Abstract
Objective
To determine the combined effect of oxygen level and glucose concentration on cell viability, ATP production, and matrix synthesis of temporomandibular joint (TMJ) disc cells.
Design
TMJ disc cells were isolated from pigs aged 6-8 months and cultured in a monolayer. Cell cultures were preconditioned for 48 hours with 0, 1.5, 5, or 25mM glucose DMEM under 1%, 5%, 10%, or 21% O2 level, respectively. The cell viability was measured using the WST-1 assay. ATP production was determined using the Luciferin-Luciferase assay. Collagen and proteoglycan synthesis were determined by measuring the incorporation of [2, 3-3H]proline and [35S]sulfate into the cells, respectively.
Results
TMJ disc cell viability significantly decreased (P<0.0001) without glucose. With glucose present, decreased oxygen levels significantly increased viability (P<0.0001), while a decrease in glucose concentration significantly decreased viability (P<0.0001). With glucose present, decreasing oxygen levels significantly reduced ATP production (P<0.0001) and matrix synthesis (P<0.0001). A decreased glucose concentration significantly decreased collagen synthesis (P<0.0001). The interaction between glucose and oxygen was significant in regards to cell viability (P<0.0001), ATP production (P=0.00015), and collagen (P=0.0002) and proteoglycan synthesis (P<0.0001).
Conclusions
Although both glucose and oxygen are important, glucose is the limiting nutrient for TMJ disc cell survival. At low oxygen levels, the production of ATP, collagen, and proteoglycan are severely inhibited. These results suggest that steeper nutrient gradients may exist in the TMJ disc and it may be vulnerable to pathological events that impede nutrient supply.
Keywords: Temporomandibular joint (TMJ) disc, cell metabolism, oxygen level, glucose concentration, tissue nutrition
INTRODUCTION
The temporomandibular joint (TMJ) is a load-bearing joint, consisting of the condyle of the mandibular bone and the fossa eminence of the temporal bone, separated by a fibrocartilaginous disc. Temporomandibular joint disorders (TMJD) affect approximately 35 million people in the United States with tremendous morbidity and financial cost, yet its etiology remains poorly understood 1. In approximately 30% of TMJD patients, mechanical dysfunction of the TMJ disc, especially displacement due to tissue degeneration, is a common event 2. The mean age of onset of degenerative changes in the TMJ is between 18 and 44 years 3, which for unknown reasons is a decade earlier than in post-cranial joints 4. In contrast to other joints, attempts to surgically reconstruct the TMJ is often unsuccessful and may result in severe disabilities 5. Thus, research to understand the pathophysiology of TMJ disc degeneration for earlier diagnosis and management are essential.
The mechanical function of the TMJ disc is determined by the composition and structure of its extracellular matrix (ECM). The TMJ disc has a distinctive ECM composition when compared to hyaline cartilage and other fibrocartilaginous tissues [e.g., the intervertebral disc (IVD)]. The TMJ disc is comprised primarily of water with a significant amount of collagen type I and a small amount of proteoglycan 6, 7. The normal human TMJ disc is a large avascular structure 8, so the nutrients required by the disc cells for maintaining a healthy matrix are supplied by synovial fluid at the margins of the disc as well as through nearby blood vessels at the connection to the posterior bilaminar zone 9. The balance between the rate of nutrient transport through the matrix and the rate of consumption by disc cells establishes a concentration gradient across the TMJ disc. In articular cartilage, these gradients of essential nutrients can profoundly affect chondrocyte viability, energy metabolism, matrix synthesis, and the response to inflammatory factors 10-13. Studies have shown that oxygen and glucose play critical roles in the metabolism of chondrocytes and are essential for both adenosine triphosphate (ATP) production and matrix synthesis 14. In the IVD, cellular energy metabolism is dominated by anaerobic glycolysis, thus glucose levels play a significant role in ATP production and matrix protein synthesis 15, 16. A disrupted nutrient supply has long been implicated in the development of IVD disc degeneration, including cartilage end-plate calcification and a further decrease in oxygen and glucose levels. In TMJ disc cells, recent studies have shown that hypoxia with inflammation modulates the gene expression of tenascin-C and matrix metalloproteinases 17, 18. However, unlike the chondrocytes and IVD cells, the effect of essential nutrients (e.g., oxygen and glucose) on the energy metabolism and matrix synthesis of TMJ disc cells is still largely unknown.
Our recent studies have shown that solute diffusivities in the TMJ disc are much lower than the values in articular cartilage and the IVD 19-21, and compressive mechanical strain can further impede solute diffusion in the TMJ disc. Moreover, our cell metabolic studies have shown that the TMJ disc has a higher cell density and higher oxygen consumption rates compared to articular cartilage and the IVD 22. Therefore, it is likely that a steeper nutrient gradient may exist in TMJ discs and thus, it is more vulnerable to pathological events which impede nutrient supply, including sustained joint loading due to jaw clenching and bruxism. To understand the biological consequence of a limited nutrient supply, it is necessary to examine the impact of nutrient levels on TMJ disc cells.
The objective of this study was to examine the combined effect of oxygen level and glucose concentration on TMJ disc cell viability, energy metabolism, and matrix protein synthesis. Specifically, the cell viability, ATP production, and radioactive proline and sulfate incorporation (i.e., collagen and proteoglycan synthesis) were measured in porcine TMJ disc cells under defined oxygen levels and glucose concentrations.
