaques in individuals with coronary heart disease [66]. Likewise, inducible reconstitution of ApoE in mice expressing a hydromorphic ApoE allele or that lack ApoE demonstrated diminished atherosclerotic plaques and foam cell content [67, 68]. On the other hand, aggravation of atherosclerosis is associated with increased accumulation of macrophages, which possibly lost their ability to leave the plaque. Notably, in the later stage of disease, but not in the early, the clearance of apoptotic cells—a mechanism referred to as efferocytosis—is impaired [69]. As a result, accumulating apoptotic cells cause secondary necrosis, which in turn aggravates the inflammatory response [70]. Both observations have led to the idea that plaque macrophages are removed from the plaque during regression, a hypothesis also supported by studies demonstrating that macrophages may actively exit some inflammatory compartments by migration, such as from the peritoneal cavity or adipose tissue [71, 72]. Macrophage removal from plaque could occur by active emigration or cell death. To distinguish between these possibilities in the context of atherosclerosis, a surgical model was developed: The atherosclerotic aortic arch was isolated and transplanted into either atherosclerosis-prone ApoE−/− or wildtype mice [65]. When the atherosclerotic aortic arch (marked by an allogeneic marker) was transplanted into wildtype mice, macrophages rapidly disappeared, and their disappearance correlated with the appearance of marker-positive cells in lymph nodes. These findings suggested active migration of plaque macrophages to lymph nodes in an atheroprotective milieu. This effect seemed to be dependent on the chemokine receptor CCR7 and to be modulated by the cholesterol load of macrophages [64, 73]. However, newer studies using bead-tracking techniques optimized for exact quantification of cellular trafficking showed that the egress of macrophages is only a minor factor or even dispensable for the loss of macrophages from the plaque [74]. Instead, lowered monocyte recruitment to the plaque in an environment of normalized circulating lipids—a factor required to induce plaque regression—and a stable, unchanged rate of apoptosis among plaque macrophage were the likely explanations for decreasing macrophage numbers in the plaque during regression. Notably, the authors of this study used a viral transduction by adenovirus to restore ApoE in an ApoE−/− mouse, a strategy that could bear potential side effects to bias the conclusion of the study [74]. Later studies confirmed that macrophage death is the main contributor to macrophage removal in the peritoneal cavity. In one study, a minor proportion of cells emigrated to lymph nodes [75]. These findings suggest that macrophage death, not egress from the plaque, accompanies plaque regression.
The many faces of plaque macrophages
Macrophages in vivo form two major subsets, M1 and M6 [76, 77]. M1 macrophages kill pathogens by NO produced by iNOS. M2 macrophages promote would heal by converting arginine to ornithine via arginase-1 [177]. The M1 polarization of macrophages is further promoted by IFN-γ and such macrophages are known as classically activated [78]. M2 macrophages can be stabilized and further driven to express more arginase-1 by IL-4, also known as alternatively activated macrophages, which express not only arginase-1 but also CD206 (Mannose receptor, MR) [79]. In vitro studies have suggested some possible inducers and signaling molecules predisposing for either one of the phenotypes, including TLR ligands, IFN-γ, GM-CSF (M1), or IL-4 and some fatty acid species (M2) [80]. However, the in vivo cues are largely unknown. Genetic predisposition of the host, tissue-derived cues, and pathogen-derived molecules like TLR ligands is suspected modifiers of macrophage phenotype [77]. NFκB and NLRP3 pathways are dominant in M1 macrophages [81] and PPARγ and Nr4a1 in M2 macrophages [82]. The distinct transcriptome of M1 and M2 macrophages includes IL-12 and Tumor Necrosis Factor (TNF)-α in M1, which supports a TH1 adaptive immune response, and IL-10 in M2, which supports a TH2 adaptive immune response. One view holds that M1 and M2 macrophages dominate in the atherosclerotic plaque at different stages. The few available in vivo studies suggest that M1, M2, and other macrophages exist in plaques side-by-side [83].
Many in vitro studies have suggested that macrophage polarization could be more complex. Some plaque macrophages show reduced expression of the scavenger receptor CD163 for the hemoglobin-haptoglobin complex, which is characteristic of M4 macrophages induced by the chemokine CXCL4 [84, 85]. A fourth macrophage phenotype can be identified by its expression of the anti-inflammatory enzyme heme oxygenase (HOX) -1, a unique phenotype, induced by oxidized phospholipids, called Mox [80, 83]. In vitro, some macrophages express high levels of IL-10 and have been termed regulatory macrophages (Mreg) [86, 87], but their in vivo relevance for atherosclerosis remains unclear. The description of macrophage phenotypes in mouse or human atherosclerosis remains incomplete [13, 88, 89]. Although there is no requirement for adaptive immune cytokines to induce M1 or M2 polarization [90], it is reasonable to speculate that immune cells, such as T or B cells, help regulate the macrophage phenotype by cytokine expression, e.g., by secretion of the TH2 cytokine IL-4 to induce a protective M2 phenotype.
Certain macrophage phenotypes may predispose toward distinct clinical outcomes: Some newer studies show that macrophages with specific functional repertoires localize within distinct parts of the plaque, an effect likely mediated by the site-specific microenvironment: While M1 macrophages predominate in rupture-prone regions of human plaques, M2 macrophages seem to inhabit the adventitia. Notably, areas of plaque hemorrhage show elevated expression of the hemoglobin receptor CD163 (Mhem phenotype) [91]—while the fibrous cap of atheromata, as well as foam cells, showed no clear predisposition for M1/M2/M4/Mox/Mhem/Mreg phenotypes [90, 92]. Based on these data, it is reasonable to propose that M1 macrophages may predispose for later clinical events, but this remains to be confirmed by clinical studies. Older findings suggested that plaque instability is associated with macrophage-rich lesions showing higher expression of extracellular matrix destabilizing metalloproteinases (MMPs) [20, 93]. Indeed, M1 and M2 polarized macrophages have distinct expression patterns of MMPs: Murine M1-type macrophages show increased gene expression for MMP-13, -14, -25 and lowered expression of MMP-19 and TIMP-2, while M2 macrophages increase expression of MMP-19. A similar pattern was observed in human macrophages [90, 94, 95]. All these data should be considered with caution, because in vitro polarized macrophages are not directly relevant to atherosclerosis.