MATERIALS AND METHODS
Cell isolation and culture
A total of nine porcine heads (American Yorkshire, male, aged 6-8 months) were collected from a local abattoir within 2 hours of slaughter (i.e., three porcine heads on three independent experimental days). Both left and right TMJs were removed en bloc with the capsule intact from each porcine head. The six TMJ discs were pooled together and harvested under sterile conditions and then digested overnight at 37°C with 0.1% (w/v) collagenase II (Worthington Biochemical Corp., Lakewood, NJ) in standard 25 mM glucose DMEM (HyClone) containing 10% fetal bovine serum (FBS) (Invitrogen). Digestions were strained through a 70μm filter, washed with PBS, and re-suspended in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin (Gibco Brl) and 25 μg/mL ascorbic acid. Isolated TMJ disc cells were plated at 1×104 cells/cm2 at 21% O2 and 5% CO2 at 37°C in 25 mM glucose DMEM. The media was changed every 2 days, and upon reaching confluence typically within 2 weeks, first-passage (P1) cells were detached with trypsin-EDTA (Invitrogen). Cells were re-plated at a 1:2 ratio and cultured in a monolayer to second passage (P2) for use in experiments. After cell viability was quantified by trypan blue exclusion (0.4% in buffered saline solution), the P2 cells were seeded at 1×104 cells into 96 wells. At 90% confluence, the culture medium was replaced by DMEM plus 10% FBS at 4 different glucose concentrations. These mediums were prepared by the supplementation of glucose-free DMEM with 0, 1.5, 5, or 25 mM glucose and the osmolality was measured within the range of 290 -310 mosmol (Vapro Vapor Pressure Osmometer, Elitech Group). The FBS (Invitrogen) was filtered by the manufacturer until glucose levels were < 5 mg/dL, which equates to approximately 0.27 mM glucose, therefore, the presence of glucose due to the presence of FBS in the testing medium is minimal. A 25 mM glucose concentration is normally adopted for in vitro cell culture, and the typical glucose concentration in plasma is 5 mM. Although the exact glucose environment has not been determined, it can be expected that the glucose concentration in TMJ disc tissue can range from 0 to 5 mM. Studies on intervertebral discs have shown that the oxygen level and glucose concentration can be as low as almost 0 inside the tissue 15, 16. For each glucose concentration, cells were further cultured under various oxygen levels (1%, 5%, 10%, and 21% O2) for 48 hours in a triple gas incubator in which N2 was used to reduce O2 levels 18.
WST-1 assay for examining metabolically active cell viability
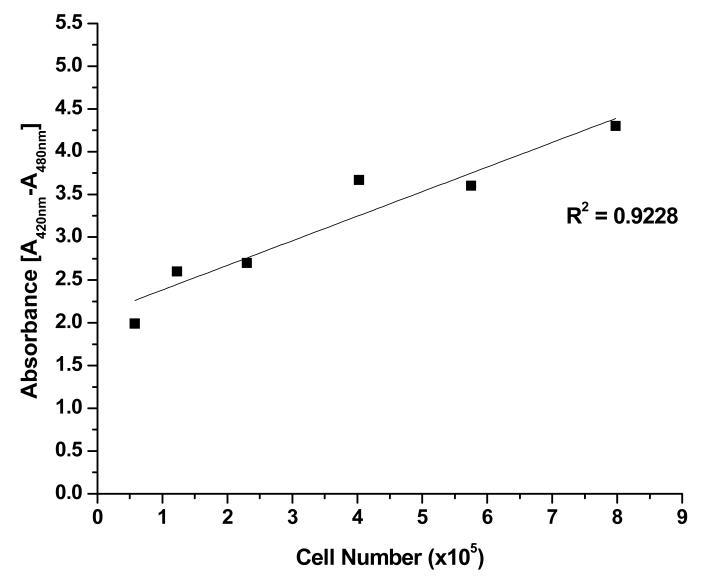
Cell viability of the preconditioned experimental groups was measured after 48 hours using the WST-1 kit (Roche Molecular Biochemicals, Mannheim, Germany). Water-soluble tetrazolium salt, 4-(3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1, 3-benzen disulfonate (WST-1), has been demonstrated to be a simple and rapid measurement of cell viability with extremely low cytotoxicity. A ten percent working solution was made by mixing one part volume of the cell viability reagent WST-1 with nine parts volume of media. Quantification of the formazan dye produced by metabolically active cells was done via a scanning multi-well spectrophotometer (420-480nm) 23. Absorbance values collected from cells cultured at 25 mM glucose and 21% oxygen level were considered the control measurement due to the initial in vitro expansion culture conditions. All other absorbance values from other cell culture conditions were normalized to the control. A standard curve was performed to show the relationship between different numbers of seeded porcine TMJ disc cells and absorbance.
ATP measurement
Levels of intra- and extracellular ATP of the preconditioned experimental groups after 48 hours were determined using the Luciferin-Luciferase kit (PerkinElmer, Wellesley, MA). At the end of an oxygenated or hypoxic incubation period, 100 μL of the cell suspension was mixed with 50 μL of a mammalian cell lysis solution (0.1M alkaline solution to inactivate endogenous ATPases and to stabilize the released ATP) in a 96-well microplate and mixed for 5 minutes. The mixture was then combined with 50 μL of the substrate (Luciferase/Luciferin) solution and mixed for an additional 5 minutes. The plate was allowed to dark-adapt for 10 minutes in the luminometer before luminescence counting was initiated. The total per viable cell based ATP production was then calculated by normalizing to the WST-1 absorbance values. 24 The total ATP production per viable cell was then normalized to the control (25 mM glucose and 21% oxygen level).