Besides plaque stability, certain pathologies could theoretically benefit from modulating phenotypic functions of macrophages. For instance, tissue repair—an effector function associated with the M2 phenotype—holds great promise in tissue remodeling after myocardial infarction. Indeed, it was shown that modulation of master transcription factors predisposing for a certain polarization, such as the M1 phenotype driving Interferon regulatory factor (IRF) 5 could represent such a strategy. Silencing IRF5 induced a shift from the inflammatory M1 phenotype toward the M2 phenotype in heart macrophages improving outcome after myocardial infarction [96]. Likewise, genetic inhibition of IRF5 in a combined atherosclerosis/lupus model was protected from both pathologies, but macrophage-specific function was not tested [97]. These findings are encouraging attempts to understand the impact of polarization-specific transcription factors and distinct functional properties.
The cellular origin of macrophage polarization
Macrophage heterogeneity and function may not only be caused by the microenvironment as new evidence suggests [98, 99], but also by origin. Two models have been proposed as follows: 1) Macrophage subsets are pre-defined by circulating monocyte progenitors—CCR2+ Ly6Chigh (human: CD14++) inflammatory or CCR2low Ly6Clow patrolling (human: CD14dim) monocytes—as previously suggested [100] or 2) by conversion from one subset into another. Hanna et al. have previously demonstrated that the orphan nuclear receptor Nr4a1 (also known as Nur77) is required for differentiation of patrolling Ly6low monocytes in the bone marrow. Nr4a1−/− mice lack Ly6Clow monocytes [101], suggesting Nr4a1 is as master transcription factor for the development of this monocyte population. Further studies showed that genetic deficiency of Nr4a1 accelerated atherosclerosis and skewed the phenotype of macrophages toward a pro-inflammatory phenotype with a high expression of TNF-α, nitric oxide, and reduced expression of arginase-1 [82].
Newer studies suggest that alternatively activated macrophages (just like M1 macrophages) originate from Ly6Chigh, not Ly6low monocytes [82]: During myocardial infarction, only Ly6high monocytes enter the inflamed myocardium, convert to macrophages, and prolong post-infarct healing [102]. Apparently, Nr4a1 is needed to facilitate conversion toward a protective phenotype, an observation based on the finding that Nr4a1−/− mice developed an inflammatory macrophage phenotype that was associated with worsened outcome in myocardial infarction [82]. This is also suggested by studies on human lesional macrophages, where over-expression of Nr4a1 decreased their pro-inflammatory gene expression in vitro [103]. This new concept is further supported by observations in different pathologies, such as liver injury, which propose that reparative macrophages arise from recruited Ly6high monocytes [104]. Of course, observations from liver tissue or myocardium cannot directly be translated to the atherosclerotic plaque and future studies will have to clarify the exact origin of these cells. All these findings suggest that Ly-6Chi monocytes may be able to turn into Ly-6Clow monocytes, a concept supported by lineage tracking studies [105].
The need for an integrated model of myeloid cell dynamics in the plaque
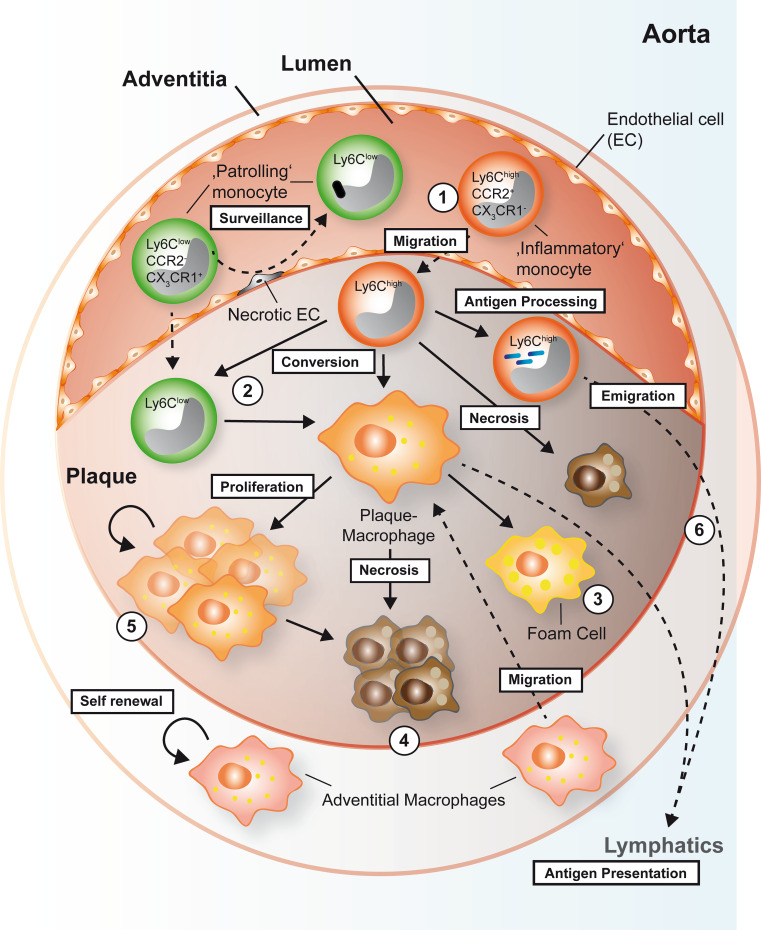
How can such new data be integrated into an updated model of macrophage origin and fate in the plaque? Firstly, the role of local proliferation of plaque macrophages versus differentiation from blood monocytes will have to be understood quantitatively. Secondly, macrophage effector function, proliferation, and cell death may depend on the specific microenvironment [98]. Thirdly, different, maybe apparently divergent mechanisms may coexist. In line with this notion, intestinal macrophages not only proliferate in vitro [106] but are also replenished from blood monocytes in vivo [107] and have been shown to be eliminated by apoptosis and migration to lymph nodes [71]. Most likely, different mechanisms exist at the same time, triggered, and amplified by the specific environment. While macrophage accumulation was considered to be in almost linear relationship to monocyte infiltration in the past, future models will have to integrate and quantify the different relative impacts of distinct macrophage progenitors in a spatial and time-dependent view [108], also incorporating the loss of macrophages by apoptosis or emigration, and possible transdifferentiation from SMC or precursors in the adventitia [109] (Fig. 1).
Fig. 1.
Proposed origin and fate of myeloid cells in the atherosclerotic plaque in mice. Early plaque macrophages originate from inflammatory Ly6Chigh monocytes that transmigrate into the plaque and differentiate (1). A second monocyte subset, patrolling Ly6Clow monocyte is thought to patrol the vessel wall to remove debris and dead endothelial cells. However, Ly6Clow monocyte may also contribute to the pool of plaque macrophages by transmigration and differentiation into macrophages. Ly6Clow monocytes in the plaque may also originate by conversion from Ly6Chigh monocytes to give rise to macrophages (2). Alternatively, it has also been speculated whether plaque macrophages may stem from migrated adventitial macrophages that are possibly derived from the primitive yolk sac during embryogenesis and that may have the potential to self-renew. Depending on the context, macrophages may ingest lipids and turn into foam cells (3) or die (4). It has recently been reported that the pool of plaque macrophages can independently be maintained in the later stages of disease by direct proliferation in situ (5). Besides their differentiation into macrophages, Ly6Chigh monocytes may transmigrate into the plaque, engulf, and present antigens by MHC-II, before leaving the plaque and migrating into peripheral lymph nodes (6). Likewise, dendritic cells (not shown) and macrophages can migrate into the lymphatic system to present antigens to T cells and initiate an immune response
The footprints of autoimmunity in atherosclerosis
A large body of recent evidence supports the hypothesis that atherosclerosis is an autoimmune disease, driven by the deposition and modification of lipoproteins in the vessel wall and their detection by specific T cells and antibodies. Particularly, 4 major findings support this hypothesis: (1) T cells infiltrate the aorta, accumulate, and show a restricted repertoire of T cell receptor (TCR), (2) Activation of T cells in the plaque is sustained by interaction with plaque resident antigen-presenting cells (APCs) and requires presentation of specific antigens, (3) In different species, auto-antibodies to lipid antigens and their protein moieties are atheroprotective and associated with better disease outcome, and (4) Outcome of murine atherosclerosis can be modulated by immunizing against some known antigens. The above-mentioned evidence builds the theoretical basis of considering atherosclerosis as autoimmune disease (a comprehensive review is found in Ref. [110]).
The antigen-specific T cell orchestrates plaque inflammation
About 59 % of cells within advanced human atherosclerotic lesions are macrophages, ~38 % of all cells represent CD3+ T cells depending on plaque morphology, while Natural Killer (NK) cells (~1 %) and B cells (~2 %) are only present at minor frequencies [111]. T cells can be detected in all stages of the atherosclerotic plaque [7] and based on their commitment to T helper cell (TH) lineages, T cells can either act as pro- or anti-inflammatory cells [9]. Notably, most T cells in the plaque are of the TH1 lineage and express the pro-inflammatory cytokines IFN-γ, IL-2, IL-3, TNF, and LT. These cytokines fuel plaque inflammation by stimulating macrophages and other cells resident in the plaque [112]. IFN-γ is also present in the human atherosclerotic plaque. Genetic knock-out of IFN-γ, its receptor, or of its lineage-defining transcription factor T-bet reduced atherosclerosis in mice [113–115], while the contribution of the TH2 or TH17 lineage is less important or controversial [116–124].
CD4+ T cells appear to respond to certain antigens in the atherosclerotic plaque: T cells from human and murine plaques show a restricted TCR repertoire, suggesting that those T cells accumulate and proliferate in the plaque, which bear a specific TCR detecting the antigen [125, 126]. Native and modified (ox-) LDL, as well as apoB-100, the main protein component of LDL, represents the most promising candidates for such antigens [127–129], but also heat shock proteins (HSPs) and some pathogens were proposed [110, 130]. Notably, T cells isolated from an atherosclerotic aorta specifically recognize oxLDL [129]. Antigen-experienced CD44hiCD62L- CD4+ T cells isolated from atherosclerotic mice interacted extensively with dendritic cells in situ in a novel ex vivo imaging approach of explanted aortas [8]. Transfer of a crude T cell suspension responding to oxLDL ex vivo aggravated atherosclerotic disease after adoptive transfer in a model in of scid/ApoE−/− mice [131]. Cytokine expression of auto-reactive T cells was dependent on antigen presentation by MHC-II on APCs and binding to TCR [128], suggesting a specific antigen-driven immune response in the plaque against those autoantigens.
Naturally occurring T-regulatory cells (nTregs) are gatekeepers of self-tolerance. Although some high affinity self-recognizing T cells are removed in the thymus by negative selection, it is important to recognize that essentially all T cells in an organism not exposed to pathogens are (positively) selected for self-antigens [132, 133]. The effect of Tregs has been extensively tested in the setting of atherosclerotic disease [134]. From a functional point of view, nTregs are considered anti-inflammatory immune cells, classically defined by co-expression of IL-2 receptor, CD25, the forkhead transcription factor (FoxP)-3, and the co-stimulatory molecule CTLA-4 [135]. nTregs are generated in the thymus, selected by self-peptides. Other T cell subsets with potential regulatory function include CD4 T cells that have acquired FoxP3, called induced T-regulatory cells (iTregs). These cells are induced in the periphery (outside the thymus) and can easily be converted to other T cell subsets, i.e., they are unstable. Other CD4 T cells secrete the anti-inflammatory IL-10 (Tr1 cells) and can act as immune-modulating cells by expressing TGF-β. IL-10 and TGF-β are known to inhibit atherosclerosis in mouse models [136–138]. In adoptive transfer experiments, a population of CD4+ CD25+ T cells (containing Tregs) was protected from atherosclerosis [139]. However, newer studies employing a model of Treg depletion by diphtheria toxin receptor under control of the Foxp3 were uninterpretable, because the blood lipid profile of these mice changed significantly [140]. IL-2 antibody complexes are thought to induce Tregs and showed beneficial effects in atherosclerosis [141]. These findings are intriguing since Tregs are required to limit the pro-inflammatory effects of auto-reactive T cells [132]. Indeed, loss of Tregs can induce severe autoimmune disease [139]. A thrilling, but yet unproven presumption based on these and other findings from immunization experiments [142, 143] is that some of the specific T cell clones reactive to ApoB-100 are Tregs. Several other important questions remain: Does a natural repertoire of T cells detecting specific antigens exist? Which circumstances may limit or expand this cell subset? Which exact peptide epitopes within the proposed antigens are recognized by specific TCRs on these cells?