[2, 3-3H] Proline Incorporation Assay
Cellular synthesis of collagen was determined by measuring the incorporation of radioactivity (derived from [2, 3-3H] proline) into collagen. The TMJ disc cells were exposed to 20 μCi/mL (2, 3-3H) proline in 2 mL of medium for the final 24 hours of the total 48 hour incubation. The cell layer was washed three times with PBS and homogenized with a polyton in 0.2% Triton-X 100 and 50 mM Tris/HCl. The cell homogenate was digested with 0.02% collagenase and incubated for 4 hours at 37°C. After incubation, 10% TCA /0.5% tannic acid was added to each sample, centrifuged at 4,000 rpm at 4°C for 10 minutes and washed three times with 10% TCA/0.5% tannic acid. The precipitates were each solubilized in 50 mM Tris/HCl and the radioactivity was measured in a scintillation counter. The per viable cell based proline incorporation was calculated by normalizing to the WST-1 absorbance values. 25. The radioactive proline incorporation per viable cell (i.e. collagen synthesis per viable cell) was then normalized to the control (25 mM glucose and 21% oxygen level).
[35S] Sulfate Incorporation Assay
Cellular synthesis of proteoglycan was determined by measuring the incorporation of radioactive [35S]sulfate into the GAGs. The matrix-forming TMJ disc cells were exposed to 5.0 μCi/mL [35S]sulfate in 2 mL of medium for the final 4 hours of the total 48 hour incubation. The cell layer was washed three times with PBS and solubilized with 2 mg/mL of Pronase E in 5 mM CaCl2 and 0.2 M Tris/HCl at 56°C for 3 hours. The precipitates incorporating [35S]sulfate were collected on glass-fiber filters and washed three times with cetyl pyrinium chloride (CPC). The radioactivity of the cells precipitated with CPC was measured in a scintillation counter. The per viable cell based sulfate incorporation was calculated by normalizing to the WST-1 absorbance values. 25. The radioactive sulfate incorporation per viable cell (i.e. proteoglycan synthesis per viable cell) was then normalized to the control (25 mM glucose and 21% oxygen level).
Statistical analysis
The measurements of each outcome (i.e., cell viability, ATP production, collagen synthesis, and proteoglycan synthesis) were performed in triplicate and repeated in three independent experiments (n=9) under a defined combination of oxygen level and glucose concentration. The results were presented as the mean with 95% confidence intervals. One-way and two-way analysis of variance (ANOVA) and Tukey’s post hoc tests were performed to determine the singular and combined effect of oxygen level (1%, 5%, 10%, or 21%) and glucose concentration (0, 1.5, 5, or 25 mM) on TMJ cell viability, ATP production, collagen synthesis, and proteoglycan synthesis. SPSS 16.0 software (SPSS Inc., Chicago, IL) was used for examining all statistical analyses and significant differences were reported at P-values < 0.05.
RESULTS
Metabolically active cell viability
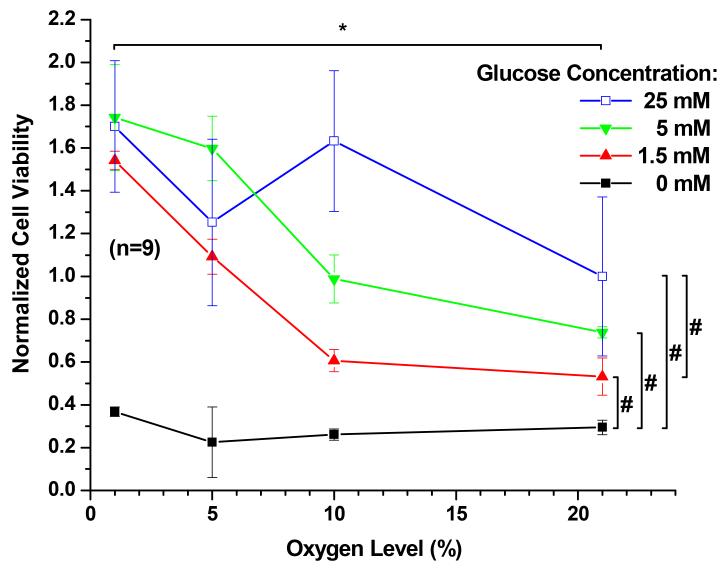
The relationship between plated porcine TMJ disc cell number and WST-1 reagent absorbance was determined to be linear, as shown in Figure 1. Glucose and oxygen concentrations had a significant impact on cellular viability (Figure 2). In the presence of glucose, an increase of oxygen level significantly decreased cell viability (P < 0.0001) with the average viability at 21% oxygen being approximately 2.1 times lower than that at 1% oxygen. In contrast, an increase of glucose concentration significantly increased TMJ disc cell viability (P < 0.0001), although the magnitude of impact is smaller compared to that of the oxygen level. The average viability in 25 mM glucose was about 1.5 times higher than that in 1.5 mM. In the absence of glucose, cell viability significantly dropped (P < 0.0001). Therefore, the following measurements of ATP production and radioactive [2, 3-3H] proline and [35S] sulfate incorporation were conducted in the presence of glucose at concentrations of 1.5 mM, 5 mM, and 25 mM. The interaction between oxygen and glucose was significant (P < 0.0001).