Antigen presentation by subsets of macrophages and dendritic cells drives specific immune responses
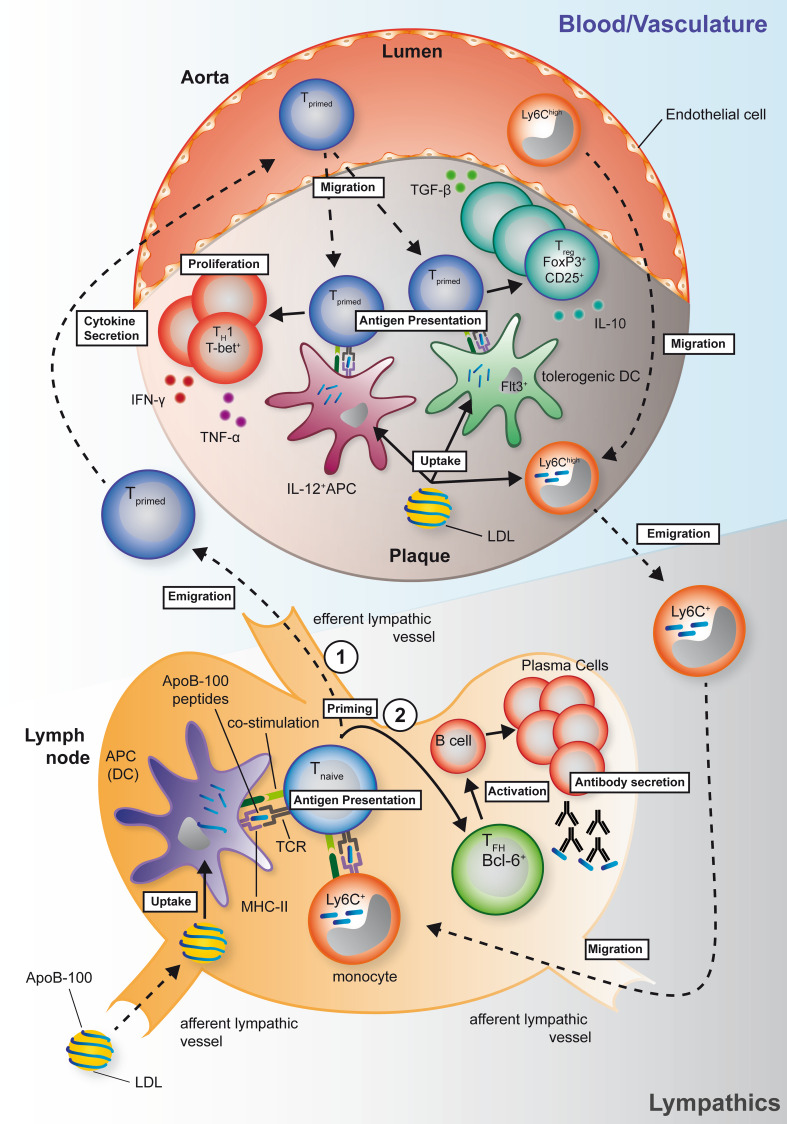
Antigen presentation is a prerequisite to mature and polarize naïve T cells toward their effector status and the major determinant of antigen specificity in adaptive immunity. To induce a specific immune response against proteins, antigenic peptides must be presented on Major Histocompatibility Complex (MHC)-II (I-Ab in C57Bl/6 mice) on antigen-presenting cells (APCs) to allow binding of the T cell receptor (TCR) [144] in the context of CD4. In atherosclerosis, CD4+ T cell activation and secretion of the pro-inflammatory cytokines IFN-γ and TNF-α critically depend on interaction and antigen presentation by APCs [8, 145]. Notably, the interaction of APCs and T cells in the atherosclerotic plaque depends on cognate antigens, emphasizing that T cells in the plaque are antigen specific [8]. It is less clear to which extent the interaction with T cells requires specific APCs to maintain or break self-tolerance in the setting of atherosclerosis. However, it has been proposed that distinct lineages of DCs may contribute to or antagonize the autoimmune response in atherosclerosis [146]. Different subtypes of DCs with distinct functional repertoires have been identified in the atherosclerotic plaque. Depending on origin, lineage, and expression of surface markers, at least three different DC subtypes have been defined. All of these show expression of MHC-II and CD11c: Monocyte-derived DCs/macrophages that respond to M-CSF and express CD11b, conventional DCs (cDCs) which can either express CD11b or CD8 or CD103, and PDCA and B220 plasmacytoid DCs (reviewed in Ref. [146]). It is noteworthy that DCs are already present in the intima of a healthy aorta in mice and humans [147, 148], although the subsets have not been identified. In advanced plaques, antigen presentation is predominantly carried out by cDCs and macrophages, which show co-expression of CD11b and CD11c and predispose for a TH1 priming of T cells by secretion of IL-12 and CCL17 (a review summarizing these findings is provided in Ref. [149]). It has been proposed that the polarization of an immune response—immunogenic or tolerogenic—will be guided during initial antigen presentation [146, 150]. Various mediators can skew the polarization of the immune response by modulating DC activation. While anti-inflammatory signals as IL-10 and TGF-β [151] will skew the response toward a tolerogenic response, IL-6 induces a TH17, and IL-12 a TH1 response. In atherosclerosis, disruption of tolerogenic pathways in dendritic cells, for instance, by deletion of the receptor for cytokine fms-like tyrosine kinase 3 ligand (Flt3L), Flt3, can reduce Tregs and the net effect is pro-atherogenic [152]. Surprisingly, some signaling pathways involved in DC maturation and T-effector (Teff) cell generation, such as those initiated by toll-like receptors (TLRs), can result in both, immunogenic and tolerogenic pathways. For instance, genetic deletion of MyD88, a master TLR adapter, resulted in a decrease of both Teff and Treg [153]. Unexpectedly, the decrease of Tregs, and not of Teffs, determined the outcome in this study with enlarged atherosclerotic lesions and increased recruitment of myeloid cells into the plaque. Moreover, ex vivo pulsing of dendritic cells with ApoB-100 aggravated the immune response with enhanced infiltration of CD4+ T cells, and increased IFN-γ and Il-2 in the aorta. DCs that were primed with IL-10 were atheroprotective [154]. However, transferring peptide-pulsed DCs was also atheroprotective without prior cytokine treatment in another study [155], rendering these results difficult to interpret. Besides monocyte-derived and cDCs, pDCs have recently been tested in an elegant experimental approach, in which the transcription factor Tcf4 was abrogated selectively in CD11c+ cells to reduce pDCs numbers. Mice with such pDC reduction were protected from atherosclerosis and had fewer TH1 polarized T cells [156]. Taken together, these results clearly indicate that antigen presentation is a fundamental part of atherogenesis and may be accessible to therapeutical modulation. However, the assessment of distinct APC subsets in atherosclerosis has been difficult with respect to its heterogeneity and incomplete tools to inhibit antigen presentation in a disease-specific manner. For instance, diphtheria toxin-guided depletion in CD11c+ DCs also abrogates CD11c-expressing M1-macrophages [157]. It is not known whether antigen presentation in the vessel wall is crucial for atherosclerosis. It is likely that priming of naïve T cells occurs in peripheral draining lymph nodes and not in the aorta itself [149]. However, there is good evidence that the recall response re-activating antigen-experienced CD4+ T cells occurs in the atherosclerotic vessel wall [8] (Fig. 2). Interestingly, cholesterol removal from the plaque also occurs by afferent lymphatics, thus providing a plausible path also for (modified) LDL to reach draining lymph nodes [158]. Whether initial priming and proliferation of T cells followed by tissue homing and second antigen-exposure in non-lymphoid tissue to generate fully polarized effector cells [150] remains to be tested.