Figure 1.
WST-1 standard curve showing the linear relationship between plated porcine TMJ disc cell number and absorbance. R2 = 0.9228.
Figure 2.
The cell viability of porcine TMJ disc cells. The data shown were means and 95% confidence intervals. Experiments were performed in triplicate on three independent experimental days (n=9). Absorbance values were normalized to the control (25 mM glucose and 21% oxygen level). The effects of oxygen levels (1%, 5%, 10%, and 21%) and glucose concentrations (0, 1.5, 5, and 25 mM) were assessed. An increase of glucose concentration significantly increased TMJ disc cell viability (P < 0.0001). In the presence of glucose, an increase of oxygen level significantly decreased cell viability (P < 0.0001). The interaction between oxygen and glucose was significant (P < 0.0001). Significance (P < 0.05) of oxygen level effect and glucose level effect are denoted by a * and #, respectfully.
ATP measurement
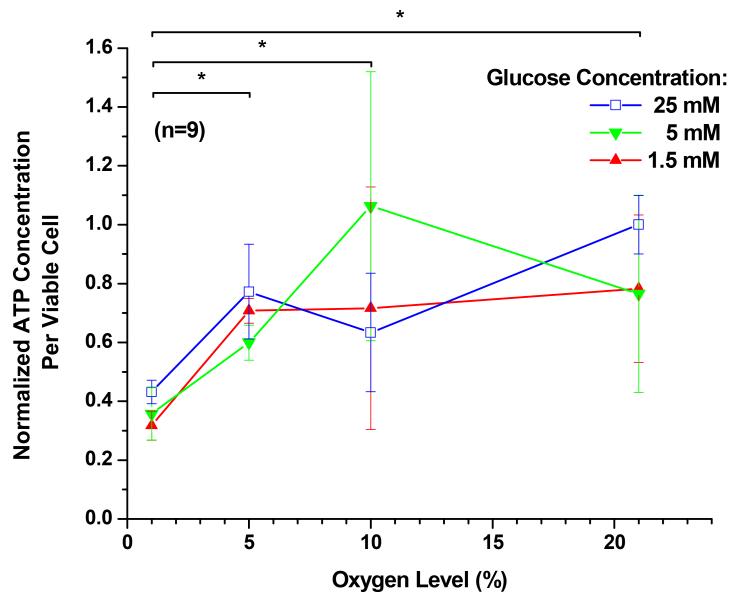
The effects of oxygen level and glucose concentration on intra- and extracellular ATP production are shown in Figure 3. In the presence of each of the glucose concentrations, an increase of oxygen level significantly increased the measurement of intra- and extracellular ATP (P < 0.0001). The average ATP measurement at 21% oxygen was about 2.3 times higher than that at 1% oxygen. However, an increase of glucose concentration from 1.5 mM to 25 mM had no significant impact on the average ATP measurement (P = 0.1388). The interaction between oxygen and glucose was significant (P = 0.00015).
Figure 3.
The measurement of ATP per viable cell of porcine TMJ disc cells. The data shown were means and 95% confidence intervals. Experiments were performed in triplicate on three independent experimental days (n=9). The total per viable cell based ATP production was calculated by normalizing to the WST-1 absorbance values. The total ATP production per viable cell was then normalized to the control (25 mM glucose and 21% oxygen level). The effects of oxygen levels (1%, 5%, 10%, and 21%) and glucose concentrations (1.5, 5, and 25 mM) were assessed. An increase of glucose concentration from 1.5 mM to 25 mM had no significant impact on the average ATP measurement (P = 0.1388). In the presence of each of the glucose concentrations, an increase of oxygen level significantly increased intra- and extracellular ATP production (P < 0.0001). The interaction between oxygen and glucose was significant (P = 0.00015). Significance (P < 0.05) of oxygen level effect is denoted by a *.
[2, 3-3H] Proline Incorporation
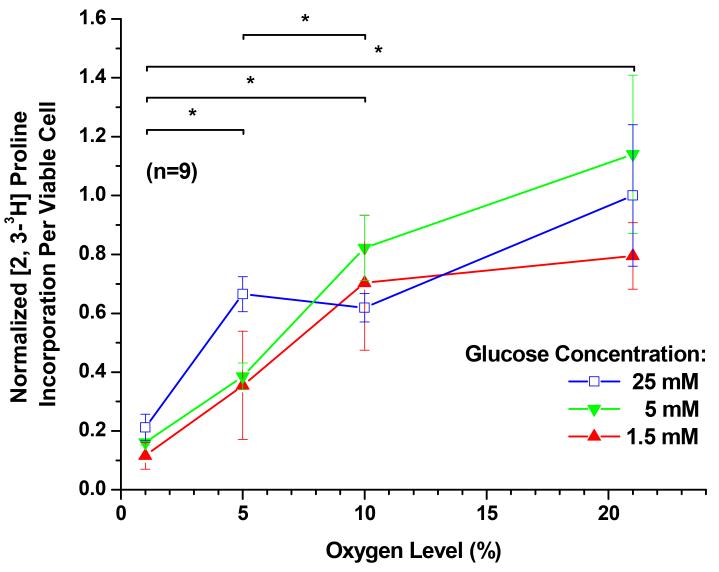
The effects of oxygen level and glucose concentration on radioactive proline incorporation, and thus collagen synthesis, are shown in Figure 4. In the presence of glucose, a reduced oxygen level resulted in a significant reduction of collagen synthesis within all glucose concentrations (P < 0.0001). The average collagen synthesis dropped 6 fold from 21% oxygen to 1% oxygen. Although the effect of glucose concentration on collagen synthesis was statistically significant (P < 0.0001), the magnitude of impact was much smaller compared to that of the oxygen level. At the 21% oxygen level, an increase of glucose concentration from 1.5 mM to 5 mM significantly increased average collagen synthesis by 27% (P = 0.033) and further increase of the glucose concentration had no significant effect (P = 0.187). The interaction between oxygen and glucose was significant (P = 0.0002).