Fig. 2.
Hypothesized mechanisms of T cell-dependent autoimmunity in mice. Generation of T cell clones and antibodies that recognize self-peptides from ApoB-100, the protein moiety of low-density lipoprotein (LDL), have been proposed as main pathways of T cell-dependent autoimmunity. Antigen-specific, pro-atherogenic T cells are primed in peripheral lymph nodes (lower schematic), but remain incompletely differentiated after first presentation of self-peptides by dendritic cells or Ly6C+ monocytes migrating from the plaque. (1) A primed T cell may leave the lymph node and home to the plaque to allow for terminal differentiation: In the plaque, presentation by IL-12+ APCs to primed T cells is thought to elicit a pro-atherogenic TH1 type immune response with the lead cytokines TNF-α and IFN-γ. Conversely, presentation by Flt3+ tolerogenic dendritic cells can induce an atheroprotective response with conversion of the primed T cell into Treg cells and secretion of the anti-inflammatory mediators IL-10 and TGF-β (upper schematic). (2) Alternatively, a primed T cell can differentiate into a follicular-helper T cells (TFH), which induces generation and secretion of ApoB-100-specific IgG antibodies by plasma cells in the lymph node or tertiary lymphoid organs (lower schematic)
Protective humoral autoimmunity in atherosclerosis
TH1-committed T cells and their response to antigens drive atherosclerotic disease, presumably by expression of pro-inflammatory and pro-atherogenic mediators. Several observations, however, propose the co-existence of a protective limb of autoimmunity in atherosclerosis: Naturally occurring IgM auto-antibodies, which recognize oxidized low-density lipoprotein (LDL) cholesterol or parts of its main protein moiety, ApoB-100, are inversely correlated with atherosclerotic disease [159–161], its complications and risk factors [162]. While IgG antibodies could originate from plasma cells derived from B cells specifically activated by follicular-helper T cells (TFH), which involves maturation of B cells and Ig-class switch from IgM to IgG [145], IgM auto-antibodies are expressed by a specific B cell subset, B1a cells, independent of TFH cell help [163]. B1a cells are thought to recognize self-antigens and respond with secretion of IgM antibodies. Those IgM antibodies have been functionally implicated in atheroprotection. Binder et al. demonstrated that immunization of mice with heat-inactivated S. pneumoniae antigen, which shares epitopes with oxidized LDL, increased IgM levels and diminished atherosclerosis [164]. Also, mice with a deficiency in secreting IgM presented increased levels of atherosclerosis [165]. Vice versa, treatment with polyclonal IgM was protected from atherosclerotic disease [166]. Some IgM antibodies can bind to oxLDL and may inhibit uptake of the antigen by macrophages [167, 168]. Interestingly, IgG antibodies to native and oxidized LDL, are positively correlated with atherosclerotic disease in mice and humans and accumulate in atherosclerotic lesions [169, 170]. Conversely, higher titers of IgM antibodies to oxLDL predict better outcomes [171, 172]. Antibodies directed against peptide epitopes of ApoB-100 are inversely correlated with disease outcome [173].
A vaccine against atherosclerosis
Following the observation that T cells can specifically recognize autoantigens in the context of atherosclerosis, several vaccination strategies have been proposed in recent years [130]: Immunization of rabbits with MDA-modified LDL, which contains MDA-modified ApoB-100, protected from atherosclerosis [174], as well as with murine LDL, MDA-modified LDL in mice [175], and AGE-modified LDL in mice [176]. Additionally, a peptide derived from human ApoB-100, p210 (ApoB-1003136-3155) was found to bind to IgM and IgG antibodies from human sera [177]. Vaccination with p210 protected mice from atherosclerosis and aortic aneurysm formation [178, 179]. While the efficacy of immunization strategies against different antigens, such as naïve and modified LDL, ApoB-100 or peptides derived from ApoB-100, has been demonstrated in various species and animal models [110] (Table 1), the functional properties of antigen recognition and its exact cellular and functional consequences, as required for the ultimate goal of defining a clinical vaccination strategy in humans, remain enigmatic. For instance, it has been shown that vaccination against some peptides, e.g., p210, conferred atheroprotection in mice, an effect linked to T cell responses in some studies [142, 143, 179–181]. However, the tested peptide does not bind to mouse MHC-II (I-Ab), thus excluding a CD4+ T cell restricted mode of action. To circumvent these limitations we have recently applied the first systematic screening to determine peptide sequences in mouse ApoB-100 with sufficient affinity to I-Ab [182]. Two candidate peptides with high affinity to MHC-II were identified and induced effective CD4+ T cell proliferation. Immunizing against those peptides with a prime in complete Freud’s adjuvant (CFA) and three subsequent booster injections in incomplete Freud’s adjuvant (IFA) reduced murine atherosclerosis. Mechanistically, we detected more IL-10 transcripts in aortas of immunized mice, suggesting that this cytokine may be causal in atheroprotection [182]. This is supported by other studies showing that T cells with properties of anti-inflammatory T-regulatory cells confer atheroprotection in immunized animals, likely by Il-10 expression [142, 143, 183].
Table 1.