Figure 4.
The [2, 3-3H] proline incorporation per viable cell (i.e. collagen synthesis per viable cell) of porcine TMJ disc cells. The data shown were means and 95% confidence intervals. Experiments were performed in triplicate on three independent experimental days (n=9). The per viable cell based proline incorporation was calculated by normalizing to the WST-1 absorbance values. The [2, 3-3H] proline incorporation per viable cell was then normalized to the control (25 mM glucose and 21% oxygen level). The effects of oxygen levels (1%, 5%, 10%, and 21%) and glucose concentrations (1.5, 5, and 25 mM) were assessed. Although the effect of glucose concentration on collagen synthesis was statistically significant (P < 0.0001), the magnitude of impact was much smaller compared to that of the oxygen level. At the 21% oxygen level, an increase in glucose concentration from 1.5 mM to 5 mM significantly increased collagen synthesis (P = 0.033), while further increase of the glucose concentration had no significant effect (P = 0.187). In the presence of glucose, a reduced oxygen level resulted in a significant reduction of collagen synthesis within all glucose concentrations (P < 0.0001). The interaction between oxygen and glucose was significant (P = 0.0002). Significance (P < 0.05) of oxygen level effect is denoted by a *.
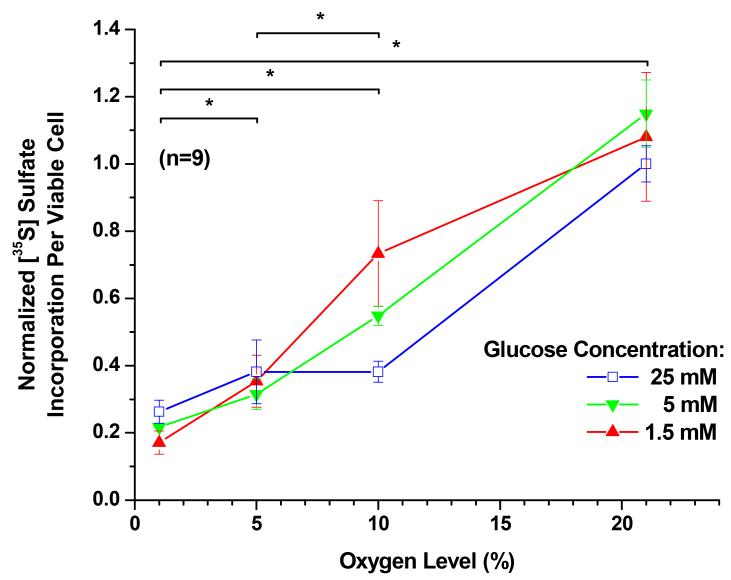
[35S] Sulfate Incorporation
The effects of oxygen level and glucose concentration on radioactive sulfate incorporation, and thus proteoglycan synthesis, are shown in Figure 5. In the presence of all glucose mediums, reduced oxygen level resulted in a significant reduction of proteoglycan synthesis (P < 0.0001). The average proteoglycan synthesis dropped 5 fold from 21% oxygen to 1% oxygen. However, an increase in glucose concentration from 1.5 mM to 25 mM, had no significant effect on average proteoglycan synthesis (P = 0.0821). The interaction between oxygen and glucose was significant (P < 0.0001).
Figure 5.
The [35S] sulfate incorporation per viable cell (i.e. proteoglycan synthesis per viable cell) of porcine TMJ disc cells. The data shown were means and 95% confidence intervals. Experiments were performed in triplicate on three independent experimental days (n=9). The per viable cell based sulfate incorporation was calculated by normalizing to the WST-1 absorbance values. The [35S] sulfate incorporation per viable cell was then normalized to the control (25 mM glucose and 21% oxygen level). The effects of oxygen levels (1%, 5%, 10%, and 21%) and glucose concentrations (1.5, 5, and 25 mM) were assessed. An increase in glucose concentration from 1.5 mM to 25 mM had no significant effect on average proteoglycan synthesis (P = 0.0821). In the presence of all glucose mediums, reduced oxygen level resulted in a significant reduction of proteoglycan synthesis (P < 0.0001). The interaction between oxygen and glucose was significant (P < 0.0001). Significance (P < 0.05) of oxygen level effect is denoted by a *.
DISCUSSION
The present findings show that different oxygen levels and glucose concentrations affect the viability of porcine TMJ disc cells. In the absence of glucose, cell viability significantly decreased. In the presence of glucose, decreased oxygen levels increased porcine TMJ disc cell viability. In agreement with other studies on cartilaginous tissues, specifically using bovine nucleus pulposus cells, the viability of the intervertebral disc cells began to decrease when glucose levels fell below 0.5 mM 15, 26. It thus appears that glucose may be the limiting nutrient for the survival of TMJ disc cells, rather than oxygen, as has previously been theoretically proposed for intervertebral disc cells 27. Articular cartilage chondrocytes have been shown to have higher proliferation rates at decreased oxygen levels. Proliferating chondrocytes are well adapted to hypoxic conditions within their avascular environment with glycolysis as the main energy source 28. Inside the chondrocyte, a molecule of glucose is converted into glucose 6-phosphate, which is the building block for the synthesis of N-acetylglucosamine, chondroitin sulphate, and hyaluronan 16 as well as maintaining cell viability. Due to its avascular nature, articular cartilage obtains its critical nutrients, including oxygen and glucose, from the surrounding synovial fluid by diffusion mechanisms 29. Articular cartilage consumes very little oxygen 30, 31, both because it has very few cells and because those cells utilize a predominantly glycolytic metabolism. Similar to articular cartilage chondrocytes, the porcine TMJ disc cells live in an avascular tissue environment and rely on diffusion mechanisms for nutrient supply. Accordingly, as shown in this study, TMJ disc cells appear to be able to survive in the extracellular matrix with limited nutrients and a low oxygen level. In regards to in vitro culture, isolated cells of the TMJ disc were previously observed to have an order of magnitude faster proliferation time than chondrocytes obtained from hyaline cartilage 28, suggesting TMJ disc cells are more active than chondrocytes in monolayer. Such differences may potentially be due to differences in cell type and maturity, as well as culture conditions.
In our present findings in porcine TMJ disc cells, a decrease in oxygen level resulted in a decrease in the measurement of ATP in all glucose concentrations. Similar oxygen dependent results have been observed in bovine articular cartilage chondrocytes, whereby, the rate of glycolysis falls as oxygen levels drop 12, 32, contributing to the fall in ATP and, hence, matrix synthesis. Both articular cartilage chondrocytes and IVD cells obtain their energy primarily through the Embden-Meyerhof-Parnas (EMP) pathway glycolysis, even in the presence of high oxygen 12, 16. The switch between the two forms of respiration, aerobic versus anaerobic, utilized by animal cells was first noted by Pasteur in the late 19th century 33, 34. In mammalian cells, glucose and glutamine are two major energy sources. Glucose is either converted anaerobically to lactate by glycolysis, yielding 2 moles of ATP per molecule of glucose, or aerobically to carbon dioxide and water by the tricarboxylic acid cycle (TCA), also known as oxidative phosphorylation, yielding 36 moles of ATP per molecule of glucose. Both articular cartilage chondrocytes and IVD cells, which utilize glycolytic metabolism in both high and low levels of oxygen, have extremely low oxygen consumption rates. In contrast, Kuo et al. reported a significantly higher oxygen consumption rate of porcine TMJ disc cells 22, compared to articular cartilage chondrocytes 27 and IVD cells 35, which in combination with our present findings, may be related to oxidative phosphorylation, and thus, a significant Pasteur effect for TMJ disc cells, but further investigation into the energy metabolism is necessary to confirm. Future studies to determine the effects of hypoxia and glucose concentrations in media on other key metabolites, such as glucose consumption and lactic acid production, in the TMJ disc cells may provide more information. Therefore, it appears that an aerobic to an anaerobic environmental shift would push porcine TMJ disc cells to utilize both glycolysis and oxidative phosphorylation pathways, respectively to maintain their metabolism.
Cell morphological studies have shown that the porcine TMJ disc contains an inhomogeneous distribution of a mixed cell population of fibroblast-like cells and chondrocyte-like cells 18, which are distinct from hyaline cartilage chondrocytes 36. The chondrocyte-like cells in the TMJ disc do not appear to exhibit the distinct pericellular capsule typical of articular cartilage chondrocytes 37. In addition, there are significant differences in organelle content between articular cartilage chondrocytes and chondrocyte-like cells in the TMJ disc, which likely suggests differences in cell behavior. The chondrocyte-like cells in TMJ discs have a greater number of mitochondria, both suggesting a higher metabolic activity than articular cartilage chondrocytes 36 and supporting our finding that ATP production may be higher in the TMJ disc cells.
In our present studies, a decrease in oxygen level resulted in a decrease in collagen and proteoglycan synthesis rates in all glucose concentrations, suggesting that the porcine TMJ cells begin to decrease cellular differentiation in the presence of low oxygen as long as sufficient glucose was present. Previous studies have investigated the effects of hypoxia on cellular metabolism in the IVD and articular cartilage and have found that it leads to a dramatic fall in the synthesis of the extracellular matrix 38, 39.
As matrix synthesis is closely coupled to intracellular ATP levels 40, 41, it thus appears that an increase in glycolysis under hypoxia is insufficient to prevent a fall in intracellular ATP concentrations and hence cannot prevent a fall in matrix synthesis. When no Pasteur effect was observed in bovine nucleus pulposus cells, the synthesis of sulfated GAGs was reduced 26 as oxygen level decreased. The Pasteur effect may even be harmful in a sense, whereby an increase in the rate of glycolysis under hypoxic conditions may lead to the depletion of an already limited supply of glucose in avascular tissues. If an absence of glucose does indeed cause cell death as indicated in this study, increasing the rate of glycolysis in cells may lead to more widespread cell death. Moreover, as a result of increased glycolysis, lactic acid production will increase in the hypoxic regions in the center of the TMJ disc. This fall in pH may cause a further decrease in cell viability, which will be detrimental to the TMJ disc.