Selected studies testing vaccination protocols in murine atherosclerosis
| References | Species/genotype | Antigen | Adjuvant | Route | Atherosclerotic lesions | Proposed effector cells | Proposed lead cytokine | Effect on immunoglobulins |
|---|---|---|---|---|---|---|---|---|
| Klingenberg et al. [176] | Mus musculus, ApoE−/− | p210 (human ApoB-100) | Cholera-toxin B subunit (CTB) | i.n. | Aortic root ↓ |
FoxP3 + ↑ (aorta and spleen) |
IFN-γ ↓ IL17 ↓ (lung mucosa) IL-10 ↑ (spleen) |
IgG anti-p210 ↑, no change in IgM anti-ApoB-100 |
| Herbin et al. [140] | Mus musculus, ApoE−/− | Mix of p210, p240, MDA-P210 (of human ApoB-100) | None | s.c. | Aortic root ↓ |
FoxP3+CD25+↑ (lymph nodes) |
IFN-γ ↓ IL-10 ↓ IL-4 ↓ |
No change in IgG, IgM |
| Hermansson et al. [150] | Mus musculus, huB100tg LDLR−/− | Human ApoB-100 pulsed DCs | IL-10 | i.v. | Aorta (en face) ↓ | CD4+↑ (aorta) |
Il-12 ↓ IL-10 ↑ TGF-β ↑ FoxP3 ↑ (spleen) |
IgG anti-ApoB-100 ↑, no change in IgM |
| Tse et al. [178] | Mus musculus, ApoeE−/− | P3, P6 (mouse ApoB-100) | 1 × CFA prime, 4x IFA boost | s.c. (prime), i.p. (boost) | Aortic root and en face ↓ | None |
Il-10 ↑ (aorta) |
IgG anti-P3, -P6 ↑ |
| Wigren et al. [139] | Mus musculus, ApoE−/− | p210 (human ApoB-100) | Alum | s.c. (3x) | Descending aorta ↓ |
CD25 + ↑ (blood) |
IFN-γ ↓ IL-10 ↓ IL-4 ↓ (spleen) |
Not tested |
i.n. intranasal, s.c. subcutaneous, i.p. intraperitoneal
However, mechanisms of atheroprotection after vaccination are controversial. Depending on antigen, route, and dose, a plethora of different mechanisms has been suggested (Table 1). Also, several methodological questions remain unanswered in some studies. Only a few systematic studies have tested and compared appropriate adjuvants, routes, and doses. One study has revealed that the adjuvant alum has atheroprotective effects even when given alone [184]. Also, there is some controversy about whether atheroprotection is carried out by a humoral immune response. The function of plasma cell-derived IgG antibodies against some epitopes after vaccination is not clear. Such antibodies can clear pathogenic antigens [185], but may be an epiphenomenon since epitopes recognized by such antibodies may not be accessible in atherosclerotic lesions and do not bind LDL [182]. This is consistent with older reports, demonstrating increased IgG antibodies against immunized antigens without protection against atherosclerosis [175]. Also, some newer studies indicate that vaccination with ApoB-100 may raise IgG antibodies to antigens and TH2 signature immune response without being atheroprotective [186]. Some newer evidence also promoted the concept that over-activation of TFH cells can aggravate atherosclerotic disease, lead by the observation that deletion of to the mouse ortholog of MHC-I HLA-E, Qa-1, resulted in uncontrolled TFH proliferation and generation of enlarged tertiary lymphoid organs (TLOs) in the aorta, which was dependent on the ICOS-ICOSL pathway [187]. Whether this increase was due to enhanced IgG production was not tested. However, recent data also propose that some B cell effector functions in atherosclerosis may be independent of antibody secretion, such as enhanced cytokine secretion [188–190], or of triggering a TH1 response [188] (an excellent review about the complexity of B cell function in atherosclerosis is provided by Ref. [191]).
Clinical perspective and concluding remarks
A substantial body of evidence identifies inflammatory and immunologic mechanisms as a driving force behind atherosclerosis and its clinical sequelae. Yet clinical treatment strategies to improve outcome are largely limited to inhibition of platelet aggregation and lowering of lipids. While these may enfold additional immune-modulatory, pleiotropic actions resulting in lower clinical event rates [192] and plaque regression [66], therapies genuinely targeting plaque inflammation and immunology are absent. Two novel strategies, inhibition of interleukin (IL) -1 by a monoclonal antibody called canakinumab [193] and the application of low-dose methotrexate [194], are currently tested in large clinical trials in a collective of high-risk patients with coronary heart disease. These may shed more light to the question whether modulation of inflammation results into lowering of clinical events. Abundant CD4+ T cells in human and mouse atherosclerotic lesions suggest an autoimmune component of atherosclerosis. There is evidence for protective autoimmunity conferred by Tregs and antibodies. Protective autoimmunity can potentially be harnessed to prevent or treat atherosclerosis by vaccination against autoantigens.
References
- 1.Virchow R (1859) Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. Verlag von August Hirschwald, Berlin
- 2.Ross R. The pathogenesis of atherosclerosis–an update. New Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Jonasson L, Holm J, Claesson-Welsh L. Class ii mhc antigen expression in the atherosclerotic plaque: smooth muscle cells express hla-dr, hla-dq and the invariant gamma chain. Clin Exp Immunol. 1986;64:261–268. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein e. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 5.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein e-deficient mice created by homologous recombination in es cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-G. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H, Ley K (2013) Atheroprotective vaccination with mhc-ii restricted peptides from apob-100. Front Immunol 4:493 [DOI] [PMC free article] [PubMed]
- 12.Hansson GK, Nilsson J. Vaccination against atherosclerosis? Induction of atheroprotective immunity. Semin Immunopathol. 2009;31:95–101. doi: 10.1007/s00281-009-0151-x. [DOI] [PubMed] [Google Scholar]
- 13.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koltsova EK, Hedrick CC, Ley K. Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms. Curr Opin Lipidol. 2013;24:371–380. doi: 10.1097/MOL.0b013e328363d298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 20.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newby AC. Metalloproteinases promote plaque rupture and myocardial infarction: a persuasive concept waiting for clinical translation. Matrix Biol. 2015;44–46C:157–166. doi: 10.1016/j.matbio.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 22.van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970;7:125–141. [PubMed] [Google Scholar]
- 23.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 25.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerrity RG. The role of the monocyte in atherogenesis: I. transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle S, Chodaczek G, Ray N, Ley K. Intravital live cell triggered imaging system reveals monocyte patrolling and macrophage migration in atherosclerotic arteries. J Biomed Optics. 2015;20:26005. doi: 10.1117/1.JBO.20.2.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf D, Ley K. Waking up the stem cell niche: how hematopoietic stem cells generate inflammatory monocytes after stroke. Circ Res. 2015;116:389–392. doi: 10.1161/CIRCRESAHA.114.305678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein e knockout mice with ccr2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol. 2005;25:1014–1019. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 39.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 40.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in cd11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/S0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of whhl and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.ATV.10.5.680. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of th2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor a or cd36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of sr-a and cd36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makinen PI, Lappalainen JP, Heinonen SE, Leppanen P, Lahteenvuo MT, Aarnio JV, Heikkila J, Turunen MP, Yla-Herttuala S. Silencing of either sr-a or cd36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res. 2010;88:530–538. doi: 10.1093/cvr/cvq235. [DOI] [PubMed] [Google Scholar]
- 50.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:272–279. doi: 10.1161/ATVBAHA.114.303565. [DOI] [PubMed] [Google Scholar]
- 52.Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR (2014) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature [DOI] [PMC free article] [PubMed]
- 53.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randolph GJ. A macrophage revolution-and beyond. Immunol Rev. 2014;262:5–8. doi: 10.1111/imr.12232. [DOI] [PubMed] [Google Scholar]
- 55.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz C. Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 57.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allahverdian S, Pannu PS, Francis GA. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc Res. 2012;95:165–172. doi: 10.1093/cvr/cvs094. [DOI] [PubMed] [Google Scholar]