In the avascular TMJ disc, the oxygen and glucose gradient may be steeper than that of articular cartilage, because of its higher cell density and rates of nutrient consumption 22 and lower solute diffusivities through the extracellular matrix 19-21. This suggests that TMJ disc cells are more susceptible to pathological changes which impede nutrient supply, such as sustained joint loading due to jaw clenching or bruxism. In the TMJ disc, it is unlikely that hypoxia occurs alone, being that local concentrations of oxygen, glucose, and lactic acid are determined by the balance between solute transport and cellular metabolism. Therefore, changes in the concentrations of these solutes cannot occur independently in vivo. In pathological conditions such as degenerate osteoarthritis, a further decrease in oxygen level may occur. Because of the role of hypoxia in modulating metabolic pathways, which in turn affects growth rates and the production of free ATP during hypoxia, these metabolic alterations will have important consequences for extracellular matrix turnover.
Possible limitations to this study include the loss of phenotype that in vitro inhomogeneous TMJ disc cells may experience when extracted from their ECM as well as during P1 and P2 culture. Previous literature has investigated the effect of high oxygen monolayer-expanded in vitro culture of articular chondrocytes and has found a cellular induction to an oxidative phenotype and metabolism 42. Therefore, it is necessary to investigate the effect of glucose and oxygen on cellular viability, proliferation, and metabolism in TMJ explants in a future study. The TMJ disc has been shown to have a significantly lower proteoglycan content 9,43 and thus, a much lower fixed charge density than articular cartilage. As a result, we expect the physiological osmolality of the TMJ disc to be around 300 mosmol. In regards to the osmolality of the medium with varying glucose concentrations, the range of measured osmolality from 290 - 310 mosmol does create a slight variation and thus, in future studies, it would be beneficial to examine this possible effect by utilizing osmotic balancing through the addition of mannitol. In addition, while the pig is the best experimental model of the TMJ after comparison to sheep, cows, dogs, cats, rabbits, rats, and goats, 44 it is necessary to investigate human TMJ discs in a future study.
In summary, we investigated the effects of glucose and oxygen on cell viability and cellular metabolism. Our results showed that in the absence of glucose, cell viability significantly decreased, suggesting that glucose may be the limiting nutrient for the survival of TMJ disc cells. In the presence of glucose, a decrease of oxygen level significantly increased cell viability. In contrast, an increase of glucose concentration significantly increased TMJ disc cell viability, although the magnitude of impact is smaller compared to that of the oxygen level. In addition, in the presence of glucose, a decrease of oxygen level significantly decreased intra- and extracellular ATP production and matrix synthesis. Our results suggest that TMJ disc cells utilize different metabolic mechanisms compared to other cartilage types, such as the IVD and articular cartilage. A possible reason is that the TMJ disc has multiple cell phenotypes that contribute to their cell behavior. The maintenance of oxygen and glucose homeostasis in the TMJ disc is essential for many vital cellular functions including viability and differentiation. Sustained mechanical loading on the TMJ disc will induce both oxygen and glucose concentrations to decrease towards the center of the TMJ disc. The results of this study therefore support the idea that a fall in nutrient supply due to pathological joint loading might be one pathway to disc degeneration. Future studies will determine the effects of hypoxia and glucose concentrations in media on other key metabolites, such as glucose consumption and lactic acid production, and energy metabolic pathways in the TMJ disc cells as well as explants in health and disease.
Acknowledgements
This project was supported by NIH grants DE021134, DE018741, and AR055775, a NSF Graduate Research Fellowship to SEC, a NIH F31 training grant DE020230 to JK, and a NIH F31 training grant DE023482 to GJW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
The authors made substantial contributions in designing the study (SEC, MJK, HY), gathering and analyzing the data (SEC, LZ, JK, GJW, YW, HY), and drafting the article (SEC, MJK, HY).
Conflict of Interest
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
REFERENCES
- 1.Stowell AW, Gatchel RJ, Wildenstein L. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. J Am Dent Assoc. 2007;138:202–208. doi: 10.14219/jada.archive.2007.0137. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14:T20–32. e21–23. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage. 2013;21:789–795. doi: 10.1016/j.joca.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashi A, Saha S, Christensen RW. Temporomandibular joint disorders: artificial joint replacements and future research needs. J Long Term Eff Med Implants. 2006;16:459–474. doi: 10.1615/jlongtermeffmedimplants.v16.i6.60. [DOI] [PubMed] [Google Scholar]
- 6.Berkovitz BK, Robertshaw H. Ultrastructural quantification of collagen in the articular disc of the temporomandibular joint of the rabbit. Arch Oral Biol. 1993;38:91–95. doi: 10.1016/0003-9969(93)90161-e. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Scott PG. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch Oral Biol. 1996;41:845–853. doi: 10.1016/s0003-9969(96)00040-4. [DOI] [PubMed] [Google Scholar]
- 8.Piette E. Anatomy of the human temporomandibular joint. An updated comprehensive review. Acta Stomatol Belg. 1993;90:103–127. [PubMed] [Google Scholar]
- 9.Detamore MS, Athanasiou KA. Structure and function of the temporomandibular joint disc: implications for tissue engineering. J Oral Maxillofac Surg. 2003;61:494–506. doi: 10.1053/joms.2003.50096. [DOI] [PubMed] [Google Scholar]
- 10.Cernanec J, Guilak F, Weinberg JB, Pisetsky DS, Fermor B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002;46:968–975. doi: 10.1002/art.10213. [DOI] [PubMed] [Google Scholar]
- 11.Grimshaw MJ, Mason RM. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthritis Cartilage. 2001;9:357–364. doi: 10.1053/joca.2000.0396. [DOI] [PubMed] [Google Scholar]
- 12.Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321(Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin G, Andriamanalijaona R, Grassel S, Dreier R, Mathy-Hartert M, Bogdanowicz P, et al. Effect of hypoxia and reoxygenation on gene expression and response to interleukin-1 in cultured articular chondrocytes. Arthritis Rheum. 2004;50:3549–3560. doi: 10.1002/art.20596. [DOI] [PubMed] [Google Scholar]
- 14.Heywood HK, Bader DL, Lee DA. Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. J Cell Physiol. 2006;206:402–410. doi: 10.1002/jcp.20491. [DOI] [PubMed] [Google Scholar]
- 15.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 17.Tojyo I, Yamaguchi A, Nitta T, Yoshida H, Fujita S, Yoshida T. Effect of hypoxia and interleukin-1beta on expression of tenascin-C in temporomandibular joint. Oral Dis. 2008;14:45–50. doi: 10.1111/j.1601-0825.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi A, Tojyo I, Yoshida H, Fujita S. Role of hypoxia and interleukin-1beta in gene expressions of matrix metalloproteinases in temporomandibular joint disc cells. Arch Oral Biol. 2005;50:81–87. doi: 10.1016/j.archoralbio.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Ann Biomed Eng. 2013;41:2349–2357. doi: 10.1007/s10439-013-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi C, Wright GJ, Ex-Lubeskie CL, Bradshaw AD, Yao H. Relationship between anisotropic diffusion properties and tissue morphology in porcine TMJ disc. Osteoarthritis Cartilage. 2013;21:625–633. doi: 10.1016/j.joca.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C, Kuo J, Bell PD, Yao H. Anisotropic solute diffusion tensor in porcine TMJ discs measured by FRAP with spatial Fourier analysis. Ann Biomed Eng. 2010;38:3398–3408. doi: 10.1007/s10439-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. Regional cell density distribution and oxygen consumption rates in porcine TMJ discs: an explant study. Osteoarthritis Cartilage. 2011;19:911–918. doi: 10.1016/j.joca.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varghese S, Theprungsirikul P, Sahani S, Hwang N, Yarema KJ, Elisseeff JH. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthritis Cartilage. 2007;15:59–68. doi: 10.1016/j.joca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Sato EF, Nishikawa M, Yamano Y, Inoue M. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis Rheum. 2001;44:96–104. doi: 10.1002/1529-0131(200101)44:1<96::AID-ANR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Ueki M, Tanaka N, Tanimoto K, Nishio C, Honda K, Lin YY, et al. The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng. 2008;36:793–800. doi: 10.1007/s10439-008-9462-7. [DOI] [PubMed] [Google Scholar]
- 26.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341–349. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine (Phila Pa 1976) 1991;16:444–449. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Rajpurohit R, Koch CJ, Tao Z, Teixeira CM, Shapiro IM. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol. 1996;168:424–432. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 31.Zscharnack M, Poesel C, Galle J, Bader A. Low Oxygen Expansion Improves Subsequent Chondrogenesis of Ovine Bone-Marrow-Derived Mesenchymal Stem Cells in Collagen Type I Hydrogel. Cells Tissues Organs. 2008 doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]
- 32.Gibson JS, Milner PI, White R, Fairfax TP, Wilkins RJ. Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch. 2008;455:563–573. doi: 10.1007/s00424-007-0310-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 34.Wernike E, Li Z, Alini M, Grad S. Effect of reduced oxygen tension and long-term mechanical stimulation on chondrocyte-polymer constructs. Cell Tissue Res. 2008;331:473–483. doi: 10.1007/s00441-007-0500-9. [DOI] [PubMed] [Google Scholar]
- 35.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–496. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 36.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, et al. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64:243–248. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkovitz BK, Pacy J. Ultrastructure of the human intra-articular disc of the temporomandibular joint. Eur J Orthod. 2002;24:151–158. doi: 10.1093/ejo/24.2.151. [DOI] [PubMed] [Google Scholar]
- 38.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 39.Hansen U, Schunke M, Domm C, Ioannidis N, Hassenpflug J, Gehrke T, et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941–949. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee RB, Wilkins RJ, Razaq S, Urban JP. The effect of mechanical stress on cartilage energy metabolism. Biorheology. 2002;39:133–143. [PubMed] [Google Scholar]
- 41.Baker MS, Feigan J, Lowther DA. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J Rheumatol. 1989;16:7–14. [PubMed] [Google Scholar]
- 42.Heywood HK, Lee DA. Low oxygen reduces the modulation to an oxidative phenotype in monolayer-expanded chondrocytes. J Cell Physiol. 2010;222:248–253. doi: 10.1002/jcp.21946. [DOI] [PubMed] [Google Scholar]
- 43.Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J Biomech. 2010;43:1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stembirek J, Kyllar M, Putnova I, Stehlik L, Buchtova M. The pig as an experimental model for clinical craniofacial research. Lab Anim. 2012;46:269–279. doi: 10.1258/la.2012.012062. [DOI] [PubMed] [Google Scholar]