- 60.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microrna-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease. New Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 67.Raffai RL, Loeb SM, Weisgraber KH. Apolipoprotein e promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- 68.Zeng H, Horie K, Madisen L, et al. An inducible and reversible mouse genetic rescue system. PLoS Genet. 2008;4:e1000069. doi: 10.1371/journal.pgen.1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 71.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 72.Ramkhelawon B, Hennessy EJ, Menager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ, Moore KJ. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20:377–384. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122:2714–2722. doi: 10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/m-2 macrophages and the th1/th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 77.Mills CD, Ley K. M1 and m2 macrophages: the chicken and the egg of immunity. J Innate Immunity. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 80.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1120–1126. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duewell P, Kono H, Rayner KJ, et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. Nr4a1 (nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gleissner CA, Shaked I, Little KM, Ley K. Cxc chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gleissner CA, Shaked I, Erbel C, Bockler D, Katus HA, Ley K. Cxcl4 downregulates the atheroprotective hemoglobin receptor cd163 in human macrophages. Circ Res. 2010;106:203–211. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fleming BD, Mosser DM. Regulatory macrophages: setting the threshold for therapy. Eur J Immunol. 2011;41:2498–2502. doi: 10.1002/eji.201141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 89.Erbel C, Tyka M, Helmes CM, Akhavanpoor M, Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, Katus HA, Gleissner CA. Cxcl4-induced plaque macrophages can be specifically identified by co-expression of mmp7+ s100a8+ in vitro and in vivo. Innate Immunity. 2015;21:255–265. doi: 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- 90.Hayes EM, Tsaousi A, Di Gregoli K, Jenkinson SR, Bond AR, Johnson JL, Bevan L, Thomas AC, Newby AC. Classical and alternative activation and metalloproteinase expression occurs in foam cell macrophages in male and female apoe null mice in the absence of t and b lymphocytes. Front Immunol. 2014;5:537. doi: 10.3389/fimmu.2014.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boyle JJ. Heme and haemoglobin direct macrophage mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol. 2012;23:453–461. doi: 10.1097/MOL.0b013e328356b145. [DOI] [PubMed] [Google Scholar]
- 92.Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Libby P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann NY Acad Sci. 1995;748:501–507. doi: 10.1111/j.1749-6632.1994.tb17348.x. [DOI] [PubMed] [Google Scholar]
- 94.Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-kappab. PLoS One. 2012;7:e42507. doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson JL, Jenkins NP, Huang WC, Di Gregoli K, Sala-Newby GB, Scholtes VP, Moll FL, Pasterkamp G, Newby AC. Relationship of mmp-14 and timp-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediators Inflamm. 2014;2014:276457. doi: 10.1155/2014/276457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor irf5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watkins AA, Yasuda K, Wilson GE, et al. Irf5 deficiency ameliorates lupus but promotes atherosclerosis and metabolic dysfunction in a mouse model of lupus-associated atherosclerosis. J Immunol. 2015;194:1467–1479. doi: 10.4049/jimmunol.1402807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of t-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 101.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor nr4a1 (nur77) controls bone marrow differentiation and the survival of ly6c- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed rnai targeting ccr2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors nur77, nurr1, and nor-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential ly-6c expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 106.Sakai M, Miyazaki A, Hakamata H, Sasaki T, Yui S, Yamazaki M, Shichiri M, Horiuchi S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J Biol Chem. 1994;269:31430–31435. [PubMed] [Google Scholar]
- 107.Bain CC, Bravo-Blas A, Scott CL. Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Randolph GJ. Proliferating macrophages prevail in atherosclerosis. Nat Med. 2013;19:1094–1095. doi: 10.1038/nm.3316. [DOI] [PubMed] [Google Scholar]
- 109.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015;48:152–160. doi: 10.3109/08916934.2014.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of t cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.ATV.6.2.131. [DOI] [PubMed] [Google Scholar]
- 112.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 113.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci USA. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the ldlr-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 115.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. Ifn-gamma potentiates atherosclerosis in apoe knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Erbel C, Akhavanpoor M, Okuyucu D, et al. Il-17a influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193:4344–4355. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein e-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin ii-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng X, Taleb S, Wang J, et al. Inhibition of il-17a in atherosclerosis. Atherosclerosis. 2010;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 120.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17a deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 121.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of il-17a attenuates atherosclerotic lesion development in apoe-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 122.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L A critical function of th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 185:5820–5827 [DOI] [PMC free article] [PubMed]
- 123.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The il-17a/il-17ra axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Palma R, Del Galdo F, Abbate G, et al. Patients with acute coronary syndrome show oligoclonal t-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation. 2006;113:640–646. doi: 10.1161/CIRCULATIONAHA.105.537712. [DOI] [PubMed] [Google Scholar]
- 126.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal t cell expansions in atherosclerotic lesions of apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17. doi: 10.1161/01.ATV.20.1.10. [DOI] [PubMed] [Google Scholar]
- 127.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 128.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shah PK, Chyu KY, Dimayuga PC, Nilsson J. Vaccine for atherosclerosis. J Am Coll Cardiol. 2014;64:2779–2791. doi: 10.1016/j.jacc.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 131.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of cd4+ t cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 132.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory t cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 133.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory t cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory t cells. Arterioscler Thromb Vasc Biol. 2015;35:280–287. doi: 10.1161/ATVBAHA.114.303568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hori S, Nomura T, Sakaguchi S. Control of regulatory t cell development by the transcription factor foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 136.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.RES.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 137.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of tgf-beta signaling in t cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory t cell type 1 response reduces the development of atherosclerosis in apolipoprotein e-knockout mice. Circulation. 2003;108:1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 139.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 140.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A, Agrotis A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands cd4+ cd25+ foxp3+ regulatory t cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 142.Wigren M, Kolbus D, Duner P, Ljungcrantz I, Soderberg I, Bjorkbacka H, Fredrikson GN, Nilsson J. Evidence for a role of regulatory t cells in mediating the atheroprotective effect of apolipoprotein b peptide vaccine. J Intern Med. 2011;269:546–556. doi: 10.1111/j.1365-2796.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- 143.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory t-cell response to apolipoprotein b100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 144.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 145.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (t and b cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114:1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 146.Zernecke A. Dendritic cells in atherosclerosis: evidence in mice and humans. Arterioscler Thromb Vasc Biol. 2015;35:763–770. doi: 10.1161/ATVBAHA.114.303566. [DOI] [PubMed] [Google Scholar]
- 147.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bobryshev YV, Lord RS. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol. 1995;58:307–322. doi: 10.1679/aohc.58.307. [DOI] [PubMed] [Google Scholar]
- 149.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547. doi: 10.1016/j.it.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ley K. The second touch hypothesis: T cell activation, homing and polarization. F1000Research. 2014;3:37. doi: 10.12688/f1000research.3-37.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lievens D, Habets KL, Robertson AK, et al. Abrogated transforming growth factor beta receptor ii (tgfbetarii) signalling in dendritic cells promotes immune reactivity of t cells resulting in enhanced atherosclerosis. Eur Heart J. 2013;34:3717–3727. doi: 10.1093/eurheartj/ehs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 153.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein b-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 155.Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, van Berkel TJ, Toes RE, Kuiper J. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in ldl receptor-deficient mice. Cardiovasc Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]
- 156.Sage AP, Murphy D, Maffia P, et al. Mhc class ii-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic t cell immunity. Circulation. 2014;130:1363–1373. doi: 10.1161/CIRCULATIONAHA.114.011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 158.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin m type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 160.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of igg and igm autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 161.Hulthe J, Bokemark L, Fagerberg B. Antibodies to oxidized ldl in relation to intima-media thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol. 2001;21:101–107. doi: 10.1161/01.ATV.21.1.101. [DOI] [PubMed] [Google Scholar]
- 162.Dotevall A, Hulthe J, Rosengren A, Wiklund O, Wilhelmsen L. Autoantibodies against oxidized low-density lipoprotein and c-reactive protein are associated with diabetes and myocardial infarction in women. Clin Sci (Lond) 2001;101:523–531. doi: 10.1042/cs1010523. [DOI] [PubMed] [Google Scholar]
- 163.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between streptococcus pneumoniae and oxidized ldl. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 165.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin m is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, Chan LF, Lio WM, Cercek B, Shah PK, Chyu KY. Immune-modulation by polyclonal igm treatment reduces atherosclerosis in hypercholesterolemic apoe-/- mice. Atherosclerosis. 2012;220:59–65. doi: 10.1016/j.atherosclerosis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 167.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class b type i as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 168.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized ldl correlate with arterial accumulation and depletion of oxidized ldl in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.ATV.21.1.95. [DOI] [PubMed] [Google Scholar]
- 170.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain igg that recognizes epitopes of oxidized ldl arteriosclerosis and thrombosis: . J Vasc Biol Am Heart Assoc. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 171.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of igg and igm autoantibodies and immune complexes to oxidized ldl with markers of oxidation and inflammation and cardiovascular events: results from the epic-norfolk study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsimikas S, Miyanohara A, Hartvigsen K, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sjogren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A. High plasma concentrations of autoantibodies against native peptide 210 of apob-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J. 2008;29:2218–2226. doi: 10.1093/eurheartj/ehn336. [DOI] [PubMed] [Google Scholar]
- 174.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (ldl) receptor-deficient rabbits with homologous malondialdehyde-modified ldl reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of ldl receptor-deficient mice with homologous malondialdehyde-modified and native ldl reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.ATV.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 176.Zhu L, He Z, Wu F, Ding R, Jiang Q, Zhang J, Fan M, Wang X, Eva B, Jan N, Liang C, Wu Z. Immunization with advanced glycation end products modified low density lipoprotein inhibits atherosclerosis progression in diabetic apoe and ldlr null mice. Cardiovasc Diabetol. 2014;13:151. doi: 10.1186/s12933-014-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fredrikson GN, Bjorkbacka H, Soderberg I, Ljungcrantz I, Nilsson J. Treatment with apo b peptide vaccines inhibits atherosclerosis in human apo b-100 transgenic mice without inducing an increase in peptide-specific antibodies. J Intern Med. 2008;264:563–570. doi: 10.1111/j.1365-2796.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 178.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoe-null mice by immunization with apob-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 179.Honjo T, Chyu KY, Dimayuga PC, Yano J, Lio WM, Trinidad P, Zhao X, Zhou J, Chen S, Cercek B, Arditi M, Shah PK. Apob-100-related peptide vaccine protects against angiotensin ii-induced aortic aneurysm formation and rupture. J Am Coll Cardiol. 2015;65:546–556. doi: 10.1016/j.jacc.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 180.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK. Intranasal immunization with an apolipoprotein b-100 fusion protein induces antigen-specific regulatory t cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 181.Chyu KY, Zhao X, Dimayuga PC, Zhou J, Li X, Yano J, Lio WM, Chan LF, Kirzner J, Trinidad P, Cercek B, Shah PK. Cd8+ t cells mediate the athero-protective effect of immunization with an apob-100 peptide. PLoS ONE. 2012;7:e30780. doi: 10.1371/journal.pone.0030780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H, Ley K. Atheroprotective vaccination with mhc-ii restricted peptides from apob-100. Front Immunol. 2013;4:493. doi: 10.3389/fimmu.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, Vandaele M, Bleton J, Tchapla A, Kaveri SV, Rudling M, Nicoletti A. Atheroprotective effect of adjuvants in apolipoprotein e knockout mice. Atherosclerosis. 2006;184:330–341. doi: 10.1016/j.atherosclerosis.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 185.Gonen A, Hansen LF, Turner WW, et al. Atheroprotective immunization with malondialdehyde-modified ldl is hapten specific and dependent on advanced mda adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55:2137–2155. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Engelbertsen D, Rattik S, Knutsson A, Bjorkbacka H, Bengtsson E, Nilsson J. Induction of t helper 2 responses against human apolipoprotein b100 does not affect atherosclerosis in apoe−/− mice. Cardiovasc Res. 2014;103:304–312. doi: 10.1093/cvr/cvu131. [DOI] [PubMed] [Google Scholar]
- 187.Clement M, Guedj K, Andreata F, et al. Control of the t follicular helper-germinal center b-cell axis by cd8+ regulatory t cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131:560–570. doi: 10.1161/CIRCULATIONAHA.114.010988. [DOI] [PubMed] [Google Scholar]
- 188.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator b cells aggravate atherosclerosis by stimulating t helper-1 adaptive immunity. Circulation. 2014;129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. Baff receptor deficiency reduces the development of atherosclerosis in mice—brief report. Arterioscler Thromb Vasc Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 190.Zouggari Y, Ait-Oufella H, Bonnin P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 193.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 194.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the cardiovascular inflammation reduction trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(199–207):e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]